Abstract
In this study we accept 25 families in Diaporthales based on phylogenetic analyses using partial ITS, LSU, rpb2 and tef1-α gene sequences. Four different families associated with canker and dieback of tree hosts are morphologically treated and phylogenetically compared. These include three new families (Diaporthostomataceae, Pseudomelanconidaceae, Synnemasporellaceae), and one new genus, Dendrostoma (Erythrogloeaceae). Dendrostoma is newly described from Malus spectabilis, Osmanthus fragrans and Quercus acutissima having fusoid to cylindrical, bicellular ascospores, with three new species namely D. mali, D. osmanthi and D. quercinum. Diaporthostomataceae is characterised by conical and discrete perithecia with bicellular, fusoid ascospores on branches of Machilus leptophylla. Pseudomelanconidaceae is defined by conidiogenous cells with apical collarets and discreet annellations, and the inconspicuous hyaline conidial sheath when mature on Carya cathayensis, compared to morphologically similar families Melanconidaceae and Juglanconidaceae. Synnemasporellaceae is proposed to accommodate fungi with synnematous conidiomata, with descriptions of S. toxicodendri on Toxicodendron sylvestre and S. aculeans on Rhus copallina.
Keywords: Ascomycota, phylogeny, Sordariomycetes, taxonomy
INTRODUCTION
Diaporthales represents an important order in Sordariomycetes containing taxa that are mainly isolated as endophytes, saprobes or plant pathogens on various hosts. The order is characterised by perithecia with elongate beaks, often forming within stromatic tissues, deliquescent paraphyses and asci that generally deliquesce, become detached from the perithecial wall when mature, and have a characteristic refractive apical annulus (Rossman et al. 2007). Members of diaporthalean fungi are responsible for several diseases causing severe damage in plants with economic importance. The most notorious is chestnut blight caused by Cryphonectria parasitica (Cryphonectriaceae) that devastated American chestnut (Castanea dentata) populations in North America (Anagnostakis 1987, Gryzenhout et al. 2006). Other common diseases include ash anthracnose due to Gnomoniella fraxinii and birch canker caused by Cryptosporella platyphylla (Gnomoniaceae) (Redlin & Stack 1988, Fan et al. 2016a), stem-end rot of citrus fruits infected by Diaporthe citri and walnut canker by Diaporthe rostrata (Diaporthaceae) (Huang et al. 2013, 2015, Fan et al. 2015b, Guarnaccia & Crous 2017), willow and walnut canker disease caused by Cytospora chrysosperma (Cytosporaceae) (Fan et al. 2014, 2015a), birch dieback disease resulting from Melanconis stilbostoma (Melanconidaceae) (Fan et al. 2016b), walnut dieback disease by Juglanconis juglandina and J. oblonga (in Juglanconidaceae) (Voglmayr et al. 2017), foliar diseases of Eucalyptus by Harknessia spp. (Harknessiaceae) (Crous et al. 2012), and foliar, fruit and stem diseases by Coniella spp. (Schizoparmaceae) (Alvarez et al. 2016, Marin-Felix et al. 2017).
