Abstract
Progression and recurrence of breast cancer, as well as reduced survival of patients with breast cancer, are associated with chronic stress, a condition known to impact the hypothalamic-pituitary axis and the autonomic nervous system. Preclinical and clinical evidence support the involvement of the sympathetic nervous system in the control of bone remodeling and in pathologies of the skeleton, including bone metastasis. In experimental mouse models of skeletal metastasis, administration of the βAR agonist isoproterenol (ISO), used as a surrogate of norepinephrine, the main neurotransmitter of sympathetic neurons, was shown to favor bone colonization of metastatic breast cancer cells via an increase bone marrow vascularity. However, successful extravasation of cancer cells into a distant organ is known to be favored by an activated endothelium, itself stimulated by inflammatory signals. Based on the known association between high sympathetic outflow, the expression of inflammatory cytokines and bone metastasis, we thus asked whether βAR stimulation in osteoblasts may alter the vascular endothelium to favor cancer cell engraftment within the skeleton. To address this question, we used conditioned medium (CM) from PBS or ISO-treated bone marrow stromal cells (BMSCs) in adhesion assays with bone marrow endothelial cells (BMECs) or the endothelial cell line C166. We found that ISO treatment in differentiated BMSCs led to a robust induction of the pro-inflammatory cytokines interleukin-1 beta (IL-1β) and interleukin-6 (IL-6). The CM from ISO-treated BMSCs increased the expression of E- and P-selectin in BMECs and the adhesion of human MDA-MB-231 breast cancer cells to these cells in short-term static and dynamic adhesion assays, and a blocking antibody against IL-1β, but not IL-6, reduced this effect. Direct IL-1β treatment of BMECs had a similar effect, whereas the impact of IL-6 treatment on the expression of adhesion molecules by BMECs and on the adhesion of cancer cells to BMECs was negligible. Collectively, these in vitro results suggest that in the context of the multicellular and dynamic bone marrow environment, sympathetic activation and subsequent βAR stimulation in osteoblasts may profoundly remodel the density but also the activation status of bone marrow vessels to favor the skeletal engraftment of circulating breast cancer cells.
Keywords: Bone metastasis, Endothelial activation, Sympathetic nervous system, Adhesion molecules, Selectins, Interleukin-1beta
Highlights
-
•
β2AR activation in osteoblasts increases the expression of pro-inflammatory cytokines IL-1β and IL-6.
-
•
IL-1β promotes the adhesion of breast cancer cells to endothelium via an endothelial increase in E- and P-selectin expression.
-
•
IL-1β blockade and selectin inhibition inhibits breast cancer cell adhesion to endothelial cells.
1. Introduction
Breast cancer, the most common cancer diagnosed among women, frequently metastasizes to bone, causing osteolytic lesions, pain, fractures and poor life quality [1], [2], [3]. Current treatment options for breast cancer-associated skeletal-related events mainly target the action of osteoclasts with bisphosphonates [4], [5], but the 5-year survival rate associated with the use of this class of drugs is improved in postmenopausal women only [6]. In addition, the use of adjuvant denosumab in an intense dosing schedule did not improve bone metastasis-free survival in patients with early-stage breast cancer who were receiving optimal locoregional and standard-of-care systemic adjuvant therapy [7]. Hence, despite the overall success of treating primary breast cancer, new treatments options are needed to reduce recurrence and increase the survival of women with advanced breast cancer.
Metastatic cancer cells circulate throughout the body at very early stages of the disease and can lodge into distant organs in a dormant stage for years until unknown mechanisms trigger their re-entry into the cell cycle and growth. These early disease stages represent an opportunity for treatment [8], as disrupting the cross-talk between disseminated cancer cells and their microenvironment might prevent progression to macrometastases and recurrence. Cancer patients, including those with triple-negative breast cancer (TNBC), have increased survival metrics when their treatment regimen includes a β-blocker [9], [10], [11], [12]. β-blockers are a widely used drug for the treatment of high blood pressure, arrhythmia and anxiety, and work by blocking the communication between sympathetic nerves and target cells. Activation of the hypothalamic-pituitary axis (HPA) and of the sympathetic nervous system (SNS) are hallmarks of prolonged stress, and recent studies have shown that chronic stress exacerbates cancer progression in animal models of prostate [13], ovarian [14], [15] and breast cancers [16], [17], [18], as well as in some cancer patient and survivor cohort studies [19], [20], [21], [22], [23], [24]. Patients diagnosed with cancer experience significant psychological stress at the time of diagnosis and all along treatment [25], [26], [27], [28], and the threat of relapse is likely to contribute to long-term stress and decline in health-related quality of life [29], [30]. Prolonged stress is known to have deleterious effects on the physiology of multiple tissues, via the release of norepinephrine (NE) from nerve terminals, which then binds post-synaptic adrenergic receptors (βAR) on target cells, including bone-forming cells [31]. During normal bone remodeling in mice, osteoblasts respond to the synthetic βAR agonist isoproterenol (ISO) by an increase in RANKL and VEGF production, leading to increased osteoclastogenesis and vascular bone density in vivo, respectively [18], [32]. We have shown in the context of preclinical bone metastasis models that these SNS-induced changes in the bone marrow environment promote breast cancer bone colonization following intracardiac injection of MDA-MB-231 breast cancer cells, by favoring breast cancer cell migration (in a RANKL-dependent fashion) and by increasing the density of bone marrow blood vessel (in a VEGF-dependent fashion) [18], [32]. Independent studies have shown that chronic SNS activation promotes breast primary tumor growth and metastasis via changes in stromal homeostasis as well, which involved an influx of pro-inflammatory macrophages and an increase in primary tumor vasculature density [16], [33].
The vasculature is a crucial component for primary tumor growth, but also for the metastatic process in distant organs. Interactions with endothelial cells must occur in order for tumor cells to extravasate from the circulatory system and to establish at a secondary site [34], [35]. The bone is a highly vascularized tissue, and osteoblasts are known regulators of angiogenesis during skeletal development and bone regeneration [36], [37]. Our previous findings suggest SNS activation effectively increases bone marrow vessel density in adult mice [32]. The extravasation process of cancer cells is very similar to lymphocytes diapedesis after injury or infection [38], [39]. During the resulting inflammation, endothelial cells are activated by inflammatory cytokines to express adhesion molecules and synthesize chemokines that are presented on their luminal surface [40]. Similar to the leukocyte extravasation process, tumor cells are recruited to secondary sites by chemotactic gradients of cytokines and growth factors [41]. Inflammatory mediators such as interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide (LPS) for instance have been shown to favor cancer cell interactions with endothelial cells by altering the levels of adhesion proteins present on the endothelium [42]. The activated endothelium expresses inducible adhesion molecules such as E-selectin, P-selectin, ICAM1, VCAM1 and multiple β-integrins, all of which can facilitate arrest, docking, and extravasation of metastatic breast and prostate tumor cells into bone [43], [44], [45]. Selectins are members of the carbohydrate-binding proteins family. These molecules are involved in adhesive interactions between endothelium and leukocytes or platelets within the blood circulatory system. There are three members of the selectin family: P-, E-, and L-selectin. The expression of these adhesion molecules on the endothelium is temporally coordinated to ensure efficient inflammatory response [46]: whereas L-selectin mediates fast rolling of leukocytes on the endothelium, P- and E-selectins support rolling at lower velocities [40]. Selectins binds to various classes of molecules (mucins, sulfated glycolipids, glycosaminoglycans) and most of these molecules were shown to be functional selectin ligands in vivo [47]. Prostate [48], colorectal [49], pancreatic [50] and breast cancer cells [51] expressing high levels of ligands for selectins have been shown to exhibit more aggressive oncogenic properties in vitro, in vivo, and in patient samples.
