Abstract
Most members of the oomycete genus Phytophthora are primary plant pathogens. Both soil- and airborne Phytophthora species are able to survive adverse environmental conditions with enduring resting structures, mainly sexual oospores, vegetative chlamydospores and hyphal aggregations. Soilborne Phytophthora species infect fine roots and the bark of suberized roots and the collar region with motile biflagellate zoospores released from sporangia during wet soil conditions. Airborne Phytophthora species infect leaves, shoots, fruits and bark of branches and stems with caducous sporangia produced during humid conditions on infected plant tissues and dispersed by rain and wind splash. During the past six decades, the number of previously unknown Phytophthora declines and diebacks of natural and semi-natural forests and woodlands has increased exponentially, and the vast majority of them are driven by introduced invasive Phytophthora species. Nurseries in Europe, North America and Australia show high infestation rates with a wide range of mostly exotic Phytophthora species. Planting of infested nursery stock has proven to be the main pathway of Phytophthora species between and within continents. This review provides insights into the history, distribution, aetiology, symptomatology, dynamics and impact of the most important canker, decline and dieback diseases caused by soil- and airborne Phytophthora species in forests and natural ecosystems of Europe, Australia and the Americas.
Keywords: disease management, epidemic, forest dieback, invasive pathogens, nursery infestation, root rot
INTRODUCTION
The oomycete genus Phytophthora belongs to the Peronosporaceae, order Peronosporales, class Peronosporomycetes, kingdom Stramenipila (Dick 2001, Hulvey et al. 2010, Beakes et al. 2014, Thines & Choi 2016). Initially, based on phylogenetic analysis of ITS rDNA sequences from 50 Phytophthora species, the genus was structured in 10 phylogenetic clades (Cooke et al. 2000). Although increasing numbers of Phytophthora species and increasing numbers of nuclear and mitochondrial gene regions were used in subsequent phylogenetic studies, the basic structure of the genus remained unaltered (Martin & Tooley 2003, Kroon et al. 2004, 2012, Blair et al. 2008, Martin et al. 2014, Yang et al. 2017). However, recently the number of clades was expanded to 12 in order to accommodate the growing number of species in the P. quercina clade and the unique position of P. lilii (Rahman et al. 2015, Jung et al. 2017b). Several phylogenetic studies demonstrated that the genus Phytophthora is monophyletic with the 19 downy mildew genera residing within Phytophthora (Cooke et al. 2000, Kroon et al. 2004, Göker et al. 2007, Runge et al. 2011, Martin et al. 2014, Thines & Choi 2016). Recently, a closely related sister genus of Phytophthora, Nothophytophthora, which shares many phenotypic and ecological characters with Phytophthora, has been described from natural ecosystems in Asia, Europe and South America (Jung et al. 2017d).
Most Phytophthora (Greek for ‘plant destroyer’) species have a hemibiotrophic or necrotrophic lifestyle as primary plant pathogens, although for many aquatic Phytophthora species from phylogenetic Clades 6 and 9 a lifestyle as saprophytes and opportunistic necrotrophic pathogens seems likely (Erwin & Ribeiro 1996, Brasier et al. 2003, Jung et al. 2011, Nechwatal et al. 2013). In contrast, all c. 600 downy mildew species are host specific, obligate biotrophic plant pathogens (Göker et al. 2007, Runge et al. 2011, Beakes et al. 2012, Thines & Choi 2016). Phytophthora species are renowned as primary parasites on thousands of tree, shrub and crop species across the world. Depending on whether the lifecycle occurs mainly above- or below-ground a distinction is made between soilborne Phytophthora species causing fine root losses, root and collar rots and bleeding bark cankers, and airborne Phytophthora species causing leaf necrosis, shoot blights, fruit rots and also bleeding bark cankers (Erwin & Ribeiro 1996). However, several Phytophthora species have both a soil- and an airborne lifecycle. Phytophthora cactorum, for example, is causing root and collar rot in strawberries and a range of fruit and forest trees but can also infect the foliage and shoots of many ornamental plants and cause aerial bleeding cankers on European beech trees (Mircetich & Matheron 1983, Wilcox & Ellis 1989, Erwin & Ribeiro 1996, Jung 2009, Jung et al. 2016). In P. pseudosyringae the rate of sporangial caducity is highly variable between isolates enabling the pathogen to cause both fine root infections and aerial bleeding cankers on oaks, beech and other forest trees (Wickland et al. 2008, Jung et al. 2003b, 2013b, Jung 2009, Scanu & Webber 2016, Hansen et al. 2017).
It was estimated that, on a global scale, more than 66 % of all fine root diseases and more than 90 % of all collar rots of woody plants are caused by Phytophthora species (Tsao 1990). However, in many cases, abiotic factors or secondary pathogens instead of the primary Phytophthora pathogens are considered as the causal agents of disease. The reasons for such misidentifications are mainly based on the specific lifecycles of Phytophthora spp. Highly specific isolation methods are required in order to break dormancy of resting spores and exclude fungi and other oomycetes like Pythium or Phytopythium which are usually much faster growing than Phytophthora species (Ribeiro 1978, Tsao 1983, Erwin & Ribeiro 1996, Jung et al. 1996, De Cock et al. 2015). Phytophthora-specific isolation approaches include, amongst others, a wide range of specific baiting tests, Phytophthora-specific isolation media containing various antibiotics and fungicides, low or high incubation temperature depending on the Phytophthora species, leaching of polyphenols from necrotic bark samples, and drying and re-moistening of infested soil samples (Tsao 1983, 1990, Jeffers & Aldwinckle 1987, Erwin & Ribeiro 1996, Jung et al. 1996, 2013a, 2016, 2017c, Jung 2009). Another problem for Phytophthora isolations can be fluctuations of inoculum levels depending on the phase of the disease. When first symptoms become visible in the crown of a mature tree, the destruction of the fine root system is already in an advanced stage resulting in a continuous decrease of Phytophthora inoculum. As a consequence, a secondary disease process caused by high populations of secondary pathogenic and saprophytic fungi is masking the primary cause of the disease (Tsao 1990, Erwin & Ribeiro 1996, Jung et al. 1996). Similarly, isolation tests of Phytophthora species from bleeding bark lesions are only reliable at the active advancing lesion fronts whereas slightly older parts of the lesions are quickly colonised by secondary pathogenic fungi preventing the isolation of the primary Phytophthora pathogen (Erwin & Ribeiro 1996, Jung & Blaschke 2004, Jung 2009). In recent years, an array of highly sensitive, high-throughput, species-specific molecular detection methods have been developed, which facilitate the diagnosis of Phytophthora diseases significantly, and are particularly useful for routine screening of high numbers of samples for harmful and emerging Phytophthora pathogens (Schubert et al. 1999, Nechwatal et al. 2001, Schena et al. 2006, Martin et al. 2012, Scibetta et al. 2012, Sikora et al. 2012, Than et al. 2013, King et al. 2015, Schenck et al. 2016). Recent metagenomic approaches provide an efficient tool for large-scale surveys of Phytophthora diversity (Vettraino et al. 2012, Català et al. 2015, Sapkota & Nicolaisen 2015, Burgess et al. 2017).
Detailed descriptions and schematic illustrations of the lifecycles of airborne and soilborne Phytophthora species were given by several authors (Hickman 1958, Ribeiro 1978, Erwin & Ribeiro 1996, Jung 1998, Hansen et al. 2000, Agrios 2005, Grünwald et al. 2008). Both soil- and airborne Phytophthora species are able to survive unsuitable environmental conditions over several years with dormant resting structures (oospores, chlamydospores and hyphal aggregations) in the soil or in infected plant tissues. When environmental conditions become suitable (high moisture and temperature higher than the minimum temperature required by the respective Phytophthora species) the resting spores germinate by forming sporangia. In soilborne Phytophthora species, the sporangia release motile, biflagellate zoospores into the soil water which are then chemotactically attracted by a gradient of organic acids released from the elongation zone of young fine roots. In airborne species, the caducous sporangia are spread by wind and rain splash onto above-ground plant tissues where they either germinate directly or release zoospores. After penetrating the rhizodermis, exodermis or periderm of roots or the cuticle and epidermis of leaves, shoots and fruits Phytophthora grows as a hemibiotroph or necrotroph inter- and intracellular in the infected tissue with typical coralloid to irregular, non-septate hyphae. Nutrient depletion, competition by secondary antagonistic fungi or strong defence reactions by the host plant stimulate the production of resting structures. After decomposition of the necrotic tissue by saprophytes the resting spores are released into the soil, and the cycle starts again. Via the continuous production of sporangia on infected roots, leaves and fruits, Phytophthora pathogens can prolifically increase and disseminate their inoculum from initially very low levels during a relatively short time of favourable environmental conditions. Therefore, Phytophthora-induced fine root, leaf and fruit diseases are considered to be multicyclic (Ribeiro 1978, Erwin & Ribeiro 1996, Grünwald et al. 2008, Jung et al. 2013b). As a consequence of the root and bark damage caused by soilborne spp., the crowns of affected trees develop non-specific symptoms of drought and malnutrition, including increased crown transparency, sparse ramification and stunted growth of lateral shoots leading to whip-like branch structures and clustering of leaves at the end of branches, small-sized, often chlorotic foliage, wilting, dieback of branches, crown-dieback and eventually mortality (Erwin & Ribeiro 1996, Jung et al. 1996, 2000, 2013b, Jung 2009). However, in mature trees it can take decades of inoculum build-up and progressive destruction of the fine root system before the crowns begin to show visible symptoms (Ribeiro 1978, Tsao 1990, Erwin & Ribeiro 1996, Jung et al. 1996, 2000). Predisposing factors, such as waterlogging or planting of trees on sites not suitable for the species, as well as contributing factors which either reduce the vitality of the tree (e.g., extreme droughts or defoliations) or favour the pathogen (e.g., excess soil moisture following heavy rain, flooding or irrigation), can accelerate the disease process or make it possible in the first place (Davison 1988, Brasier et al. 1993, Marçais et al. 1993, Erwin & Ribeiro 1996, Jung et al. 1996, 2013b, Jung 2009). For European oak decline, a conceptual model was presented by Jönsson (2006) which included the complex interactions between soilborne Phytophthora species and various biotic and abiotic factors. Airborne Phytophthora diseases usually progress gradually upwards and in severe cases cause complete defoliation within a few months (Buddenhagen & Young 1957, Erwin & Ribeiro 1996, Durán et al. 2008). Extent and progress of soil- and airborne Phytophthora diseases are strongly depending on both long-term climatic and short-term weather conditions as demonstrated by the current declines and diebacks of oak and beech stands in Europe (Brasier et al. 1993, Brasier & Scott 1994, Jung et al. 1996, 2000, 2013b, Jung 2009, this review), the leaf fall disease of rubber in India and Malaysia (Agnihothrudu 1975, Erwin & Ribeiro 1996), the epidemics of P. ramorum on oaks and tanoaks in the western USA and on larch trees in the UK (Rizzo et al. 2002, 2005, Brasier et al. 2010, Grünwald et al. 2012a, Harris & Webber 2016, this review), and the needle cast and defoliation caused by P. pinifolia on Pinus radiata in Chile (Durán et al. 2008, this review).
In 1996, 50 Phytophthora species were known to science (Erwin & Ribeiro 1996). During the past two decades, more than 100 new Phytophthora species have been described or informally designated (Brasier 2009, Jung et al. 2011, 2017a, b, c, Hansen et al. 2012, Scanu et al. 2015, Yang et al. 2017, Burgess et al. 2018). This exponential increase has been caused by several factors:
1. more researchers have been studying Phytophthora diversity in natural ecosystems while research in the past had been focussed on agricultural crops. A conservative estimation predicted that there may be another 200–600 unknown Phytophthora species in natural ecosystems awaiting their detection (Brasier 2009);
2. phylogenetic analyses of nuclear and mitochondrial gene regions allow to discriminate complexes of morphologically similar but phylogenetically distinct species (Jung et al. 2003b, 2011, 2017b, c, Jung & Burgess 2009, Hong et al. 2009, 2011, Bezuidenhout et al. 2010, Rea et al. 2010, Ginetti et al. 2014, Henricot et al. 2014, Burgess et al. 2018);
3. the exponential increase in imports of living plants from overseas in combination with an outdated list-based plant biosecurity approach and notoriously understaffed plant protection services enables the continuous accidental introduction of exotic Phytophthora species (and other pests and pathogens) to Europe and other continents (Brasier 2008, Liebhold et al. 2012, Hantula et al. 2013, Santini et al. 2013, Eschen et al. 2015a, b, 2017, Jung et al. 2016); and
4. hybridisations between phylogenetically close Phytophthora species which due to geographic separation did not build up reproductive barriers and accidentally met after the introduction of one or both parents. Well-known examples of the latter are P. ×alni, P. ×cambivora, P. ×pelgrandis, P. ×serendipita and P. ×stagnum, all infecting woody plants, and undescribed hybrids in the complex of vegetable infecting Phytophthora species from Clade 8b (Brasier et al. 2004, Man in ‘t Veld et al. 2012, Bertier et al. 2013, Yang et al. 2014, Husson et al. 2015, Jung et al. 2017c).
A Europe-wide survey, conducted in almost 2 000 nursery stands of 730 nurseries and in 2 500 young forest, horticultural and ornamental plantings, demonstrated widespread Phytophthora infestations. More than 80 % of the nursery stands in more than 90 % of the tested nurseries, and 2/3 of the tested young plantings were infested with in total 68 different Phytophthora species, of which 44 were unknown to science before 1990 (Jung et al. 2016). Based on these results, a calculation suggested that across Europe between 1990 and 2010 approximately 680 000 new afforestations with a total area of almost 5 million hectares had been established with Phytophthora-infested nursery stock. In the same period, the area of potentially Phytophthora-infested re-forestations may have exceeded 17 million hectares (Jung et al. 2016). Apart from only a few potentially native Phytophthora species from Clades 3 and 6, the vast majority of these 68 Phytophthora taxa are considered alien invasive pathogens in Europe, based on an accumulating body of indirect evidence. These include high aggressiveness towards native European tree, crop and ornamental plant species, occurrence in healthy, undisturbed natural ecosystems in other continents, presence of both mating types of heterothallic Phytophthora species in natural ecosystems of other continents, low genetic variability of European Phytophthora populations, and close phylogenetic relatedness to non-native Phytophthora species (Jung et al. 2016). Also in Australia and the USA, Phytophthora infestations of nursery stock are common (Hardy & Sivasithamparam 1988, MacDonald et al. 1994, Davison et al. 2006, Schwingle et al. 2007, Yakabe et al. 2009, Bienapfl & Balci 2014, Parke et al. 2014, Yang et al. 2014, Simamora et al. 2015).
The potentially high number of unknown Phytophthora species and the unknown origin of many known aggressive Phytophthora species in combination with the high Phytophthora-infestation rates of nursery stock and the increasing complexity, intensity and volume of the international plant trade (Brasier 2008, Dehnen-Schmutz et al. 2010, Drew et al. 2010, Liebhold et al. 2012, Jung et al. 2016, Chapman et al. 2017, Eschen et al. 2017) pose a serious threat to the health and sustainability of natural ecosystems, managed forests and crop production systems on a global scale.
Before 1950, the only known forest diseases caused by Phytophthora pathogens were ink disease of chestnuts in Europe and the USA and littleleaf disease of pines in the USA (Crandall et al. 1945, Zak 1957, Tainter & Baker 1996). During the past six decades, the number of previously unknown Phytophthora declines and diebacks of forests and natural ecosystems and the number of described species and informally designated taxa of Phytophthora have increased exponentially (Fig. 1; Brasier 2009). Most of these diseases are driven by exotic Phytophthora species which remain unnoticed in their native environment and often were unknown to science prior to their introduction to other continents. However, in their new environments they became invasive and threatened a non-adapted flora which due to a lack of co-evolution contains a high number of susceptible plant species (Shearer & Tippett 1989, Marks & Smith 1991, Erwin & Ribeiro 1996, Jung et al. 2000, 2013b, 2016, Rizzo et al. 2002, Shearer et al. 2004, Brasier 2008, Grünwald et al. 2012a). This review provides insights into the history, distribution, aetiology, symptomatology, dynamics and impact of the most important canker, decline and dieback diseases caused by soil- and airborne Phytophthora species in forests and natural ecosystems of Europe, Australia and the Americas.
Fig. 1.
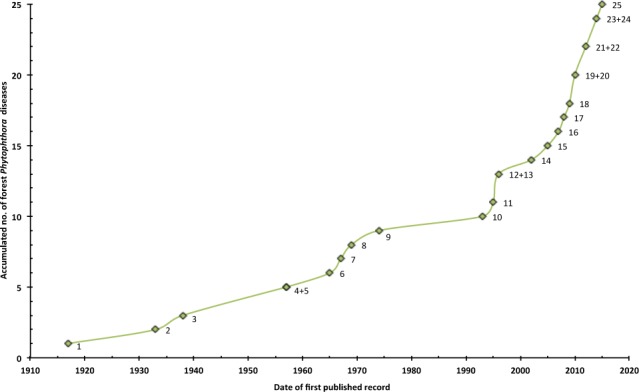
Accumulated number of important Phytophthora declines and diebacks of forests and natural ecosystems over time; 1 = ink disease of Castanea sativa in Europe (observation of first typical symptoms in 1838); 2 = ink disease of Castanea dentata in the USA (observation of first typical symptoms in 1824); 3 = decline of Fagus sylvatica in the UK; 4 = littleleaf disease of pines in the USA; 5 = decline and mortality of Chamaecyparis lawsoniana in the Pacific Northwest; 6 = jarrah dieback in Western Australia (WA; observation of first typical symptoms in the 1920s); 7 = ink disease of C. crenata and chestnut hybrids in Korea; 8 = eucalypt dieback in Victoria (observation of first typical symptoms in 1935); 9 = kauri dieback in New Zealand; 10 = Mediterranean oak decline; 11 = Alnus mortality in Europe; 12 = temperate European oak decline; 13 = decline of F. sylvatica in mainland Europe; 14 = Sudden Oak Death in California and Oregon; 15 = mortality of Austrocedrus chilensis in Argentina (observation of first typical symptoms in 1948); 16 = oak decline in the Eastern USA; 17 = needle cast and defoliation of Pinus radiata in Chile; 18 = dieback of Eucalyptus gomphocephala in WA; 19 = dieback of E. rudis in WA; 20 = Sudden Larch Death in the UK; 21 = dieback of Nothofagus spp. in the UK; 22 = mortality of Juniperus communis in the UK; 23 = red needle cast of P. radiata in New Zealand; 24 = leaf and twig blight of Ilex aqufolium in Corsica and Sardinia; 25 = dieback of Mediterranean maquis vegetation.
SOILBORNE PHYTOPHTHORA DISEASES IN FORESTS AND WOODLANDS
Ink disease of chestnuts worldwide
Ink disease, caused by Phytophthora species, is one of the most destructive diseases affecting Castanea sativa worldwide. In Europe, typical symptoms were first reported in Portugal in 1838 (Crandall et al. 1945, Crandall 1950) and since then it has become widespread across the continent with an increase in incidences during the last decades (Vannini & Vettraino 2001, Vettraino et al. 2005, Černý et al. 2008, Jung et al. 2013b, Tziros & Diamandis 2014). In the United States, ink disease was the main problem of C. dentata before the chestnut blight epidemic (Crandall et al. 1945, Crandall 1950). Ink disease symptoms in the crown are best observed during the vegetative growing season. The trees initially show small-sized and chlorotic foliage followed by increasing transparency, defoliation and dieback of the whole crown, eventually leading to extensive dieback and mortality of trees (Fig. 2a–d). Dry leaves and fruits often persist on dead trees over the winter. These symptoms are caused by extensive root losses and dark-brown and flame shaped necrosis in the inner bark developing from the main roots into the collar (Fig 2e–g). Blue to black exudates, oozing from the necrotic tissues through cracks in the bark, are often visible on the stem, collar and roots. The disease was named after the black exudates from necrotic roots staining the surrounding soil (Fig. 2e). Infected chestnut stumps can lose their ability to resprout due to the destruction of the entire root system. Ink disease also occurs on chestnut seedlings in nurseries and new plantations which usually show a rapid or gradual wilting (Jung et al. 2016).
Fig. 2.

Ink disease symptoms on Castanea sativa caused by Phytophthora spp. a. Extensive dieback and mortality caused by P. cinnamomi, P. ×cambivora and P. castanetorum in Portugal; b. patch dieback and mortality caused by P. ×cambivora in Portugal; c. chlorosis, microphylly, thinning, dieback and mortality of planted chestnut trees caused by P. cinnamomi in Chile; d. microphylly, severe thinning and dieback caused by P. cinnamomi and P. ×cambivora in Portugal; e. surface roots with bleeding lesions and black staining of surrounding soil caused by P. ×cambivora in Portugal; f, g. typical flame-shaped necrotic lesions of the inner bark caused by P. ×cambivora on the collar of a mature (f) and a young tree (g) in Italy. — Photos: a–e: T. Jung; f, g: B. Scanu.
The hybrid species P. ×cambivora has been the main species associated with ink disease in central and south-eastern Europe, while P. cinnamomi seems to be more widespread in Atlantic regions such as England and France and in the USA (Day 1938, Crandall et al. 1945, Crandall 1950, Vettraino et al. 2001, 2005, Martins et al. 2007, Černý et al. 2008, Jung et al. 2016). In Portugal, P. cinnamomi and P. ×cambivora often co-occur resulting in particularly high disease incidences and mortality rates (Fig. 1a, b) (Lopes-Pimentel 1946, 1947, T. Jung & M. Horta Jung unpubl. data). Due to its intolerance to low temperatures, the current distribution of P. cinnamomi in European forests is limited to areas with an average minimum soil temperature above 1.4 °C (Marçais et al. 2004, Vettraino et al. 2005). In accordance with the intensification of P. cinnamomi activity in Europe predicted by the CLIMEX model for increasing average temperatures (Brasier & Scott 1994, Burgess et al. 2017), P. cinnamomi is currently spreading in Italy to chestnut areas characterised by mild winters (Vettraino et al. 2005). Also in Chile, P. cinnamomi is currently threatening planted forests of C. sativa (Fig. 1c) (Jung et al. 2018). Several other Phytophthora species of minor impact were found associated with declining chestnuts in Europe, including P. cactorum, P. cryptogea, P. gonapodyides, P. megasperma, P. nicotianae, P. plurivora, P. pseudosyringae, P. sansomeana and P. syringae (Vettraino et al. 2005, Černý et al. 2008, Perlerou et al. 2010, Scanu et al. 2010, Jung et al. 2013b). The recently described P. castanetorum, a close relative of P. quercina, was isolated alongside other Phytophthora spp. from diseased chestnut trees in Italy and Portugal (Jung et al. 2017b). In Oregon, P. ×cambivora causes root rot, girdling basal stem cankers and mortality of golden chinquapin (Chrysolepis chrysophylla) which is closely related to the genus Castanea. The concentration of affected trees along roads suggests recent introduction and spread of the pathogen (Saavedra et al. 2007). In Japan and South Korea, P. castaneae (previously P. katsurae) was repeatedly found causing bleeding bark lesions and mortality of C. crenata and the chestnut hybrid C. crenata ×C. mollissima (Uchida 1967, Lee et al. 2009, Oh & Parke 2012). Phytophthora castaneae, together with P. cinnamomi, was also associated with bleeding bark lesions and mortality of Castanopsis carlesii in subtropical forests of Taiwan (Jung et al. 2017a).
Ink disease incidence is strictly related to climatic and site condition as well as human activities (Fonseca et al. 2004, Robin et al. 2006, Martins et al. 2007, Vannini et al. 2010). Heavy or continuous rain during the vegetative season, soil compaction and disturbance by tillage practices, physical restrictions to root expansion, poor soil fertility, vehicle movement along roads, and human recreational activities in forests are the main predisposing and contributing factors for disease development (Fonseca et al. 2004, Vannini et al. 2005, Martins et al. 2007). Planting of infested nursery stock and movement of contaminated substrates are the main pathways of short and long-distance inoculum dispersal (Jung et al. 2016). The existence of genetic variability in susceptibility to P. ×cambivora in C. sativa and resistance to P. cinnamomi in some clones of C. sativa and in many clones of the Euroasiatic chestnut hybrids C. crenata ×C. sativa and C. molissima ×C. sativa could provide the basis for a resistance screening programme necessary for a long-term management of ink disease in Europe (Robin et al. 2006, Miranda-Fontaíña et al. 2007, Costa et al. 2011, Santos et al. 2015, 2017a, b).
Oak declines and diebacks in Europe and North America
Episodically recurring declines of oak (Quercus spp.) stands have been reported since the early 1900s in both temperate and Mediterranean regions of Europe and in the USA (Staley 1965, Delatour 1983, Ragazzi et al. 1989, Schütt 1993, Gottschalk & Wargo 1996, Abrams 2003). In Europe, the current phase of oak decline started in the 1980s and is still ongoing (Delatour 1983, Brasier et al. 1993, Jung et al. 1996, 2000, 2013b, Vettraino et al. 2002, Balci & Halmschlager 2003a, b). A wide range of abiotic and biotic factors, including frost, drought, air pollutants, decreased groundwater levels, silvicultural mismanagement, insect defoliators, bark borers, fungal species like Ophiostoma and Ceratocystis spp., bacteria, mycoplasma-like organisms and viruses, were discussed as predisposing and inciting factors of this phenomenon (Manion 1981, Delatour 1983, Nihlgård 1985, Nienhaus 1987, Oleksyn & Przybyl 1987, Oosterbaan & Nabuurs 1991, Siwecki & Liese 1991, Ahrens & Seemüller 1994, Schlag 1995, Ragazzi et al. 1995, Thomas et al. 2002). However, none of these potential agents accounted for more than local or regional decline episodes. Due to similarities in aetiology between Mediterranean oak decline and eucalypt dieback in Western Australia (WA) caused by P. cinnamomi (Shearer & Tippett 1989, this review), and earlier records of P. cinnamomi from declining chestnuts and oaks in Portugal (Lopes-Pimentel 1946, 1947, this review), the possible involvement of P. cinnamomi in Iberian oak decline was suggested in 1991 and soon after confirmed (Brasier et al. 1993). Another study demonstrated the association of several known and previously unknown Phytophthora species with declining oak forests in central Europe and temperate and Mediterranean regions of Italy and Slovenia (Jung et al. 1996). Numerous surveys in oak stands throughout Europe uncovered a diverse assemblage of Phytophthora taxa including eight known species, P. cactorum, P. ×cambivora, P. cinnamomi, P. cryptogea, P. drechsleri, P. gonapodyides, P. megasperma, P. syringae; and 14 previously unknown Phytophthora taxa, P. bilorbang, P. chlamydospora, P. europaea, P. gallica, P. multivora, P. plurivora, P. pseudosyringae, P. psychrophila, P. quercina, P. ramorum, P. tyrrhenica, P. uliginosa, P. taxon forest soil and P. taxon river soil (Brasier et al. 1993, Brasier 1996, Jung et al. 1996, 1999, 2000, 2002, 2003b, 2013b, 2017b, Robin et al. 1998, Gallego et al. 1999, Hansen & Delatour 1999, Sanchez et al. 2002, Vettraino et al. 2002, Balci & Halmschlager 2003a, b, Jönsson et al. 2003b, 2005, Moreira & Martins 2005, Brown & Brasier 2007, Jung & Nechwatal 2008, Corcobado et al. 2010, Camilo-Alves et al. 2013, Scanu et al. 2013, T. Jung, M. Horta Jung & S.O. Cacciola unpubl. data). Several of these Phytophthora species, including P. ×cambivora, P. cinnamomi, P. cryptogea, P. drechsleri, P. multivora, P. plurivora and P. ramorum, are introduced invasive pathogens in Europe whereas most other species are considered to be native or of cryptic origin (Jung et al. 2016, 2017b). Recently, the presence of P. cinnamomi and other Phytophthora species has also been demonstrated in declining oak stands in Western Algeria (H. Smahi & B. Scanu unpubl. data). Against the background of almost ubiquitous infestations of oak stands in European nurseries with P. cinnamomi, P. quercina, P. plurivora and 13 other Phytophthora spp. (Jung et al. 2016), the massive afforestation during the previous three decades, stimulated by both national and EU subsidy programmes, may have contributed to the widespread Phytophthora infestations of oak woodlands across Europe.
In Mediterranean regions, the most affected species are Quercus suber and Q. ilex and, to a lesser extent, Q. cerris, Q. faginea, Q. pubescens and Q. pyrenaica (Brasier et al. 1993, Gallego et al. 1999, Sanchez et al. 2002, Vettraino et al. 2002, Pérez-Sierra et al. 2013, T. Jung & M. Horta Jung unpubl. data). In temperate regions, stands of Q. petraea and Q. robur usually show similar disease incidences. Generally, in oak decline a rapid death of previously healthy-looking trees and a slow chronic decline and dieback are distinguished although there are gradual transitions between both scenarios (Delatour 1983, Brasier et al. 1993, Jung et al. 1996, 2000, Gallego et al. 1999, Vettraino et al. 2002, Camilo-Alves et al. 2013). Rapid or acute death of oaks occurs mainly in Mediterranean regions and is usually caused by an interaction between excessive root losses and collar rot cankers by P. cinnamomi and severe summer droughts (Brasier et al. 1993, Gallego et al. 1999, Sanchez et al. 2002, Scanu et al. 2013, Jung et al. 2013b). Affected trees and whole stands of Q. suber and Q. ilex rapidly develop crown dieback and often collapse within the same year (Fig. 3a–c). Bark lesions at main roots and the collar may occur in severely infected oak trees, with black exudates oozing from the outer bark and necrotic lesions and staining of the underlying phloem and xylem tissues (Fig. 3e, f), often girdling the stem. Chronic decline and dieback are characterized by a progressive crown thinning, branch dieback, leaf chlorosis and abundant pro- liferation of epicormic shoots (Fig. 3d). These crown symptoms are caused by extensive losses of both fine roots and lateral small woody roots and callusing cankers on suberized roots (Fig. 3g–i). The ability of the root system to absorb and transport water and nutrients is increasingly hampered, eventually leading to a slow death of the trees (Jung et al. 1996, 2000, 2013b, Jönsson et al. 2005). However, the interaction with several abiotic stress factors, including prolonged droughts, waterlogging, fluctuating water tables, sandy or shallow soils and unseasonal heavy rain, and opportunistic pathogens and pests, can accelerate the disease progress and cause rapid wilting and mortality of trees (Brasier et al. 1993, Jung et al. 1996, 2000, 2003a, Vettraino et al. 2002, Balci & Halmschlager 2003a, b, Jönsson et al. 2003a, 2005, Jönsson 2006, Moreira & Martins 2005).