The classification of Diaporthales has changed drastically over the past decades because of the plasticity and variability in morphology. The order Diaporthales and Valsales were first introduced by Nannfeldt (1932), based on subfamilies Eu-Diaportheen and Valseen in Diaportheaceae proposed by Von Höhnel (1917). Later, Diaporthaceae and ‘Valsaceae’ (now Cytosporaceae, and referred to as such below) were recognised in the Diaporthales by Von Arx & Müller (1954). Kobayashi (1970) proposed Diaporthaceae (including Valsa = Cytospora) in a wide concept including all taxa accepted in Diaporthales by Barr (1978). Wehmeyer & Hanlin (1975) accepted three families within this order, including non-allantoid spored Gnomoniaceae and Diaporthaceae separated on the presence or absence of a stroma, and Cytosporaceae with allantoid ascospores. Barr (1978) arranged four families (Gnomoniaceae, Melanconidaceae, Pseudovalsaceae and Cytosporaceae) in Diaporthales based on beak position of ascomata and thin or firm ascospore walls without special emphasis on allantoid or non-allantoid ascospores. Families within the Diaporthales have been segregated by several mycologists to utilise various criteria: stromatic tissues, arrangement of ascomata in the stroma or substrate, and ascospore shape, e.g., four families (Cytosporaceae, Endoxylaceae, Gnomoniaceae and Melanconidaceae) by Monod (1983), three families (Cytosporaceae, Melanconidaceae and Phyllachoraceae) by Cannon (1988), while Hawksworth et al. (1995) merged the Cytosporaceae, Gnomoniaceae and Melanconidaceae proposed by Barr (1990) to Cytosporaceae and Melanconidaceae. These changes and confusions suggested that phenotypic characters alone were unable to provide sufficient evidence to resolve phylogenetic and evolutionary patterns among these fungi.
Molecular studies on fungi began in the early 1990s, and since then ribosomal DNA sequence data were accepted as the standard gene loci for fungi (Berbee & Taylor 1992, Schoch et al. 2012). Zhang & Blackwell (2001) recognised three lineages in Diaporthales, while Castlebury et al. (2002) postulated six major lineages, namely the Cryphonectria-Endothia complex, Cytosporaceae s.str., Diaporthaceae s.str., Gnomoniaceae s.str., Melanconidaceae s.str. and the Schizoparme complex. When Rossman et al. (2007) reviewed the Diaporthales, nine families were recognised, i.e., Cryphonectriaceae, Cytosporaceae, Diaporthaceae, Gnomoniaceae, Melanconidaceae, Pseudovalsaceae, Schizoparmeaceae, Sydowiellaceae and Togniniaceae. Kirk et al. (2008) added Melogrammataceae and listed 10 families in this order, whereas Jaklitsch & Voglmayr (2012) placed Melogrammataceae within Xylariales rather than Diaporthales. Subsequently, the Pseudoplagiostomataceae, Harknessiaceae, Macrohilaceae and Tirisporellaceae were also added to the Diaporthales (Cheewangkoon et al. 2010, Crous et al. 2012, 2015, Suetrong et al. 2015). Voglmayr & Jaklitsch (2014) resurrected Stilbosporaceae, while the Togniniaceae and Tirisporellaceae were reallocated to the Togniniales and Tirisporellales (Gramaje et al. 2015, Jones et al. 2015). Later, Lamproconiaceae and Juglanconidaceae were proposed as new families in this order (Norphanphoun et al. 2016, Voglmayr et al. 2017). The recent outline of Diaporthales published by Senanayake et al. (2017) used morphological and phylogenetic evidence to introduce seven new families and accepted a total of 21 families in the order. In spite of these changes, the phylogenetic placement of many genera in the Diaporthales remains unknown, and many families still wait to be elucidated.
During the trips to collect forest pathogens that cause canker or dieback diseases in China, several diaporthalean taxa associated with various disease symptoms were collected in Jiangxi and Zhejiang Provinces, China. Because the higher-level phylogeny of many genera within the Diaporthales remains largely unresolved, this project was initiated to address this issue. In this paper, we propose three new families and one new genus as well as several new species.
MATERIALS AND METHODS
Isolation
Fresh specimens of diaporthalean fungi were collected from infected branches of seven hosts during collection trips in China (Table 1). A total of 20 isolates were established by removing a mucoid spore mass from ascomata or conidiomata, spreading the suspension on the surface of 1.8 % potato dextrose agar (PDA), and incubating at 25 °C for up to 24 h. Single germinating conidia/ascospores were removed and plated onto fresh PDA plates. Specimens and isolates were deposited in the Key Laboratory for Silviculture and Conservation of the Ministry of Education in the Beijing Forestry University (BJFU) and the working Collection of X.L. Fan (CF) housed at the BJFU. Axenic cultures are maintained in the China Forestry Culture Collection Centre (CFCC).