In this study, we investigated the putative impact of sympathetic nerve activation on the adhesive properties of the activated bone endothelium for metastatic breast cancer cells, via in vitro assays designed to probe the communication and interaction between osteoblasts, endothelial cells and breast cancer cells.
2. Materials and methods
2.1. Cell lines
Human GFP+ MDA-MB-231 and murine GFP+ 4T1 mammary tumor cells were cultured with 10% FBS DMEM High Glucose (ThermoFisher, #1965118), BMSCs with 10% FBS α-MEM (Fisher scientific, #SH3026501), mouse C166 endothelial cells and BMECs with complete ECM (ScienCell, #1001) at 37 °C and 8% CO2.
2.2. Primary mouse bone marrow stromal cells
Hindlimbs from WT C57BL/6 mice were used to prepare primary mouse bone marrow stromal cells (BMSCs). Femur and tibia were stripped of skin and muscles, distal and proximal epiphyses were cut off, and each bone was inserted into a punctured 0.5 mL tube placed into a 1.5 mL tube. Tubes were centrifuged for 4 min at 4000 g. Resulting pellets were resuspended in complete α-MEM (Fisher Scientific, #SH3026501), and cells were plated at 1×106 cells/mL. Cultures were grown in 10% FBS α-MEM for 7 days and then switched to an osteogenic medium (10% α-MEM containing 50 µg/mL ascorbic acid [Sigma, #A5950] and 10 mM β-glycerophosphate [Sigma, #G9891-25 G]) for 7 more days.
2.3. Primary mouse bone marrow endothelial cells
Primary mouse bone marrow endothelial cells (BMECs) were harvested as described for BMSCs. Flushed cells were resuspended in complete ECM (ScienCell, #1001). Tissue culture dishes were coated for 20 min at 37 °C with 0.8 μg/cm2 fibronectin (Gibco, #33016015) then cells were plated at 3×106 cells/mL. Cultures were then grown in complete ECM for 7 days.
2.4. Gene expression assay
For all gene expression assays, total RNA was extracted from cells using TRIzol (Invitrogen, #15596-026). Following DNAse I treatment (ThermoFisher, #18068015), cDNA was generated using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, #4368813). Real-time PCR was performed using SYBR Green Supermix (Biorad, #1708884) gene expression assays on a Biorad CFX96 Real-Time System with appropriated primers (see Supplementary Table 1). Amplification specificity was verified by the presence of a single peak on the melting curve of the amplicon. Gene expression was analyzed by the ΔΔCt method.
2.5. Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, then blocked in 1% bovine serum albumin for 1 h at room temperature. Immunodetection of CD62E, CD31, and endomucin was performed using rat anti-CD62E (1:50, BD Pharmingen, #553749), mouse anti-CD31 (1:100, Abcam, ab24590), or rat anti-endomucin (1:100, Santa Cruz, sc65495) antibody at a 1:100 dilution overnight at 4 °C, and donkey anti-rat Alexa594 (Invitrogen, #A21209) or goat anti-mouse Alexa488 (Abcam, ab150113) secondary antibody at a 1:1,000 dilution for 1 h at room temperature. Nucleus staining was performed using 2 μg/mL Hoescht solution (Invitrogen, #H3569), and immunofluorescence was preserved in FluorSave (Millipore, #345789). Images were acquired with a Leica fluorescent microscope (Leica, Germany).
2.6. Adhesion assays
Endothelial cells (C166 or BMEC) were suspended in serum-free medium at 1 × 106 cells/mL and then incubated with 5 µL of Vybrant DiI per mL of cell suspension (Invitrogen, #V22889) for 20 min at 37 °C. The cells were washed of excess dye twice prior to plating.
2.6.1. Coverslip assay
Endothelial cells were grown to confluence in a 24-well plate on a fibronection-coated glass coverslip (VWR, #89167-106). Complete medium was then removed and replaced with growth factor-free medium in control wells or growth factor-free medium supplemented with rIL-1β (10 ng/mL) or BMSC-derived CM for 4 h. GFP+ tumor cells were resuspended in 10% DMEM High Glucose (Thermo Fisher, #1965118). 3 × 104 cancer cells/well were incubated for 3.5 h at 37 °C on the different endothelial cell monolayers. Non-adherent cells were washed with PBS (VWR, #45000-434), then cells fixed with 4% PFA and counted.
2.6.2. Gravity assay
Endothelial cells were grown to confluence on a 96-well clear bottom black plate. After 4 h of cytokine treatment, tumor cells (3×105 cells/mL) were incubated for 1 h on the endothelial cell monolayer, then the plate was turned upside-down. After 1.5 h, adherent tumor cells were fixed with 4% PFA and counted.
2.6.3. Flow chamber assay
Endothelial cells were grown to confluence in endothelial cell medium in a fibronectin-coated flow chamber (Ibidi, µ-Slide I Luer #80176). After incubation with rIL-1β or BMSC-derived CM for 6 h, cancer cells (5×105 cells/mL) were infused into a flow chamber at 1 dyn/cm2 shear stress for 5 min. The unbound cells were washed off for 5 min with DMEM then PBS, and fixed with 4% PFA prior to counting.
For all adhesion assays, pictures were taken using a fluorescence microscope (Leica, Germany) and analyzed by ImageJ by counting GFP + adherent tumor cells on 10 random fields per slide in a blinded fashion by two investigators. The Otsu's method, an automatic clustering-based image thresholding, was used as previously described [52], with slight modifications. Briefly, the GFP filter image was set in greyscale (8-bit), Otsu Threshold was used to highlight all of the individual tumor cells, then the Analyze Particles option was run (between 0.001 - 0.05 inches to ignore regions that were particularly small or large) to detect and measure all the foreground regions as individual objects. Particular care was taken to insure the endothelial cell layers were confluent in all wells/slides, and cell cultures were authenticated by the expression of marker genes (Supplementary Fig. 1). Results are expressed as fold change compared to either PBS or PBS-CM level set up at 1.
2.7. Statistics
All data are presented as means ± the standard error mean (SEM). For experiments comparing two groups a non-parametric Mann-Whitney was used and for experiments comparing more than two groups a Kruskal-Wallis test was used. For all analyses, a p-value ≤ 0.05 was considered significant.