Fig. 3.

Oak decline symptoms caused by Phytophthora spp. a. Extensive dieback and mortality of Quercus suber trees caused by P. cinnamomi in Portugal; b. progressive dieback and wilting of a mature Q. suber caused by P. cinnamomi and P. quercina in Sardinia (Italy); c. sudden death of a Q. ilex due to P. cinnamomi in a savannah-like ecosystem in Spain; d. Q. robur trees in Germany showing chlorosis, thinning and dieback of crowns, abundant proliferation of epicormic shoots, and mortality due to severe fine root destructions caused by P. plurivora and P. quercina; e. bleeding collar lesion with flame-shaped staining of the underlying xylem caused by P. cinnamomi on a mature Q. suber in Sardinia; f. root and collar rot caused by P. cinnamomi on a young Q. suber in a forest plantation in Sardinia; g, h. small woody roots of a mature Q. suber in Italy showing severe losses of fine roots and lateral roots and black necrotic lesions due to P. cinnamomi infections; i. small woody root of a mature Q. robur in Germany with severe losses of lateral roots and a callusing bark canker caused by P. plurivora and P. quercina. — Photos: a–d, i: T. Jung; e–h: B. Scanu.
The distribution of Phytophthora species and their impact on oak trees depends on the site conditions, in particular soil drainage and pH. In a study of fine root systems in 35 forest stands in Germany, the oak-specific P. quercina and other Phytophthora spp. were frequently detected in the rhizosphere of mature Q. robur and Q. petraea on sites with a mean soil-pH higher than 3.5 and sandy-loamy to clayey soil texture (Jung et al. 2000). In infested forests, crown transparency and several fine root parameters were significantly correlated. Oak trees with P. quercina and other Phytophthora spp. in their rhizospheres had significantly higher losses of fine roots and of small woody roots, reduced crown density and a threefold higher decline risk than oaks without Phytophthora. In contrast, no Phytophthora species could be recovered from forests on well-drained sandy to sandy-loamy soils with a mean pH below 3.9, and root and crown conditions of oak trees were not correlated in these non-infested stands (Jung et al. 2000). Also in Austria, Italy, Turkey and Sweden, significant associations between presence of P. quercina and decline of oaks were demonstrated (Vettraino et al. 2002, Balci & Halmschlager 2003a, b, Jönsson et al. 2005).
In soil infestation tests, P. cinnamomi, P. quercina, P. ×cambivora, P. plurivora and P. uliginosa caused severe losses of fine roots and small woody roots, necrotic root lesions and mortality of Q. ilex, Q. petraea, Q. robur and Q. suber seedlings (Jung et al. 1996, 1999, 2002, 2003a, b, 2017c, Robin et al. 1998, Gallego et al. 1999, Sanchez et al. 2002, Jönsson et al. 2003a, Pérez-Sierra et al. 2013, Corcobado et al. 2017).
The primary role of P. cinnamomi in the decline of oak woodlands across Mediterranean countries, including Portugal, Spain and southern regions of France and Italy was demonstrated by numerous studies (Brasier et al. 1993, Brasier 1996, Robin et al. 1998, Gallego et al. 1999, Sanchez et al. 2002, Vettraino et al. 2002, Moreira & Martins 2005, Camilo-Alves et al. 2013, Jung et al. 2013b, Scanu et al. 2013). For many years, P. cinnamomi was considered the only Phytophthora species associated with Mediterranean oak decline (Camilo-Alves et al. 2013). However, the Phytophthora diversity in Mediterranean oak ecosystems is considerably higher than previously assumed. Several other Phytophthora species have recently been isolated from declining Mediterranean oaks, including P. gonapodyides from Q. ilex in Extremadura (Spain), P. psychrophila, P. quercina and P. syringae from Q. ilex and Q. faginea in two protected areas in Italy and eastern Spain, and the newly described species P. tyrrhenica from both Q. ilex and Q. suber in Sardinia and Sicily (Corcobado et al. 2010, Pérez-Sierra et al. 2013, Linaldeddu et al. 2014, Scanu et al. 2015, Jung et al. 2017b). Pathogenicity of all these Phytophthora species to the respective oak species was demonstrated, and their involvement in the declines suggested. In addition, in co-infection experiments P. cinnamomi, P. gonapodyides and P. quercina caused severe mortality of young Q. ilex seedlings which might explain the widespread lack of seedling recruitment in Mediterranean oak forests (Corcobado et al. 2017).
Several studies of Mediterranean oak decline have shown the interaction between P. cinnamomi and a combination of site factors, including the presence of shallow or compacted soils (Moreira & Martins 2005) and prolonged summer droughts (Brasier et al. 1993, Gallego et al. 1999). Mediterranean oak forests are delicate ecosystems, which are adversely impacted by human activities and global climate changes (Desprez-Loustau et al. 2010). The Mediterranean basin, which includes most of the natural range of Q. suber and Q. ilex, is considered one of the hot spots of future climate changes (Pachauri & Reisinger 2007). In this context, the predicted increase of P. cinnamomi activity with rising average temperatures (Brasier & Scott 1994, Brasier 1996, Burgess et al. 2017) could intensify root and collar rot incidences and further destabilise Mediterranean oak forests. Climate changes, in particular a rise of mean winter temperatures, a seasonal shift of precipitation from summer into wintertime, and a tendency towards heavy rain and prolonged droughts, have also been discussed as triggering factors for the current Phytophthora-related oak decline in temperate regions of Europe which, compared to previous oak decline episodes, is exceptional regarding its epidemic extent and long duration (Jung et al. 1996, 2000, 2003a, 2013b).
Also in the USA, episodes of oak decline with a symptomatology similar to European oak declines are occurring since decades (Staley 1965, Gottschalk & Wargo 1996, Abrams 2003). However, despite P. cinnamomi has been reported since the 1920s in North America, the involvement of Phytophthora species was not investigated conclusively (Balci et al. 2007). By the 1950s, P. cinnamomi was widespread in the north-eastern USA, but the significance of this species in tree health was only evaluated in connection with the demise of American chestnut (C. dentata). Today, in eastern US oak forests, P. cinnamomi is common, but due to its sensitivity to deep frost, its distribution is limited to below 40° northern latitude. In contrast, most other Phytophthora species are not restricted climatically but have a scattered distribution. The assemblage of Phytophthora species associated with oak stands includes P. cryptogea, P. europaea, P. quercetorum, P. pini, P. plurivora, P. ×cambivora and P. taxon ohioensis (Balci et al. 2007, 2008a, 2010, McConnell & Balci 2014b). Phytophthora cactorum and P. heveae occur only in southern oak forests (Meadows et al. 2011). All these species proved to be pathogenic to oaks in pathogenicity tests, but due to its widespread occurrence, P. cinnamomi has long been suspected as the main driver of oak decline in eastern US forests. Due to cold winter temperatures limiting canker development on stems, P. cinnamomi is mainly infecting the root system. Necrotic lesions on larger roots or bleeding stem cankers are only sporadically found on oaks in the southern USA, California and Mexico (Mircetich et al. 1977, Tainter et al. 2000, Wood & Tainter 2002, Balci et al. 2008b). Several recent studies investigated the role of P. cinnamomi in oak decline events in eastern US forests and provided insights into the epidemiology of the disease. Most significantly, both in the field and in pathogenicity tests P. cinnamomi-associated fine root losses were shown to be related to the propagule density of the pathogen. It was demonstrated that fine root losses are driving oak decline events in moist low-elevation stands where inoculum levels of the pathogen are higher and in areas like plant hardiness zones 6 and 7 where fine root regeneration is limited due to climatic constraints (McConnell & Balci 2014a, b).
Decline and mortality of Alnus species in Europe
In Europe, four Alnus species are indigenous. They are all characterised by having a symbiosis with the nitrogen-fixing actinomycete Frankia alni which is living in root nodules and enabling the trees to colonise extreme sites (Claessens 2003). The most widespread species is common alder (Alnus glutinosa) which, due to its ability to withstand permanently waterlogged conditions and prolonged flooding, typically colonises swamps and the banks and flood-plains along slow-flowing lowland streams. Grey alder (A. incana) is mainly distributed along fast-flowing white-water rivers and on dry rocky slopes, whereas the shrubby green alder (A. viridis) forms the subalpine tree line on heavy soils in the Alps and in the mountains of eastern Europe. The natural distribution of the Italian alder (A. cordata) is restricted to Corsica and southern Italy where it colonises moderately dry sites as a pioneer species. Alders have always been considered relatively non-problematic regarding their susceptibility to pests and pathogens. However, in 1993 a previously unknown lethal root and collar rot of A. glutinosa was recorded in southern Britain, which occurred mainly along riverbanks, but also in orchard shelterbelts and forest plantings (Brasier et al. 1995). In subsequent years, the disease was also found on A. incana and A. cordata, and in various countries including Austria, Belgium, Czech Republic, Croatia, Estonia, France, Germany, Hungary, Ireland, Italy, Lithuania, Norway, Poland, Portugal, Spain, Sweden, Switzerland and the Netherlands (Gibbs et al. 1999, 2003, Streito et al. 2002, Santini et al. 2003, Nagy et al. 2003, Jung & Blaschke 2004, Schumacher et al. 2006, Černŷ & Strnadová 2010, Solla et al. 2010, Jung et al. 2013b, 2016, Redondo et al. 2015, Trzewik et al. 2015, Kanoun-Boulé et al. 2016, M. Horta Jung & T. Jung unpubl. data). Affected trees show small-sized, sparse and often chlorotic foliage, a thinning and dieback of the crown, early and often excessive fructification, and eventually death (Fig. 4a, b). Young trees die within a few months while mature trees with large stem diameters take several years before they die (Gibbs et al. 1999, 2003, Streito et al. 2002, Jung & Blaschke 2004). Mortality rates can reach almost 100 % in permanently waterlogged swamps (Fig. 4a; Jung & Blaschke 2004), on sites with long retention of flood water such as oxbows and stretches behind bridges (Gibbs et al. 1999, Jung & Blaschke 2004) and in direct contact to slow-flowing stream water (Fig. 4b). A disease model, based on data from 35 rivers in north-eastern France, demonstrated that the disease incidence was increasing with decreasing distance of the stem base to the midwater line, with increasing river width, summer temperature of river water and clay content of the riverbank, as well as with slower water flow rates (Thoirain et al. 2007). Crown dieback and mortality are caused by root rot and collar rot lesions which can girdle the stem and extend up to 3 m from the stem base. Lesions are characterised by tarry or rusty exudate spots on the surface of the bark and flame-shaped orange-brown lesions of the inner bark (Fig. 4c–h). Along streams, waterborne inoculum, i.e. zoospores released from sporangia produced on infected alder tissues, is the main source of root and collar infections. Zoospores infect the collar region usually via the non-suberized adventitious roots and through the large lenticels (Gibbs et al. 2003, Jung & Blaschke 2004). During exceptionally high floods, infections can take place higher up the stem producing isolated aerial bark lesions (Fig. 4d). On non-flooded sites, above-ground bark lesions only develop when the pathogen progresses from infected main roots into the collar (Fig. 4f). If trees survive the first year after the infection occurred, lesions may progress in the following spring leaving the older parts sunken and surrounded by callusing tissues (Fig. 4f, i). Often the xylem underneath necrotic lesions shows a flame-shaped staining (Fig. 4j). Infected trees usually produce epicormic shoots in the vicinity of the bark lesions (Fig. 4g).
Fig. 4.

Symptoms of Phytophthora root and collar rot of Alnus spp. in Europe. a. Severe dieback and mortality of a mature A. glutinosa stand caused by P. ×alni in a seasonally flooded swamp forest in Bavaria, Germany; b. extensive dieback and mortality caused by P. ×alni in a riparian stand of A. glutinosa at Rio Alva in Portugal; c. bleeding bark lesion caused by P. ×alni on a surface root of mature riparian A. glutinosa in Portugal; d. bleeding aerial bark lesion on a riparian A. glutinosa at Rio Miño in Galicia, Spain, caused by simultaneous infections of P. ×alni and P. ×multiformis in 2 m stem height during an extreme spring flood; e, f. collar rot lesions caused by P. ×alni on A. glutinosa (e) and A. incana (f) planted on a non-flooded site in Bavaria, Germany, with rusty and tarry spots on the outer bark and flame-shaped, orange-brown necrotic lesions of the inner bark; inactive older lesion areas from the previous year are sunken and surrounded by callussing tissues (arrows); g. black exudates oozing from multiple collar rot lesions caused by P. ×multiformis during a severe flood on a riparian young A. glutinosa in Sardinia, Italy, reacting with the production of epicormic shoots; h. same tree as in (g) with multiple, fresh necrotic lesions of the inner bark caused by P. ×multiformis; i. inactive sunken collar rot lesion with surrounding callusing tissues (arrows) caused by P. siskiyouensis on a young A. cordata in the UK; j. flame-shaped staining of the xylem tissue underneath an active collar rot lesion caused by P. siskiyouensis on a young A. cordata in the UK. — Photos: a–f: T. Jung; g, h: B. Scanu; i, j: A. Pérez-Sierra.
The causal organisms of the unknown lethal rot on Alnus species were identified as a previously unknown swarm of interspecific hybrids originally described as Phytophthora alni s.lat. comprising three subspecies: P. alni ssp. alni (PAA), P. alni ssp. uniformis (PAU) and P. alni ssp. multiformis (PAM) (Brasier et al. 2004). Originally, it was hypothesised that P. ×cambivora and an unknown species close to P. fragariae were the progenitors of the hybrids (Brasier et al. 1999, 2004). However, allele-specific analyses of four single-copy nuclear genes and mtDNA of a European collection of isolates of PAA, PAM, PAU, P. ×cambivora and P. fragariae shed more light on the parental origin of the hybrids:
1. PAU is most likely a distinct non-hybrid species;
2. PAA resulted from a hybridisation between PAU and PAM; and
3. PAM is an ancient hybrid.
Thus, multiple hybridization events must have occurred (Ioos et al. 2006). These results were further confirmed using allele-specific real-time PCR for the quantification of allele copy numbers of three single-copy nuclear genes to assess ploidy levels, and flow cytometry for the determination of genome sizes (Husson et al. 2015). Consequently, the allotriploid PAA, the allotetraploid PAM and the diploid PAU were renamed as P. ×alni, P. ×multiformis and P. uniformis, respectively (Husson et al. 2015). It is suggested that P. ×multiformis and P. uniformis are of exotic, but different, origin and that the hybridisation that created P. ×alni occurred in a European nursery where both accidentally introduced parental species met (Brasier et al. 2004, Jung et al. 2017c). Interestingly, P. uniformis is common in streams and alder stands in Alaska and Oregon without causing noticeable damage (Adams et al. 2010, Navarro et al. 2015). Using a microsatellite analysis, Aguayo et al. (2013) demonstrated higher genetic diversity of P. uniformis isolates from Alaska compared to their European counterparts indicating a North American origin of this species.
In pathogenicity tests, P. ×alni was significantly more aggressive to the bark of mature A. glutinosa logs than P. ×multiformis and P. uniformis (Brasier & Kirk 2001). Phytophthora ×alni, P. ×multiformis and P. uniformis were shown to be non-pathogenic to a range of other tree species indicating their host specificity to Alnus spp. (Brasier & Kirk 2001, Santini et al. 2003). In a comparative pathogenicity trial with different Alnus spp., the ranking of susceptibility to P. ×alni was A. glutinosa > A. incana and A. cordata > A. viridis > A. rubra (Jung & Blaschke 2006). Despite being only moderately aggressive to European alder species in pathogenicity trials, P. uniformis is primarily driving the root and collar rot epidemic of A. glutinosa and A. incana in Sweden and in the Bavarian Alps, Germany (Jung & Blaschke 2004, Redondo et al. 2015). This is most likely due to the significantly higher frost tolerance of P. uniformis compared to P. ×alni (Černý et al. 2012, J. Schumacher & T. Jung unpubl. data).
In Bavaria, root and collar rot and extensive mortality of A. glutinosa and A. incana was found in riparian forests along more than 20 000 km of rivers and streams. In the catchments of 58 of 60 river systems studied in detail, the primary source of inoculum could be traced back to numerous, young infested alder plantings established on the riverbanks or on forest sites draining into the rivers (Jung & Blaschke 2004). A similar association was found in 26 river systems in Austria (Jung et al. 2009, 2013b). The presence of the disease in more than 800 young forest and riparian alder plantings in Bavaria (Jung & Blaschke 2004), and the findings of P. ×alni and P. uniformis in 20 and 6.3 %, respectively, of the 64 nursery fields of Alnus spp. examined in seven European countries (Jung et al. 2016), clearly demonstrated that the rapid spread and the epidemic extent of this disease in Europe was primarily driven by the widespread planting of infested nursery stock. A range of other Phytophthora species, including P. cactorum, P. gonapodyides, P. lacustris, P. plurivora and P. polonica, are occasionally isolated from small root and collar lesions of Alnus spp. without playing a significant role in this epidemic (Gibbs et al. 2003, Jung & Blaschke 2004, Belbahri et al. 2006, Kanoun-Boulé et al. 2016). However, the recent finding of the introduced pathogen P. siskiyouensis causing collar rot and mortality of A. cordata in the UK (Fig. 4i, j) might pose an additional threat to alder stands in Europe. Phytophthora siskiyouensis was previously reported causing collar rot lesions on planted trees of A. glutinosa and A. cordata in Victoria, Australia, and in California, respectively, and from A. rhombifolia and A. rubra in riparian forests in Oregon (Smith et al. 2006, Rooney-Latham et al. 2009, Sims et al. 2015a). In underbark inoculation tests, P. siskiyouensis caused similar lesion lengths on A. rubra as P. uniformis (Navarro et al. 2015).
Variation in susceptibility of alder trees to P. ×alni, observed both in the field and in pathogenicity tests, could be the basis for a successful resistance screening programme which would allow sustainable long-term management of diseased alder stands. For example, in Bavaria, susceptibility of A. glutinosa trees, growing in permanent or seasonal contact to infested water on the banks of five rivers with long disease history, was tested and significantly higher tolerance to P. ×alni was found in many healthy-looking trees as compared to declining trees with root and collar rot symptoms (Jung & Blaschke 2006). Also in Belgium, considerable variation in susceptibility to P. ×alni was found among A. glutinosa trees randomly selected along 34 watercourses (Chandelier et al. 2016).
Decline and mortality of Chamaecyparis lawsoniana in Europe and North America
Chamaecyparis lawsoniana (Lawson’s cypress or Port-Orford-cedar, POC) has a limited geographical distribution in humid regions of south-western Oregon and northern California. It grows on a wide range of soil types and sites as a major overstorey component in mixed forests with Abies concolor, A. magnifica, Notholithocarpus densiflora, Pinus monticola, Pseudotsuga menziesii, Tsuga heterophylla and Sequoia sempervirens at altitudes from sea level up to 2 000 m (Zobel et al. 1985, Jimerson et al. 2001). POC is also one of the most common ornamental trees worldwide used for amenity plantings, shelterbelts and in hedgerows. The first reports of diseased POC came in 1923 from an ornamental nursery in Seattle, USA, outside of the natural range of the species, and since the early 1950s decline and mortality have been observed in natural forest stands in Oregon. The association with a previously unknown Phytophthora species, P. lateralis, was established by Tucker & Milbradt in 1942. Phytophthora lateralis is a soilborne pathogen which abundantly produces chlamydospores enabling its survival during dry and hot summers. Homothallic production of oogonia containing oospores was mentioned in the original description (Tucker & Milbradt 1942) but the sexual stage could not be confirmed in later studies (Hansen et al. 2000, Brasier et al. 2012). Phytophthora lateralis is a slow growing low-temperature species reproducing and infecting in the Pacific Northwest (PNW; California to British Columbia) mainly during cool and wet conditions in spring, autumn and summer (Hansen et al. 2000). Phylogenetically, P. lateralis is the closest known relative of P. ramorum, the causal agent of ‘Sudden Oak Death’ in California and Oregon and ‘Sudden Larch Death’ in the UK (Brasier et al. 2012, Yang et al. 2017). Phytophthora lateralis is highly aggressive to POC (Hansen et al. 2000, Robin et al. 2015). The only other tree species occasionally infected by P. lateralis in forests in Oregon is Taxus brevifolia but its susceptibility to the pathogen is much lower compared to POC (Murray & Hansen 1997, Hansen et al. 2000). Similar to P. cinnamomi in WA, the main pathway of P. lateralis in the USA is along roads and paths with infested soil particles adhering to tyres of vehicles and boots (Hansen et al. 2000, Goheen et al. 2012). Once introduced to a forest, P. lateralis shows efficient and rapid spread downhill and along rivers and streams (Hansen et al. 2000, Jimerson et al. 2001, Jules et al. 2002). Phytophthora lateralis has spread throughout the total natural range of POC causing a devastating decline with high mortality rates, which can reach more than 90 % on riparian sites with devastating effects on stream ecology (Hansen et al. 2000, Jimerson et al. 2001, Jules et al. 2002).
Outside the PNW, P. lateralis was for the first time isolated from POC in a nursery in France and the infestation was considered eradicated (Hansen et al. 1999). However since 2005, P. lateralis has been causing severe decline and mortality of thousands of C. lawsoniana trees growing in shelterbelts in Brittany, France (Robin et al. 2011). Soon after, P. lateralis has also been recovered from declining POC and C. pisifera trees at numerous sites in forests, parks and shelterbelts in England, Scotland and Northern Ireland (Green et al. 2013, Schlenzig et al. 2014). In Europe and the Pacific Northwest, the symptoms of POC decline include chlorosis, wilting and bronzing of foliage, thinning and dieback of the crown and finally death of affected trees (Fig. 5a, b) (Hansen et al. 2000, Robin et al. 2011, Green et al. 2013). These crown symptoms and mortality are caused by root rot and basal stem lesions with resinous exudates on the outer bark and flame-shaped red-brown necrosis of the underlying phloem and cambium (Fig. 5c–e; Hansen et al. 2000, Robin et al. 2011, Green et al. 2013). While seedlings get killed within a few weeks, large trees usually die within one year after appearance of first crown symptoms (Hansen et al. 2000). In both Brittany and the UK, P. lateralis was also isolated from aerial bark lesions on stems and branches, and in Brittany also from necrotic foliage suggesting aerial infections (Robin et al. 2011, Green et al. 2013). Foliage infections and aerial bark cankers on stems and large branches, supposedly originating from rain and wind splash inoculum, were also observed earlier in the USA (Roth et al. 1957). In a Scottish nursery, P. lateralis was isolated from pale-green discoloured foliage of declining seedlings of Thuja occidentalis originally imported from a nursery in France (Schlenzig et al. 2011). In the Netherlands, P. lateralis was detected on POC nursery stock (Brasier et al. 2012).
Fig. 5.

Symptoms of Port-Orford-cedar (Chamaecyparis lawsoniana) decline and mortality caused by Phytophthora lateralis in the UK (a–e) and of kauri (Agathis australis) dieback caused by P. agathidicida in New Zealand (f–h). a, b. Shelterbelts of C. lawsoniana containing recently dead trees with sparse brown foliage (white arrows) and declining trees with chlorotic foliage and increased transparency (red arrows); c. C. lawsoniana stem with resinous exudates indicating necrotic lesion of the inner bark caused by P. lateralis; d. flame-shaped reddish brown inner bark lesion on a C. lawsoniana caused by P. lateralis, extending from the main roots to more than 1 m stem height; e. flame-shaped reddish brown lesion front caused by P. lateralis on a C. lawsoniana tree (detail from d); f. mature A. australis in a diverse kauri forest showing advanced thinning and dieback of the crown; g. massive stem of a mature A. australis with tongue-shaped resinous bark lesion caused by P. agathidicida, extending from the main roots up to 2 m stem height; h. resin exudations marking the front of the inner bark lesion caused by P. agathidicida on a mature kauri stem (detail from g). — Photos: a–e: A. Pérez-Sierra; f–h: T. Jung.
Due to the high aggressiveness of P. lateralis to POC and the occurrence of several endemic Chamaecyparis species in eastern Asia with comparatively high resistance to P. lateralis, an Asian origin of this pathogen has long been suggested (Tucker & Milbradt 1942, Zobel et al. 1985, Hansen et al. 2000). Recently, P. lateralis was isolated at several sites in natural, high-altitude cloud forests of Taiwan from soil in wet seeps and from necrotic foliage of mature C. obtusa var. formosana with generally healthy crowns and non-damaged fine root systems (Brasier et al. 2010, 2012, Webber et al. 2012). A population genetic study using microsatellites demonstrated that isolates from France, the Netherlands and the majority of the UK isolates were identical to isolates from the PNW, whereas the Taiwanese isolates belonged to two distinct evolutionary lineages, designated as TWJ and TWK. Several isolates from Scotland constituted a separate UK lineage which might have resulted from a hybridisation between the PNW and the Taiwanese lineages (Vettraino et al. 2017). The lineages also show considerable phenotypic and morphometric differences (Brasier et al. 2012). In all lineages short preformed sporangial pedicels occur enabling aerial spread and infections of foliage and bark (Brasier et al. 2012). In comparative underbark shoot-dip and root-dip inoculation trials the PNW and TWJ lineages showed higher aggressiveness to POC than the TWK and the UK lineages (Robin et al. 2015). Based on the results of these studies, it was concluded that the European PNW isolates were recently introduced from the USA with infested nursery stock (Vettraino et al. 2017). The exact origin of the PNW lineage in Asia still remains to be found.
The detection of heritable genetic resistance to the PNW lineage of P. lateralis in the natural population of POC (Hansen et al. 1989) stimulated a successful long-term resistance screening programme (Hansen et al. 2000, 2011, Oh et al. 2006, Sniezko et al. 2006, 2011). Using stem-dip and root-dip infection trials resistance was found in 1 600 of 12 000 trees tested (Oh et al. 2006). In greenhouse trials survival rate of seedlings and rooted cuttings from resistant parent trees varied between 25 and 100 % compared to 0–10 % survival of seedlings from susceptible parents (Sniezko et al. 2006). In a long-term outplanting trial on a high disease impact site, seedlings and rooted cuttings from the five most resistant families showed after 16 years 20–80 % survival compared to less than 8 % in progenies from the three most susceptible families (Oh et al. 2006). Several POC families resistant to the PNW lineage of P. lateralis were also challenged with the UK, TWJ and TWK lineages and no breakdown of resistance was observed (Robin et al. 2015). These results are promising regarding the re-establishment of this important tree species on dieback sites. The successful POC resistance screening programme could serve as a role model for the management of other Phytophthora diseases of forest trees.
Kauri dieback in New Zealand
Kauri (Agathis australis), a particularly long-lived ancient conifer species in the Araucariaceae, can reach 5 m stem diameter and up to 50 m height. Naturally, it grows widely on the North Island of New Zealand as a keystone species in diverse, mixed lowland forests below 600 m altitude together with other conifers, including Dacrycrydium cuppressinum, Phyllocladus trichomanoides, Podocarpus totara, P. laetus and Prumnopitys ferruginea (Nicholls 1976, Wardle 1991, Steward & Beveridge 2010). Due to excessive logging in the past, the current distribution of kauri is largely fragmented and relatively pristine old growth stands occupy less than 5 % of the pre-European area (Ahmed & Ogden 1987).