Table 1.
Details of the strains included for molecular study.
| Species | Culture | Location | Host | GenBank accession numbers |
|||
|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1-α | ||||
| Apiosporopsis carpinea | CBS 771.79 | Switzerland | Carpinus betulus | NA | AF277130 | NA | NA |
| Apiosporopsis sp. | Masuya 11Af2-1 | Japan | Alnus firma | NA | AB669034 | NA | NA |
| Apoharknessia insueta | CBS 111377 | Brazil | Eucalyptus pellita | JQ706083 | AY720814 | NA | NA |
| CBS 114575 | Colombia | Eucalyptus sp. | NA | AY720813 | NA | NA | |
| Asterosporium asterospermum | MFLU 15-3555 | Italy | Fagus sylvatica | NA | MF190062 | MF377615 | NA |
| CBS 112404 | Italy | Fagus sylvatica | NA | AB553745 | NA | NA | |
| KT2138 | Japan | Fagus crenata | NA | AB553744 | NA | NA | |
| Auratiopycnidiella tristaniopsidis | CBS 132180 = CPC 16371 | Australia | Tristaniopsis laurina | JQ685516 | JQ685522 | NA | NA |
| Cainiella johansonii | Kruys 731 | Sweden | Dryas octopetala | NA | JF701920 | NA | NA |
| Chapeckia nigrospora | AR 3809 | USA | Betula sp. | JF681957 | EU683068 | NA | NA |
| Chiangraiomyces bauhiniae | MFLUCC 17-1669 | Thailand | Bauhinia sp. | MF190118 | MF190064 | MF377604 | NA |
| MFLUCC 17-1670 | Thailand | Bauhinia sp. | MF190119 | MF190065 | MF377603 | NA | |
| Chrysocrypta corymbiae | CBS 132528 | Australia | Corymbia sp. | JX069867 | JX069851 | NA | NA |
| Coniella diplodiella | CBS 111858 = CPC 3708 | France | Vitis vinifera | AY339323 | AY339284 | KX833423 | KX833603 |
| Coniella koreana | CBS 143.97 | Korea | NA | KX833584 | AF408378 | KX833490 | KX833684 |
| Coniella musaiensis var. hibisci | AR 3534 = CBS 109757 | South Africa | Hibiscus sp. | KX833589 | AF408337 | NA | KX833689 |
| Coniella straminea | CBS 149.22 = CPC 3932 | USA | Fragaria sp. | AY339348 | AF362569 | KX833506 | KX833704 |
| Coniella wangiensis | CBS 132530 = CPC 19397 | Australia | Eucalyptus sp. | JX069873 | JX069857 | KX833509 | KX833705 |
| Coryneum depressum | AR 3897 | Austria | Quercus cerris | NA | EU683074 | NA | NA |
| Coryneum modonium | AR 3558 | Austria | Castanea sativa | NA | EU683073 | NA | NA |
| Coryneum umbonatum | AR 3541 | Austria | Quercus cerris | NA | EU683072 | NA | NA |
| MFLUCC 15-1110 | Italy | Quercus sp. | MF190121 | MF190067 | MF377610 | NA | |
| MFLUCC 13-0658 | Italy | Quercus sp. | MF190120 | MF190066 | MF377609 | NA | |
| Cryphonectria macrospora | AR 3444 = CBS 109764 | Russia | Quercus mongolica | EU199182 | AF408340 | EU220029 | NA |
| Cryphonectria nitschkei | AR 3433 = CBS 109776 | Russia | Quercus mongolica | DQ120761 | AF408341 | NA | NA |
| Cryphonectria parasitica | ATCC 38755 | USA | Castanea dentata | AY141856 | EU199123 | DQ862017 | EU222014 |
| Cryptodiaporthe aesculi | CBS 109765 = AFTOL-ID 1238 | Austria | Aesculus hippocastanum | DQ323530 | AF408342 | EU199138 | GU354004 |