3. Results
3.1. βAR stimulation in bone marrow stromal cells promotes the release of factor(s) that favor adhesion between tumor and endothelial cells
The extravasation of metastatic cancer cells in distant organs requires successive steps of adhesive interactions with endothelial cells, mediated by cell adhesion molecules expressed by the endothelium and cancer cells. Femurs of mice treated with the βAR agonist isoproterenol (ISO) are characterized by higher vascularity than PBS controls [32], thus increasing the likelihood of circulating metastatic tumor cells to arrest in the bone microenvironment. This effect is mediated by the β2AR in osteoblasts and is VEGF-dependent. However, whether ISO alters the adhesive properties of these new vessels in a way that promotes tumor cell extravasation and bone metastasis remains unknown. To address this question, we assessed if the conditioned medium (CM) from β2AR + bone marrow stromal cells (BMSCs) differentiated for 14 days in osteogenic medium, and treated with ISO for 24 h, promoted tumor cell interaction with endothelial cells. Two endothelial cell populations were used in this study: CD31 and endomucin-positive primary bone marrow-derived endothelial cells (BMECs, Supplementary Fig. S1A and S1B) were chosen based on their skeletal origin and direct relevance to the bone metastatic process, and the endothelial cell line C166, which represents a pure population of endothelial cells [53].
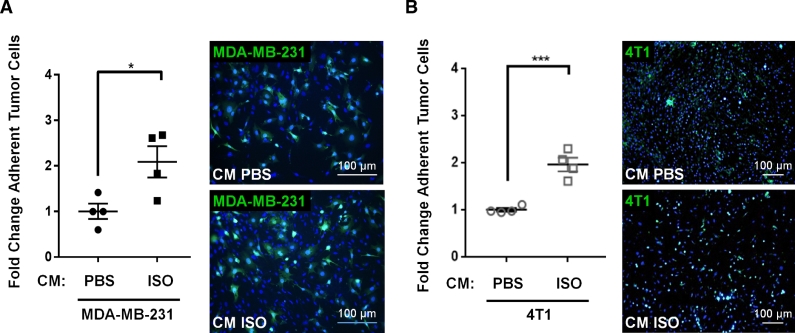
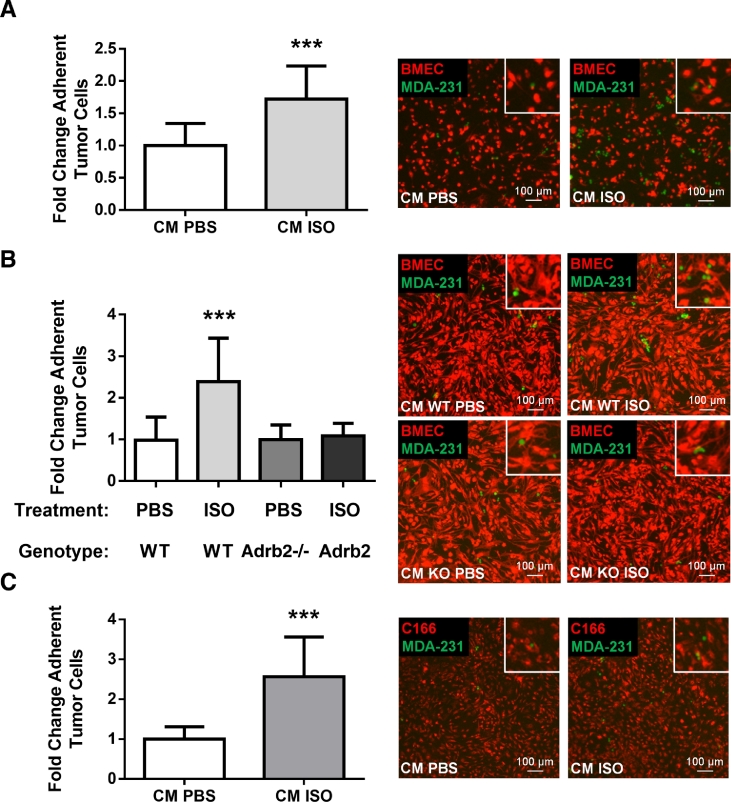
In a short-term (3.5 h) adhesion assay under static conditions, the CM from differentiated BMSCs treated with ISO doubled the number of human MDA-MB-231 breast cancer cells attached to a confluent monolayer of BMECs (Fig. 1A), compared to the CM from PBS-treated BMSCs. The same result was observed with mouse 4T1 mammary tumor cells (Fig. 1B), and when using MDA-MB-231 in a more stringent flow-resistance adhesion assay used to better represent physiological flow conditions (Fig. 2A and Supplementary Fig. S2A). Cancer cell adhesion nearly doubled in the CM from ISO-treated BMSCs compared to PBS control, and showed negligible adhesion in the flow chamber when BMECs were omitted (Supplementary Figs. S2B and S2C), thus confirm the existence of true cell-cell interactions stimulated by the CM from ISO treated BMSCs. In this dynamic flow assay, the CM from ISO-treated BMSCs lacking the β2AR did not stimulate the number of adherent MDA-MB-231 cancer cells on BMECs (Fig. 2B), thus demonstrating that the stimulatory effect of ISO, a β1/β2 non-selective βAR agonist, on tumor-endothelial cell adhesion, is mediated by the β2AR expressed in BMSCs, specifically. The use of the C166 mouse endothelial cell line in the flow assay to generate the endothelial layer recapitulated what was observed with the more heterogenous BMEC population (Fig. 2C). These results thus reveal that β2AR activation in osteoblasts stimulates the release of soluble factor(s) that favor the adhesion of tumor cells to bone marrow endothelial cells.
Fig. 1.