In 1972, Phytophthora isolates were recovered from a dying kauri stand on the Great Barrier Island off the coast of the North Island and morphologically identified as P. heveae (Gadgil 1974). Apparently, not much attention had been paid to managing this local disease outbreak until dieback and mortality of kauri stands were observed 30 years later on the North Island (Beever et al. 2009, Scott & Williams 2014, Bellgard et al. 2016). Symptoms include thinning, chlorosis and dieback of crowns (Fig. 5f), fine root losses and tongue-shaped collar rot lesions with abundant resin exudations extending several meters up the trunk and also into the main roots (Fig. 5g, h) (Beever et al. 2009, Bellgard et al. 2016). A homothallic Phytophthora species, informally designated as Phytophthora taxon Agathis (PTA) and later described as P. agathidicida, was consistently isolated from necrotic bark lesions and from rhizosphere soil (Beever et al. 2009, Scott & Williams 2014, Weir et al. 2015). Although the smooth-walled oogonia of P. agathidicida resemble those of P. heveae, multigene phylogenetic analysis placed it closer to P. castaneae within Phytophthora Clade 5, together with P. cocois from Hawaii and P. heveae (Weir et al. 2015, Yang et al. 2017). Since its closest relatives P. castaneae and P. heveae are of Southeast Asian and Australasian origin (Arentz 1986, Brown 1999, Ko et al. 2006, Jung et al. 2017a) it is likely that P. agathidicida is also indigenous to this area (Beever et al. 2009, Weir et al. 2015, Bellgard et al. 2016). One original isolate, obtained in 1972 from a dying kauri tree on the Great Barrier Island, could be assigned to P. agathidicida and in 2006 this pathogen could again be isolated from kauri collar rot at this original outbreak site (Beever et al. 2009). It is assumed that P. agathidicida did not spread from the Great Barrier Island to kauri stands of the North Island but has rather been present there for decades and collar rot and dieback symptoms have simply been overseen (Beever et al. 2009). A reassessment of the dieback area on the Great Barrier Island revealed an annual rate of disease extension of approximately 3 m, comparable to P. cinnamomi in Western Australia (Strelein et al. 2006, Beever et al. 2009). Other Phytophthora species like P. cryptogea, P. kernoviae, P. multivora and P. nicotianae are occasionally recovered from kauri soils, while P. cinnamomi is widespread in kauri forests. Although in general P. cinnamomi is not associated with kauri dieback it can be infrequently associated with scattered mortality of individual kauri trees under particularly favouring conditions for the pathogen (Podger & Newhook 1971, Beever et al. 2009, Waipara et al. 2013, Horner & Hough 2014). In both underbark inoculation and soil infestation trials, P. agathidicida showed much higher aggressiveness to A. australis than P. cinnamomi, P. cryptogea and P. multivora, causing large girdling lesions and high mortality (Horner & Hough 2014). Recent surveys demonstrated a wide distribution of collar rot, dieback and mortality of kauri throughout much of the natural range in the North Island, and P. agathidicida has been isolated at many sites from collar rot lesions confirming this pathogen being the causal agent of this epidemic (Waipara et al. 2013, Scott & Williams 2014). Phytophthora agathidicida infects trees of all ages and, besides killing mature trees, poses a serious threat to kauri regeneration, hence, resulting in the long-term in an altered forest composition from kauri-dominated forests to forests dominated by Podocarpus spp., D. dacrydioides and P. trichomanoides (Beever et al. 2009, Bellgard et al. 2016).
Decline and mortality of Austrocedrus chilensis and Juniperus communis in Argentina and Europe
Austrocedrus chilensis (Cordilleran cypress or Chilean cedar) is a dioecious evergreen conifer, member of the Cupressaceae, and native to the mountains of South Chile and South Argentina. It grows in a wide range of soil types and under different environmental conditions between S36°30’ and S43°35’ latitude on the eastern slopes of the Andes in Argentina and between S32°39’ and S44° latitude on the western slopes of the Andes in Chile (Veblen et al. 1995). Besides its ecological importance, A. chilensis is valued for its high timber quality and straight stems used for construction and woodworking (La Manna & Rajchenberg 2004). Decline and mortality of Cordilleran cypress, commonly known as ‘mal del ciprés’, was first detected in 1948 (Havrylenko et al. 1989, Greslebin et al. 2005) and has since spread throughout its whole range in Argentina. Chlorosis, progressive withering, defoliation, and finally the death of affected trees characterise the decline. Trees can die rapidly and in these cases the foliage turns from chlorotic to red. Such epidemics cause high mortality at landscape level (Fig. 6a–d). Earlier studies discussed several hypotheses regarding the causal agents of the decline including root infections by Pythiaceous pathogens, root decay by basidiomycete fungi, lack or dysfunction of mycorrhizae of Cordilleran cypress, global warming, droughts, and poor drainage and increased precipitation (Havrylenko et al. 1989, Rajchenberg et al. 1998, Filip & Rosso 1999). Although several studies of biotic and abiotic factors tried to elucidate the origin and causes of this decline, it was not before 2007 that a previously unknown Phytophthora species, P. austrocedri (synonym P. austrocedrae), was first described and associated with the mortality of A. chilensis in Argentina (Greslebin et al. 2007). Phytophthora austrocedri is a homothallic species with very slow growth and a low optimum growth temperature of 17.5 °C. It was placed in Clade 8 where the most closely related species are the soilborne P. syringae and P. obscura, both causing root rots, cankers and foliar and shoot blights on numerous trees and ornamentals (Grünwald et al. 2012b). Phytophthora syringae and P. ×cambivora were occasionally recovered from the rhizosphere of declining trees but are not involved in the decline (Greslebin et al. 2005, 2007). Phytophthora austrocedri was isolated from roots and from collar and stem lesions of symptomatic A. chilensis trees. Lesions extend from the roots up the trunk and are characterised by resin exudations at the surface of the bark and brown, flame-shaped lesions of the underlying phloem and cambium (Fig. 6e). Sometimes, a staining of the superficial sapwood underneath bark lesions can be observed. Pathogenicity tests on seedlings, saplings and adult trees demonstrated high aggressiveness of P. austrocedri to A. chilensis confirming the association of this pathogen with the decline in Argentina (Greslebin & Hansen 2010). The virtually clonal population structure of P. austrocedri and its aggressive behaviour on Cordilleran cypress, strongly suggested that it is an alien invasive pathogen in Argentina (Vélez et al. 2013).
Fig. 6.

Decline and dieback symptoms caused by Phytophthora austrocedri on Austrocedrus chilensis in Argentina (a–e) and on Juniperus communis in the UK (f, g). a. Extensive mortality of A. chilensis at landscape level; b. decline, dieback and mortality of A. chilensis at stand level; c. A. chilensis trees with thinning and dieback of crowns; d. healthy, declining chlorotic and dead A. chilensis (from right to left); e. mature A. chilensis stem with flame-shaped necrotic lesion of the inner bark; f. long-dead and recently killed J. communis with red-brown foliage; g. tongue-shaped orange-brown lesion extending from the main roots into the collar of a mature J. communis. — Photos: a–d: T. Jung; e–g: A. Pérez-Sierra.
Juniperus communis (common juniper) is another dioecious evergreen conifer from the Cupressaceae, with a wide holarctic, boreo-temperate distribution ranging from N30° in North Africa to northern Asia, North America and Europe (Preston et al. 2002, Thomas et al. 2007). In 2010, first reports of a serious decline of common juniper came from a National Nature Reserve in northern England (Britain). By 2013, the disease was detected at 19 sites in northern England and Scotland. Dead and dying juniper trees are scattered throughout the affected areas, mainly concentrated on wet, flat ground but also extending uphill across drier slopes. Affected trees and shrubs show fading green colour, reddening or browning foliage on individual branches or the whole crown, and eventually defoliation and tree death (Fig. 6f). Crown symptoms and mortality are caused by orange-brown tongue-shaped lesions in the phloem at the stem collar and along upper roots (Fig. 6g). In some cases, a distinct yellowing of healthy phloem in advance of the lesion margin can be observed on infected trees. Phytophthora austrocedri was confirmed as the cause of this dieback and mortality (Green et al. 2012). This pathogen was also identified on two other non-native conifer species in the UK, Xanthocyparis nootkatensis (Nootka cypress or Alaskan yellow cedar) (Green et al. 2016) and POC, both like A. chilensis and J. communis from the Cupressaceae family. British isolates from different geographical sites were analysed and also showed limited genetic diversity (Green et al 2015). In Germany, a single isolate of P. austrocedri was obtained from a young J. horizontalis ‘Glauca’ in a nursery (Werres et al. 2014).
Recently, Henricot et al. (2017) compared Argentinian, British and German isolates of P. austrocedri to clarify the epidemiological and evolutionary relationships between them. Morphological and physiological parameters did not differ significantly between the different populations and in cross-infection experiments both Argentinian and British isolates were pathogenic to the two main hosts A. chilensis and J. communis. However, phylogenetic analyses of sequences from two mitochondrial and five nuclear gene regions showed that all British isolates were near identical but phylogenetically distinct from the Argentinian and German isolates which share the same genotype. These results indicate that British isolates and Argentinian/German isolates of P. austrocedri constitute two evolutionarily distinct lineages originating from the same as-yet-unidentified source population (Henricot et al. 2017).
At the end of 2017, a new case of P. austrocedri was reported on a new host, Cupressus sempervirens (Italian cypress) in northern Iran (Mahdikhani et al. 2017). The symptoms in affected trees were consistent with those reported on other hosts and included bronzed foliage and an orange-brown lesion in the phloem around the collar. To date, decline and canker diseases caused by P. austrocedri have been reported from three continents, South America, Europe and western Asia, and so far the host range has been confined to the Cupressaceae family.
Diebacks of natural ecosystems in Australia
Apart from tropical regions in north-eastern Queensland and cool-temperate Tasmania, the climate in Australia is characterised by hot and dry summers and relatively low annual precipitations. In the south-west of WA, soil temperatures during summer often exceed 30 °C, and soil water potentials and soil water contents often drop below -6 Mpa (-60 bar) and 1 %, respectively (Shearer & Tippett 1989, Lamont & Bergl 1991, Enright & Lamont 1992, Collins et al. 2011). Although such conditions are generally not believed to favour survival, spread and infection via zoospores of soilborne oomycetes, Australia hosts an impressive diversity of Phytophthora species. Since 2009, 19 previously unknown Phytophthora species were described from natural ecosystems in WA, including P. amnicola, P. arenaria, P. bilorbang (previously P. taxon oak soil), P. balyanboodja, P. boodjera, P. condilina, P. constricta, P. cooljarloo, P. elongata, P. fluvialis, P. gibbosa, P. gregata, P. kwongonina, P. litoralis, P. mooyotj, P. multivora, P. pseudorosacearum, P. thermophila and P. versiformis (Scott et al. 2009, Rea et al. 2010, 2011, Jung et al. 2011, Crous et al. 2011, 2012, 2014, Aghighi et al. 2012, Simamora et al. 2015, Paap et al. 2017, Burgess et al. 2018). A metagenomic survey of Phytophthora diversity at 640 natural sites across Australia demonstrated presence of 68 Phytophthora phylotypes, of which 21 were potentially new taxa and another 25 were previously not found in natural ecosystems or were new introductions to Australia (Burgess et al. 2017). With presence at 44.7 and 34.2 % of the sites, respectively, P. multivora and P. cinnamomi were by far the most common species. Interestingly, these two introduced wide-host range pathogens also have the most deleterious impact on Australian natural ecosystems.
During the long isolation from other continents, a diverse and largely endemic flora evolved in Australia which, due to a lack of co-evolution, contains a high proportion of plant species susceptible to non-native introduced Phytophthora species. Alone in the south-western Botanical Province of WA, 40 % of the 5 710 endemic plant species from 39 families are susceptible to P. cinnamomi (Shearer et al. 2004). Recently, pathogenicity of P. cinnamomi and 21 other Phytophthora taxa from WA, most of them recently described or informally designated new species, to seven native plant species from WA, including Eucalyptus marginata (jarrah), Banksia grandis, B. littoralis, B. occidentalis, Casuarina obesa, Corymbia calophylla (marri) and Lambertia inermis, was demonstrated (Belhaj et al. 2018). Eucalyptus marginata and the three Banksia species were susceptible to the highest number of Phytophthora taxa whereas C. calophylla showed the highest resistance to all 21 Phytophthora taxa (Belhaj et al. 2018). Australia has a long history of diebacks of various eucalypt forests, Banksia woodlands and heathlands, which are particularly severe in the Mediterranean climates of WA (Shearer & Tippett 1989, Shearer & Dillon 1995, 1996, Shearer et al. 2004) and Victoria (Weste & Marks 1987, Marks & Smith 1991, Laidlaw & Wilson 2003, Weste 2003, Cahill et al. 2008). In WA, the most widespread forest type is the dry sclerophyll jarrah forest which is largely dominated in the overstorey by E. marginata, while the understorey contains a diverse flora mainly from the Proteaceae, Dilleniaceae, Epacridaceae, Fabaceae and Xanthorrhoeaceae (Shearer & Tippett 1989). First symptoms of dieback and mortality in the jarrah forest were reported during the 1920s but it took 40 years until the association with the presence of P. cinnamomi in the rhizosphere was established (Podger et al. 1965, Podger 1972, Shearer & Tippett 1989). Fifty years of intense research has produced a rich body of knowledge on the aetiology, site relations and dynamics of jarrah dieback and numerous other diebacks in WA and eastern Australia, the ecology, host range and pathways of P. cinnamomi, and the role that other Phytophthora spp. are playing. Although P. elongata, P. gibbosa, P. gregata, P. litoralis, P. multivora, P. thermophila and P. versiformis can cause local dieback and mortality of understorey species and sometimes also of E. marginata (P. multivora, P. elongata), the dieback of the jarrah forest is mainly driven by two clonal lineages of P. cinnamomi from the A2 mating type (Shea 1975, Shea et al. 1982, Shearer & Tippett 1989, Dobrowolski et al. 2003). The main pathway of P. cinnamomi in the jarrah forest and other natural ecosystems in WA is the accidental transport of infested soil adhering to vehicles and boots and the use of infested gravel material for building of forest roads (Shea et al. 1983, Shearer & Tippett 1989, Marks & Smith 1991). A few months after the introduction of P. cinnamomi to a site, first symptoms of chlorosis and wilting appear on highly susceptible understorey species, in particular B. grandis, Xanthorrhoea preissii and Macrozamia riedlei, hence, they are used as indicator species for presence of P. cinnamomi (Shea 1979, Shearer & Tippett 1989). The pathogen usually moves through the forest via zoospores and root-to-root contact with more or less sharply defined infection fronts (Weste & Marks 1987, Shearer & Tippett 1989). An extensive study at 55 sites across WA using aerial photography demonstrated mean disease progression rates of 1.4 m per year for uphill extension and 9.5 m per year across slopes (Strelein et al. 2006). Downhill progression is much faster reaching up to 400 m per year in Victoria (Weste & Law 1973). After several years, the majority of midstorey and understorey species are affected by dieback and mortality and highly susceptible species can be eliminated while the E. marginata overstorey shows severe thinning, chlorosis, wilting and dieback of the canopy and mortality of scattered trees or in groups up to several hectares in size (Fig. 7a) (Podger 1972, Shearer & Tippett 1989, Jung et al. 2013a). Although in jarrah the rate of symptom expression is varying depending on the site conditions, other tree species, in particular marri and bullich (E. megacarpa) can persist much longer. In E. marginata, P. cinnamomi causes excessive fine root losses, which interact with the extreme summer droughts leading to gradual decline and eventually dieback and mortality (Podger 1972, Shea 1975, Crombie et al. 1987, Shearer & Tippett 1989). In addition, P. cinnamomi can infect large woody roots and produce bark lesions, which on sites with impeded drainage due to a concrete lateritic hardpan close to the surface may girdle the vertical roots just above the lateritic layer resulting in acute wilting and mortality (Shea et al. 1982, Tippett et al. 1983, Shearer & Tippett 1989). Collar infections on jarrah are rare and mostly restricted to waterlogged sites or young trees (Fig. 7f; Shearer & Tippett 1989). Burgess et al. (1999) demonstrated increased susceptibility of jarrah stems to P. cinnamomi infection under conditions of root hypoxia. About 20 % of the 64 000 km2 covered by the jarrah forest are already infested by P. cinnamomi and the structural and floristic changes it caused are unprecedented (Fig. 7a). Recently, it has been shown that P. cinnamomi survives the hot and dry summer conditions in WA within infected fine roots and small woody roots of woody host and non-host species and annual herbs. Particularly thick-walled oospores produced by selfing of the A2 mating type, stromata-like hyphal aggregations and intracellular hyphae encased by callose sheaths (lignitubers) serve as long-term survival structures, while the thin-walled chlamydospores enable short-term survival between consecutive rain events (Crone et al. 2013, Jung et al. 2013a).
Fig. 7.

Diebacks of natural ecosystems caused by Phytophthora spp. in the south-west of Western Australia (WA). a. Severe dieback and mortality of jarrah (Eucalyptus marginata) forest with extensive elimination of the previously diverse understorey caused by P. cinnamomi; b. high mortality of tuart (E. gomphocephala) on calcaric sandy soils in the Swan Coastal Plain caused by extensive fine root losses due to P. multivora infections; c. severe dieback and mortality of riparian flooded gum (E. rudis) stand within the jarrah forest caused by P. elongata and P. multivora; d. extensive dieback and collapse of a Banksia woodland caused by P. cinnamomi; e. dieback and mortality of mature red tingle (E. jacksonii) caused by P. cinnamomi and P. cryptogea in a humid relic forest at the south coast of WA; f. girdling collar rot lesion caused by P. cinnamomi on a young E. marginata in the jarrah forest; g. bleeding collar rot lesion caused by P. cinnamomi on a mature Banksia grandis tree in an open Banksia woodland. — Photos: all T. Jung.
Across its native range and in particular in the Swan coastal plane south of Perth, tuart (E. gomphocephala) forests, growing on dry and sandy limestone sites, are suffering for almost 20 years from a severe decline characterised by thinning and dieback of the crowns and high mortality rates (Fig. 7b; Edwards 2004, Archibald 2006, Scott et al. 2009). Several environmental factors have initially been considered responsible for tuart decline until a firm association with the previously undescribed pathogen P. multivora was established (Scott et al. 2009). In pathogenicity tests, P. multivora demonstrated high aggressiveness to the fine root system of E. gomphocephala (Scott et al. 2012). In the tuart forest, P. multivora causes progressive fine root losses which exacerbate the severe drought stress during summer on the sandy soils predisposing affected trees to attacks by stem borers (Scott et al. 2009). Other tree species like Agonis flexuosa (peppermint) are also affected, but to a lesser extent (Scott et al. 2009). Phytophthora multivora has a wide host range in WA and in other continents whereas in South Africa it is widespread in natural ecosystems without causing visible disease symptoms suggesting long-term coevolution (Scott et al. 2009, Oh et al. 2013, Jung et al. 2016). The acidophilic P. cinnamomi is generally absent from these calcaric sites in WA and, hence, is not involved in tuart decline. Also in the south-west of WA, riparian gallery forests of flooded gum (E. rudis) along many rivers and streams are showing since the 1970s increasingly severe thinning and dieback of crowns with high levels of mortality (Fig. 7c). For a long time, attacks by psyllids and leaf miners and various environmental factors, including rising salinity levels of river water due to agricultural mismanagement, have been discussed as causal agents, but a satisfactory disease aetiology was not established (Curry 1981, Abbott 1999, Yeomans 1999, Clay & Majer 2001). However, recently P. elongata and P. multivora were regularly isolated along many rivers from the rhizosphere of dying riparian E. rudis trees with extensive fine root losses (Edwards et al. 2010). It seems likely that this widespread devastating decline is driven by root damage caused by these invasive Phytophthora species interacting with other biotic and abiotic factors including insect defoliations, droughts and salinity. Phytophthora elongata has a clonal population structure in WA and its origin is still unknown. It was most likely introduced to the jarrah forest during the 1970s with infested nursery stock used for rehabilitation plantings of mine sites, and subsequently spread into streams and river systems (Rea et al. 2010).
In WA, highly diverse Banksia woodlands and Kwongan heathlands constitute the climax vegetation in the northern and southern sandplains and on other sites too dry for supporting forest growth. In these ecosystems P. cinnamomi and many other Phytophthora spp. are causing fine root losses in susceptible plant species leading to dieback and bleeding collar lesions (Fig. 7g) which may girdle the plants resulting in acute wilting and mortality (Fig. 7b; Scott et al. 2009, Shearer et al. 2009, Rea et al. 2011, Jung et al. 2013a). Pathogenicity of 22 Phytophthora taxa to three Banksia species and L. inermis was recently demonstrated (Belhaj et al. 2018). While the invasive P. cinnamomi usually moves through these ecosystems in clearly visible infection fronts with high mortality rates, potentially indigenous species like P. arenaria from Clade 4, P. constricta from Clade 9, P. balyanboodja, P. condilina, P. cooljarloo, P. kwongonina and P. pseudorosacearum from Clade 6a and P. gibbosa, P. gregata, P. litoralis and P. thermophila from Clade 6b cause scattered or patchy dieback which is usually associated with episodic, unseasonal heavy rain events (Rea et al. 2011, Jung et al. 2011, Burgess et al. 2018).
In the humid south-western-most corner of WA, a tertiary relic forest dominated by particularly tall and long-lived tree species like karri (E. diversicolor), red tingle (E. jacksonii), yellow tingle (E. guilfoylei), Rates tingle (E. brevistylis) and marri is growing in a small area which is mostly protected within the Walpole-Nornalup National Park. For about a decade, numerous red tingle trees are suffering from a severe dieback and mortality which is associated with root infections by P. cinnamomi and P. cryptogea (Fig. 7e; T. Jung unpubl.). Due to the small natural habitat of red tingle this disease might threaten the survival of this endangered iconic tree species in nature.
In Victoria, severe diebacks occur in eucalypt forests and Banksia woodlands, in particular in the Grampians, in East and South Gippsland, the Wilsons Promontary and the Brisbane Ranges National Parks (Marks & Smith 1991). Symptomatologies, disease aetiologies, dynamics and site relations are largely similar to the situation in WA (Weste & Marks 1987, Marks & Smith 1991, Wilson et al. 2000, Weste 2001, Cahill et al. 2008). A 30-years study at 13 sites in representative eucalypt forests, woodlands and heathlands across Victoria demonstrated a gradual decline of P. cinnamomi inoculum to very low levels with progressing elimination of highly susceptible plant species from infested sites and their natural replacement by highly resistant species. After 20–30 years, substantial regeneration and survival of 30–40 previously eliminated susceptible plant species like Xanthorrhoea australis was observed, while the crowns of affected overstorey trees showed no recovery (Weste 2003). Future surveys are needed to assess whether the ecosystem recoveries will be sustainable or whether P. cinnamomi inoculum levels will increase again resulting in new dieback cycles.
In the tropical rainforests of northern Queensland, an extensive Phytophthora survey revealed presence of 10 Phytophthora species at 55 % of the 1 897 sites tested. More than 13 000 isolates were obtained of which 86 % and 9 % were P. cinnamomi and P. heveae, respectively. The remaining isolates belonged to P. boehmeriae, P. castaneae, P. citricola s.lat., P. cryptogea, P. drechsleri, P. meadii, P. nicotianae and P. palmivora (Brown 1999). Phytophthora cinnamomi was more frequently isolated from dead and dying than from healthy forests, and at nine sites this pathogen was associated with patch dieback of rainforest trees. All other Phytophthora spp. showed no association with disease (Brown 1999). The absence of large-scale dieback of forests might be explained by the constantly warm and humid climate which is favouring the trees more than P. cinnamomi, or indicate long-term coevolution between the flora and the pathogen, which could have spread from New Guinea to Queensland via a landbridge during the pleistocene. However, the presence of the A1 mating type at only four of the 646 sites infested by P. cinnamomi does not support the latter hypothesis, since in Papua New Guinea the A1 mating type is more widespread and considered an ancient introduction whereas the A2 mating type was most likely introduced in modern times (Arentz 1983, 2017, Arentz & Simpson 1986).
A recent survey in the diverse Gondwana rainforests of southern Queensland and northern New South Wales demonstrated the presence of eight Phytophthora species, including P. cinnamomi, P. cryptogea, P. frigida, P. heveae, P. macrochlamydospora, P. multivora, the previously unknown P. gondwanensis and another unknown Phytophthora taxon (Scarlett et al. 2015). Due to their wide host ranges and their high virulence, P. cinnamomi and P. multivora might pose a serious threat to the Gondwana rainforests which contain more than 200 rare and endangered plant species (Crous et al. 2015, Scarlett et al. 2015). Also in New South Wales, both P. multivora and P. cinnamomi were recovered from declining trees of the ‘living fossil’ Wollemia nobilis (Wollemi pine) in its only natural site and their high aggressiveness to this critically endangered tree species has been demonstrated (Puno et al. 2015).
Decline and dieback of the Mediterranean maquis vegetation
Mediterranean-type ecosystems, with their characteristic and unique climatic regimes of mild wet winters and warm and dry summers, occur just in five regions of the world: California, Central Chile, the Mediterranean Basin, South Africa and south-western and South Australia (Peel et al. 2007). These Mediterranean climate regions harbour a remarkable and globally significant level of plant diversity and endemism, accounting for almost 20 % of all plant species in the world (Myers et al. 2000, Cowling et al. 2006). In response to the climate, similar woody, shrubby plants, with evergreen sclerophyll leaves, have developed in communities of varying density. The names for the shrub vegetation vary by region because of language and plant structure, including ‘maquis’ and ‘garrigue’ in the Mediterranean Basin, ‘chaparral’ in California, ‘matorral’ in Chile, ‘fynbos’ or ‘renosterveld’ in South Africa and ‘mallee’ scrubs and shrublands and ‘kwongan’ heathlands in Australia.
The maquis vegetation in the Mediterranean Basin is characterised by scrub, sparse grass and scattered evergreen trees with a maximum size of 2–3 m, which differ in structure and species richness depending on local conditions (Spano et al. 2013). Since 2010, in the National Park of La Maddalena archipelago, off the northern coast of Sardinia, extensive dieback and mortality of a wide range of plant species, typical of the Mediterranean maquis in the archipelago as well as in other maquis sites in Sardinia, has been recorded across slopes downhill of roads and trekking paths (Fig. 8a, b) (Scanu et al. 2015). The main species affected are Arbutus unedo, Asparagus albus, Cistus sp., Erica spp., Juniperus phoenicea, J. oxycedrus, Pistacia lentiscus and Rhamnus alaternus (Scanu et al. 2015). Amongst the most susceptible species, J. phoenicea, J. oxycedrus and A. unedo show a wide range of symptoms including partial or complete dieback of the crown and abnormal production of epicormic shoots, and reddening or browning of drying foliage on dying and recently dead trees and shrubs (Fig. 8c, d). Crown symptoms are associated with extensive losses of both lateral small woody roots and fine roots, opening cankers and the presence of basal phloem lesions extending from the main roots up the stems (Fig. 8e). Root and collar rot on juniper trees and shrubs frequently occurs in low-laying areas with seasonal waterlogging. In wetlands, other tree/shrub species like P. lentiscus and R. alaternus also show severe crown thinning, dieback of single branches and mortality (Fig. 8b, f), which are caused by root and collar rot. Ground layer species such as A. albus (Fig. 8g) and Cistus spp. are also commonly affected showing chlorosis, wilting and dieback.
Fig. 8.

Decline and dieback symptoms on maquis vegetation caused by Phytophthora spp. in the Mediterranean basin. a. Extensive dieback and mortality of shrub species due to P. cinnamomi; b. Pistacia lentiscus showing severe dieback and mortality caused by P. ornamentata; c. mature tree of Juniperus oxycedrus with severe wilting and red discoloration of the foliage; d. large orange-brown lesion extending from the main root into the collar of a mature J. phoenicea; e. young Arbutus unedo recently killed by P. cinnamomi; f. chlorosis, wilting and dieback of Rhamnus alaternus caused by P. cinnamomi; g. chlorosis, wilting and dieback of Asparagus albus caused by P. asparagi. — Photos: a–c, e–g: B. Scanu; d: T. Jung.
An unexpected high diversity of Phytophthora species has been found associated with the decline and dieback of this diverse ecosystem. In total 10 Phytophthora species were isolated from rhizosphere soil samples collected from declining Mediterranean maquis vegetation and stream catchments in the National Park of La Maddalena archipelago, including P. asparagi, P. bilorbang, P. cinnamomi, P. crassamura, P. gonapodyides, P. melonis, P. ornamentata, P. parvispora, P. pseudocryptogea and P. syringae (Scanu et al. 2015). The most common species detected were P. asparagi and P. bilorbang, both from Clade 6 (Jung et al. 2011, Aghighi et al. 2012). While P. bilorbang appears to be a common species in natural environments (Aghighi et al. 2012, Sims et al. 2015b), P. asparagi was previously only reported from horticultural and ornamental plants (Cunnington et al. 2005, Saude et al. 2008, Jung et al. 2016). Likewise, also P. melonis was previously only known from agriculture causing a severe disease of members of the Cucurbitaceae in Asia (Ho et al. 2007). This suggests the possible introduction of both P. asparagi and P. melonis with infested plant material (Moralejo et al. 2009, Jung et al. 2016). Two previously unknown species, P. crassamura and P. ornamentata have been described from J. phoenicea and P. lentiscus, respectively, in Sardinia (Scanu et al. 2015). While P. crassamura is widespread across continents with different climatic conditions (Brasier et al. 2003, Burgess et al. 2009), P. ornamentata seems to be restricted to the Mediterranean Basin (Scanu et al. 2015), although it has recently been detected from C. calophylla plants in Australia (G.E.St.J. Hardy pers. comm.). In pathogenicity trials, all Phytophthora species demonstrated pathogenicity to J. phoenicea and P. lentiscus (Scanu et al. 2015). On J. phoenicea the most aggressive pathogens were P. asparagi and P. bilorbang, killing 50 % and 37.5 % of inoculated plants, respectively, while P. cinnamomi caused 100 % mortality on P. lentiscus. Phytophthora asparagi, P. crassamura, P. bilorbang, P. melonis and P. ornamentata were also highly aggressive to P. lentiscus (Scanu et al. 2015).
Phytophthora cinnamomi was isolated from R. alaternus showing severe dieback and wilting (Scanu et al. 2015) and together with its closest relative P. parvispora, this pathogen was also isolated from A. unedo, another common species in Mediterranean maquis ecosystems (Scanu et al. 2014a). Also in Portugal, P. cinnamomi has been isolated from declining and wilting A. unedo plants in Mediterranean maquis vegetation (T. Jung & M. Horta Jung unpubl. data). Phytophthora cinnamomi was also found causing a severe decline and mortality of Erica umbellata, a small heather species native to the western Iberian Peninsula and northern Morocco, in a protected natural area in Extremadura (Spain) (Acedo et al. 2013). Several understory Ericaceae and Cistaceae scrubs commonly occurring in declining Mediterranean oak stands in Spain and Portugal were also found to be infected by P. cinnamomi (Brasier et al. 1993, Moreira & Martins 2005). These plant species are suspected to act as a reservoir of pathogen inoculum, contributing to the spatial distribution of P. cinnamomi (Moreira & Martins 2005). The frequent detection of P. cinnamomi from Mediterranean plant species in recent years indicates that this pathogen is currently spreading into the maquis vegetation ecosystems. Against the background of a predicted increase of P. cinnamomi activity under current climate change projections (Brasier & Scott 1994, Burgess et al. 2017), the severe destructions of the root system caused by P. cinnamomi in pathogenicity tests on A. unedo, E. umbellata, J. phoenicea and P. lentiscus (Acedo et al. 2013, Scanu et al. 2014a, 2015) suggest that this polyphagous pathogen has the potential to threaten the native maquis vegetation in the Mediterranean basin on a large scale.
Decline and dieback of European beech in Europe and the USA
European beech (Fagus sylvatica) is characterised by high shade tolerance and growth capacity, a wide climatic and geological amplitude ranging from atlantic to continental climate and from moderately dry to periodically wet soils with pH ranging from < 3 to > 7. Due to its high competitiveness, European beech would naturally dominate more than 50 % of the forests at hilly to mountainous sites in Western and Central Europe and in mountain areas of Eastern and Southern Europe (Walentowski et al. 2004, Ellenberg & Leuschner 2010).
Since the mid 1990s, forests and amenity stands of European beech across the entire natural range in Europe are increasingly threatened by a severe decline and dieback (Motta et al. 2003, Jung et al. 2003b, 2005, 2009, 2013b, Cacciola et al. 2005, Hartmann et al. 2006, Orlikowski et al. 2006, Brown & Brasier 2007, Munda et al. 2007, Vettraino et al. 2008, Černý et. al. 2009, Jung 2009, Schmitz et al. 2009, Stępniewska & Dłuszyński 2010, Telfer et al. 2015). Also in the USA and in Chile, European beech plantations experience a similar decline and mortality (Jung et al. 2005, 2018, Nelson 2009, Weiland et al. 2010). The symptoms are typical for Phytophthora diseases and comprise small-sized and often yellowish foliage, thinning and dieback of crowns, extensive losses of fine roots and lateral roots, collar rot, and aerial bleeding cankers along the stem up to the canopy (Fig. 9). A local epidemic with similar symptoms was recorded in the 1930s in the UK and could be associated to Phytophthora infections (Day 1938). Severe destructions of the fine root system are common in affected forests and lead to a slow chronic decline, whereas bleeding collar rot and aerial bark cankers have a scattered or patchy distribution and usually cause rapid mortality (Jung et al. 2005, 2009, 2013b).
Fig. 9.

Decline and dieback symptoms caused by Phytophthora spp. on Fagus sylvatica. a. Scattered mortality and patch dieback and chlorosis (arrow) due to root and collar rot and aerial bleeding cankers caused by Phytophthora plurivora and P. ×cambivora in a mountain forest in the Bavarian Alps. Germany; b. severe dieback and mortality due to root and collar rot caused by P. ×cambivora in Austria; c. chlorosis, microphylly, thinning and dieback due to root losses caused by P. cactorum and P. plurivora in Bavaria; d. small woody roots with extensive losses of lateral roots and fine roots caused by P. cactorum and P. plurivora in Bavaria; e. bleeding collar rot lesion and aerial bleeding canker caused by P. ×cambivora in a mountain forest in the Bavarian Alps; f. bleeding collar rot lesion caused by P. ×cambivora in a Swedish forest; g. collar rot lesion with orange- to dark-brown exudates on the outer bark and flame-shaped lesion front in the inner bark caused by P. plurivora in Sweden. — Photos: all T. Jung.
Several independent surveys in 17 European countries showed that more than 80 % of the almost 300 beech stands growing on a wide range of geological substrates with soil pH ranging from 3.3 to 7.8 were infested with in total 15 Phytophthora taxa. Most widespread were P. ×cambivora, P. plurivora and, in urban situations, also P. cactorum. Phytophthora ×cambivora was frequently encountered on acidic heavy soils, while P. plurivora was more common in calcaric soils. Other species had a scattered distribution like P. chlamydospora, P. europaea, P. gonapodyides, P. pseudosyringae, P. psychrophila, P. quercina, P. syringae, P. tubulina and P. uliginosa, or were restricted to areas with mild winters like P. cinnamomi in Portugal and the UK (Jung & Blaschke 1996, Balci & Halmschlager 2003a, Jung et al. 2003b, 2005, 2013b, 2017b, Motta et al. 2003, Cacciola et al. 2005, Hartmann et al. 2006, Orlikowski et al. 2006, Brown & Brasier 2007, Munda et al. 2007, Vettraino et al. 2008, Černý et. al. 2009, Jung 2009, Schmitz et al. 2009, Stępniewska & Dłuszyński 2010, Telfer et al. 2015). The airborne pathogens P. kernoviae and P. ramorum exclusively occurred in the UK where the humid climate allows the continuous production of caducous sporangia on infected foliage of adjacent Rhododendron ponticum plants and their aerial dispersal onto neighbouring beech stems where they cause extensive bleeding lesions (Brasier et al. 2005, Brown & Brasier 2007, Jung et al. 2013b). In the eastern USA, P. cactorum, P. plurivora and its close relative P. pini (previously P. citricola I) are causing root and collar rot and mortality on mature F. sylvatica in amenity plantings (Jung et al. 2005, Weiland et al. 2010). Phytophthora cactorum, P. ×cambivora, P. kernoviae, P. plurivora and P. ramorum proved to be highly aggressive to the bark and root systems of both mature and young beech trees (Fleischmann et al. 2002, Brasier & Jung 2003, Jung et. al. 2003b, 2017b, Brown & Brasier 2007, Vettraino et al. 2008, Weiland et al. 2010). In Europe, all five species are considered as exotic invasive pathogens (Jung 2009, Jung & Burgess 2009, Jung et al. 2013b, 2016).
Detailed investigations in Germany, Austria and the UK showed that aerial bleeding cankers on stems of mature beech trees are in most cases not following the classical pattern of the phenomenon ‘Beech Bark Disease’. The latter is considered as complex interaction of predisposing drought stress, colonisation of the bark by the scale insect Cryptococcus fagisuga, infection of the colonised bark by the secondary parasite Neonectria coccinea and, eventually, invasion of the stem by wood decay fungi (Parker 1974, Lonsdale & Wainhouse 1987). However, this aetiology is apparently restricted to young pole-stage stands (Lonsdale & Wainhouse 1987) while in mature stands Phytophthora pathogens are the primary disease agents (Jung et al. 2005, 2013b, Brown & Brasier 2007, Jung 2009). Both soilborne Phytophthora species, in particular P. plurivora, P. ×cambivora and P. gonapodyides, and the airborne P. cactorum, P. kernoviae, P. pseudosyringae and P. ramorum can infect stem bark and shoots of beech via rain- or wind-splash dispersal of sporangia or progression from root lesions into the collar (Jung et al. 2005, 2013b, Brown & Brasier 2007, Jung 2009, Nechwatal et al. 2011). After establishment in the bark, Phytophthora pathogens can spread non-symptomatically in the xylem causing multiple aerial cankers along the stem (Brown & Brasier 2007).
The onset of the Phytophthora epidemics in Austria, Germany and Sweden was triggered by the succession of continuously wet conditions during the growing season followed by extreme drought in the same or the following year (Jung 2009, Jung et al. 2013b, T. Jung, T. Corcobado & T. Cech unpubl., T. Jung & J. Witzell unpubl. data).
A large-scale survey of nursery stands and young plantings of F. sylvatica in Europe revealed that 80 % of the nursery stands and almost 100 % of the plantings tested were infested with 9 and 11 Phytophthora species, respectively (Jung et al. 2016). Most common were P. ×cambivora, P. plurivora and P. cactorum demonstrating the importance of the nursery pathway for the spread of this devastating disease into beech stands.
AIRBORNE PHYTOPHTHORA DISEASES OF FORESTS AND WOODLANDS
Dieback and mortality of Nothofagus species in the UK and Chile
The genus Nothofagus is native to the Southern Hemisphere, where it constitutes an important component of temperate rainforests and mountainous forests (Kirkpatrick & DellaSala 2011, Tecklin et al. 2011) and represents an important economic and silvicultural resource (Heenan & Smissen 2013). Since the early 1900s, several Nothofagus species have been planted in the UK due to their fast growth and high wood quality (Danby 1991, Webber et al. 2011). Apart from very occasional infections by P. ramorum (Webber 2008), no other pests and pathogens have been recorded on Nothofagus. However, in 2009 severe dieback and mortality of Nothofagus trees, associated with bleeding cankers on stems and main branches, were reported in the UK (Scanu et al. 2012). Due to the similarity to aerial cankers caused by P. ramorum on F. sylvatica trees in the UK (Brown & Brasier 2007) this pathogen was initially thought to be the causal agent of this new disease outbreak. However, isolations from necrotic bark tissues revealed the presence of a homothallic Phytophthora species that was subsequently identified as P. pseudosyringae (Scanu & Webber 2016).
Nothofagus obliqua and N. alpina are the main species affected in the UK. Both species are affected by severe dieback and mortality of mature and young trees (Fig. 10a–c). At early stages, trees show symptoms of crown thinning, wilting and branch dieback (Fig. 10b, c), which are often associated with abundant proliferation of epicormic shoots on stems and branches. These symptoms are caused by up to 2 m long bleeding bark lesions on the main stem (Fig. 10d). Necrotic lesions often progress deep into the xylem (Fig. 10e) resulting in multiple bleeding aerial lesions along the stem and in branches, which may girdle the trunk and branches entirely, causing branch and crown dieback and wilting foliage. Up to 60 discrete lesions can be observed on a single tree and these can reach 16 m above ground level (Scanu & Webber 2016). In the canopy, shoot blight and necrosis on small branches, twigs and leaves are common (Fig. 10f–i). During dry conditions, lesion growth on small branches and twigs ceases, resulting in sunken cankers with cracking bark (Fig. 10f) and necrotic underlying xylem tissues. Although the pathogen was detected in rhizosphere soil samples, symptoms of root infection are largely absent (Scanu & Webber 2016).
Fig. 10.

Disease symptoms caused by Phytophthora pseudosyringae on Nothofagus spp. in the UK. a, b. Severe dieback and mortality of N. obliqua and N. alpina in mixed plantations in the UK; c. N. obliqua trees showing severe thinning, wilting and dieback of the crown and recent mortality; d. large, aerial bleeding canker with red-brown flame-shaped phloem lesion on the main stem of N. obliqua; e. deep xylem lesion on N. obliqua; f. sunken canker with cracking bark on a young N. alpina twig; g, h. N. obliqua twigs with dark necrotic bark lesions and orange-brown discoloration of the underlying necrotic phloem tissue (h); i. N. obliqua leaves with necrotic lesions resulting from aerial infections. — Photos: all B. Scanu.
Field observations and the high sporulation rate of P. pseudosyringae on Nothofagus foliage strongly indicate that aerial bark infections occur directly through penetration of intact bark with caducous sporangia produced on infected foliage and dispersed by wind and rain (Scanu & Webber 2016). At infected sites, aerial lesions are often observed on other host plants, including F. sylvatica and Vaccinium myrtillus (Beales et al. 2009, Denman et al. 2009b), most probably due to massive airborne inoculum coming from infected overstorey Nothofagus trees (Scanu & Webber 2016). Phytophthora pseudosyringae is characterised by having partial caducity of sporangia ranging from 10 to 90 % (Jung et al. 2003b). This enables the pathogen to be aerially dispersed and cause foliar and bark infections, as well as being a soil, water and root inhabitant. Previously in Europe, P. pseudosyringae was shown to be mainly involved in root and collar infections of alder, beech, chestnut and oak trees (Jung et al. 2003b, Cacciola et al. 2005, Denman et al. 2009b, Jung 2009, Scanu et al. 2010). However, in the humid forests of northern California and south-western Oregon, P. pseudosyringae has a similar lifestyle as P. ramorum, causing foliar infections on Umbellularia californica and aerial bark cankers on Notholithocarpus densiflorus and Quercus agrifolia (Wickland et al. 2008).
More recently, P. pseudosyringae has been detected associated with dieback and partial defoliation of N. obliqua in its natural range in central southern Chile (Fajardo et al. 2017). Main symptoms were similar to those reported from Nothofagus in Britain, with typical bleeding stem lesions and crown dieback, but twig and foliage infection were absent (Fajardo et al. 2017). Disease incidence seems to be lower in Chile compared to the UK (10 % vs up to 70 %). In inoculation trials under the bark of mature logs, and in zoospore inoculation trials of detached leaves, N. obliqua was highly susceptible to P. pseudosyringae isolates from the UK, while N. alpina was less susceptible (Scanu & Webber 2016). In both stem inoculation and soil infestation trials, isolates of P. pseudosyringae from Chile were highly aggressive to stems and roots of both N. obliqua and N. alpina (Fajardo et al. 2017). Nothofagus dombeyi was also shown to be susceptible to P. pseudosyringae in artificial inoculation experiments, although no natural infection has been reported so far (Fajardo et al. 2017).
The severity of P. pseudosyringae outbreaks on Nothofagus in the UK and its recent detection in native Nothofagus in South America, and the presence of P. pseudosyringae in the international nursery trade (Jung et al. 2016) highlight the risk of further spread of the pathogen into the Gondwanan Nothofagus forests in Chile, New Zealand and Tasmania. Previous records of Phytophthora spp. infecting Nothofagus spp. in their countries of origin include P. cinnamomi, associated with severe dieback and mortality of Nothofagus trees in the southern highlands of Papua New Guinea (Arentz 1983), and P. citrophthora and P. nicotianae, causing dieback of N. macrocarpa seedlings in a nursery in Chile (Valencia et al. 2011). Many natural forest ecosystems in the temperate regions of the southern Hemisphere, in particular in Chile, Argentina, New Guinea, Tasmania and New Zealand, are climatically favourable for inoculum production, spread and infections by airborne Phytophthora species (Scanu & Webber 2016), which may pose a serious risk to these native forests.
‘Sudden Oak Death’ and ‘Sudden Larch Death’ in the USA and the UK
‘Sudden Oak Death’ (SOD) is one of the most destructive epidemics of forest trees worldwide. This disease was first recorded in 1995 in Marine County, California, and subsequently spread across the relatively narrow coastal strip from Monterey county in central California to south-western Oregon (Goheen et al. 2002, Rizzo et al. 2002). In this area grow diverse temperate rainforests with an overstorey dominated by conifers like Sequoia sempervirens, Abies grandis, Pseudotsuga menziesii and Tsuga heterophylla and a mid- and understory layer of various broadleaved tree species including Notholithocarpus densiflorus (tanoak), Quercus agrifolia (coast live oak), Umbellularia californica (California bay laurel), Acer macrophyllum, Alnus rubra and Arbutus menziesii (Abrams & Ferris 1960, Knapp 1965, Lanner 1999).
The disease rapidly reached epidemic proportions in forests and in urban-forest interfaces in California, with a large number of N. densiflorus, Q. agrifolia, and to a lesser extent Q. kellogii and Q. parvula trees showing severe wilting and high mortality (Fig. 11a). First reports from forests in Oregon came in 2002 (Goheen et al. 2002). The causal organism was the heterothallic airborne pathogen P. ramorum which was first described from ornamental Rhododendron spp. and Viburnum tinus in Europe (Werres et al. 2001). The scale of devastation in California prompted national and international regulatory actions and local eradication efforts (Rizzo et al. 2005, Grünwald et al. 2012a, Hansen et al. 2012). Within a decade, extensive research had produced a rich body of knowledge on disease aetiology, infection biology, host range, pathways and population structure of P. ramorum. Over the last 20 years, the host list of P. ramorum has grown continuously exceeding to date 150 host species ranging from herbaceous plants and ferns to woody shrubs and trees (Grünwald et. al. 2012a). Extensive pathogenicity trials in Oregon demonstrated that 80 % of 49 native tree and shrub species tested were susceptible to P. ramorum (Hansen et al. 2005). Host lists, distribution of the pathogen in California and any new research findings are periodically updated on the web page www.suddenoakdeath.org.
Fig. 11.

Sudden Oak Death and Sudden Larch Death symptoms caused by Phytophthora ramorum in the USA (a–e) and in the UK (f, g), respectively. a. Severe wilting and mortality of tanoaks (Notholithocarpus densiflorus) in a mixed coastal forest with Sequoia sempervirens in California; b. necrotic lesions on leaves of California bay laurel (Umbellularia californica) in California; c. necrotic lesions on tanoak shoot in California; d. bleeding bark lesions on a tanoak stem in California; e. tanoak stem in California with multiple red-brown necrotic lesions of the inner bark; f. severe defoliation and mortality of a Japanese larch (Larix kaempferi) plantation in the UK; g. phloem lesion on a branch of a Japanese larch in the UK showing brown discoloration of older necrotic parts and a maroon-red advancing margin. — Photos: a, b, d: T. Jung; c, e: Y. Balci; f, g: A. Pérez-Sierra.
While the host list is extensive, the susceptibility and role of different hosts in disease development varies considerably and relatively few plant species are actually killed by P. ramorum. One important distinction is that between the ‘leaf or sporulation hosts’ and ‘canker hosts’ which are dead-end hosts due to the inability of P. ramorum to produce sporangia on infected bark (Garbelotto et al. 2003, Davidson et al. 2005, Rizzo et al. 2005, Grünwald et al. 2008). Several plant species like N. densiflorus fall into both categories. In the humid coastal forests of northern California and south-western Oregon, P. ramorum demonstrates the typical multicyclic infection pattern of Phytophthora diseases. The pathogen is infecting with its caducous sporangia and zoospores the leaves and young shoots of a wide range of herbaceous and woody species, causing necrotic lesions and shoot blight (Fig. 11b, c). On infected necrotic and symptomless tissues prolific numbers of sporangia are produced and dispersed via rain and wind splash onto other leaves, branches and stems (Rizzo et al. 2002, 2005, Grünwald et al. 2008, Denman et al. 2009a). Infectious propagules are produced readily on petioles and leaves in the crowns and are spread aerially as demonstrated by the isolations of P. ramorum from canopy drip baited in rainwater traps (Davidson et al. 2005, Hansen et al. 2012). Efficient aerial spread and infections occur within 10 m distance of an infected host (Davidson et al. 2005, Hansen et al. 2008), although the sporadic appearance of isolated disease outbreaks suggests that long-distance dispersal of sporangia with strong blowing winds and fog may sometimes incite new disease foci in distances of more than 4 km from infected trees (Peterson et al. 2015).
Foliar infections, in contrast to bark cankers, do not usually result in plant death, thus plant species that have susceptible foliage only are not eliminated, enabling continuous inoculum production (Garbelotto et al. 2017, Lione et al. 2017). Due to its common occurrence in coastal forests and the high sporulation capacity of P. ramorum on its leaves, California bay laurel is the main driver of inoculum build-up and, hence, the epidemic in California, whereas infected tanoak leaves are the most important source of inoculum in south-western Oregon (Davidson et al. 2005, Rizzo et al. 2002, 2005, Hansen et al. 2008, Peterson et al. 2015). The existence of genetically identical P. ramorum populations in forests separated by distances exceeding 50 km, strongly indicates long-distance spread of the pathogen via infested nursery stock and infested substrate adhering to boots and tyres (Davidson et al 2005, Mascheretti et al. 2008, Filipe et al. 2012, Grünwald et al. 2012a). Long-term survival and long-distance spread in infested soil particles is achieved with thick-walled chlamydospores produced abundantly in infected plant tissues (Werres et al. 2001, Rizzo et al. 2002, Shishkoff 2007, Grünwald et al. 2008). Phytophthora ramorum can also be isolated from soil and streams, but propagules in such ecological niches are not contributing to disease development (Peterson et al. 2014). On stems and branches of N. densiflorus, Q. agrifolia and other oak species, P. ramorum sporangia cause infections leading to bleeding cankers with red-brown lesions of the underlying phloem and cambium (Fig. 11d, e). Due to multiple cankers girdling large branches and the stem, affected trees die within a short time, often in considerable numbers (Fig. 11a).
Using data on host susceptibility, host species distribution and reproduction, dispersal, and climate suitability of P. ramorum, Meentemeyer et al. (2004) developed a rule-based model of P. ramorum establishment and spread risk in California plant communities. This model demonstrated high accuracy when spread risk predictions were compared to data about presence, absence and severity of the disease from field surveys. In 2017, citizen science – based SOD Blitz surveys documented in California a three-fold increase in infection rates following the ending of a long period of drought in 2015 (Meentemeyer et al. 2015, Anonymous 2017). This increase in disease incidence during the wet years 2016 and 2017 and the recovery of susceptible tree species during the preceding long-lasting drought are correlated with the El Niño-Southern Oscillation (ENSO) cycle, an ocean-atmosphere phenomenon which originates in the tropical Pacific (Ropelewski & Halpert 1987, 1989). This is highlighting the importance of climatic parameters in the development of disease epidemics by airborne Phytophthora pathogens like P. ramorum.
Two other airborne Phytophthora species, P. nemorosa and P. pseudosyringae, are widely distributed in forests in California and Oregon. Both species cause leaf necrosis on foliar hosts like California bay laurel and cankers on oaks and tanoaks indistinguishable from the symptoms caused by P. ramorum. However, cankers associated with P. nemorosa and P. pseudosyringae are less common, have a scattered distribution and much lower mortality levels compared to P. ramorum, possibly suggesting host-pathogen coevolution (Wickland et al. 2008, Hansen et al. 2012, Kozanitas et al. 2017). Results from a population genetic AFLP analysis supported this hypothesis for P. nemorosa while P. pseudosyringae appears to be of European origin (Linzer et al. 2009).
Although P. ramorum was detected in Europe in the early 1990s, its impact has long been restricted to Rhododendron spp., Viburnum spp. and other ornamentals in nurseries and amenity plantings (Werres et al. 2001, Ivors et al. 2006, Vercauteren et al. 2010, Jung et al. 2016). Using a climate matching model (CLIMEX) revised according to the Californian risk ranking model of Meentemeyer et al. (2004) to Europe, a high risk of P. ramorum infections has been predicted for the Atlantic regions along the western parts of the British islands, Portugal, Spain and North-western France (Anonymous 2007). In accordance with this prediction, P. ramorum has since 2003 been causing aerial bleeding lesions on individual trees of F. sylvatica, Aesculus hippocastanum and Q. rubra growing in close proximity to heavily infected R. ponticum foliage in the UK (Brasier et al. 2004, Denman et al. 2006, Brown & Brasier 2007, Webber 2008). In August 2009, in south-western England, extensive mortality on mature and juvenile Japanese larch (Larix kaempferi) was detected for the first time and found being associated with P. ramorum but not with infected Rhododendron foliage (Brasier & Webber 2010, Webber et al. 2010). The disease rapidly reached epidemic proportions and was soon named ‘Sudden Larch Death’ (SLD) (Brasier & Webber 2010). Larch trees (Larix spp.) are fast growing deciduous conifers from the family Pinaceae which are distributed in all cold-temperate and boreal zones of the northern hemisphere. In the UK, Japanese larch, European larch (L. decidua) and their hybrid (L. ×leptolepis) are all grown commercially for their durable timber with high mechanical strength and decay resistance. In 2010, the disease was also detected on Japanese larch in Scotland, Wales and Northern Ireland, and in 2011 P. ramorum was confirmed on European larch and on hybrid larch across the UK. This was the first record of P. ramorum causing lethal infection on a commercially important conifer species anywhere in the world. By the end of 2013, more than 3 million trees growing on over 10 000 hectares had been felled or were under notice to fell (Forest Research, unpubl. data). In Northern Ireland, it was recently demonstrated that the felling and removal of larch trees significantly reduces the inoculum of P. ramorum in the soil and successfully eradicates the pathogen from infested sites (O’Hanlon et al. 2017). SLD has a serious impact on the UK larch industry. Landowners suffer from significant losses through the destruction of immature crops, implementation of biosecurity measures and decreased timber values as the market is flooded with surplus supplies of felled larch (Harris 2014).
Initial symptoms of SLD on affected trees include purple or pale needle discolouration, wilting of short shoots, aborted buds, defoliation, dieback of branches or death of the entire crown (Fig. 11f). Crown symptoms are most pronounced in autumn. Resin bleeding can be observed on bark lesions of lateral shoots, branches and trunks of affected trees with brown discoloration and a deep pink to maroon-red margin of the underlying necrotic phloem (Fig. 11g) (Brasier & Webber 2010, Webber et al. 2010). On infected needles of all three larch species, P. ramorum produces very high numbers of 800 to almost 1 800 sporangia per cm2 making larch the best of all sporulation hosts known to date (Harris & Webber 2016). This massive sporulation on the infected needles is driving multicyclic infections of needles and bark resulting in rapid and large-scale mortality of larch (Fig. 11f). In addition, sporangia from infected larch needles cause massive infection pressure on other tree species growing under-neath or adjacent to infected larch trees (Brasier & Webber 2010, Harris & Webber 2016). Underneath infected larch trees, P. ramorum infections have been detected on broadleaved woody species such as Betula pendula, C. sativa, F. sylvatica, Nothofagus spp. and R. ponticum, and on other conifers such as Abies procera, A. grandis, Picea sitchensis, P. menziesii and T. heterophylla (Brasier & Webber 2010, Webber et al. 2010, Harris & Webber 2016). Climate and weather play a key role in the distribution and intensity of the disease on larch.
Initially, apart from the UK, the Republic of Ireland had been the only other country where larch trees were suffering from P. ramorum infections. However, in 2017, P. ramorum was for the first time detected affecting Japanese larch in mainland Europe in Brittany (France) (http://ephytia.inra.fr/fr/C/24935/Forets-Phytophthora-ramorum).
Phytophthora ramorum resides, together with P. lateralis, P. foliorum and P. hibernalis, within phylogenetic Clade 8c (Werres et al. 2001, Grünwald et al. 2008, Yang et al. 2017). There are four known clonal lineages named after the continent of their first appearance as EU1 and EU2 from Europe and NA1 and NA2 from North America (Ivors et al. 2006, Grünwald et al. 2008, Van Poucke et al. 2012). All European isolates of the EU1 and EU2 lineages belong to the A1 mating type while the North American NA1 and NA2 lineages contain exclusively A2 isolates (Brasier & Kirk 2004, Werres & Kaminski 2005, Vercauteren et al. 2011). In interlineage pairing tests of A1 and A2 isolates the mating frequency is extremely low and the resulting oospores have aberrant genome sizes and unusually high abortion rates, suggesting the presence of reproductive barriers (Brasier & Kirk 2004, Boutet et al. 2010, Vercauteren et al. 2011, Franceschini et al. 2014). This is supported by the results of a coalescence analysis indicating that the EU1, NA1 and NA2 lineages are separated since 165 000–500 000 yr (Goss et al. 2009). The apparent lack of sexual reproduction explains the clonal structure of the P. ramorum populations in Europe and the USA. In North America, three of the four known clonal lineages of P. ramorum are currently recognised: NA1, NA2 and EU1. The NA1 lineage most likely arrived in California in the 1990s via infected nursery stock imported from an unknown exotic origin, and has since spread throughout California and southwest Oregon as primary driver of the SOD epidemic (Grünwald et al. 2012a). Interestingly, the Oregon outbreak could genetically not be linked to the Californian NA1 population and, apparently, originates from a separate introduction of infested nursery plants (Prospero et al. 2007, Mascheretti et al. 2008, Grünwald et al. 2012a). The NA2 lineage was most likely introduced to nurseries in the PNW and occurs only infrequently in Californian forests (Ivors et al. 2006, Goss et al. 2011, Grünwald et al. 2012a). The EU1 lineage was introduced from Europe to the PNW where it is thriving in ornamental nurseries and eventually spread to California (Goss et al. 2011, Grünwald et al. 2012a). In the UK, the majority of the isolates from larch belong to the widespread EU1 lineage, while the EU2 lineage occurs currently only in Northern Ireland and in a small area in south-western Scotland (Van Poucke et al. 2012). The EU2 lineage shows faster growth and tolerates higher temperatures than the EU1 lineage (Franceschini et al. 2014). In addition, the EU2 lineage is also significantly more aggressive to larch bark tissue than the EU1 lineage (Harris et al. 2015) and, therefore, is likely to kill affected trees more rapidly (King et al. 2015). In 2016, P. ramorum was detected in several streams running through diverse mountain forests in northern Vietnam. Extensive mating tests demonstrated that the Vietnamese population contained both mating types, which together with the absence of apparent leaf symptoms and bleeding stem lesions in these forests suggest potential endemism of P. ramorum to Southeast Asia (T. Jung, M. Horta Jung, C.M. Brasier unpubl. data).
Leaf and twig blight of Ilex aquifolium in Europe and North America
Native to Atlantic regions of Europe and to southern Europe and western Asia, English holly (Ilex aquifolium) is an important component of the understorey vegetation in temperate Fagaceae forests and in cool and humid mountain ecosystems in Mediterranean regions (Pignatti 1982). This species is also widely planted as an ornamental plant and in hedgerows across Europe, and it is grown extensively in the PNW, USA for the production of holly cuttings and young trees.
In 1954, a previously unknown Phytophthora species was consistently isolated from black leaf spots, twig blight, berry infections and limb and trunk cankers of English holly orchards in Oregon and later also Washington, which were previously attributed to ascomycete fungi like Diaporthe crustosa, Vialaea insculpta and Fusarium spp. (Buddenhagen & Young 1957). This new species was described as P. ilicis, and a detailed description of the disease aetiology was given by Buddenhagen & Young (1957). In severe cases, the whole crown became completely defoliated with leaf shedding starting from the lower branches. No root or collar infection of P. ilicis were reported and the pathogen has never been detected from river water (Hansen et al. 2017).