Confluent and adherent primary mouse bone marrow endothelial cells (BMECs) were treated for 24 h with the conditioned medium (CM) from vehicle or ISO-treated BMSCs prior to addition of GFP-positive (+ ) MDA-MB-231 (A) or GFP + 4T1 (B) cells. After 3.5 h, wells were washed in PBS and the relative adhesion of cancer cells to the endothelial monolayer was quantified by fluorescence-labeled cell count assay. Results are graphed as fold change relative to the CM of PBS-treated BMECs (n = 4 independent experiments, * = p < 0.05, *** = p < 0.001). A and B, right panel: Representative images of tumor cells attached to BMEC monolayers after treatment (blue: DAPI nuclear staining, green: GFP + tumor cells). Scale bar = 100 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
(A) DiI-labeled BMECs (red) were grown on flow chamber pre-coated with fibronectin (0.8 μg/cm2) until 100% confluent and were then stimulated with the CM from PBS or ISO-treated bone marrow stromal cells (BMSCs) for 6 h prior to perfusion of GFP + MDA-MB-231 cancer cells at 1 dyn/cm2 shear stress for 5 min. The number of adherent tumor cells was calculated following fluorescence-labeled cell count of ten observation fields. The results are graphed as fold change relative to CM PBS (n = 3 independent experiments, *** = p < 0.001). Representative images are shown for each condition. Scale bar = 100 µm. (B) The 24 h CM from WT or Adrb2-deficient BMSCs following treatment with either PBS or ISO was collected and used for BMECs adhesion assay as described in 2A (n = 3 independent experiments, *** = p < 0.001). (C) C166 endothelial cells were labeled with Vybrant DiI prior to injection in the flow chamber. Then, adhesion assay was performed as described in 2A (n = 3 independent experiments, *** = p < 0.001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. βAR stimulation in bone marrow stromal cells promotes the expression of adhesion molecules by BMECs
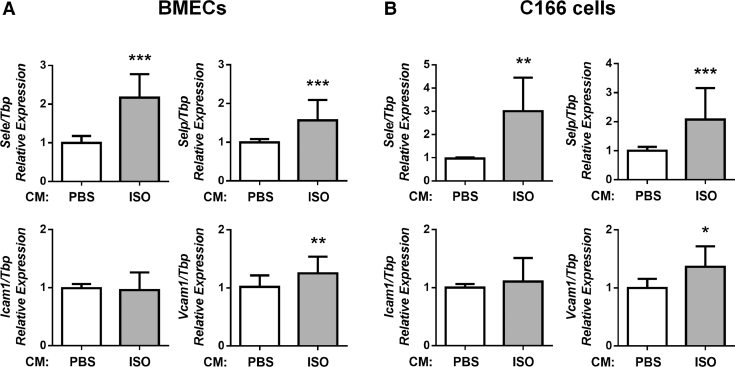
The increased adhesion of cancer cells to endothelial cells treated with the CM from ISO-treated BMSCs suggested that the latter caused the endothelium layer to switch from a basal to activated phenotype [54], [55]. To address whether a soluble factor from βAR-stimulated osteoblasts activates endothelial cells, we incubated BMECs in the 24hr-CM from PBS or ISO-treated BMSCs for 1 h and measured the expression of adhesion molecules typical of an activated endothelium [42], [56], [57]. In response to the CM from ISO-treated BMSCs, we were able to detect a significant increase in the expression of Sele, Selp and to a lesser extent Vcam1 compared to the PBS control, whereas Icam1 expression was unchanged (Fig. 3A). Very similar results were obtained with the C166 mouse endothelial cell line (Fig. 3B). βAR-stimulated BMSCs thus can trigger an activated phenotype in BMECs in vitro, which is associated with increased adhesive properties for breast cancer cells (Figs. 1 and 2).
Fig. 3.
BMECs (A) or C166 cells (B) were treated for 1 h with the CM from PBS or ISO-treated BMSCs. The CM from ISO-treated BMSCs induced a 2-fold increase of Sele and Selp expression compared to the CM from PBS-treated BMSCs, whereas Vcam1 level was increased only by 20% and Icam1 expression did not change significantly in both BMECs and C166 cells (qPCR, n = 3 independent experiments, * = p < 0.05, ** = p < 0.01, *** = p < 0.001).
3.3. ISO treatment increases IL-1β and IL-6 levels in BMSCs in a β2AR-dependant manner
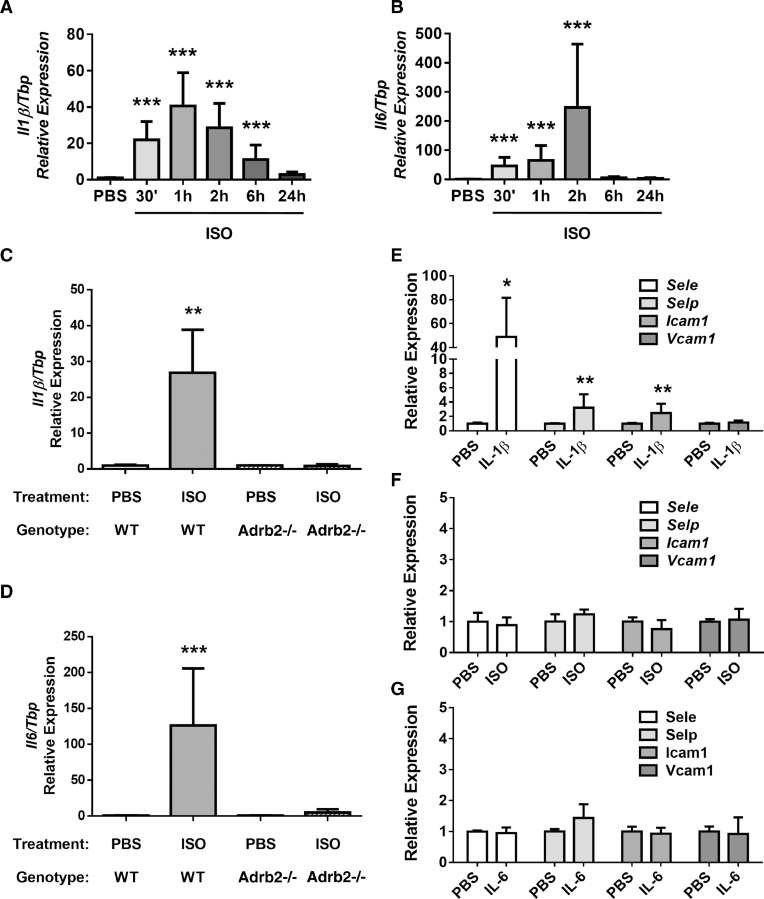
Pro-inflammatory cytokines can switch the endothelium into an activated state [42], [56], [57], and chronic stress is known to be associated with a pro-inflammatory environment in multiple tissues [58]. We thus focused our attention on pro-inflammatory cytokines expressed in osteoblasts in response to βAR stimulation. Upon ISO treatment, BMSCs displayed a rapid (1–2 h) and robust (40–200 times) induction of Il1β and Il6 expression (Fig. 4A and B), which was blunted in ISO-treated BMSCs prepared from mice lacking the β2AR (Fig. 4C and D). The expression of other inflammatory cytokines such as Il8 and Il10 was not increased in ISO-treated BMSCs, except for Mcp1, which showed a 2-fold induction (Supplementary Fig. S3).
Fig. 4.
ISO (10 µM) increased expression of the pro-inflammatory cytokines Il1β (A) and Il6 (B) in mouse BMSCs within 2 h (qPCR, n = 5 independent experiments, *** = p < 0.001). Il1β (C) and Il6 (D) expression was increased in WT BMSCs but not in BMSCs extracted from Adrb2-deficient mice following 2 h of ISO treatment (qPCR, n = 4 independent experiments, ** = p < 0.01, *** = p < 0.001). One hour treatment with murine rIL-1β (10 ng/mL, E) but neither ISO (10 µM, F) nor murine rIL-6 (50 ng/mL, G) increased expression of Sele, Selp, and Icam1 in BMECs (qPCR, n = 5 independent experiments, * = p < 0.05, ** = p < 0.01).
3.4. Recombinant IL-1β increases the expression of Sele and Selp in BMECs
The above results suggested that IL-1β and/or IL-6 secreted by osteoblasts following βAR stimulation activate bone marrow endothelial cells and increase their adhesive properties for breast cancer cells, via the induction of E- and P-selectin expression. However, endothelial cells are known to express β1ARs and β2ARs for their control of vasomotor function [59], [60]. We, therefore, sought to tease out the relative contribution of IL-1β, IL-6 and βARs stimulation to the increase in adhesion molecules observed in BMECs stimulated by the CM of ISO-treated osteoblasts. For that purpose, BMECs were directly treated with recombinant mouse IL-1β (10 ng/mL), recombinant mouse IL-6 (10 ng/mL) or ISO (10 µM) for 1 h and gene expression was measured. Treatment of BMECs with rIL-1β led to a robust increase in the expression of Sele, Selp, and Icam1, whereas ISO treatment had no significant effect (Fig. 4E and F and Supplementary Fig. S4A). Recombinant IL-6 had no effect on Sele and Selp expression across multiple time points (Fig. 4G and Supplementary Fig. S4B). Direct ISO treatment of BMECs, however, increased expression of Il6, thus revealing an autocrine activation loop in BMECs in response to βAR stimulation (Supplementary Fig. S4C).