Thirty years later, P. ilicis was recovered from Ilex spp. in parks and gardens of the UK, where it caused infections along hedges, and also from symptomatic nursery stock (Strouts et al. 1989). The pathogen was considered introduced to the country, probably during the 20th century (Tubby & Webber 2010). More recently, the pathogen has been reported causing twig blight on ornamental I. aquifolium in Galicia, Spain (Pintos et al. 2012) and in Germany from nursery plants (https://gd.eppo.int/reporting/article-5866).
Based on these reports, the geographic distribution of P. ilicis seemed to be restricted to cool-temperate regions (Buddenhagen & Young 1957, Strouts et al. 1989, Pintos et al. 2012). However, a recent study demonstrated the widespread occurrence of P. ilicis on I. aquifolium in natural forests of the Tyrrhenian islands Corsica and Sardinia (Scanu et al. 2014b). The symptomatology matches previous descriptions in the USA and Europe, including severe defoliation of the whole crown (Fig. 12a) and bleeding cankers on the main stem and branches (Fig. 12b). Necrotic twig lesions are often observed around the axillary node (Fig. 12c, e) and where the petiole is inserted (Fig. 12f), most likely due to the accumulation of zoospores produced by sporangia that emerge through stomata on the leaf surface and by growth of the pathogen through the petiole (Scanu et al. 2014b). Black leaf spots occur in the early decline stages (Fig. 12g), starting from branches close to the ground. Necrotic twig lesions develop as cankers with brownish orange, suberized and cracking epidermal tissues during the dry season (Fig. 12d, e). In these tissues, P. ilicis forms oospores, which allow the pathogen to survive dry summer conditions and germinate in the following wet season. Having caducous sporangia and a low optimum temperature for growth of 20 °C, P. ilicis infections occur mainly during the cool rainy period, from October to May, while the disease is completely inactive during summer (Buddenhagen & Young 1957, Scanu et al. 2014b).
Fig. 12.

Disease symptoms caused by Phytophthora ilicis on Ilex aquifolium in mountain forests of the Mediterranean islands Corsica and Sardinia. a. Mature trees showing complete defoliation and severe dieback; b. bleeding stem canker; c. fresh twig lesion around an axillary node and dead infected leaves; d. inactive twig canker with cracking surface due to the production of brown suberised tissue during the dry season; e. necrotic twig lesion originating from the progression of P. ilicis from the infected leaf through the petiole into the twig; f. leaf necrosis and progression of P. ilicis through the petiole into the twig; g. black necrotic leaf spots indicating multiple fresh infections. — Photos: all B. Scanu.
Since all previous records of the species worldwide came from horticultural, parks, gardens and nurseries, the widespread distribution of P. ilicis in natural ecosystems of Corsica and Sardinia strongly indicates endemism of this pathogen in the Mediterranean basin (Scanu et al. 2014b, Hansen et al. 2017). This hypothesis is supported by the fact that two close relatives of P. ilicis, P. pseudosyringae and P. psychrophila, are most likely also native in Europe (Jung et al. 2002, 2003b, 2016, Linzer et al. 2009, Pérez-Sierra et al. 2013, Hansen et al. 2017).
Needle cast and defoliation of Pinus radiata in Chile
Pinus radiata (Monterey pine) has a scattered limited natural distribution along the Pacific coast of central California and Baja Californica in Mexico (Rogers 2004, Rogers et al. 2006). On a global scale, it is one of the most common plantation trees in Mediterranean regions, in particular in Australia, Chile, New Zealand, South Africa and Spain (Rogers et al. 2006, Richardson et al. 2007). In 2004, a needle cast and defoliation disease, named ‘Daño foliar del pino’, was reported for the first time from 70 ha coastal P. radiata plantations in the Arauco province of central Chile (Durán et al. 2008). By 2006, the affected area had increased to almost 60 000 ha, with varying levels of damage. In 2007 the disease area decreased to less than 2 000 ha and has remained at that level (Durán et al. 2008, 2010, Ahumada et al. 2013). Currently, the disease occurs between Constitución and Valdivia, exclusively in areas with high humidity during most of the year due to their proximity to the Pacific coast (Ahumada et al. 2013). ‘Daño foliar del pino’, one of the most important foliar diseases affecting Monterrey pine, is caused by the previously unknown P. pinifolia which was the first Phytophthora species reported to cause needle infections on Pinus spp. (Durán et al. 2008). The pathogen has only been found in Chile where it exclusively affects P. radiata (Durán et al. 2008, 2010, Ahumada et al. 2012, 2013). The considerable variation of disease incidences and affected areas over the years is related to the El Niño cycle and has been explained using favourable days calculated with the Hyre model. With 141 d, the peak of favourable conditions was recorded in 2006, which correlated with the highest disease incidence recorded until now, while each of the following years had less than 60 favourable days coinciding with low incidence of the disease (Ahumada et al. 2013). Due to the lack of preformed sporangial pedicels, P. pinifolia is not a true airborne species. However, as in P. constricta in Western Australia, sporangia break off with relative ease enabling an aerial lifestyle (Durán et al. 2008, Rea et al. 2011). Phytophthora pinifolia survives non-favourable dry conditions in infected needles on the ground. After onset of humid conditions in spring or early winter, the pathogen infects needles on lower branches via sporangia formed on infected needles on the ground and spreading via rain and wind splash onto healthy needles. If humid conditions persist, P. pinifolia produces new sporangia on infected needles causing multicyclic infections and moving gradually up in the canopy (Fig. 13a–f) (Durán et al. 2008). In seedlings and trees younger than three years old, total defoliation occurs frequently resulting in plant death. In addition, the pathogen can progress in the shoots and the stem causing girdling lesions, leading to quick death of the trees. In trees older than three years the infection progress and the symptoms are similar but bark lesions are not girdling the stem and mortality is rare (Ahumada et al. 2012). On trees older than six years, the disease can affect all needles except those less than one year old (Fig. 13c–f). Needle infection is usually characterized by the presence of a black band (translucent areas) and a pale-green to greyish discolouration (Fig. 13g) turning brown at the end of spring (Ahumada et al. 2013). Long-dead needles have a pale greyish colour (Fig. 13e). The symptoms are generally best observed during the rainy season, from May to November, but heavy rain at the end of summer can induce an early appearance of symptoms.
Fig. 13.

Needle cast and defoliation caused by Phytophthora pinifolia on Pinus radiata in the Valdivian region of Chile. a. Mature plantation with extensive red-brown discolouration of infected foliage; b. mature plantation with largely defoliated crowns due to massive needle infections by P. pinifolia; c. extensive defoliation of mature crowns resulting from the progressive browning and shedding of infected needles which started at the lowest branches and spared only the youngest needles; d. recently killed tree with severe defoliation; e. dying young tree in a dense stand with greyish discolouration of infected needles; f. upper crown typically showing transparency due to shedding of older infected needles, pale-green discolouration of freshly infected needles and uninfected, dark-green young needles; g. needle showing typical black bands (translucent areas; arrow) within the pale-green to greyish area of infected tissue. — Photos: a–f: T. Jung; g: A. Durán.
Since 2008, another needle disease, named Red Needle Cast, with similar symptoms and disease aetiology has been reported in P. radiata plantations in New Zealand. However, in this case the airborne Phytophthora species P. pluvialis from Clade 3 was shown to be the causal agent (Dick et al. 2014).
Phytophthora pinifolia belongs to Clade 6, which contains mostly aquatic species and opportunistic soil- and waterborne pathogens of woody plants (Jung et al. 2011, Burgess et al. 2018). Currently, the only known foliage-infecting member of Clade 6 is P. pinifolia (Durán et al. 2008, Burgess et al. 2018). An amplified fragment length polymorphism (AFLP) analysis demonstrated a clonal population structure of P. pinifolia in Chile indicating a single clonal introduction of the pathogen (Durán et al. 2010). A character shared between P. pinifolia and many other Clade 6 species is the sterile breeding system (Durán et al. 2008) which is responsible for maintaining the clonal population structure of P. pinifolia in Chile and reduces the ability of the pathogen to adapt to changes in P. radiata resistance.
An integrated management approach for the needle disease caused by P. pinifolia includes the selection of tolerant P. radiata clones, species replacement in high-risk areas and fungicide application (Ahumada et al. 2013). Using a disease model, areas with high, medium and low risk of disease development have been identified. In high-risk areas, P. radiata plantations are being replaced by alternative species, in particular Eucalyptus spp., while in medium-risk areas tolerant P. radiata clones are planted. Alternatively, fungicides like mefenoxam and metalaxyl (phenylamides) and the fungi-static potassium phophite have proven suitable for disease control, reducing symptoms by up to 90 % and plant mortality to less than 5 % (Ahumada et al. 2013). Fungicides are mainly applied in one- or two-years old plantations to reduce the heavy infection pressure.
Despite the significantly reduced disease incidence since the peak in 2006 and the development of successful management strategies (Ahumada et al. 2013), P. pinifolia continues to be an important threat to the P. radiata industry worldwide. In Chile, the introduction of new genotypes or the appearance of mutations in the existing clonal population (Durán et al. 2010) could produce new pathotypes able to overcome the current genetic resistance or pesticide effectiveness, as has been reported for other Phytophthora spp. (Gisi & Cohen 1996, Goodwin et al. 1996). Since the origin of P. pinifolia is still unknown, the pathogen also poses a serious threat to natural populations of P. radiata and other pine species in their native ranges (Widmer & Dodge 2015).
CONCLUDING REMARKS
Concern about Phytophthora pathogens in natural and forest ecosystems began in the 1960s in Australia, where the invasive P. cinnamomi has been spreading for more than one century threatening some of the world’s richest plant communities in Western Australia and Victoria (Podger et al. 1965, Podger & Newhook 1971, Shearer & Tippett 1989, Marks & Smith 1991, Shearer et al. 2004, Hardham 2005). During the past 60 years, the detection of previously unknown Phytophthora diseases in natural and semi-natural ecosystems has increased exponentially (Fig. 1). Since the 1990s, the finding of P. cinnamomi and many other soilborne Phytophthora spp. involved in the oak declines in southern and central Europe (Brasier et al. 1993, Jung et al. 1996, 2000), the discovery of the airborne P. ramorum causing ‘Sudden Oak Death’ in the western USA (Rizzo et al. 2002), the resurgence of chestnut ink disease and the widespread alder mortality across Europe due to the P. ×alni hybrid complex (Vettraino et al. 2001, Brasier et al. 2004, Jung & Blaschke 2004, Husson et al. 2015), have raised the interest by many plant pathologists for this important diseases causing genus. Consequently, in 1999 the International Union of Forestry Research Organizations (IUFRO) Working Party 7.02.09 ‘Phytophthora Diseases of Forest Trees’ (https://www.iufro.org/science/divisions/division-7/70000/70200/70209/) was established which has subsequently become the main platform for researchers studying all aspects of Phytophthora pathogens and the diseases they are causing in forests and other natural woody ecosystems. This review provides the first comprehensive overview of the history, distribution, aetiology, symptomatology, dynamics and impact for the main diseases caused by Phytophthora species on woody host plants in natural ecosystems on a global scale, hence giving a baseline from which to identify and compare similar disorders in the future.
Most of the diseases presented in this review are caused by exotic invasive Phytophthora pathogens with a clear link between the ‘plants-for-planting’ pathway and subsequent impacts in natural ecosystems (Jules et al. 2002, Jung & Blaschke 2004, Brasier 2008, Chadfield & Pautasso 2012, Jung et al. 2016). There is an accumulating body of indirect and partly also direct evidences that P. cinnamomi, P. lateralis, P. plurivora and P. ramorum originate from Southeast and eastern Asia (Shearer & Tippett 1989, Brasier et al. 1993, 2010, 2012, Chang et al. 1996, Hansen et al. 2000, 2012, Jung et al. 2000, 2016, 2017a, b, c, Rizzo et al. 2002, Shearer et al. 2004, Goss et al. 2009, Hardham 2005, Jung 2009, Jung & Burgess 2009, Brasier & Webber 2010, Webber et al. 2010, 2012, Franceschini et al. 2014, Arentz 2017). Also for P. agathidicida in New Zealand P. austrocedri in Argentina and the UK, P. acerina and P. cactorum in Europe, P. elongata in Australia, P. kernoviae in the UK, P. multivora in Australia and Europe, P. pinifolia in Chile, P. ×cambivora in Europe and North America, and for the parents of P. ×alni, i.e., P. ×multiformis and P. uniformis, the high aggressiveness to native woody species, low genetic variability of pathogen populations and co-existence with healthy native vegetation in other continents, respectively, indicate exotic origin (Crandall et al. 1945, Jung et al. 2000, 2002, 2003b, 2016, 2017b, c, Brasier & Kirk 2001, Vettraino et al. 2001, 2005, Jung & Blaschke 2004, 2006, Brasier et al. 2005, Greslebin et al. 2007, 2010, Saavedra et al. 2007, Beever et al. 2009, Jung 2009, Scott et al. 2009, Durán et al. 2010, Rea et al. 2010, Green et al. 2013, Vélez et al. 2013, Ginetti et al. 2014, Henricot et al. 2014, Scott & Williams 2014, Weir et al. 2015). Recently, several studies identified the international plant trade as the main pathway for the introduction of invasive forest diseases into North America and Europe (Liebhold et al. 2012, Santini et al. 2013, Chapman et al. 2017). Accordingly, Jung et al. (2016) demonstrated almost ubiquitous infestations of nurseries and young plantings across Europe with a wide range of Phytophthora species. This study and similar results from Australia and the USA (Hardy & Sivasithamparam 1988, MacDonald et al. 1994, Davison et al. 2006, Schwingle et al. 2007, Yakabe et al. 2009, Bienapfl & Balci 2014, Parke et al. 2014, Yang et al. 2014, Simamora et al. 2015) leave no doubt that plant production facilities are the major source of Phytophthora spread into the wider environment. Due to the application of fungicides or fungistatic chemicals, nursery plants infected by Phytophthora spp. often appear visually symptomless and, hence, pass unnoticed through the phytosanitary controls acting as ‘inoculum reservoirs’ in the nurseries and resulting in accidental Phytophthora spread (Pérez-Sierra & Jung 2013, Bienapfl & Balci 2014, Migliorini et al. 2015, Jung et al. 2016). Another danger, arising from the intensified international nursery trade, is the accidental encounter of closely related allopatric Phytophthora species, which due to geographic separation have not build up reproductive barriers and readily hybridise. Such interspecific hybrids may differ in host range and virulence from the parental species as demonstrated by P. ×alni (Brasier & Kirk 2001, Brasier et al. 2004, Husson et al. 2015), thus making predictions about the potential effects of an ongoing invasion even more difficult.
Once introduced to a new suitable environment, a Phytophthora pathogen will inevitably spread, actively via root-to-root infection and with motile zoospores in soil and surface water and passively through the movement of infested soil or infested water. Therefore, the control and management of Phytophthora pathogens and diseases are mainly focused on the prevention of their introduction, and on slowing down their spread once they are introduced. Prevention of primary Phytophthora introductions can be achieved by testing nursery stock using classical isolation methods, sensitive high-throughput molecular detection methods and temporary outplanting in quarantine-facilities. When managing natural ecosystems, the only cost-effective and ethically acceptable approach is the use of plants from certified pathogen-free production facilities (Parke & Grünwald 2012). Preventative system approaches for the production of Phytophthora-free nursery stock in nurseries have been repeatedly suggested by the scientific community to the regulators (Brasier 2008, Parke & Grünwald 2012, Jung et al. 2016). Parke & Grünwald (2012) highlighted the importance to define the hazards for Phytophthora contamination within a nursery and employ best management practices to reduce the risk of infestation for all pathogens and pests. This systems approach demonstrated to improve the control of pathogens and pests in nursery production and prevent the movement of exotic pathogens or pests in the nursery trade. Training nursery growers to identify early symptoms minimizes risks. Thermo-sterilisation of potting media and the filtering or disinfestation of irrigation water also helps to reduce the risk of Phytophthora introductions (Stewart-Wade 2011, Pérez-Sierra & Jung 2013).
The current approach to prevent the movement of pests and pathogens via the nursery trade is mainly based on international plant biosecurity protocols, including certification, endpoint inspections for list-based pests and pathogens, and quarantine measures. However, these methods have largely failed to prevent the arrival of invasive and exotic pathogens (Brasier 2008, Liebhold et al. 2012, Santini et al. 2013, Jung et al. 2016, Eschen et al. 2017). For plants moving through the nursery trade, a phytosanitary certificate, indicating the production facility is free of regulated Phytophthora spp., is mandatory. In the absence of a certificate, a visual inspection of plants has to be performed (Brasier 2008). However, visual inspections of symptoms are costly and often ineffective, especially since they are based on lists of known harmful species despite the fact that the majority of introduced aggressive Phytophthora species were unknown to science before they caused serious damages in their new environments. In addition, the regular use of fungicides and fungistatic chemicals in nurseries is decreasing disease incidences without eliminating the Phytophthora pathogens. This practice is masking the presence of the pathogens, which are surviving with enduring resting structures, resulting in plantings of visually healthy nursery stock that develop disease symptoms in subsequent years (Jung & Blaschke 2004, Brasier 2008, Pérez-Sierra & Jung 2013, Migliorini et al. 2015, Jung et al. 2016). In a critical review, Brasier (2008) underlined the need for a scientific revision of the international plant biosecurity protocols. The scientific community recommended replacing the list-based species-by-species regulation approach by a more efficient pathway regulation approach based on pathway risk analyses and combined with a strict quarantine system (Keller et al. 2007, Jung et al. 2016).
In natural ecosystems, there are a number of possible strategies to mitigate the impact of Phytophthora species. Phosphite (phosphoric acid) applications are the most common and most successful method for controling dieback of trees and forests resulting from extensive fine root losses caused by Phytophthora infections and slowing down disease spread (Shearer & Tippett 1989, Fernandez-Escobar et al. 1999, Pilbeam et al. 2000, Hardy et al. 2001, Tynan et al. 2001, Smith 2003, Shearer & Fairman 2007, Barrett & Rathbone 2018). Several early studies demonstrated that treatments with phosphite indirectly control Phytophthora diseases by acting on host physiology and on host-pathogen interactions (Fenn & Coffey 1984, Guest & Grant 1991), but the exact mode of action had long been a mystery. Recent studies have elucidated that phosphite induces systemic activity against Phytophthora spp. in plants mainly by priming plants for a rapid and intense response to infection via up-regulation of several defence-related genes in the jasmonate, salicylic acid, ethylene and auxin signalling pathways (Eshragi et al. 2011, 2014a, Dalio et al. 2014). However, it was suggested that phosphite-induced resistance to P. cinnamomi in susceptible Arabidopsis thaliana ecotypes and natural resistance of tolerant ecotypes may be triggered through different signaling pathways (Eshragi et al. 2014b). Generally, phosphite appears to be more effective for soilborne than for airborne Phytophthora diseases, although phosphite applications can significantly reduce canker size in tanoaks infected by P. ramorum (Garbelotto et al. 2009). The efficacy of the treatments is higher when plants are physiologically active (Pilbeam et al. 2000). Phosphite can be applied by injecting a water dilution directly into the trees (Fernandez-Escobar et al. 1999) or through foliar applications, with or without surfactants, although not all evergreen plants with waxy leaves absorb phosphite (Shearer & Fairman 2007). Application rates vary depending on the application mode, the host plants and the region (Hardy et al. 2001, Smith 2003, Shearer et al. 2006, Garbelotto et al. 2009). Overdosing commonly leads to phytotoxicity (Thao & Yamakawa 2009). Although phosphite applications can slow down disease spread and protect individual plants (Hardy et al. 2001), they cannot completely halt Phytophthora spread and disease progression in native vegetation (Shearer et al. 2004). Silvicultural and site-specific management measures, like soil amendments and reducing host plant density, have a proven effect in reducing disease incidence and/or slowing down further disease spread (Erwin & Ribeiro 1996, Pérez-Ramos et al. 2008). Thinning should be more intensive in those areas predicted to be more easily infested, such as areas downhill or downstream from outbreaks. If multiple plant species are present at an infested site, selective thinning of the best sporulation host will have the most significant impact on disease spread (Serrano et al. 2010, Fichtner et al. 2011). Containment and eradication of soilborne Phytophthora species from spot infestations in natural ecosystems can be achieved by a combination of robust treatments including host plant destruction with herbicides, application of selective fungicides, fumigation with strong biocides like metham-sodium, and mechanical root barriers (Dunstan et al. 2009). However, due to the lack of legal authorisations and for environmental reasons, such treatments are not realistic options for the control of Phytophthora diseases in natural areas of most countries.
On the long-term, increasing the genetic resistance of susceptible tree species against Phytophthora spp. seems to be the most promising sustainable management approach for stabilising declining natural ecosystems and reintroducing susceptible tree species at sites with high disease impact. The successful long-term resistance screening programme of C. lawsoniana concerning P. lateralis, can serve as a role model for other Phytophthora diseases (Hansen et al. 2000, 2011, Oh et al. 2006, Sniezko et al. 2006, 2011). Also for the pathosystems Castanea / P. cinnamomi and P. ×cambivora, A. glutinosa / P. ×alni, and E. marginata / P. cinnamomi the natural occurrence of genetic resistance has been demonstrated (Jung & Blaschke 2006, Robin et al. 2006, Miranda-Fontaíña et al. 2007, Costa et al. 2011, Santos et al. 2015, 2017a, b, Shearer et al. 2014, Chandelier et al. 2016).
Recently, the potential origin of several invasive Phytophthora species, including P. cinnamomi, P. lateralis, P. plurivora and P. ramorum, in Southeast Asia has been unravelled (Brasier et al. 2010, 2012, Huai et al. 2013, Jung et al. 2017a, c, T. Jung, C.M. Brasier, M. Horta Jung unpubl. data). Comparative phenotypic and molecular studies of the invasive and native populations of these pathogens will advance our understanding of their adaptation and evolution after introduction to new environments. In addition, future studies of the behaviour and ecological role of these pathogens and their interaction with the native vegetation in their centres of origin will help to elucidate the factors that shaped their outstanding invasiveness. This knowledge will help improving management concepts for the diseases these pathogens are causing. Moreover, tolerant or resistant species from tree genera affected by these pathogens in other continents may be found in the centres of origin which could be used in resistance screening programmes. Finally, natural antagonists which may be used as biological control agents in invasive situations, may be detected in the future.
Whether and how the projected future changes in temperature and precipitation patterns will affect the spread and activity of Phytophthora pathogens on the global and the regional scales remains unknown. However, using the CLIMEX model a significant increase in the activity of P. cinnamomi and the area suitable for this pathogen has been proposed (Brasier & Scott 1994, Brasier 1996, Burgess et al. 2017). Due to the known interaction between Phytophthora-caused fine root losses and droughts, and the multicyclic spread of Phytophthora zoospores and sporangia during persisting humid conditions, rising temperatures, in particular during winter, and increased summer droughts alternating with periods of unseasonal heavy rain, predicted by several models and extrapolations of climatic trends of the 20th century (Schönwiese et al. 1994, Rapp & Schönwiese 1995, Houghton et al. 1996, Watson et al. 1996, 1998, Pachauri & Reisinger 2007, Battles et al. 2008, Giannakopoulos et al. 2009, Ozturk et al. 2015), will most likely intensify root and collar rot incidences. This will further destabilise Phytophthora-infested natural ecosystems. Therefore, effective plant biosecurity protocols to prevent further introductions and spread of Phytophthora pathogens, management concepts and control measures to mitigate the impact of invasive Phytophthora diseases in natural ecosystems, and resistance screening programmes are urgently required.
Acknowledgments
The authors are grateful to the Czech Ministry for Education, Youth and Sports and the European Regional Development Fund for financing the Project Phytophthora Research Centre Reg. No. CZ.02.1.01/0.0/0.0/15_003/0000453.
REFERENCES
- Abbott I. 1999. “Dieback” in flooded gum. Western Wildlife 3: 3. [Google Scholar]
- Abrams L, Ferris R. 1960. Illustrated flora of the Pacific states. Stanford University Press, Stanford, California, USA. [Google Scholar]
- Abrams MD. 2003. Where has all the white oak gone? BioScience 53: 927–939. [Google Scholar]
- Acedo A, Cardillo E, Pérez MC, et al. 2013. First report of Phytophthora cinnamomi associated with mortality of Erica umbellata natural shrubs in Spain. New Disease Reports 28: 8. [Google Scholar]
- Adams GC, Catal M, Trummer L. 2010. Distribution and severity of alder Phytophthora in Alaska. In: Frankel SJ, Kliejunas T, Palmieri KM. (eds), Proceedings of the Sudden Oak Death Fourth Science Symposium, General Technical Report PSW-GTR-229: 29–49. USDA Forest Service, Albany, California, USA. [Google Scholar]
- Aghighi S, Hardy GEStJ, Scott J, et al. 2012. Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. European Journal of Plant Pathology 133: 841–855. [Google Scholar]
- Agnihothrudu V. 1975. Abnormal leaf fall of rubber. In: Raychaudhuri SP, Varma A, Bhargava KS, et al. (eds), Advances in mycology and plant pathology: 223–230. Harsh Kumar at Sagar Printers, New Delhi, India. [Google Scholar]
- Agrios GN. 2005. Plant pathology. 5th edition. Academic Press, San Diego, California. [Google Scholar]
- Aguayo J, Adams GC, Halkett F, et al. 2013. Strong genetic differentiation between North American and European populations of Phytophthora alni subsp. uniformis. Phytopathology 103: 190–199. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Ogden J. 1987. Population dynamics of the emergent conifer Agathis australis (D.Don) Lindl. (kauri) in New Zealand. I. Population structures and tree growth rates in mature stands. New Zealand Journal of Botany 25: 217–229. [Google Scholar]
- Ahrens U, Seemüller E. 1994. Detection of mycoplasma-like organisms in declining oaks by polymerase chain reaction. European Journal of Forest Pathology 24: 55–63. [Google Scholar]
- Ahumada R, Rotella A, Poisson MA, et al. 2013. Phytophthora pinifolia: the cause of Daño Foliar del Pino on Pinus radiata in Chile. In: Lamour K. (ed), Phytophthora: A global perspective: 159–165. CABI, Wallingford, UK. [Google Scholar]
- Ahumada R, Rotella A, Slippers B, et al. 2012. Potential of Phytophthora pinifolia to spread via sawn green lumber: a preliminary investigation. Southern Forests: a Journal of Forest Science 74: 211–216. [Google Scholar]
- Anonymous. 2007. Final report of the project ‘Risk analysis for Phytophthora ramorum, a newly recognised pathogen threat to Europe and the cause of Sudden Oak Death in the USA (RAPRA)’. Forest Research, Farnham, UK. [Google Scholar]
- Anonymous. 2017. California Oak Mortality Task Force Report to the Board of Forestry November 2017. www.suddenoakdeath.org/wp-content/uploads/2017/11/COMTF-Report-November-2017-4.pdf. Last accessed 28 January 2018.
- Archibald R. 2006. Fire and the persistence of tuart woodlands. PhD thesis, Murdoch University, Perth, Australia. [Google Scholar]
- Arentz F. 1983. Nothofagus dieback on Mt. Giluhe, Papua New Guinea. Pacific Science 37: 453–458. [Google Scholar]
- Arentz F. 2017. Phytophthora cinnamomi A1: An ancient resident of New Guinea and Australia of Gondwanan origin? Forest Pathology 47: e12342 doi: https://doi.org/10.1111/efp.12342. [Google Scholar]
- Arentz F, Simpson JA. 1986. Distribution of Phytophthora cinnamomi in Papua New Guinea and notes on its origin. Transactions of the British Mycological Society 87: 289–295. [Google Scholar]
- Balci Y, Balci S, Blair JE, et al. 2008a. Phytophthora quercetorum sp. nov., a novel species isolated from eastern and north-central USA oak forest soils. Mycological Research 112: 906–916. [DOI] [PubMed] [Google Scholar]
- Balci Y, Balci S, Eggers J, et al. 2007. Phytophthora spp. associated with forest soils in Eastern and North-Central U.S. oak ecosystems. Plant Disease 91: 705–710. [DOI] [PubMed] [Google Scholar]
- Balci Y, Balci S, MacDonald WL. et al. 2008b. Relative susceptibility of oaks to seven species of Phytophthora isolated from oak forest soils. Forest Pathology 38: 394–409. [Google Scholar]
- Balci Y, Halmschlager E. 2003a. Incidence of Phytophthora species in oak forests in Austria and their possible involvement in oak decline. Forest Pathology 33: 157–174. [Google Scholar]
- Balci Y, Halmschlager E. 2003b. Phytophthora species in oak ecosystems in Turkey and their association with declining oak trees. Plant Pathology 52: 694–702. [Google Scholar]
- Balci Y, Long RP, Mansfield M, et al. 2010. Involvement of Phytophthora species in white oak (Quercus alba) decline in southern Ohio. Forest Pathology 40: 430–442. [Google Scholar]
- Barrett S, Rathbone D. 2018. Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology. In press. doi: https://doi.org/10.1111/aec.12574.