To determine the functional contribution of IL-1β to the increased cancer cell adhesion on endothelial cells, we then tested the effect of this pro-inflammatory cytokine in adhesion assays under static and flow conditions. For these experiments, we used the C166 murine endothelial cell line which responded to rIL-1β by a robust expression of E-selectin surface expression (Fig. 5A). Under dynamic conditions in a flow chamber adhesion assay (Fig. 5B) and in two static adhesion assays (Fig. 5C and D), we observed a 2-3-fold increase in the number of MDA-MB-231 cancer cells attached to an endothelial monolayer of C166 cells following pre-treatment of the endothelial layer with rIL1β (10 ng/mL, 6 h). The increase in Sele, Selp and Icam1 expression induced by rIL-1β in endothelial cells is thus associated with a functional increase in their adhesive properties for cancer cells.
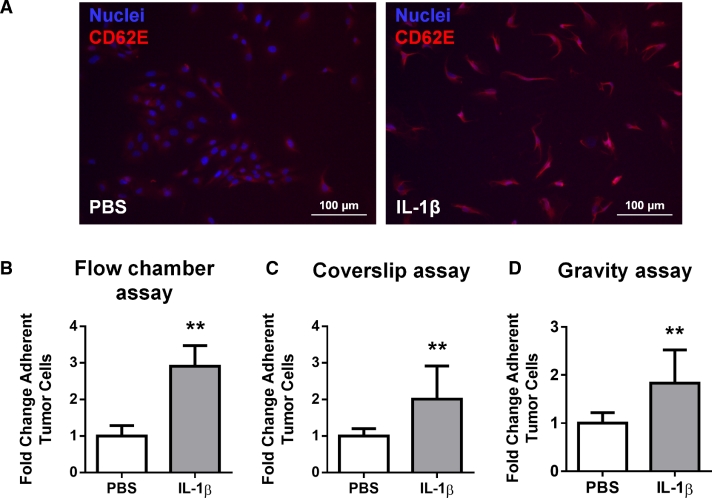
Fig. 5.
(A) C166 endothelial cells were treated for 6 h with rIL-1β (10 ng/mL) before immunofluorescence staining for E-selectin (blue: Hoechst nuclear staining, red: E-selectin/CD62E). Scale bar = 100 µm. (B–D) Murine rIL-1β (10 ng/mL) increased the adhesion of GFP + MDA-MB-231 cancer cells to a C166 monolayer 3-fold compared to vehicle-treated endothelial cells in flow conditions (flow chamber assay, B) and 2-fold in static conditions (in a coverslip assay, C and in a gravity assay, D). Quantification of adherent tumor cells was calculated by fluorescence-labeled cell count assay and graphed as fold change relative to PBS (n = 3 independent experiments, ** = p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. IL-1β mediates the increase in tumor cell adhesion to endothelial cells induced by the CM from ISO-treated BMSCs
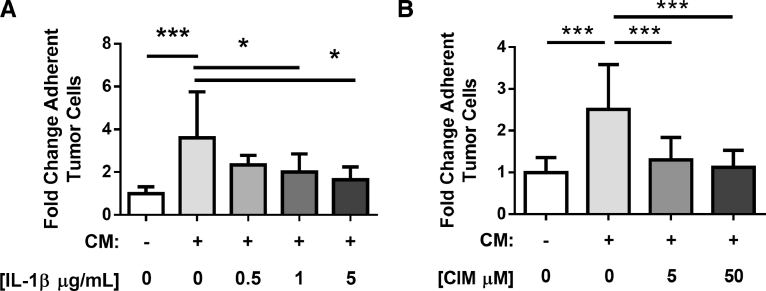
To determine if IL-1β in the CM from ISO-treated osteoblasts increases the activation status of C166 endothelial cells and the adhesion of MDA-MB-231 cancer cells to these cells, we used a loss-of-function strategy to block this cytokine or the increase in selectin expression in endothelial cells induced by exposure to the CM of ISO-treated BMSCs. In this experiment, confluent layers of C166 endothelial cells were pre-incubated in flow chambers with the CM from PBS or ISO-treated osteoblasts for 6 h, in the presence of an IL-1β neutralizing antibodies or an IgG control antibody or cimetidine, an inhibitor of selectin expression [61]. Then, MDA-MB-231 cancer cells were circulated in the flow chambers for 5 min. In this dynamic flow adhesion assay again, the CM from ISO-treated BMSCs (in the presence of an IgG antibody control) induced a 3–4 fold induction of MDA-MD-231-cell adhesion, but preincubation with an IL-1β-neutralizing antibody or cimetidine led to a significant reduction in the number of adherent MDA-MB-231 cells (Fig. 6A and B).
Fig. 6.
C166 cells were treated for 6 h with the CM of PBS (-) or ISO (+ ) treated BMSCs along with an IL-1β neutralizing antibody or an IgG antibody control (A) or cimetidine (B), prior to addition of GFP + MDA-MB-231 cells in the flow chamber (n = 3 independent experiments, * = p < 0.05, *** = p < 0.001). Number of adherent tumor cells was calculated by fluorescence-labeled cell count assay and graphed as fold change relative to untreated group.
4. Discussion
Successful dissemination of circulating cancer cells from primary epithelial tumors to distant organs requires arrest into the vasculature and extravasation. This step is dependent on the expression of adhesion ligands that allow cells to roll along the endothelium by interacting with corresponding binding partners present on blood vessels [62]. Cancer cells colonize the bone marrow environment through a distinct E-selectin and SDF1-positive sinusoidal vasculature [63], [64]. In this study, we used a series of in vitro assays to show that β2AR stimulation in osteoblasts, analogous to an increase in sympathetic outflow to bone in vivo, triggers the release of osteoblastic soluble factors that favor the adhesion between breast cancer cells and bone marrow-derived endothelial cells. We show that this effect involves IL-1β released by osteoblasts and the upregulation of E- and P-selectin expression by endothelial cells.