- Battles JJ, Robards T, Das A, et al. 2008. Climate change impacts on forest growth and tree mortality: a data-driven modeling study in the mixed-conifer forest of the Sierra Nevada, California. Climatic Change 87: 193–231. [Google Scholar]
- Beakes GW, Glockling SL, Sekimoto S. 2012. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 249: 3–19. [DOI] [PubMed] [Google Scholar]
- Beakes GW, Honda T, Thines M. 2014. Systematics of the Stramenipila: Labyrinthulomycota, Hyphochytridiomycota, and Oomycota. In: McLaughlin DJ, Spatafora J. (eds), Systematics and Evolution: 39–97. Springer, New York. [Google Scholar]
- Beales P, Giltrap PM, Webb KM, et al. 2009. A further threat to UK heathland bilberry Vaccinium myrtillus by Phytophthora pseudosyringae. Plant Pathology 59: 406. [Google Scholar]
- Beever RE, Waipara NW, Ramsfield TD, et al. 2009. Kauri (Agathis australis) under threat from Phytophthora? In: Goheen EM, Frankel SJ. (eds), Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09, General Technical Report PSW-GTR-221: 74–85. USDA Forest Service, Pacific Southwest Research Station, Albany, California. [Google Scholar]
- Belbahri L, Moralejo E, Calmin G, et al. 2006. Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiological Letters 261: 165–174. [DOI] [PubMed] [Google Scholar]
- Belhaj R, McComb J, Burgess TI, et al. 2018. Pathogenicity of 21 newly described Phytophthora species against seven Western Australian native plant species. Plant Pathology. In press. doi: https://doi.org/10.1111/ppa.12827.
- Bellgard SE, Pennycook SR, Weir BS, et al. 2016. Phytophthora agathidicida. Forest Phytophthoras 6 (1). doi: https://doi.org/10.5399/osu/fp.5.1.3748. [Google Scholar]
- Bertier L, Leus L, D’hondt L, et al. 2013. Host adaptation and speciation through hybridization and polyploidy in Phytophthora. PloS ONE 8: e85385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezuidenhout CM, Denman S, Kirk SA, et al. 2010. Phytophthora taxa associated with cultivated Agathosma, with emphasis on the P. citricola complex and P. capensis sp. nov. Persoonia 25: 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienapfl JC, Balci Y. 2014. Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Disease 98: 134–144. [DOI] [PubMed] [Google Scholar]
- Blair JE, Coffey MD, Park S-Y, et al. 2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45: 266–277. [DOI] [PubMed] [Google Scholar]
- Boutet X, Vercauteren A, Heungens K, et al. 2010. Oospore progenies from Phytophthora ramorum. Fungal Biology 114: 369–378. [DOI] [PubMed] [Google Scholar]
- Brasier CM. 1996. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Annales des Sciences Forestière 53: 347–358. [Google Scholar]
- Brasier CM. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57: 792–808. [Google Scholar]
- Brasier C[M] 2009. Phytophthora biodiversity: How many Phytophthora species are there? In: Goheen EM, Frankel SJ. (eds), Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09, General Technical Report PSW-GTR-221: 101–115. USDA Forest Service, Pacific Southwest Research Station, Albany, California. [Google Scholar]
- Brasier CM, Beales PA, Kirk SA, et al. 2005. Phytophthora kernoviae sp. nov. an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in Britain. Mycological Research 109: 853–859. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM. 1999. Origins of a new Phytophthora pathogen through interspecific hybridisation. Proceedings of the National Academy of Sciences, USA 96: 5878–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM, et al. 2003. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides – P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycological Research 107: 277–290. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Denman S, Brown A, et al. 2004. Sudden Oak Death (Phytophthora ramorum) discovered on trees in Europe. Mycological Research 108: 1107–1110. [Google Scholar]
- Brasier CM, Franceschini S, Vettraino AM, et al. 2012. Four phenotypically and phylogenetically distinct lineages in Phytophthora lateralis. Fungal Biology 116: 1232–1249. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Jung T. 2003. Progress in understanding Phytophthora diseases of trees in Europe. In: McComb JA, Hardy GEStJ, Tommerup I. (eds), Phytophthora in Forests and Natural Ecosystems: 4–18. Murdoch University, Perth, Australia. [Google Scholar]
- Brasier CM, Kirk SA. 2001. Comparative aggressiveness of standard and variant hybrid alder phytophthoras, Phytophthora cambivora and other Phytophthora species on bark of Alnus, Quercus and other woody hosts. Plant Pathology 50: 218–229. [Google Scholar]
- Brasier CM, Kirk SA. 2004. Production of gametangia by Phytophthora ramorum in vitro. Mycological Research 108: 823–827. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Kirk SA, Delcan J, et al. 2004. Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycological Research 108: 1172–1184. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Robredo F, Ferraz JFP. 1993. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathology 42: 140–145. [Google Scholar]
- Brasier CM, Rose J, Gibbs JN. 1995. An unusual Phytophthora associated with widespread alder mortality in Britain. Plant Pathology 44: 999–1007. [Google Scholar]
- Brasier CM, Scott JK. 1994. European oak declines and global warming: a theoretical assessment with special reference to the activity of Phytophthora cinnamomi. Bulletin OEPP/EPPO Bulletin 24: 221–234. [Google Scholar]
- Brasier CM, Vettraino AM, Chang TT, et al. 2010. Phytophthora lateralis discovered in an old growth Chamaecyparis forest in Taiwan. Plant Pathology 59: 595–603. [Google Scholar]
- Brasier C[M], Webber J. 2010. Sudden larch death. Nature 466: 824–825. [DOI] [PubMed] [Google Scholar]
- Brown AV, Brasier CM. 2007. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathology 56: 227–241. [Google Scholar]
- Brown B. 1999. Occurrence and impact of Phytophthora cinnamomi and other Phytophthora species in rainforests of the Wet Tropics World Heritage Area, and of the Mackay region, Qld. In: Gadek PA. (ed), Patch deaths in tropical Queensland rainforests: association and impact of Phytophthora cinnamomi and other soil borne organisms: 41–76. Cooperative Research Centre for Tropical Rainforest Ecology and Management, Cairns, Australia. [Google Scholar]
- Buddenhagen IW, Young RA. 1957. Leaf and twig disease of English holly caused by Phytophthora ilicis n. sp. Phytopathology 47: 95–100. [Google Scholar]
- Burgess T[I], McComb JA, Colquhoun I, et al. 1999. Increased susceptibility of Eucalyptus marginata to stem infection by Phytophthora cinnamomi resulting from root hypoxia. Plant Pathology 48: 797–806. [Google Scholar]
- Burgess TI, Simamora AV, White D, et al. 2018. New species from Phytophthora Clade 6a: evidence for recent radiation. Persoonia 41: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TI, Webster JL, Ciampini JA, et al. 2009. Re-evaluation of Phytophthora species isolated during 30 years of vegetation health surveys in Western Australia using molecular techniques. Plant Disease 93: 215–223. [DOI] [PubMed] [Google Scholar]
- Burgess TI, White D, McDougall KM, et al. 2017. Distribution and diversity of Phytophthora across Australia. Pacific Conservation Biology 23: 1–13. [Google Scholar]
- Cacciola SO, Motta E, Raudino F, et al. 2005. Phytophthora pseudosyringae the causal agent of bleeding cankers of beech in central Italy. Journal of Plant Pathology 87: 289. [Google Scholar]
- Cahill DM, Rookes JE, Wilson BA, et al. 2008. Turner Review No. 17. Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56: 279–310. [Google Scholar]
- Camilo-Alves C, Da Clara MI, Ribeiro N. 2013. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: a review. European Journal of Forest Research 132: 411–432. [Google Scholar]
- Català S, Pérez-Sierra A, Abad-Campos P. 2015. The use of genus-specific amplicon pyrosequencing to assess Phytophthora species diversity using eDNA from soil and water in Northern Spain. PLoS ONE 10: e0119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Černý K, Filipová N, Strnadová V. 2012. Influence of low temperature and frost duration on Phytophthora alni subsp. alni viability. Forest Systems 21: 337–342. [Google Scholar]
- Černý K, Gregorová B, Strnadová V, et al. 2008. Phytophthora cambivora causing ink disease of sweet chestnut recorded in the Czech Republic. Czech Mycology 60: 265–274. [Google Scholar]
- Černý K, Strnadová V. 2010. Phytophthora alder decline: disease symptoms, causal agent and its distribution in the Czech Republic. Plant Protection Science 46: 12–18. [Google Scholar]
- Černý K, Strnadová V, Gregorova B, et al. 2009. Phytophthora cactorum causing bleeding canker of common beech, horse chestnut, and white poplar in the Czech Republic. Plant Pathology 58: 394. [Google Scholar]
- Chadfield V, Pautasso M. 2012. Phytophthora ramorum in England and Wales: which environmental variables predict county disease incidence? Forests Pathology 42: 150–159. [Google Scholar]
- Chandelier A, Husson C, Druart P, et al. 2016. Assessment of inoculation methods for screening black alder resistance to Phytophthora × alni. Plant Pathology 65: 441–450. [Google Scholar]
- Chang TT, Wang WW, Wang WY. 1996. Use of random amplified polymorphic DNA markers for the detection of genetic variation in Phytophthora cinnamomi in Taiwan. Botanical Bulletin of the Academia Sinica 37: 165–171. [Google Scholar]
- Chapman D, Purse BV, Roy HE, et al. 2017. Global trade networks determine the distribution of invasive non-native species. Global Ecology and Biogeography 26: 907–917. [Google Scholar]
- Claessens H. 2003. The alder populations of Europe. In: Gibbs JN, Van Dijk C, Webber JF. (eds), Phytophthora disease of alder in Europe. Forestry Commission Bulletin 126: 5–14. Edinburgh, UK. [Google Scholar]
- Clay R, Majer J. 2001. Flooded Gum (Eucalyptus rudis) decline in the Perth Metropolitan area: A preliminary assessment. Curtin University of Technology, Perth, WA: School of Environmental Biology Bulletin No. 19. [Google Scholar]
- Collins S, McComb JA, Howard K, et al. 2011. The long term survival of Phytophthora cinnamomi in mature Banksia grandis killed by the pathogen. Forest Pathology 42: 28–36. [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM, et al. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology 30: 17–32. [DOI] [PubMed] [Google Scholar]
- Corcobado T, Cubera E, Pérez-Sierra A, et al. 2010. First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Disease Reports 22: 33. [Google Scholar]
- Corcobado T, Miranda-Torres JJ, Martín-García J, et al. 2017. Early survival of Quercus ilex subspecies from different populations after infections and co-infections by multiple Phytophthora species. Plant Pathology 66: 792–804. [Google Scholar]
- Costa R, Santos C, Tavares F, et al. 2011. Mapping and transcriptomic approaches implemented for understanding disease resistance to Phytophthora cinnamomi in Castanea sp. BMC Proceedings 5: O18 doi: https://doi.org/10.1186/1753-6561-5-S7-O18. [Google Scholar]
- Cowling RM, Rundel PW, Lamont BB, et al. 2006. Plant diversity in mediterranean-climate regions. Tree 11: 362–366. [DOI] [PubMed] [Google Scholar]
- Crandall BS. 1950. The distribution and significance of the chestnut root rot Phytophthoras, P. cinnamomi and P. cambivora. Plant Disease Reporter 34: 194–196. [Google Scholar]
- Crandall BS, Gravatt GF, Ryan MM. 1945. Root disease of Castanea species and some coniferous and broadleave nursery stocks, caused by Phytophthora cinnamomi. Phytopathology 35: 162–180. [Google Scholar]
- Crombie DS, Tippett JT, Gorddard DJ. 1987. Water relations of root pruned jarrah (Eucalyptus marginata Sm.) saplings. Australian Journal of Botany 35: 653–663. [Google Scholar]
- Crone M, McComb JA, O’Brien PA, et al. 2013. Survival of Phytophthora cinnamomi as oospores, stromata and thick walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology 117: 112–123. [DOI] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, et al. 2011. Fungal Planet description sheets: 69–91. Persoonia 26: 108–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012. Fungal Planet description sheets: 107–127. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Le Roux JJ, et al. 2015. Fungal Planet description sheets: 371–399. Persoonia 35: 264–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, et al. 2014. Fungal Planet description sheets: 281–319. Persoonia 33: 212–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington JH, De Alwis S, Pascoe IG, et al. 2005. The ‘asparagus’ Phytophthora infecting members of the Agavaceae at the Royal Botanic Gardens, Melbourne. Australasian Plant Pathology 34: 413–414. [Google Scholar]
- Curry SJ. 1981. The association of insects with eucalypt dieback in Southwestern Australia. In: Old KM, Kile GA, Omart CP. (eds), Eucalypt dieback in forests and woodlands: 130–133. CSIRO, Melbourne, Australia. [Google Scholar]
- Dalio RJD, Fleischmann F, Humez M, et al. 2014. Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLOS one 9: e87860 doi: https://doi.org/10.1371/journal.pone.0087860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danby NP. 1991. Nothofagus in Wales. Quarterly Journal of Forestry 85: 103–116. [Google Scholar]
- Davidson JM, Wickland AC, Patterson H, et al. 2005. Transmission of Phytophthora ramorum in mixed evergreen forests in California. Phytopathology 95: 587–596. [DOI] [PubMed] [Google Scholar]
- Davison EM. 1988. The role of waterlogging and Phytophthora cinnamomi in the decline and death of Eucalyptus marginata in Western Australia. GeoJournal 17.2: 239–244. [Google Scholar]
- Davison EM, Drenth A, Kumar S, et al. 2006. Pathogens associated with nursery plants imported into Western Australia. Australasian Plant Pathology 35: 473–475. [Google Scholar]
- Day WR. 1938. Root-rot of sweet chestnut and beech caused by species of Phytophthora. I. Cause and symptoms of disease: Its relation to soil conditions. Forestry 12: 101–116. [Google Scholar]
- De Cock AWAM, Lodhi AM, Rintoul TL, et al. 2015. Phytopythium: molecular phylogeny and systematics. Persoonia 34: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnen-Schmutz K, Holdenrieder O, Jeger MJ, et al. 2010. Structural change in the international horticultural industry: some implications for plant health. Scientia Horticulturae 125: 1–15. [Google Scholar]
- Delatour C. 1983. Les dépérissements de chênes en Europe. Revue Forestière Francaise 15: 265–282. [Google Scholar]
- Denman S, Kirk S, Moralejo E, et al. 2009a. Phytophthora ramorum and Phytophthora kernoviae on naturally infected asymptomatic foliage. Bulletin OEPP/EPPO Bulletin 39: 105–111. [Google Scholar]
- Denman S, Rose J, Slippers B. 2009b. Phytophthora pseudosyringae found on European beech and hornbeam trees in the UK. In: Goheen EM, Frankel SJ. (eds), Proceedings of the Fourth Meeting of the IUFRO Working Party S07.02.09: Phytophthoras in Forests and Natural Ecosystems. General Technical Report PSW-GTR-221: 273–280. USDA Forest Service, Albany, California. [Google Scholar]
- Denman S, Whybrow A, Orton E, et al. 2006. Phytophthora kernoviae and P. ramorum: host susceptibility and sporulation potential on foliage of susceptible trees. Bulletin OEPP/EPPO Bulletin 36: 373–376. [Google Scholar]
- Desprez-Loustau ML, Robin C, Reynaud G, et al. 2010. Simulating the effects of a climate-change scenario on the geographical range and activity of forest-pathogenic fungi. Canadian Journal of Plant Pathology 29: 101–120. [Google Scholar]
- Dick MA, Williams NM, Bader MK, et al. 2014. Pathogenicity of Phytophthora pluvialis to Pinus radiata and its relation with red needle cast disease in New Zealand. New Zealand Journal of Forestry Science 44: 6. [Google Scholar]
- Dick MW. 2001. Straminipilous fungi: systematics of the Peronosporomycetes including accounts of the marine straminipilous protists, the plasmodiophorids and similar organisms. Kluwer Academic Publishers, Dordrecht, the Netherlands. [Google Scholar]
- Dobrowolski MP, Tommerup IC, Blakeman HD. 2003. Non-mendelian inheritance revealed in a genetic analysis of sexual progeny of Phytophthora cinnamomi with microsatellite markers. Fungal Genetics and Biology 35: 197–212. [DOI] [PubMed] [Google Scholar]
- Drew J, Anderson N, Andow D. 2010. Conundrums of a complex vector for invasive species control: a detailed examination of the horticultural industry. Biological Invasions 12: 2837–2851. [Google Scholar]
- Dunstan WA, Rudman T, Shearer BL, et al. 2009. Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biological Invasions 12: 913–925. [Google Scholar]
- Durán A, Gryzenhout M, Slippers B, et al. 2008. Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathology 57: 715–727. [Google Scholar]
- Durán A, Slippers B, Gryzenhout M, et al. 2010. AFLP analysis reveals a clonal population of Phytophthora pinifolia in Chile. Fungal Biology 114: 746–752. [DOI] [PubMed] [Google Scholar]
- Edwards K, Dunstan W, Jung T, et al. 2010. Phytophthora species associated with declining Eucalyptus rudis (Flooded gum) in Western Australia. In: Book of Abstracts from the 5th Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09, Phytophthora Diseases in Forests and Natural Ecosystems, 7–12 March, Auckland and Rotorua, New Zealand: 15. [Google Scholar]
- Edwards T. 2004. Environmental correlates and associations of tuart (Eucalyptus gomphocephala DC.) decline. Masters thesis, Edith Cowen University, Perth, Australia. [Google Scholar]
- Ellenberg H, Leuschner C. 2010. Vegetation Mitteleuropas mit den Alpen. 6th edition. Ulmer, UTB, Stuttgart. [Google Scholar]
- Enright NJ, Lamont BB. 1992. Survival, growth and water relations of Banksia seedlings on a sand mine rehabilitation site and adjacent scrub-heath sites. Journal of Applied Ecology 29: 663–671. [Google Scholar]
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. APS Press, St. Paul, Minnesota. [Google Scholar]
- Eschen R, Britton K, Brockerhoff E, et al. 2015a. International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environmental Science & Policy 51: 228–237. [Google Scholar]
- Eschen R, Douma JC, Grégoire J-C, et al. 2017. A risk categorisation and analysis of the geographic and temporal dynamics of the European import of plants for planting. Biological Invasions 19: 3243–3257. [Google Scholar]
- Eschen R, Rigaux L, Sukovata L, et al. 2015b. Phytosanitary inspection of woody plants for planting at European Union entry points: a practical enquiry. Biological Invasions 17: 2403–2413. [Google Scholar]
- Eshraghi L, Anderson JP, Aryamanesh N, et al. 2011. Phosphite primed defence responses and enhanced expression of defence genes in Arabidobsis thaliana infected with Phytophthora cinnamomi. Plant Pathology 60: 1086–1095. [Google Scholar]
- Eshraghi L, Anderson JP, Aryamanesh N, et al. 2014a. Suppression of the auxin response pathway enhances susceptibility to Phytophthora cinnamomi while phosphite-mediated resistance stimulates the auxin signalling pathway. BMC Plant Biology 14: 68 doi: https://doi.org/10.1186/1471-2229-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi L, Anderson JP, Aryamanesh N, et al. 2014b. Defence signalling pathways involved in plant resistance and phosphite-mediated control of Phytophthora cinnamomi. Plant Molecular Biology Reporter 32: 342–356. [Google Scholar]
- Fajardo SN, Valenzuela S, Dos Santos AF, et al. 2017. Phytophthora pseudosyringae associated with the mortality of Nothofagus obliqua in a pure stand in central-southern Chile. Forest Pathology 47: e12361 doi: https://doi.org/10.1111/efp.12361. [Google Scholar]
- Fenn ME, Coffey MD. 1984. Studies on the in vitro and in vivo antifungal activity of fosetyl-Al and phosphorous acid. Phytopathology 74: 606–611. [Google Scholar]
- Fernandez-Escobar R, Gallego FJ, Benlloch M, et al. 1999. Treatment of oak decline using pressurized injection capsules of antifungal materials. European Journal of Forest Pathology 29: 29–38. [Google Scholar]
- Fichtner EJ, Rizzo DM, Kirk SA, et al. 2011. Root infections may challenge management of invasive Phytophthora spp. in UK woodlands. Plant Disease 95: 13–18. [DOI] [PubMed] [Google Scholar]
- Filip GM, Rosso PH. 1999. Cypress mortality (mal del ciprés) in the Patagonian Andes: comparisons with similar forest diseases and declines in North America. European Journal of Forest Pathology 29: 89–96. [Google Scholar]
- Filipe JAN, Cobb RC, Meentemeyer RK, et al. 2012. Landscape epidemiology and control of pathogens with cryptic and long-distance dispersal: Sudden Oak Death in Northern Californian forests. PLoS Computational Biology 8: e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann F, Schneider D, Matyssek R, et al. 2002. Investigations on Net CO2 assimilation, transpiration and root growth of Fagus sylvatica infested with four different Phytophthora species. Plant Biology 4: 144–152. [Google Scholar]
- Fonseca TF, Abreu CG, Parresol BR. 2004. Soil compaction and chestnut ink disease. Forest Pathology 34: 173–183. [Google Scholar]
- Franceschini S, Webber JF, Sancisi-Frey S, et al. 2014. Gene × environment tests discriminate the new EU2 evolutionary lineage of Phytophthora ramorum and indicate that it is adaptively different. Forest Pathology 44: 219–232. [Google Scholar]
- Gadgil PD. 1974. Phytophthora heveae, a pathogen of kauri. New Zealand Journal of Forestry Science 4: 59–63. [Google Scholar]
- Gallego FJ, Perez de Algaba A, Fernandez-Escobar R. 1999. Etiology of oak decline in Spain. European Journal of Forest Pathology 29: 17–27. [Google Scholar]
- Garbelotto M, Davidson JM, Ivors K, et al. 2003. Non-oak native plants are the main hosts for the Sudden Oak Death pathogen in California. California Agriculture 57: 18–23. [Google Scholar]
- Garbelotto M, Harnik TY, Schmidt DJ. 2009. Efficacy of phosphonic acid, metalaxyl-M and copper hydroxide against Phytophthora ramorum in vitro and in planta. Plant Pathology 58: 111–119. [Google Scholar]
- Garbelotto M, Schmidt D, Swain S, et al. 2017. The ecology of infection between a transmissive and a dead-end host provides clues for the treatment of a plant disease. Ecosphere 8: e01815 doi: https://doi.org/10.1002/ecs2.1815. [Google Scholar]
- Giannakopoulos C, Le Sager P, Bindi M, et al. 2009. Climatic changes and associated impacts in the Mediterranean resulting from global warming. Global Planet Change 68: 209–224. [Google Scholar]
- Gibbs JN, Lipscombe MA, Peace AJ. 1999. The impact of Phytophthora disease on riparian populations of common alder (Alnus glutinosa) in southern Britain. European Journal of Forest Pathology 29: 39–50. [Google Scholar]
- Gibbs JN, Van Dijk C, Webber JF. (eds). 2003. Phytophthora disease of alder in Europe. Forestry Commission Bulletin 126, Edinburgh, UK. [Google Scholar]
- Ginetti B, Moricca S, Squires JN, et al. 2014. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in Northern Italy. Plant Pathology 63: 858–876. [Google Scholar]
- Gisi U, Cohen Y. 1996. Resistance to phenylamide fungicides: A case study with Phytophthora infestans involving mating type and race structure. Annual Review of Phytopathology 34: 549–572. [DOI] [PubMed] [Google Scholar]
- Goheen DJ, Mallams K, Betlejewski F, et al. 2012. Effectiveness of vehicle washing and roadside sanitation in decreasing spread potential of Port-Orford-Cedar root disease. Western Journal of Applied Forestry 27: 170–175. [Google Scholar]
- Goheen EM, Hansen EM, Kanaskie A, et al. 2002. Sudden Oak Death, caused by Phytophthora ramorum, in Oregon. Plant Disease 86: 441. [DOI] [PubMed] [Google Scholar]
- Göker M, Voglmayer H, Riethmüller A, et al. 2007. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genetics and Biology 44: 105–122. [DOI] [PubMed] [Google Scholar]
- Goodwin SB, Sujkowski LS, Fry WE. 1996. Widespread distribution and probable origin of resistance to Metalaxyl in clonal genotypes. Phytopathology 86: 793–800. [Google Scholar]
- Goss EM, Carbone I, Grünwald NJ. 2009. Ancient isolation and independent evolution of the three clonal lineages of the exotic Sudden Oak Death pathogen Phytophthora ramorum. Molecular Ecology 18: 1161–1174. [DOI] [PubMed] [Google Scholar]
- Goss EM, Larsen M, Vercauteren A, et al. 2011. Phytophthora ramorum in Canada: evidence for migration within North America and from Europe. Phytopathology 101: 166–171. [DOI] [PubMed] [Google Scholar]
- Gottschalk KW, Wargo PM. 1996. Oak decline around the world. In: Fosbroke SLC, Gottschalk KW. (eds), Proceedings of the USDA Interagency Gypsy Moth Research Forum, 16–19 January 1996, Annapolis Maryland, General Technical Report NE-230: 3–13. USDA Forest Service, Annapolis Maryland. [Google Scholar]
- Green S, Brasier CM, Schlenzig A, et al. 2013. The destructive invasive pathogen Phytophthora lateralis found on Chamaecyparis lawsoniana across the UK. Forest Pathology 43: 19–28. [Google Scholar]
- Green S, Elliot M, Armstrong A, et al. 2015. Phytophthora austrocedrae emerges as a serious threat to juniper Juniperus communis in Britain. Plant Pathology 64: 456–466. [Google Scholar]
- Green S, Hendry SJ, MacAskill GA, et al. 2012. Dieback and mortality of Juniperus communis in Britain associated with Phytophthora austrocedrae. New Disease Reports 26: 2. [Google Scholar]
- Green S, MacAskill GA, Dun H, et al. 2016. First report of Phytophthora austrocedri infecting Nootka cypress in Britain. New Disease Reports 33: 21. [Google Scholar]
- Greslebin AG, Hansen EM. 2010. Pathogenicity of Phytophthora austrocedrae on Austrocedrus chilensis and its relation with mal del ciprés in Patagonia. Plant Pathology 59: 604–612. [Google Scholar]
- Greslebin AG, Hansen EM, Sutton W. 2007. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia Argentina. Mycological Research 111: 308–316. [DOI] [PubMed] [Google Scholar]
- Greslebin AG, Hansen EM, Winton L, et al. 2005. Phytophthora species from declining Austrocedrus chilensis forests in Patagonia, Argentina. Mycologia 97: 218–228. [DOI] [PubMed] [Google Scholar]
- Grünwald NJ, Garbelotto M, Goss EM, et al. 2012a. Emergence of the Sudden Oak Death pathogen Phytophthora ramorum. Trends in Microbiology 20: 131–138. [DOI] [PubMed] [Google Scholar]
- Grünwald NJ, Goss EM, Press CM. 2008. Phytophthora ramorum: a pathogen with a remarkably wide host range causing Sudden Oak Death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology 9: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünwald NJ, Werres S, Goss EM, et al. 2012b. Phytophthora obscura sp. nov., a new species of the novel Phytophthora subclade 8d. Plant Pathology 61: 610–622. [Google Scholar]
- Guest D, Grant B. 1991. The complex action of phosphonates as antifungal agents. Biological Reviews 66: 159–187. [Google Scholar]
- Hansen E[M], Delatour C. 1999. Phytophthora species in oak forests of north-east France. Annales des Sciences Forestière 56: 539–547. [Google Scholar]
- Hansen EM, Goheen DJ, Jules ES, et al. 2000. Managing Port-Orford-cedar and the introduced pathogen Phytophthora lateralis. Plant Disease 84: 4–14. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Hamm PB, Roth LF. 1989. Testing Port-Orford-cedar for resistance to Phytophthora. Plant Disease 73: 791–794. [Google Scholar]
- Hansen EM, Kanaskie A, Prospero S, et al. 2008. Epidemiology of Phytophthora ramorum in Oregon tanoak forests. Canadian Journal of Forest Research 38: 1133–1143. [Google Scholar]
- Hansen EM, Parke JL, Sutton W. 2005. Susceptibility of Oregon forest trees and shrubs to Phytophthora ramorum: a comparison of artificial inoculation and natural infection. Plant Disease 89: 63–70. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Reeser P[W], Sutton W, et al. 2011. Methods for screening Port-Orford-cedar for resistance to Phytophthora lateralis. In: Sniezko RA, Yanchuk AD, Kliejunas JT, et al. (eds), Proceedings of the 4th International Workshop on Genetics of Host-Parasite Interactions in Forestry. General Technical Report PSW-GTR-240: 181–188. USDA Forest Service, Pacific Southwest Research Station, Albany, California. [Google Scholar]
- Hansen EM, Reeser PW, Sutton W. 2012. Phytophthora beyond agriculture. Annual Review of Phytopathology 50: 359–378. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Reeser PW, Sutton W. 2017. Ecology and pathology of Phytophthora ITS clade 3 species in forests in western Oregon, USA. Mycologia 109: 100–114. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Streito C, Delatour C. 1999. First confirmation of Phytophthora lateralis in Europe. Plant Disease 83: 587. [DOI] [PubMed] [Google Scholar]
- Hantula J, Müller M, Uusivuori J. 2013. International plant trade associated risks: laissezfaire or novel solutions. Environmental Science & Policy 37: 158–160. [Google Scholar]
- Hardham AR. 2005. Phytophthora cinnamomi. Molecular Plant Pathology 6: 589–604. [DOI] [PubMed] [Google Scholar]
- Hardy GEStJ, Barrett S, Shearer BL. 2001. The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30: 133–139. [Google Scholar]
- Hardy GE[StJ], Sivasithamparam K. 1988. Phytophthora spp. associated with container-grown plants in nurseries in Western Australia. Plant Disease 72: 435–437. [Google Scholar]
- Harris AR. 2014. The epidemiology of Phytophthora ramorum associated with Larix in the UK. Imperial College London, PhD Thesis. [Google Scholar]
- Harris AR, Scanu B, Webber J. 2015. Comparative fitness of European lineages of Phytophthora ramorum. In: Sutton W, Reeser P, Hansen EM. (eds), Proceedings of the 7th Meeting of the IUFRO Working Party 7.02.09. Phytophthora in Forests and Natural Ecosystems, 10–14 November 2014, Esquel, Argentina: 135. [Google Scholar]
- Harris AR, Webber J. 2016. Sporulation potential, symptom expression and detection of Phytophthora ramorum on larch needles and other foliar hosts. Plant Pathology 65: 1441–1451. [Google Scholar]
- Hartmann G, Blank R, Kunca A. 2006. Collar rot of Fagus sylvatica caused by Phytophthora cambivora: damage, site relations and susceptibility of broadleaf hosts. In: Brasier CM, Jung T, Osswald W. (eds), Progress in Research on Phytophthora Diseases of Forest Trees: 135–138. Forest Research, Farnham, Hampshire, UK. [Google Scholar]
- Havrylenko M, Rosso PH, Fontenla SB. 1989. Austrocedrus chilensis: contribución al estudio de su mortalidad en Argentina. Bosque 1: 29–36. [Google Scholar]
- Heenan PB, Smissen RD. 2013. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). Phytotaxa 146: 1–31. [Google Scholar]
- Henricot B, Pérez-Sierra A, Armstrong AC, et al. 2017. Morphological and genetic analyses of the invasive forest pathogen Phytophthora austrocedri reveal that two clonal lineages colonized Britain and Argentina from a common ancestral population. Phytopathology 107: 1532–1540. [DOI] [PubMed] [Google Scholar]
- Henricot B, Pérez-Sierra A, Jung T. 2014. Phytophthora pachypleura sp. nov., a new species causing root rot of Aucuba japonica and other ornamentals in the United Kingdom. Plant Pathology 63: 1095–1109. [Google Scholar]
- Hickman CJ. 1958. Phytophthora – plant destroyer. Transactions of the British Mycological Society 41: 1–13. [Google Scholar]
- Ho HH, Gallegly ME, Hong CX. 2007. Redescription of Phytophthora melonis. Mycotaxon 102: 339–345. [Google Scholar]
- Hong C, Gallegly ME, Richardson PA, et al. 2011. Phytophthora pini Leonian resurrected to distinct species status. Mycologia 103: 351–360. [DOI] [PubMed] [Google Scholar]
- Hong CX, Gallegly ME, Browne GT, et al. 2009. The avocado subgroup of Phytophthora citricola constitutes a distinct species, Phytophthora mengei sp. nov. Mycologia 90: 833–840. [DOI] [PubMed] [Google Scholar]
- Horner IJ, Hough EG. 2014. Pathogenicity of four Phytophthora species on kauri: in vitro and glasshouse trials. New Zealand Plant Protection 67: 54–59. [Google Scholar]
- Houghton JJ, Meiro Filho LG, Callander BA, et al. (eds). 1996. Climate Change 1995 – The science of climate change. Contribution of working group I to the second assessment report of the Intergovernmental Panel of Climate Change. Kattenberg & K. Maskell, Cambridge University Press. [Google Scholar]
- Huai WX, Tian G, Hansen EM, et al. 2013. Identification of Phytophthora species baited and isolated from forest soil and streams in northwestern Yunnan province, China. Forest Pathology 43: 87–103. [Google Scholar]
- Hulvey J, Telle S, Nigrelli L, et al. 2010. Salisapiliaceae – a new family of oomycetes from marsh grass litter of southeastern North America. Persoonia 25: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson C, Aguayo J, Revellin C, et al. 2015. Evidence for homoploid speciation in Phytophthora alni supports taxonomic reclassification in this species complex. Fungal Genetics and Biology 77: 12–21. [DOI] [PubMed] [Google Scholar]
- Ioos R, Andrieux A, Marçais B, et al. 2006. Genetic characterization of the natural hybrid species Phytophthora alni as inferred from nuclear and mitochondrial DNA analyses. Fungal Genetics and Biology 43: 511–529. [DOI] [PubMed] [Google Scholar]
- Ivors KL, Garbelotto M, Vries IDE, et al. 2006. Microsatellite markers identify three lineages of Phytophthora ramorum in US nurseries, yet single lineages in US forest and European nursery populations. Molecular Ecology 15: 1493–1505. [DOI] [PubMed] [Google Scholar]
- Jeffers SN, Aldwinckle HS. 1987. Enhancing detection of Phytophthora cactorum in naturally infested soil. Phytopathology 77: 1475–1482. [Google Scholar]
- Jimerson TM, White DE, Atzet T, et al. 2001. Ecological factors associated with Port-Orford-cedar. In: Betlejewski F, Casavan KC, Dawson A, et al. (eds), A range-wide assessment of Port-Orford-cedar (Chamaecyparis lawsoniana) on Federal lands. BLM/OR/WA/PL-004/004-1792: 5–32. U.S. Department of Agriculture, Forest Service and U.S. Department of Interior, Bureau of Land Management, Washington, DC. [Google Scholar]
- Jönsson U. 2006. A conceptual model for the development of Phytophthora disease in Quercus robur. New Phytologist 171: 55–68. [DOI] [PubMed] [Google Scholar]
- Jönsson U, Jung T, Rosengren U, et al. 2003a. Pathogenicity of Swedish isolates of Phytophthora quercina to Quercus robur in two different soil types. New Phytologist 158: 355–364. [Google Scholar]
- Jönsson U, Jung T, Sonesson K, et al. 2005. Relationships between Quercus robur health, occurrence of Phytophthora species and site conditions in southern Sweden. Plant Pathology 54: 502–511. [Google Scholar]
- Jönsson U, Lundberg L, Sonesson K, et al. 2003b. First records of soilborne Phytophthora species in Swedish oak forests. Forest Pathology 33: 175–179. [Google Scholar]
- Jules ES, Kauffman MJ, Ritts WD, et al. 2002. Spread of an invasive pathogen over a variable landscape: a nonnative root rot on Port Orford cedar. Ecology 83: 3167–3181. [Google Scholar]
- Jung T. 1998. Die Phytophthora-Erkrankung der Europäischen Eichenarten – Wurzel zerstörende Pilze als Ursache des Eichensterbens. Lincom Europe, Munich, Germany. [Google Scholar]
- Jung T. 2009. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. Forest Pathology 39: 73–94. [Google Scholar]
- Jung T, Blaschke H. 1996. Phytophthora root rot in declining forest trees. Phyton (Austria) 36: 95–102. [Google Scholar]
- Jung T, Blaschke M. 2004. Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies. Plant Pathology 53: 197–208. [Google Scholar]
- Jung T, Blaschke M. 2006. Phytophthora dieback of alders in Bavaria: distribution, pathways and management strategies. In: Brasier CM, Jung T, Osswald W. (eds), Progress in Research on Phytophthora Diseases of Forest Trees: 61–66. Forest Research, Farnham, Hampshire, UK. [Google Scholar]
- Jung T, Blaschke H, Neumann P. 1996. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. European Journal of Forest Pathology 26: 253–272. [Google Scholar]
- Jung T, Blaschke H, Osswald W. 2000. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathology 49: 706–718. [Google Scholar]
- Jung T, Blaschke H, Oßwald W. 2003a. Effect of environmental constraints on Phytophthora-mediated oak decline in Central Europe. In: McComb JA, Hardy GEStJ, Tommerup I. (eds), Phytophthora in Forests and Natural Ecosystems: 89–98. Murdoch University, Perth, Australia. [Google Scholar]
- Jung T, Burgess TI. 2009. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 22: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Chang TT, Bakonyi J, et al. 2017a. Diversity of Phytophthora species in natural ecosystems of Taiwan and association with disease symptoms. Plant Pathology 66: 194–211. [Google Scholar]
- Jung T, Colquhoun IJ, Hardy GEStJ. 2013a. New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. Forest Pathology 43: 266–288. [Google Scholar]
- Jung T, Cooke DEL, Blaschke H, et al. 1999. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycological Research 103: 785–798. [Google Scholar]
- Jung T, Durán A, Sanfuentes von Stowasser E, et al. 2018. Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. Forest Pathology 48: e12443 doi: https://doi.org/10.1111/EFP.12443. [Google Scholar]
- Jung T, Hansen EM, Winton L, et al. 2002. Three new species of Phytophthora from European oak forests. Mycological Research 106: 397–411. [Google Scholar]
- Jung T, Horta Jung M, Cacciola SO, et al. 2017b. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 8: 219–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Horta Jung M, Scanu B, et al. 2017c. Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Persoonia 38: 100–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Hudler GW, Jensen-Tracy SL, et al. 2005. Involvement of Phytophthora spp. in the decline of European beech in Europe and the USA. Mycologist 19: 159–166. [Google Scholar]
- Jung T, Nechwatal J. 2008. Phytophthora gallica sp. nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycological Research 112: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Jung T, Nechwatal J, Cooke DEL, et al. 2003b. Phytophthora pseudosyringae sp. nov., a new species causing root and collar rot of deciduous tree species in Europe. Mycological Research 107: 772–789. [DOI] [PubMed] [Google Scholar]
- Jung T, Orlikowski L, Henricot B, et al. 2016. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathology 46: 134–163. [Google Scholar]
- Jung T, Scanu B, Bakonyi J, et al. 2017d. Nothophytophthora gen. nov., a new sister genus of Phytophthora from natural and semi-natural ecosystems. Persoonia 39: 143–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Stukely MJC, Hardy GEStJ, et al. 2011. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: evolutionary and ecological implications. Persoonia 26: 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Vannini A, Brasier CM. 2009. Progress in understanding Phytophthora diseases of trees in Europe 2004–2007. In: Goheen EM, Frankel SJ. (eds), Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09, General Technical Report PSW-GTR-221: 3–24. USDA Forest Service, Pacific Southwest Research Station, Albany, California. [Google Scholar]
- Jung T, Vettraino AM, Cech TL, et al. 2013b. The impact of invasive Phytophthora species on European forests. In: Lamour K. (ed), Phytophthora: A global perspective: 146–158. CABI, Wallingford, UK. [Google Scholar]
- Kanoun-Boulé M, Vasconcelos T, Gaspar J, et al. 2016. Phytophthora × alni and Phytophthora lacustris associated with common alder decline in Central Portugal. Forest Pathology 46: 174–176. [Google Scholar]
- Keller RP, Lodge DM, Finnoff DC. 2007. Risk assessment for invasive species produces net bioeconomic benefits. Proceedings of the National Academy of Sciences USA 104: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Harris AR, Webber JF. 2015. In planta detection used to define the distribution of the European lineages of Phytophthora ramorum on larch (Larix) in the UK. Plant Pathology 64: 1168–1175. [Google Scholar]
- Kirkpatrick JB, DellaSala DA. 2011. Temperate rainforests of Australasia. In: DellaSala DA. (ed), Temperate and boreal rainforests of the world: 195–212. Island Press, Washington, Covelo, London. [Google Scholar]
- Knapp R. 1965. Die Vegetation von Nord- und Mittelamerika und der Hawaii-Inseln (‘The vegetation of North and Central America and of the Hawaiian islands’). Fischer, Stuttgart, Germany. [Google Scholar]
- Ko W-H, Wang SY, Ann P. 2006. The possible origin and relation of Phytophthora katsurae and P. heveae, discovered in a protected natural forest in Taiwan. Botanical Studies 47: 273–277. [Google Scholar]
- Kozanitas M, Osmundson TW, Linzer R, et al. 2017. Interspecific interactions between the Sudden Oak Death pathogen Phytophthora ramorum and two sympatric Phytophthora species in varying ecological conditions. Fungal Ecology 28: 86–96. [Google Scholar]
- Kroon LP, Brouwer H, De Cock AW, et al. 2012. The genus Phytophthora anno 2012. Phytopathology 102: 348–364. [DOI] [PubMed] [Google Scholar]
- Kroon LPNM, Bakker FT, Van den Bosch GBM, et al. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology 41: 766–782. [DOI] [PubMed] [Google Scholar]
- La Manna L, Rajchenberg M. 2004. The decline of Austrocedrus chilensis forests in Patagonia, Argentina: soil features as predisposing factors. Forest Ecology and Management 190: 345–357. [Google Scholar]
- Laidlaw WS, Wilson BA. 2003. Floristic and structural characteristics of a coastal heathland exhibiting symptoms of Phytophthora cinnamomi infestation in the eastern Otway ranges, Victoria. Australian Journal of Botany 51: 283–293. [Google Scholar]
- Lamont BB, Bergl SM. 1991. Water relations, shoot and root architecture, and phenology of three co-occurring Banksia species: no evidence for niche differentiation in the pattern of water use. Oikos 60: 291–298. [Google Scholar]
- Lanner RM. 1999. Conifers of California. Cachuma Press, Los Olivos, California, USA. [Google Scholar]
- Lee JK, Jo JW, Shin KC, et al. 2009. Isolation, identification and characterization of Phytophthora katsurae, causing chestnut ink disease in Korea. The Plant Pathology Journal 25: 121–127. [Google Scholar]
- Liebhold AM, Brockerhoff EG, Garrett LJ, et al. 2012. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and Environment 10: 135–143. [Google Scholar]
- Linaldeddu BT, Scanu B, Maddau L, et al. 2014. Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in holm oak decline on Caprera Island (Italy). Forest Pathology 44: 191–200. [Google Scholar]
- Linzer RE, Rizzo DM, Cacciola SO, et al. 2009. AFLPs detect low genetic diversity for Phytophthora nemorosa and P. pseudosyringae in the US and Europe. Mycological Research 113: 298–307. [DOI] [PubMed] [Google Scholar]
- Lione G, Gonthier P, Garbelotto M. 2017. Environmental factors driving the recovery of bay laurels from Phytophthora ramorum infections: An application of numerical ecology to citizen science. Forests 8: 293 doi: https://doi.org/10.3390/f8080293. [Google Scholar]
- Lonsdale D, Wainhouse D. 1987. Beech bark disease. Forestry Commission Bulletin 69, HMSO, London. [Google Scholar]
- Lopes-Pimentel AA. 1946. O sobreiro também é parasitado pela Phytophthora cambivora (Petri) Buis., agente da “doença da tinta” do castanheiro. Lisboa. Direcção Geral dos Serviços Florestais e Aquícolas 13: 45–49. [Google Scholar]
- Lopes-Pimentel AA. 1947. Phytophthora cinnamomi (Rands) um outro agente extremamente virulento da “doença da tinta” do castanheiro. Agronomia lusitana 9: 181–191. [Google Scholar]
- MacDonald JD, Ali-Shtayeh MS, Kabashima J, et al. 1994. Occurrence of Phytophthora species in recirculated nursery irrigation effluents. Plant Disease 78: 607–611. [Google Scholar]
- Mahdikhani M, Matinfar M, Aghaalikhani A. 2017. First report of Phytophthora austrocedri causing phloem lesions and bronzing on Cupressus sempervirens in northern Iran. New Disease Reports 36: 10 doi: https://doi.org/10.5197/j.2044-0588.2017.036.010. [Google Scholar]
- Man in ‘t Veld WA, Rosendahl KCHM, Hong C. 2012. Phytophthora × serendipita sp. nov. and P. × pelgrandis, two destructive pathogens generated by natural hybridization. Mycologia 104: 1390–1396. [DOI] [PubMed] [Google Scholar]
- Manion PD. 1981. Tree disease concepts. Prentice Hall, Inc., Englewood Cliffs, New Jersey, USA. [Google Scholar]
- Marçais B, Bergot M, Perarnaud V, et al. 2004. Prediction and mapping of the impact of winter temperatures on the development of Phytophthora cinnamomi induced cankers on red and pedunculate oak. Phytopathology 94: 826–831. [DOI] [PubMed] [Google Scholar]
- Marçais B, Dupuis F, Desprez-Loustau ML. 1993. Influence of water stress on susceptibility of red oak (Quercus rubra) to Phytophthora cinnamomi. European Journal of Forest Pathology 23: 295–305. [Google Scholar]
- Marks GC, Smith IW. 1991. The Cinnamon fungus in Victorian forests. History, Distribution, Management and Control. Lands and Forests Bulletin No. 31. Department of Conservation and Environment, Melbourne, Australia. [Google Scholar]
- Martin FN, Abad ZG, Balci Y, et al. 2012. Identification and detection of Phytophthora: reviewing our progress, identifying our needs. Plant Disease 96: 1080–1103. [DOI] [PubMed] [Google Scholar]
- Martin FN, Blair JE, Coffey MD. 2014. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genetics and Biology 66: 19–32. [DOI] [PubMed] [Google Scholar]
- Martin FN, Tooley PW. 2003. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 95: 269–284. [PubMed] [Google Scholar]
- Martins L, Castro J, Macedo W, et al. 2007. Assessment of the spread of chestnut ink disease using remote sensing and geostatistical methods. European Journal of Plant Pathology 119: 159–164. [Google Scholar]
- Mascheretti S, Croucher PJP, Vettraino A, et al. 2008. Reconstruction of the Sudden Oak Death epidemic in California through microsatellite analysis of the pathogen Phytophthora ramorum. Molecular Ecology 17: 2755–2768. [DOI] [PubMed] [Google Scholar]
- McConnell M, Balci Y. 2014a. Fine root dynamics of oak saplings in response to Phytophthora cinnamomi infection under different temperatures and durations. Forest Pathology: 45: 155–164. [Google Scholar]
- McConnell M, Balci Y. 2014b. Phytophthora cinnamomi as a contributor to white oak decline in mid-Atlantic United States forests. Plant Disease 98: 319–327. [DOI] [PubMed] [Google Scholar]
- Meadows IM, Zwart DC, Jeffers SN, et al. 2011. Effects of fuel reduction treatments on incidence of Phytophthora species in soil of a southern Appalachian Mountain forest. Plant Disease 95: 811–820. [DOI] [PubMed] [Google Scholar]
- Meentemeyer R, Rizzo D, Mark W, et al. 2004. Mapping the risk of establishment and spread of sudden oak death in California. Forest Ecology and Management 200: 195–214. [Google Scholar]
- Meentemeyer RK, Dorning MA, Vogler JB, et al. 2015. Citizen science helps predict risk of emerging infectious disease. Frontiers in Ecology and Environment 13: 189–194. [Google Scholar]
- Migliorini D, Ghelardini L, Tondine E, et al. 2015. The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Diversity and Distributions 21: 1218–1229. [Google Scholar]
- Miranda-Fontaíña ME, Fernández-López J, Vettraino AM, et al. 2007. Resistance of Castanea clones to Phytophthora cinnamomi: testing and genetic control. Silvae Genetica 56: 11–21. [Google Scholar]
- Mircetich SM, Campbell RN, Matheron ME. 1977. Phytophthora trunk canker of coast live oak and cork oak trees in California. Plant Disease Reporter 61: 66–70. [Google Scholar]
- Mircetich SM, Matheron ME. 1983. Phytophthora root and crown rot of walnut trees. Phytopathology 73: 1481–1488. [Google Scholar]
- Moralejo E, Pérez-Sierra A, Álvarez LA, et al. 2009. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathology 58: 100–110. [Google Scholar]
- Moreira AC, Martins JMS. 2005. Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. Forest Pathology 35: 145–162. [Google Scholar]
- Motta E, Annesi T, Pane A, et al. 2003. A new Phytophthora causing a basal canker on beech in Italy. Plant Disease 87: 1005. [DOI] [PubMed] [Google Scholar]
- Munda A, Zerjav M, Schroers H-J. 2007. First report of Phytophthora citricola occurring on Fagus sylvatica in Slovenia. Plant Disease 91: 907. [DOI] [PubMed] [Google Scholar]
- Murray MS, Hansen EM. 1997. Susceptibility of Pacific yew to Phytophthora lateralis. Plant Disease 81: 1400–1404. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, et al. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Nagy ZÁ, Bakonyi J, Érsek T. 2003. Standard and Swedish variant types of the hybrid alder Phytophthora attacking alder in Hungary. Pest Management Science 59: 484–492. [DOI] [PubMed] [Google Scholar]
- Navarro S, Sims L, Hansen EM. 2015. Pathogenicity to alder of Phytophthora species from riparian ecosystems in western Oregon. Forest Pathology 45: 358–366. [Google Scholar]
- Nechwatal J, Bakonyi J, Cacciola SO, et al. 2013. The morphology, behaviour and molecular phylogeny of Phytophthora taxon Salixsoil and its redesignation as Phytophthora lacustris sp. nov. Plant Pathology 62: 355–369. [Google Scholar]
- Nechwatal J, Hahn J, Schönborn A, et al. 2011. A twig blight of understorey European beech (Fagus sylvatica) caused by soilborne Phytophthora spp. Forest Pathology 41: 493–500. [Google Scholar]
- Nechwatal J, Schlenzig A, Jung T, et al. 2001. A combination of baiting and PCR techniques for the detection of Phytophthora quercina and P. citricola in soil samples from oak stands. Forest Pathology 31: 85–97. [Google Scholar]
- Nelson AH. 2009. The etiology and epidemiology of bleeding canker on European beech. Ph.D. thesis. Cornell University, Ithaca, New York, USA. [Google Scholar]
- Nicholls JL. 1976. A revised classification of the North Island indigenous forests. New Zealand Journal of Forestry 21: 105–113. [Google Scholar]
- Nienhaus F. 1987. Viren und primitive Prokaryonten in Eichen (viruses and primitive procaryotes in oaks). Österreichische Forstzeitung 98: 64–65. [Google Scholar]
- Nihlgård B. 1985. The ammonium hypothesis – an additional explanation to the forest dieback in Europe. Ambio 14: 2–8. [Google Scholar]
- O’Hanlon R, Choiseul J, Brennan JM, et al. 2017. Assessment of the eradication treatments applied to Phytophthora ramorum in Irish Larix kaempferi forests. Forest Pathology 48: e12389. [Google Scholar]
- Oh E, Gryzenhout M, Wingfield BD, et al. 2013. Surveys of soil and water reveal a goldmine of Phytophthora diversity in South African natural ecosystems. IMA Fungus 4: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Hansen EM, Sniezko RA. 2006. Port-Orford-cedar resistant to Phytophthora lateralis. Forest Pathology 36: 385–394. [Google Scholar]
- Oh E, Parke JL. 2012. Phythophthora katsurae. Forest Phytophthoras 2 (1). doi: https://doi.org/10.5399/osu/fp.2.1.3046. [Google Scholar]
- Oleksyn J, Przybyl K. 1987. Oak decline in the Soviet Union: scale and hypotheses. European Journal of Forest Pathology 17: 321–336. [Google Scholar]
- Oosterbaan A, Nabuurs GJ. 1991. Relationships between oak decline and groundwater class in the Netherlands. Plant and Soil 136: 87–93. [Google Scholar]
- Orlikowski BL, Oszako T, Szkuta G. 2006. First record of Phytophthora spp. associated with the decline of European beech stand in southwest Poland. Phytopathologia Polonica 42: 37–46. [Google Scholar]
- Ozturk T, Ceber ZP, Türkes M, et al. 2015. Projections of climate change in the Mediterranean Basin by using downscaled global climate model outputs. International Journal of Climatology 35: 4276–4292. [Google Scholar]
- Paap T, Croeser L, White D, et al. 2017. Phytophthora versiformis sp. nov., a new species from Australia related to P. quercina. Australasian Plant Pathology 46: 369–378. [Google Scholar]
- Pachauri RK, Reisinger A. (eds). 2007. Climate change 2007: Synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland. [Google Scholar]
- Parke JL, Grünwald NJ. 2012. A systems approach for management of pests and pathogens of nursery crops. Plant Disease 96: 1236–1244. [DOI] [PubMed] [Google Scholar]
- Parke JL, Knaus BJ, Fieland VJ, et al. 2014. Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 104: 1052–1062. [DOI] [PubMed] [Google Scholar]
- Parker EJ. 1974. Beech Bark Disease. Forestry Commission Forest Record 96, HMSO, London. [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11: 1633–1644. [Google Scholar]
- Pérez-Ramos IM, Zavala MA, Marañon T, et al. 2008. Dynamics of understory diversity following shrub-clearing of cork oak forests: A five-year study. Forest Ecology and Management 255: 3242–3253. [Google Scholar]
- Pérez-Sierra A, Jung T. 2013. Phytophthora in woody ornamental nurseries. In: Lamour K. (ed), Phytophthora: A global perspective: 166–177. CABI, Wallingford, UK. [Google Scholar]
- Pérez-Sierra A, López-García C, León M, et al. 2013. Previously unrecorded low temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in Eastern Spain. Forest Pathology 43: 331–339. [Google Scholar]
- Perlerou C, Tziros G, Vettraino AM, et al. 2010. Phytophthora cryptogea causing ink disease of Castanea sativa newly reported in Greece. Plant Pathology 59: 799. [Google Scholar]
- Peterson E, Hansen E, Hulbert J. 2014. Source or sink. The role of soil and water borne inoculum in the dispersal of Phytophthora ramorum in Oregon tanoak forests. Forest Ecology and Management 322: 48–57. [Google Scholar]
- Peterson EK, Hansen EM, Kanaskie A. 2015. Temporal epidemiology of Sudden Oak Death in Oregon. Phytopathology 105: 937–946. [DOI] [PubMed] [Google Scholar]
- Pignatti S. 1982. Flora d’Italia Volume 1. Edagricole-New Business Media, Milan, Italy. [Google Scholar]
- Pilbeam RA, Colquhoun IJ, Shearer B, et al. 2000. Phosphite concentration: its effect on phytotoxicity symptoms and colonisation by Phytophthora cinnamomi in three understorey species of Eucalyptus marginata forest. Australasian Plant Pathology 29: 86–95. [Google Scholar]
- Pintos C, Rial C, Aguín O, et al. 2012. First report of Phytophthora ilicis causing twig blight on holly in Spain. New Disease Reports 26: 16. [Google Scholar]
- Podger FD. 1972. Phytophthora cinnamomi, a cause of lethal disease in indigenous plant communities in Western Australia. Phytopathology 62: 972–981. [Google Scholar]
- Podger FD, Doepel RF, Zentmyer GA. 1965. Association of Phytophthora cinnamomi with a disease of Eucalyptus marginata forest in Western Australia. Plant Disease Reporter 49: 943–947. [Google Scholar]
- Podger FD, Newhook FJ. 1971. Phytophthora cinnamomi in indigenous plant communities in New Zealand. New Zealand Journal of Botany 9: 625–638. [Google Scholar]
- Preston CD, Pearman DA, Dines TD. 2002. New Atlas of the British and Irish Flora. Oxford University Press, UK. [Google Scholar]
- Prospero S, Hansen EM, Grünwald NJ, et al. 2007. Population dynamics of the Sudden Oak Death pathogen Phytophthora ramorum in Oregon from 2001 to 2004. Molecular Ecology 16: 2958–2973. [DOI] [PubMed] [Google Scholar]
- Puno VI, Laurence MH, Guest DI, et al. 2015. Detection of Phytophthora multivora in the Wollemi Pine site and pathogenicity to Wollemia nobilis. Australasian Plant Pathology 44: 205–215. [Google Scholar]
- Ragazzi A, Fedi I, Mesturino L. 1989. The oak decline: a new problem in Italy. European Journal of Forest Pathology 19: 105–110. [Google Scholar]
- Ragazzi A, Vagniluca S, Moricca S. 1995. European expansion of oak decline: involved microorganisms and methodological approaches. Phytopathologia Mediterranea 34: 207–226. [Google Scholar]
- Rahman MZ, Uematsu S, Kimishima E, et al. 2015. Two plant pathogenic species of Phytophthora associated with stem blight of Easter lily and crown rot of lettuce in Japan. Mycoscience 56: 419–433. [Google Scholar]
- Rajchenberg M, Barroetaveña C, Cwielong PP, et al. 1998. Fungal species associated with the decline of Austrocedrus chilensis in Patagonia, Argentina: preliminary results. In: Delatour L, Guillaumi JJ, Lung-Escarmant B, et al. (eds), Proceedings of the Ninth International Conference on Root and Butt Rots, vol. 89, Carcans, France, 31. August – 6. September 1997, Les Colloques: 235–244. [Google Scholar]
- Rapp J, Schönwiese CD. 1995. Trendanalyse der räumlichjahreszeitlichen Niederschlags- und Temperaturstruktur in Deutschland 1891–1990 und 1961–90. Annalen der Meteorologie 31: 33–34. [Google Scholar]
- Rea AJ, Burgess TI, Hardy GEStJ, et al. 2011. Two novel and potentially endemic species of Phytophthora associated with episodic dieback of kwongan vegetation in the south-west of Western Australia. Plant Pathology 60: 1055–1068. [Google Scholar]
- Rea AJ, Jung T, Burgess TI, et al. 2010. Phytophthora elongata sp. nov. a novel pathogen from the Eucalyptus marginata forest of Western Australia. Australasian Plant Pathology 39: 477–491. [Google Scholar]
- Redondo MA, Boberg J, Olsson CHB, et al. 2015. Winter conditions correlate with Phytophthora alni subspecies distribution in southern Sweden. Phytopathology 105: 1191–1197. [DOI] [PubMed] [Google Scholar]
- Ribeiro OK. 1978. A source book of the genus Phytophthora. Cramer, Vaduz. [Google Scholar]
- Richardson DM, Rundel PW, Jackson ST, et al. 2007. Human impacts in pine forests: past, present, and future. Annual Review of Ecology, Evolution and Systematics 38: 275–297. [Google Scholar]
- Rizzo DM, Garbelotto M, Davidson JM, et al. 2002. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Disease 86: 205–214. [DOI] [PubMed] [Google Scholar]
- Rizzo DM, Garbelotto M, Hansen EM. 2005. Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology 43: 309–335. [DOI] [PubMed] [Google Scholar]
- Robin C, Brasier CM, Reeser P, et al. 2015. Pathogenicity of Phytophthora lateralis lineages on resistant and susceptible selections of Chamaecyparis lawsoniana. Plant Disease 99: 1133–1139. [DOI] [PubMed] [Google Scholar]
- Robin C, Desprez-Loustau ML, Capron G, et al. 1998. First record of Phytophthora cinnamomi on cork and holm oaks in France and evidence of pathogenicity. Annales des Sciences Forestière 55: 869–883. [Google Scholar]
- Robin C, Morel O, Vettraino AM, et al. 2006. Genetic variation in susceptibility to Phytophthora cambivora in European chestnut (Castanea sativa). Forest, Ecology and Management 226: 199–207. [Google Scholar]
- Robin C, Piou D, Feau NF, et al. 2011. Root and aerial infections of Chamaecyparis lawsoniana by Phytophthora lateralis: a new threat for European countries. Forest Pathology 41: 417–424. [Google Scholar]
- Rogers DL. 2004. In situ genetic conservation of a naturally restricted and commercially widespread species, Pinus radiata. Forest Ecology and Management 197: 311–322. [Google Scholar]
- Rogers DL, Matheson CA, Vargas-Hernandez JJ, et al. 2006. Genetic conservation of insular populations of Monterey pine (Pinus radiata D. Don). Biodiversity and Conservation 15: 779–798. [Google Scholar]
- Rooney-Latham S, Blomquist CL, Pastalka T, et al. 2009. Collar rot on Italian alder trees in California caused by Phytophthora siskiyouensis. Online. Plant Health Progress. doi: https://doi.org/10.1094/PHP-2009-0413-01-RS.