These findings are directly related to previous observations showing that osteoblastic βAR stimulation and chronic immobilization stress in adult mice augment bone vascularity and the likelihood of metastatic cancer cells to engraft in the skeleton [32]. Although a higher bone vascular density can indeed favor the likelihood of metastatic cancer cells engraftment in this organ, the process is likely to be much more efficient with an activated endothelium promoting capture, rolling, attachment and extravasation of these cells. E-selectin is an adhesion molecule that is thought to play a major role in this process. The observation that metastasis is redirected from lung to liver in mice overexpressing E-selectin in the liver best supports this notion that E-selectin on the activated endothelium can facilitate tumor cell adhesion, extravasation and seeding into distant organs [43]. In cancer patients, an elevated serum level of E-selectin is generally recognized as a biomarker of endothelial cell activation and is associated with clinical metastasis [65], and several studies support the therapeutic potential of inhibiting metastasis by targeting selectins [66], [67]. A pro-metastatic function of activated endothelial cells released in the blood circulation has also been observed before distant organ seeding, via chaperoning of circulating tumor cells and protection of cancer cells from anoikis [68]. Therefore, the fact that protection from anoikis and extravasation into distant organs are first, critical and endothelial-dependent steps for successful metastasis suggests that targeting endothelial-tumor cell interactions represents a valid potential therapeutic window for further investigation, especially in the context of chronic stress or inflammation. The observation that βAR stimulation by ISO promotes the colonization of the mouse skeleton by osteotropic MDA-MB-231 cells via a RANKL-mediated increase in cell migration [69] also suggests that high sympathetic outflow in bone might not only increase probability for cancer cell seeding and extravasation in the bone marrow environment, but also contributes to the migration and retention of these cells in this milieu.
IL-1β is a pro-inflammatory cytokine known as “gatekeeper” of inflammation. It is not expressed in normal conditions, hence its expression by primary breast tumors makes it a potential biomarker for breast cancer patients at increased risk for developing bone metastasis [70]. IL-1β expression in human breast primary tumors correlates with disease recurrence and skeletal metastasis, and increased expression is linked with the ability of tumor cells to home to the bone microenvironment in mouse models [70]. In previous studies, IL-1β was shown to increase adherence of tumor cells to endothelial cells in vitro, and administration of IL-1β to mice increased tumor growth and the number of melanoma cancer metastatic colonies in lung [71], [72] and in bone [73]. Importantly, reducing endogenous IL-1α or IL-1β activity in a mouse model of melanoma reduced both tumor burden as well as metastases [74]. Three recombinant protein-based drugs targeting specifically IL-1β signaling have been approved for clinical use (Anakinra, Rilonacept, and Canakinumab) and other related agents are under study. These drugs are mostly used for the management of rheumatoid arthritis or other inflammatory diseases [75], but multiple clinical trials are ongoing with them in patients with advanced malignancies. One especially is focusing on Anakinra versus Denosumab in combination with everolimus (mTOR Inhibitor) for the treatment of advanced or metastatic cancers that are refractory to standard therapy [76]. The in vitro data presented herein suggest that such drugs might be beneficial in the context of breast cancer metastasis and for reducing the pro-adhesive properties of the activated bone endothelium for circulating tumor cells, although this will need to be tested experimentally in preclinical models. In another study, the IL-1R1 antagonist Anakinra was shown to reduce the number of mice with bone metastases caused by osteotropic MDA-MB-231 cells, possibly through reduction in angiogenesis and tumor growth within the bone microenvironment [77]. Tumor cell dissemination in bone appeared not to be affected by the treatment, suggesting that the impact of osteoblast-derived IL-1β on endothelial cells described in our in vitro study might be most relevant to states of high sympathetic outflow/high βAR stimulation or inflammation, consistent with IL-1β being important mainly in diseased states.
Although expression of both IL-1β and IL-6 was robustly induced by βAR stimulation in BMSCs, only IL-1β significantly stimulated short-term endothelium activation and adhesive properties. Similar findings were reported by Giavazzi and al., who demonstrated that a single injection of human recombinant IL-1β, but not IL-6, before cancer cell inoculation induced an augmentation of experimental lung metastases of A375M melanoma tumor cells in mice [71]. The increase in osteoblast-derived IL-6 induced by ISO may thus not be relevant to endothelial cell activation in the bone marrow, but certainly has the potential to stimulate osteoclast differentiation [78], [79], [80], cancer cell survival, proliferation and metabolism [81], and eventually bone destruction at later stages of the metastatic process [82].
Evidence from preclinical studies and the study herein support a model whereby vascular bone density [32], the adhesive properties of the bone endothelium (this study) and the migration of breast cancer cells toward RANKL-secreting cells [83] are under the direct control of β2-adrenergic signaling in osteoblasts. An important implication of these findings is that βAR blockade represents a low cost and potentially safe therapeutic option in the context of a long-term preventative treatment to prevent relapse in women treated for their breast tumors. This is supported by the inhibitory effect of β-blockers on bone metastasis in mice subjected to chronic immobilization stress (aka high sympathetic and HPA activity) [69] and by the longer survival of women that happened to take β-blockers at time of diagnosis [12], [84], [85].
Acknowledgments
Acknowledgments
The authors thank Dr. Xiang Zhang for providing MDA-MB-231 GFP+ cells.
Funding
This work was supported by grants from the NCI #CA168717 (FE) and 5F31CA192748 (PM).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.09.002.
Appendix. Supplementary materials
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 2.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa L., Badia X., Chow E., Lipton A., Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 4.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Clément-Demange L., Clézardin P. Emerging therapies in bone metastasis. Curr. Opin. Pharmacol. 2015;22:79–86. doi: 10.1016/j.coph.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Hadji P., Coleman R.E., Wilson C., Powles T.J., Clézardin P., Aapro M., Costa L., Body J.-J., Markopoulos C., Santini D., Diel I., Di Leo A., Cameron D., Dodwell D., Smith I., Gnant M., Gray R., Harbeck N., Thurlimann B., Untch M., Cortes J., Martin M., Albert U.-S., Conte P.-F., Ejlertsen B., Bergh J., Kaufmann M., Holen I. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann. Oncol. 2016;27:379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 7.Study of Denosumab as Adjuvant Treatment for Women With High Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (D-CARE) - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT01077154 (accessed July 18, 2018).
- 8.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm. Sin. B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palesh O., Butler L.D., Koopman C., Giese-Davis J., Carlson R., Spiegel D. Stress history and breast cancer recurrence. J. Psychosom. Res. 2007;63:233–239. doi: 10.1016/j.jpsychores.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barron T.I., Sharp L., Visvanathan K. Beta-adrenergic blocking drugs in breast cancer: a perspective review. Ther. Adv. Med. Oncol. 2012;4:113–125. doi: 10.1177/1758834012439738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald P.J. Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin. Epidemiol. 2012;4:151–156. doi: 10.2147/CLEP.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E.N., Lee R.T., Meric-Bernstam F., Sood A.K., Conzen S.D., Hortobagyi G.N., Gonzalez-Angulo A.-M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan S., Karpova Y., Baiz D., Yancey D., Pullikuth A., Flores A., Register T., Cline J.M., D'Agostino R., Danial N., Datta S.R., Kulik G. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Invest. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaker P.H., Han L.Y., Kamat A.A., Arevalo J.M., Takahashi R., Lu C., Jennings N.B., Armaiz-Pena G., Bankson J.A., Ravoori M., Merritt W.M., Lin Y.G., Mangala L.S., Kim T.J., Coleman R.L., Landen C.N., Li Y., Felix E., Sanguino A.M., Newman R.A., Lloyd M., Gershenson D.M., Kundra V., Lopez-Berestein G., Lutgendorf S.K., Cole S.W., Sood A.K. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 15.Nagaraja A.S., Dorniak P.L., Sadaoui N.C., Kang Y., Lin T., Armaiz-Pena G., Wu S.Y., Rupaimoole R., Allen J.K., Gharpure K.M., Pradeep S., Zand B., Previs R.A., Hansen J.M., Ivan C., Rodriguez-Aguayo C., Yang P., Lopez-Berestein G., Lutgendorf S.K., Cole S.W., Sood A.K. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene. 2016;35:2390–2397. doi: 10.1038/onc.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M.G., Morizono K., Karanikolas B.D.W., Wu L., Sood A.K., Cole S.W. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creed S.J., Le C.P., Hassan M., Pon C.K., Albold S., Chan K.T., Berginski M.E., Huang Z., Bear J.E., Lane J.R., Halls M.L., Ferrari D., Nowell C.J., Sloan E.K. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. BCR. 2015;17:145. doi: 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell J.P., Karolak M.R., Ma Y., Perrien D.S., Masood-Campbell S.K., Penner N.L., Munoz S.A., Zijlstra A., Yang X., Sterling J.A., Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffner K.L., Loving T.J., Robles T.F., Kiecolt-Glaser J.K. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain. Behav. Immun. 2003;17(Suppl 1):S109–S111. doi: 10.1016/s0889-1591(02)00076-4. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel D., Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol. Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 21.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross K. Mapping pathways from stress to cancer progression. J. Natl. Cancer Inst. 2008;100:914–915. doi: 10.1093/jnci/djn229. 917. [DOI] [PubMed] [Google Scholar]
- 23.Chida Y., Hamer M., Wardle J., Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 24.Vahdaninia M., Omidvari S., Montazeri A. What do predict anxiety and depression in breast cancer patients? A follow-up study. Soc. Psychiatry Psychiatr. Epidemiol. 2010;45:355–361. doi: 10.1007/s00127-009-0068-7. [DOI] [PubMed] [Google Scholar]
- 25.Couper J.W., Love A.W., Duchesne G.M., Bloch S., Macvean M., Dunai J.V., Scealy M., Costello A., Kissane D.W. Predictors of psychosocial distress 12 months after diagnosis with early and advanced prostate cancer. Med. J. Aust. 2010;193:S58–S61. doi: 10.5694/j.1326-5377.2010.tb03930.x. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor M., Christensen S., Jensen A.B., Møller S., Zachariae R. How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br. J. Cancer. 2011;104:419–426. doi: 10.1038/sj.bjc.6606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vin-Raviv N., Hillyer G.C., Hershman D.L., Galea S., Leoce N., Bovbjerg D.H., Kushi L.H., Kroenke C., Lamerato L., Ambrosone C.B., Valdimorsdottir H., Jandorf L., Mandelblatt J.S., Tsai W.-Y., Neugut A.I. Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J. Natl. Cancer Inst. 2013;105:563–572. doi: 10.1093/jnci/djt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voigt V., Neufeld F., Kaste J., Bühner M., Sckopke P., Wuerstlein R., Hellerhoff K., Sztrókay-Gaul A., Braun M., von Koch F.E., Silva-Zürcher E., Hasmüller S., Bauerfeind I., Debus G., Herschbach P., Mahner S., Harbeck N., Hermelink K. Clinically assessed posttraumatic stress in patients with breast cancer during the first year after diagnosis in the prospective, longitudinal, controlled COGNICARES study. Psychooncology. 2017;26:74–80. doi: 10.1002/pon.4102. [DOI] [PubMed] [Google Scholar]
- 29.Trentham-Dietz A., Sprague B.L., Klein R., Klein B.E.K., Cruickshanks K.J., Fryback D.G., Hampton J.M. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res. Treat. 2008;109:379–387. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher J.R., Palta M., Loconte N.K., Trentham-Dietz A., Witt W.P., Heidrich S.M., Smith M.A. Characterizing the psychological distress response before and after a cancer diagnosis. J. Behav. Med. 2013;36:591–600. doi: 10.1007/s10865-012-9453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y., Nyman J.S., Tao H., Moss H.H., Yang X., Elefteriou F. β2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology. 2011;152:1412–1422. doi: 10.1210/en.2010-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulcrone P.L., Campbell J.P., Clément-Demange L., Anbinder A.L., Merkel A.R., Brekken R.A., Sterling J.A., Elefteriou F. Skeletal Colonization by Breast Cancer Cells Is Stimulated by an Osteoblast and β2AR-Dependent Neo-Angiogenic Switch. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017;32:1442–1454. doi: 10.1002/jbmr.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulsurkar M., Li Z., Zhang Y., Li X., Zheng D., Li W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene. 2017;36:1525–1536. doi: 10.1038/onc.2016.319. [DOI] [PubMed] [Google Scholar]
- 34.Massagué J., Obenauf A.C. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoletov K., Kato H., Zardouzian E., Kelber J., Yang J., Shattil S., Klemke R. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusumbe A.P., Ramasamy S.K., Itkin T., Mäe M.A., Langen U.H., Betsholtz C., Lapidot T., Adams R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–384. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madsen C.D., Sahai E. Cancer Dissemination—Lessons from Leukocytes. Dev. Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Strell C., Entschladen F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun. Signal. CCS. 2008;6:10. doi: 10.1186/1478-811X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 41.Geng Y., Chandrasekaran S., Hsu J.-W., Gidwani M., Hughes A.D., King M.R. Phenotypic switch in blood: effects of pro-inflammatory cytokines on breast cancer cell aggregation and adhesion. PloS One. 2013;8:e54959. doi: 10.1371/journal.pone.0054959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makó V., Czúcz J., Weiszhár Z., Herczenik E., Matkó J., Prohászka Z., Cervenak L. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytom. Part J. Int. Soc. Anal. Cytol. 2010;77:962–970. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 43.Biancone L., Araki M., Araki K., Vassalli P., Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J. Exp. Med. 1996;183:581–587. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto M.S., Serres S., Anthony D.C., Sibson N.R. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro-Oncol. 2014;16:540–551. doi: 10.1093/neuonc/not222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendas G., Borsig L. Cancer Cell Adhesion and Metastasis: Selectins, Integrins, and the Inhibitory Potential of Heparins. Int. J. Cell Biol. 2012 doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalili A.A., Ahmad M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015;16:18149–18184. doi: 10.3390/ijms160818149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kansas G.S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 48.Yasmin-Karim S., King M.R., Messing E.M., Lee Y.-F. E-selectin ligand-1 controls circulating prostate cancer cell rolling/adhesion and metastasis. Oncotarget. 2014;5:12097–12110. doi: 10.18632/oncotarget.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremblay P.-L., Auger F.A., Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene. 2006;25:6563–6573. doi: 10.1038/sj.onc.1209664. [DOI] [PubMed] [Google Scholar]