- Ropelewski C, Halpert M. 1987. Global and regional scale precipitation patterns associated with the El Niño/Southern Oscillation. Monthly Weather Review 115: 1606–1626. [Google Scholar]
- Ropelewski C, Halpert M. 1989. Precipitation patterns associated with the high index phase of the Southern Oscillation. Journal of Climate 2: 268–284. [Google Scholar]
- Roth LF, Trione EJ, Ruhmann WH. 1957. Phytophthora induced root rot of native Port-Orford-cedar. Journal of Forestry 55: 294–298. [Google Scholar]
- Runge F, Telle S, Ploch S, et al. 2011. The inclusion of downy mildews in a multi-locus-dataset and its reanalysis reveals a high degree of paraphyly in Phytophthora. IMA Fungus 2: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A, Hansen EM, Goheen DJ. 2007. Phytophthora cambivora in Oregon and its pathogenicity to Chrysolepis chrysophylla. Forest Pathology 37: 409–419. [Google Scholar]
- Sanchez ME, Caetano P, Ferraz J, et al. 2002. Phytophthora disease of Quercus ilex in southwestern Spain. Forest Pathology 32: 5–18. [Google Scholar]
- Santini A, Barzanti GP, Capretti P. 2003. Susceptibility of some mesophylic hardwoods to alder Phytophthora. Journal of Phytopathology 151: 406–410. [Google Scholar]
- Santini A, Ghelardini L, De Pace C, et al. 2013. Biogeographic patterns and determinants of invasion by alien forest pathogens in Europe. New Phytologist 197: 238–250. [DOI] [PubMed] [Google Scholar]
- Santos C, Duarte S, Tedesco S, et al. 2017a. Expression profiling of Castanea genes during resistant and susceptible interactions with the oomycete pathogen Phytophthora cinnamomi reveal possible mechanisms of immunity. Frontiers in Plant Science 8: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C, Machado H, Correia I, et al. 2015. Phenotyping Castanea hybrids for Phytophthora cinnamomi resistance. Plant Pathology 64: 901–910. [Google Scholar]
- Santos C, Nelson CD, Zhebentyayeva T, et al. 2017b. First interspecific genetic linkage map for Castanea sativa × Castanea crenata revealed QTLs for resistance to Phytophthora cinnamomi. PLoS ONE 12: e0184381 10.1371/journal.pone.0184381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota R, Nicolaisen M. 2015. An improved high throughput sequencing method for studying oomycete communities. Journal of Microbiological Methods 110: 33–39. [DOI] [PubMed] [Google Scholar]
- Saude C, Hurtado-Gonzales OP, Lamour KH, et al. 2008. Occurrence and characterization of a Phytophthora sp. pathogenic to asparagus (Asparagus officinalis) in Michigan. Phytopathology 98: 1075–1083. [DOI] [PubMed] [Google Scholar]
- Scanu B, Hunter GC, Linaldeddu BT, et al. 2014a. A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. Forest Pathology 44: 1–20. [Google Scholar]
- Scanu B, Jones B, Webber JF. 2012. A new disease of Nothofagus in Britain caused by Phytophthora pseudosyringae. New Disease Reports 25: 27. [Google Scholar]
- Scanu B, Linaldeddu BT, Deidda A, et al. 2015. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 10: e0143234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu B, Linaldeddu BT, Franceschini A. 2010. First report of Phytophthora pseudosyringae associated with ink disease of Castanea sativa in Italy. Plant Disease 94: 1068. [DOI] [PubMed] [Google Scholar]
- Scanu B, Linaldeddu BT, Franceschini A, et al. 2013. Occurrence of Phytophthora cinnamomi in cork oak forests in Italy. Forest Pathology 43: 340–343. [Google Scholar]
- Scanu B, Linaldeddu BT, Pérez-Sierra A, et al. 2014b. Phytophthora ilicis as a leaf and stem pathogen of Ilex aquifolium in Mediterranean islands. Phytopathologia Mediterranea 53: 480–490. [Google Scholar]
- Scanu B, Webber JF. 2016. Dieback and mortality of Nothofagus in Britain: ecology, pathogenicity and sporulation potential of the causal agent Phytophthora pseudosyringae. Plant Pathology 65: 26–36. [Google Scholar]
- Scarlett K, Daniel R, Shuttleworth LA, et al. 2015. Phytophthora in the Gondwana Rainforests of Australia World Heritage Area. Australasian Plant Pathology 44: 335–348. [Google Scholar]
- Schena L, Hughes KJD, Cooke DEL. 2006. Detection and quantification of Phytophthora ramorum, P. kernoviae, P. citricola and P. quercina in symptomatic leaves by multiplex real-time PCR. Molecular Plant Pathology 7: 365–379. [DOI] [PubMed] [Google Scholar]
- Schenck N, Fourrier-Jeandel C, Ioos R. 2016. A robust and specific real-time PCR tool for the detection of Phytophthora lateralis in plant tissues. European Journal of Plant Pathology 146: 231–244. [Google Scholar]
- Schlag M. 1995. The condition of the phloem in declining oaks. European Journal of Forest Pathology 25: 83–94. [Google Scholar]
- Schlenzig A, Campbell R, Eden R. 2014. First report of Phytophthora lateralis on Chamaecyparis pisifera. New Disease Reports 29: 15. [Google Scholar]
- Schlenzig A, Campbell R, Mulholland V. 2011. Thuja occidentalis: a new host for Phytophthora lateralis. New Disease Reports 24: 8. [Google Scholar]
- Schmitz S, Zini J, Chandelier A. 2009. Involvement of Phytophthora species in the decline of beech (Fagus sylvatica) in the southern part of Belgium. In: Goheen EM, Frankel SJ. (eds), Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09, General Technical Report PSW-GTR-221: 320–323. USDA Forest Service, Pacific Southwest Research Station, Albany, California. [Google Scholar]
- Schönwiese CD, Rapp J, Fuchs T, et al. 1994. Observed climate trends in Europe 1891–1990. Meteorologische Zeitschrift N.F. 3: 22–28. [Google Scholar]
- Schubert R, Bahnweg G, Nechwatal J, et al. 1999. Detection and quantification of Phytophthora species which are associated with root-rot diseases in European deciduous forests by species-specific polymerase chain reaction. Forest Pathology 29: 169–188. [Google Scholar]
- Schumacher J, Leonhard S, Grundmann BM, et al. 2006. New alder disease in Spreewald biosphere reserve – causes and incidental factors of an epidemic. Nachrichtenblatt Deutscher Pflanzenschutzdienst 58: 141–147. [Google Scholar]
- Schütt P. 1993. Oak decline in Central and Eastern Europe – A critical review of a little understood phenomenon. In: Luisi N, Lerario P, Vannini A. (eds), Recent advances in studies on oak decline. Proceedings of an International Congress, Selva di Fassano, Italy, 13–18 September 1992: 235–239. [Google Scholar]
- Schwingle BW, Smith JA, Blanchette RA. 2007. Phytophthora species associated with diseased woody ornamentals in Minnesota nurseries. Plant Disease 91: 97–102. [DOI] [PubMed] [Google Scholar]
- Scibetta S, Schena L, Chimento A, et al. 2012. A molecular method to assess Phytophthora diversity in environmental samples. Journal of Microbiological Methods 88: 365–368. [DOI] [PubMed] [Google Scholar]
- Scott PM, Burgess TI, Barber PA, et al. 2009. Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia. Persoonia 22: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PM, Jung T, Shearer BL, et al. 2012. Pathogenicity of Phytophthora multivora to Eucalyptus gomphocephala and E. marginata. Forest Pathology 42: 289–298. [Google Scholar]
- Scott PM, Williams N. 2014. Phytophthora diseases in New Zealand forests. New Zealand Journal of Forestry 59: 14–21. [Google Scholar]
- Serrano MS, Fernández-Rebollo P, De Vita P, et al. 2010. Lupinus luteus, a new host of Phytophthora cinnamomi in Spanish oak-rangeland ecosystems. European Journal of Plant Pathology 128: 149–152. [Google Scholar]
- Shea SR. 1975. Environmental factors of the northern jarrah forest in relation to pathogenicity and survival of Phytophthora cinnamomi. Western Australia Forests Department Bulletin 85.
- Shea SR. 1979. Phytophthora cinnamomi – a collar rot pathogen of Banksia grandis. Australasian Plant Pathology 8: 32–34. [Google Scholar]
- Shea SR, Shearer BL, Tippett JT. 1982. Recovery of Phytophthora cinnamomi Rands from vertical roots of jarrah Eucalyptus marginata Sm. Australasian Plant Pathology 11: 25–27. [Google Scholar]
- Shea SR, Shearer BL, Tippett JT, et al. 1983. Distribution, reproduction, and movement of Phytophthora cinnamomi on sites highly conducive to Jarrah dieback in south western Australia. Plant Disease 67: 970–973. [Google Scholar]
- Shearer BL, Crane CE, Cochrane A. 2004. Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52: 435–443. [Google Scholar]
- Shearer BL, Crane CE, Fairman RG, et al. 2009. Ecosystem dynamics altered by pathogen-mediated changes following invasion of Banksia woodland and Eucalyptus marginata forest biomes of south-western Australia by Phytophthora cinnamomi. Australasian Plant Pathology 38: 417–436. [Google Scholar]
- Shearer BL, Dillon M. 1995. Susceptibility of plant species in Eucalyptus marginata forest to infection by Phytophthora cinnamomi. Australian Journal of Botany 43: 113–134. [Google Scholar]
- Shearer BL, Dillon M. 1996. Susceptibility of plant species in Banksia wood-lands on the Swan Coastal Plain, Western Australia, to infection by Phytophthora cinnamomi. Australian Journal of Botany 44: 433–445. [Google Scholar]
- Shearer BL, Fairman RG. 2007. A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australasian Plant Pathology 36: 78–86. [Google Scholar]
- Shearer BL, Fairman RG, Grant MJ. 2006. Effective concentration of phosphite in controlling Phytophthora cinnamomi following stem injection of Banksia species and Eucalyptus marginata. Forest Pathology 36: 119–135. [Google Scholar]
- Shearer BL, Michaelsen BJ, Somerford PJ, et al. 2014. Forest environment mediated intraspecific resistance of Eucalyptus marginata to Phytophthora cinnamomi. Australasian Plant Pathology 43: 245–255. [Google Scholar]
- Shearer BL, Tippett JT. 1989. Jarrah dieback: The dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forests of south-western Australia. Perth, Department of Conservation and Land Management. [Google Scholar]
- Shishkoff N. 2007. Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Disease 91: 1245–1249. [DOI] [PubMed] [Google Scholar]
- Sikora K, Verstappen E, Mendes O, et al. 2012. A universal microarray detection method for identification of multiple Phytophthora spp. using padlock probes. Phytopathology 102: 635–645. [DOI] [PubMed] [Google Scholar]
- Simamora AV, Stukely MJC, Hardy GEStJ, et al. 2015. Phytophthora boodjera sp. nov., a damping-off pathogen in production nurseries and from urban and natural landscapes, with an update on the status of P. alticola. IMA Fungus 6: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims LL, Goheen E, Kanaskie A, et al. 2015a. Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems. Northwest Science 89: 34–46. [Google Scholar]
- Sims LL, Sutton W, Reeser P, et al. 2015b. The Phytophthora species assemblage and diversity in riparian alder ecosystems of western Oregon, USA. Mycologia 107: 889–902. [DOI] [PubMed] [Google Scholar]
- Siwecki R, Liese W. (eds). 1991. Oak decline in Europe. Proceedings of an International Symposium, Kornik, Poland, 15–18 May 1990. [Google Scholar]
- Smith IW, Cunnington J, Pascoe I. 2006. Another new? species of Phytophthora on alder ‘down under’(Australia). In: Brasier CM, Jung T, Osswald W. (eds), Progress in Research on Phytophthora Diseases of Forest Trees: Poster 30. Forest Research, Farnham, Hampshire, UK. [Google Scholar]
- Smith RS. 2003. Aerial application of phosphite to protect endangered Western Australian flora. In: McComb JA, Hardy GEStJ, Tommerup I. (eds), Phytophthora in Forests and Natural Ecosystems: 194–196. Murdoch University, Perth, Australia. [Google Scholar]
- Sniezko RA, Hamlin J, Hansen EM. 2011. Operational Program to Develop Phytophthora lateralis-Resistant Populations of Port-Orford-cedar (Chamaecyparis lawsoniana). In: Sniezko RA, Yanchuk AD, Kliejunas JT, et al. (eds), Proceedings of the 4th International Workshop on Genetics of Host-Parasite Interactions in Forestry. General Technical Report PSW-GTR-240: 65–79. USDA Forest Service, Pacific Southwest Research Station, Albany, California, USA. [Google Scholar]
- Sniezko RA, Kolpak SE, Hansen EM, et al. 2006. Field survival of Phytophthora lateralis resistant and susceptible Port-Orford-cedar families. In: Brasier CM, Jung T, Osswald W. (eds), Progress in Research on Phytophthora Diseases of Forest Trees: 104–108. Forest Research, Farnham, Hampshire, UK. [Google Scholar]
- Solla A, Pérez-Sierra A, Corcobado T, et al. 2010. Phytophthora alni on Alnus glutinosa reported for the first time in Spain. Plant Pathology 59: 798. [Google Scholar]
- Spano D, Snyder RL, Cesaraccio C. 2013. Mediterranean phenology. In: Schwartz MD. (ed), Phenology: an integrative environmental science: 173–196. Springer Science & Business Media B.V., Dordrecht. [Google Scholar]
- Staley JM. 1965. Decline and mortality of red and scarlet oaks. Forest Science 11: 2–17. [Google Scholar]
- Stępniewska H, Dłuszyński J. 2010. Incidence of Phytophthora cambivora in bleeding lesions on beech stems in selected forest stands in south-eastern Poland. Phytopathologia 56: 39–51. [Google Scholar]
- Steward GA, Beveridge AE. 2010. A review of New Zealand Kauri (Agathis australis (D. Don) Lindl.): its ecology, history, growth and potential for management for timber. New Zealand Journal of Forestry Science 40: 33–59. [Google Scholar]
- Stewart-Wade SM. 2011. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: their detection and management. Irrigation Science 29: 267–297. [Google Scholar]
- Streito JC, Legrand P, Tabary F, et al. 2002. Phytophthora disease of alder (Alnus glutinosa) in France: investigations between 1995 and 1999. Forest Pathology 32: 179–191. [Google Scholar]
- Strelein G, Sage LW, Blankendaal PA. 2006. Rates of disease expansion of Phytophthora cinnamomi in the jarrah forest bioregion of southwestern Australia. In: Brasier CM, Jung T, Osswald W. (eds), Progress in Research on Phytophthora Diseases of Forest Trees: 49–52. Forest Research, Farnham, Hampshire, UK. [Google Scholar]
- Strouts RG, Rose DR, Reffold TC. 1989. Advisory services. Wales and southern England. In: Report on Forest Research 1988: 42–43. HMSO, London, UK. [Google Scholar]
- Tainter E, O’Brien GJ, Hernandez A, et al. 2000. Phytophthora cinnamomi as a cause of oak mortality in the State of Colima, Mexico. Plant Disease 84: 394–398. [DOI] [PubMed] [Google Scholar]
- Tainter FH, Baker FA. 1996. Principles of Forest Pathology. Wiley & Sons, Inc., New York. [Google Scholar]
- Tecklin D, DellaSala AD, Luebert F, et al. 2011. Valdivian temperate rainforests of Chile and Argentina. In: DellaSala DA. (ed), Temperate and boreal rainforests of the world: 132–153. Island Press, Washington, Covelo, London. [Google Scholar]
- Telfer KH, Brurberg MB, Herrero M-L, et al. 2015. Phytophthora cambivora found on beech in Norway. Forest Pathology 45: 415–425. [Google Scholar]
- Than DJ, Hughes KJD, Boonhan N, et al. 2013. A TaqMan real-time PCR assay for the detection of Phytophthora ‘taxon Agathis’ in soil, pathogen of Kauri in New Zealand. Forest Pathology 43: 324–330. [Google Scholar]
- Thao HTB, Yamakawa T. 2009. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Science and Plant Nutrition 55: 228–234. [Google Scholar]
- Thines M, Choi Y–J. 2016. Evolution, diversity and taxonomy of the Peronosporaceae, with focus on the genus Peronospora. Phytopathology 106: 6–18. [DOI] [PubMed] [Google Scholar]
- Thoirain B, Husson C, Marçais B. 2007. Risk factors for the Phytophthora-induced decline of alder in north-eastern France. Phytopathology 97: 99–105. [DOI] [PubMed] [Google Scholar]
- Thomas FM, Blank R, Hartmann G. 2002. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology 32: 277–307. [Google Scholar]
- Thomas P, El-Barghathi M, Polwart A. 2007. Biological flora of the British Isles: Juniperus communis L. Journal of Ecology 95: 1404–1440. [Google Scholar]
- Tippett JT, Shea RS, Hill TC, et al. 1983. Development of lesions caused by Phytophthora cinnamomi in the secondary phloem of Eucalyptus marginata. Australian Journal of Botany 31: 197–210. [Google Scholar]
- Trzewik A, Orlikowski LB, Oszako T, et al. 2015. The characterization of Phytophthora isolates obtained from diseased Alnus glutinsa in Poland. Baltic Forestry 21: 44–50. [Google Scholar]
- Tsao PH. 1983. Factors affecting isolation and quantitation of Phytophthora from soil. In: Erwin DC, Bartnicki-Garcia S, Tsao PH. (eds), Phytophthora. Its biology, taxonomy, ecology, and pathology. The American Phytopathological Society, St. Paul, Minnesota: 219–236. [Google Scholar]
- Tsao PH. 1990. Why many Phytophthora root rots and crown rots of tree and horticultural crops remain undetected. Bulletin OEPP/EPPO Bulletin 20: 11–17. [Google Scholar]
- Tubby KV, Webber JF. 2010. Pests and diseases threatening urban trees under a changing climate. Forestry 83: 451–459. [Google Scholar]
- Tucker CM, Milbrath JA. 1942. Root rot of Chamaecyparis caused by a species of Phytophthora. Mycologia 34: 94–103. [Google Scholar]
- Tynan KM, Wilkinson CJ, Holmes JM, et al. 2001. The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Australian Journal of Botany 49: 761–770. [Google Scholar]
- Tziros GT, Diamandis S. 2014. First report of Phytophthora cinnamomi causing ink disease on Castanea sativa in Greece. Journal of Plant Pathology 96: 415–417. [Google Scholar]
- Uchida K. 1967. Phytophthora disease of chestnut. Plant Protection 21: 383–387. [Google Scholar]
- Valencia AL, Chorbadjian RA, Latorre BA. 2011. First report of Nothofagus macrocarpa dieback caused by Phytophthora citrophthora and P. nicotianae in Chile. Plant Disease 95: 1193. [DOI] [PubMed] [Google Scholar]
- Van Poucke K, Franceschini S, Webber JF, et al. 2012. Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biology 116: 1178–1191. [DOI] [PubMed] [Google Scholar]
- Vannini A, Natili G, Anselmi N, et al. 2010. Distribution and gradient analysis of ink disease in chestnut forests. Forest Pathology 40: 73–86. [Google Scholar]
- Vannini A, Vettraino AM. 2001. Ink disease of chestnut: impact on European chestnut. Forest, Snow and Landscape Research 76: 345–350. [Google Scholar]
- Vannini A, Vettraino AM, Fabi A, et al. 2005. Monitoring ink disease of chestnut with the airborne multispectral system A.S.P.I.S. Acta Horticulturae 693: 529–533. [Google Scholar]
- Veblen TT, Burns BR, Kitzberger T, et al. 1995. The ecology of the conifers of southern South America. In: Enright NS, Hill RS. (eds), Ecology of the Southern Conifers: 120–155. Melbourne University Press, Melbourne, Australia. [Google Scholar]
- Vélez ML, Coetzee MPA, Wingfield MJ, et al. 2013. Evidence of low levels of genetic diversity for the Phytophthora austrocedrae population in Patagonia, Argentina. Plant Pathology 63: 212–220. [Google Scholar]
- Vercauteren A, Boutet X, D’hondt L, et al. 2011. Aberrant genome size and instability of Phytophthora ramorum oospore progenies. Fungal Genetics and Biology 48: 537–543. [DOI] [PubMed] [Google Scholar]
- Vercauteren A, De Dobbelaere I, Grünwald NJ, et al. 2010. Clonal expansion of the Belgian Phytophthora ramorum populations based on new microsatellite markers. Molecular Ecology 19: 92–107. [DOI] [PubMed] [Google Scholar]
- Vettraino AM, Barzanti GP, Bianco MC, et al. 2002. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. Forest Pathology 32: 19–28. [Google Scholar]
- Vettraino AM, Bonants P, Tomassini A, et al. 2012. Pyrosequencing as a tool for the detection of Phytophthora species: error rate and risk of false Molecular Operational Taxonomic Units. Letters in Applied Microbiology 55: 390–396. [DOI] [PubMed] [Google Scholar]
- Vettraino AM, Brasier CM, Webber JF, et al. 2017. Contrasting microsatellite diversity in the evolutionary lineages of Phytophthora lateralis. Fungal Biology 121: 112–126. [DOI] [PubMed] [Google Scholar]
- Vettraino AM, Jung T, Vannini A. 2008. First report of Phytophthora cactorum associated with beech decline in Italy. Plant Disease 92: 1708. [DOI] [PubMed] [Google Scholar]
- Vettraino AM, Morel O, Perlerou C, et al. 2005. Occurrence and distribution of Phytophthora species associated with ink disease of chestnut in Europe. European Journal of Plant Pathology 111: 169–180. [Google Scholar]
- Vettraino AM, Natili G, Anselmi N, et al. 2001. Recovery and pathogenicity of Phytophthora species associated with resurgence of ink disease on Castanea sativa in Italy. Plant Pathology 50: 90–96. [Google Scholar]
- Waipara N, Hill S, Hill L, et al. 2013. Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. New Zealand Plant Protection 66: 235–241. [Google Scholar]
- Walentowski H, Ewald J, Fischer A, et al. 2004. Handbuch der natürlichen Waldgesellschaften Bayerns (handbook of the natural forest types of Bavaria). Freising, Germany, Geobotanica. [Google Scholar]
- Wardle P. 1991. Vegetation of New Zealand. Cambridge, Cambridge University Press. [Google Scholar]
- Watson RT, Zinyowera MC, Moss RH, et al. (eds). 1996. Climate change 1995 – impacts, adaptations and mitigations of climate change: scientific-technical analyses. Contribution of Working Group II to the Second Assessment Report of the Intergovernmental Panel of Climate Change. Cambridge University Press. [Google Scholar]
- Watson RT, Zingowera MC, Moss RH, et al. (eds). 1998. The regional impacts of climate change. An assessment of vulnerability. Special Report of Working Group II. Cambridge University Press, New York. [Google Scholar]
- Webber JF. 2008. Status of Phytophthora ramorum and P. kernoviae in Europe. In: Frankel SJ, Kliejunas JT, Katharine M. (eds), Proceedings of the Sudden Oak Death 3rd Science Symposium. General Technical Report: PSW-GTR-214: 19–26. USDA Forest Service, Pacific Southwest Research Station, Albany, California, USA. [Google Scholar]
- Webber JF, Mullett M, Brasier CM. 2010. Dieback and mortality of plantation Japanese larch (Larix kaempferi) associated with infection by Phytophthora ramorum. New Disease Reports 22: 19. [Google Scholar]
- Webber J[F], Tilbury C, Steele H, et al. 2011. Potential impacts of pests and pathogens on short rotation forestry in Britain. In: McKay H. (ed), Short Rotation Forestry: Review of Growth and Environmental Impacts: 165–190. Forest Research Monograph 2. Forest Research, Farnham, UK. [Google Scholar]
- Webber JF, Vettraino AM, Chang TT, et al. 2012. Isolation of Phytophthora lateralis from Chamaecyparis foliage in Taiwan. Forest Pathology 42: 136–143. [Google Scholar]
- Weiland JE, Nelson AH, Hudler GW. 2010. Aggressiveness of Phytophthora cactorum, P. citricola I, and P. plurivora from European beech. Plant Disease 94: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Weir BS, Paderes EP, Anand N, et al. 2015. A taxonomic revision of Phytophthora Clade 5, including two new species, Phytophthora agathidicida and P. cocois. Phytotaxa 205: 21–38. [Google Scholar]
- Werres S, Elliot M, Greslebin A. 2014. Phytophthora austrocedrae Gresl. & E.M. Hansen. JKI Datasheets Plant Diseases and Diagnosis. http://pub.jki.bund.de/index.php/dsPDD/issue/view/3008/3204.
- Werres S, Kaminski K. 2005. Characterisation of European and North American Phytophthora ramorum isolates due to their morphology and mating behaviour in vitro with heterothallic Phytophthora species. Mycological Research 109: 860–871. [DOI] [PubMed] [Google Scholar]
- Werres S, Marwitz R, Man in ‘t Veld WAMI, et al. 2001. Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycological Research 105: 1155–1165. [Google Scholar]
- Weste G. 2001. Interaction between Phytophthora cinnamomi and Victorian native plant species growing in the wild. Australian Mycologist 20: 64–72. [Google Scholar]
- Weste G. 2003. The dieback cycle in Victorian forests: a 30-year study of changes caused by Phytophthora cinnamomi in Victorian forests, woodlands and heathlands. Australasian Plant Pathology 32: 247–256. [Google Scholar]
- Weste G, Law C. 1973. Invasion of native forest by Phytophthora cinnamomi. III: Threat to the national park, Wilson’ s Promontory, Victoria. Australian Journal of Botany 21: 31–51. [Google Scholar]
- Weste G, Marks GC. 1987. The biology of Phytophthora cinnamomi in Australasian forests. Annual Review of Phytopathology 25: 207–229. [Google Scholar]
- Wickland AC, Jensen CE, Rizzo DM. 2008. Geographic distribution, disease symptoms and pathogenicity of Phytophthora nemorosa and Phytophthora pseudosyringae in California, USA. Forest Pathology 38: 288–298. [Google Scholar]
- Widmer TL, Dodge SC. 2015. Bioassay conditions for infection of Pinus radiata seedlings with Phytophthora pinifolia zoospores. Plant Disease 99: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Wilcox WF, Ellis MA. 1989. Phytophthora root and crown rots of peach trees in the eastern Great Lakes region. Plant Disease 73: 794–798. [Google Scholar]
- Wilson BA, Aberton J, Cahill DM. 2000. Relationship between site factors and distribution of Phytophthora cinnamomi in the eastern Otway Ranges, Victoria. Australian Journal of Botany 48: 247–260. [Google Scholar]
- Wood AK, Tainter FH. 2002. First report of Phytophthora cinnamomi on Quercus laurifolia. Plant Disease 86: 441. [DOI] [PubMed] [Google Scholar]
- Yakabe LE, Blomquist CL, Thomas SL, et al. 2009. Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries. Plant Disease 93: 883–890. [DOI] [PubMed] [Google Scholar]
- Yang X, Richardson PA, Hong C. 2014. Phytophthora × stagnum nothosp. nov., a new hybrid from irrigation reservoirs at ornamental plant nurseries in Virginia. PloS ONE 9: e103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tyler BM, Hong C. 2017. An expanded phylogeny for the genus Phytophthora. IMA Fungus 8: 355–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans V. 1999. Historical and present day patterns in the decline of flooded gum (E. rudis) along Preston River, Donnybrook, southwest WA. Honours Thesis, Department of Botany, University of Western Australia, Perth. [Google Scholar]
- Zak B. 1957. Littleleaf of pine. USDA Forest Service, Forest Pest Leaflet 20.
- Zobel DB, Roth LF, Hawk GM. 1985. Ecology, pathology, and management of Port-Orford-cedar (Chamaecyparis lawsoniana). General Technical Report PNW-184. USDA, Forest Service, Pacific Northwest Forest and Range Experiment Station, Portland, Oregon. [Google Scholar]