- 50.Dallas M.R., Chen S.-H., Streppel M.M., Sharma S., Maitra A., Konstantopoulos K. Sialofucosylated podocalyxin is a functional E- and l-selectin ligand expressed by metastatic pancreatic cancer cells. Am. J. Physiol. Cell Physiol. 2012;303:C616–C624. doi: 10.1152/ajpcell.00149.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zen K., Liu D.-Q., Guo Y.-L., Wang C., Shan J., Fang M., Zhang C.-Y., Liu Y. CD44v4 Is a Major E-Selectin Ligand that Mediates Breast Cancer Cell Transendothelial Migration. PLOS ONE. 2008;3:e1826. doi: 10.1371/journal.pone.0001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trabuco J.R.C., Martins S.A.M., Prazeres D.M.F. Use of ImageJ to Recover Information from Individual Cells in a G Protein-Coupled Receptor Assay. In: Prazeres D.M.F., Martins S.A.M., editors. G Protein-Coupled Recept. Screen. Assays Methods Protoc. Springer New York; New York, NY: 2015. pp. 143–172. [DOI] [PubMed] [Google Scholar]
- 53.Wang S.J., Greer P., Auerbach R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell. Dev. Biol. Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- 54.Augustin H.G., Kozian D.H., Johnson R.C. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. BioEssays News Rev. Mol. Cell. Dev. Biol. 1994;16:901–906. doi: 10.1002/bies.950161208. [DOI] [PubMed] [Google Scholar]
- 55.Unger R.E., Krump-Konvalinkova V., Peters K., Kirkpatrick C.J. In Vitro Expression of the Endothelial Phenotype: Comparative Study of Primary Isolated Cells and Cell Lines, Including the Novel Cell Line HPMEC-ST1.6R. Microvasc. Res. 2002;64:384–397. doi: 10.1006/mvre.2002.2434. [DOI] [PubMed] [Google Scholar]
- 56.Cook-Mills J.M., Deem T.L. Active participation of endothelial cells in inflammation. J. Leukoc. Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Alcaide P., Liu L., Sun J., He A., Luscinskas F.W., Shi G.-P. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y.-Z., Wang Y.-X., Jiang C.-L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017:11. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi D.S., Fitzgerald S.M., Pitts S., Cantor K., King E., Lee S.A., Huang S.-K., Krishnaswamy G. MAPK-dependent regulation of IL-1- and beta-adrenoreceptor-induced inflammatory cytokine production from mast cells: implications for the stress response. BMC Immunol. 2004;5:22. doi: 10.1186/1471-2172-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji Y., Chen S., Li K., Xiao X., Zheng S., Xu T. The role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div. 2013;8:1. doi: 10.1186/1747-1028-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi K., Matsumoto S., Morishima T., Kawabe T., Okamoto T. Cimetidine Inhibits Cancer Cell Adhesion to Endothelial Cells and Prevents Metastasis by Blocking E-selectin Expression. Cancer Res. 2000;60:3978–3984. [PubMed] [Google Scholar]
- 62.Li J., King M.R. Adhesion receptors as therapeutic targets for circulating tumor cells. Front. Oncol. 2012:2. doi: 10.3389/fonc.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sipkins D.A., Wei X., Wu J.W., Runnels J.M., Côté D., Means T.K., Luster A.D., Scadden D.T., Lin C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Price T.T., Burness M.L., Sivan A., Warner M.J., Cheng R., Lee C.H., Olivere L., Comatas K., Magnani J., Lyerly H.K., Cheng Q., McCall C.M., Sipkins D.A. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016;8:340ra73. doi: 10.1126/scitranslmed.aad4059. 340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A.P. Mann, T. Tanaka, E-selectin: its role in cancer and potential as a biomarker, Transl. Med. 0 (201AD) 1–4.
- 66.Pantziarka P., Bouche G., Meheus L., Sukhatme V., Sukhatme V.P. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014:8. doi: 10.3332/ecancer.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natoni A., Macauley M.S., O'Dwyer M.E. Targeting selectins and their ligands in cancer. Front. Oncol. 2016:6. doi: 10.3389/fonc.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yadav A., Kumar B., Yu J.-G., Old M., Teknos T.N., Kumar P. Tumor-associated endothelial cells promote tumor metastasis by chaperoning circulating tumor cells and protecting them from anoikis. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell J.P., Karolak M.R., Ma Y., Perrien D.S., Masood-Campbell S.K., Penner N.L., Munoz S.A., Zijlstra A., Yang X., Sterling J.A., Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nutter F., Holen I., Brown H.K., Cross S.S., Evans C.A., Walker M., Coleman R.E., Westbrook J.A., Selby P.J., Brown J.E., Ottewell P.D. Different molecular profiles are associated with breast cancer cell homing compared with colonisation of bone: evidence using a novel bone-seeking cell line. Endocr. Relat. Cancer. 2014;21:327–341. doi: 10.1530/ERC-13-0158. [DOI] [PubMed] [Google Scholar]
- 71.Giavazzi R., Garofalo A., Bani M.R., Abbate M., Ghezzi P., Boraschi D., Mantovani A., Dejana E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990;50:4771–4775. [PubMed] [Google Scholar]
- 72.Lauri D., Bertomeu M.C., Orr F.W., Bastida E., Sauder D., Buchanan M.R. Interleukin-1 increases tumor cell adhesion to endothelial cells through an RGD dependent mechanism: in vitro and in vivo studies. Clin. Exp. Metastasis. 1990;8:27–32. doi: 10.1007/BF00155590. [DOI] [PubMed] [Google Scholar]
- 73.Arguello F., Baggs R.B., Graves B.T., Harwell S.E., Cohen H.J., Frantz C.N. Effect of IL-1 on experimental bone/bone-marrow metastases. Int. J. Cancer. 1992;52:802–807. doi: 10.1002/ijc.2910520522. [DOI] [PubMed] [Google Scholar]
- 74.Voronov E., Shouval D.S., Krelin Y., Cagnano E., Benharroch D., Iwakura Y., Dinarello C.A., Apte R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dinarello C.A., Simon A., van der Meer J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anakinra or Denosumab and Everolimus in Advanced Cancer - Full Text View - ClinicalTrials.gov, (n.d.).https://clinicaltrials.gov/ct2/show/NCT01624766 (accessed July 24, 2018).
- 77.Holen I., Lefley D.V., Francis S.E., Rennicks S., Bradbury S., Coleman R.E., Ottewell P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7:75571–75584. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devlin R.D., Reddy D.V., Savino R., Ciliberto G., Roodman G.D. IL-6 Mediates the Effects of IL-1 or TNF, but Not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J. Bone Miner. Res. 1998;13:393–399. doi: 10.1359/jbmr.1998.13.3.393. [DOI] [PubMed] [Google Scholar]
- 79.Kudo O., Sabokbar A., Pocock A., Itonaga I., Fujikawa Y., Athanasou N.. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 80.Axmann R., Böhm C., Krönke G., Zwerina J., Smolen J., Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 81.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 82.Ara T., DeClerck Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer Oxf. Engl. 2010;1990(46):1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drell T.L., Joseph J., Lang K., Niggemann B., Zaenker K.S., Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 84.Barron T.I., Connolly R.M., Sharp L., Bennett K., Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 85.Powe D.G., Voss M.J., Zänker K.S., Habashy H.O., Green A.R., Ellis I.O., Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.