| AR3640 = CBS 121905 | USA | Aesculus hippocastanum | EU254994 | EU255164 | EU219269 | DQ313558 | |
| LCM 447.01 | Germany | Aesculus hippocastanum | GU367076 | NA | GU367110 | GU354002 | |
| Cryptosporella betulae | AR 3524 = CBS 109763 | Austria | Betula pendula | EU199180 | AF408375 | EU199139 | EU221884 |
| Cryptosporella hypodermia | AR 3552 | Austria | Ulmus minor | EU199181 | AF408346 | EU199140 | NA |
| Cryptosporella suffusa | AR 3496 = CBS 109750 | Austria | Alnus incana | EU199207 | AF408376 | EU199163 | EU221945 |
| Cytospora cenisia | AR 3522 = CBS 109752 | Austria | Juniperus communis | NA | AF408385 | NA | NA |
| Cytospora chrysosperma | CFCC 89600 | China | Sophora japonica | KR045623 | KR045623 | KU710951 | KU710915 |
| Cytospora elaeagni | CFCC 89633 | China | Elaeagnus angustifolia | KF765677 | KF765693 | KU710956 | KU710919 |
| Cytospora leucostoma | CFCC 50468 | China | Betula platyphylla | KT732949 | KT732968 | NA | NA |
| Cytospora nivea | AR 3512 | Austria | Salix purpurea | NA | AF408367 | NA | NA |
| Cytospora sacculus | AR 3416= CBS 109756 | Russia | Quercus mongolica | NA | AF408386 | NA | NA |
| AR 3426 = CBS 109777 | Austria | Quercus robur | NA | AF408387 | NA | NA | |
| Dendrostoma mali | CFCC 52102* | China | Malus spectabilis | MG682072 | MG682012 | MG682032 | MG682052 |
| Dendrostoma osmanthi | CFCC 52106* | China | Osmanthus fragrans | MG682073 | MG682013 | MG682033 | MG682053 |
| CFCC 52107* | China | Osmanthus fragrans | MG682074 | MG682014 | MG682034 | MG682054 | |
| CFCC 52108* | China | Osmanthus fragrans | MG682075 | MG682015 | MG682035 | MG682055 | |
| CFCC 52109* | China | Osmanthus fragrans | MG682076 | MG682016 | MG682036 | MG682056 | |
| Dendrostoma quercinum | CFCC 52103* | China | Quercus acutissima | MG682077 | MG682017 | MG682037 | MG682057 |
| CFCC 52104* | China | Quercus acutissima | MG682078 | MG682018 | MG682038 | MG682058 | |
| CFCC 52105* | China | Quercus acutissima | MG682079 | MG682019 | MG682039 | MG682059 | |
| Diaporthe decedens | AR 3459 = CBS 109772 | Austria | Corylus avellana | KC343059 | AF408348 | NA | NA |
| Diaporthe detrusa | AR 3424 = CBS 109770 | Austria | Berberis vulgaris | KC343061 | AF408349 | NA | KC343787 |
| Diaporthe eres | AR 3538 = CBS 109767 | Austria | Acer campestre | KC343075 | AF408350 | NA | KC343801 |
| Diaporthella corylina | CBS 121124 | China | Corylus sp. | KC343004 | NA | NA | NA |
| Diaporthella sp. | CN 5 | Italy | Corylus avellana | KP205483 | NA | NA | NA |
| CN13 | Italy | Corylus avellana | KP205484 | NA | NA | NA | |
| Diaporthosporella cercidicola | CFCC 51994 | China | Cercis chinensis | KY852492 | KY852515 | NA | NA |
| CFCC 51995 | China | Cercis chinensis | KY852493 | KY852516 | NA | NA | |
| CFCC 51996 | China | Cercis chinensis | KY852494 | KY852517 | NA | NA | |
| Diaporthostoma machili | CFCC 52100* | China | Machilus leptophylla | MG682080 | MG682020 | MG682040 | MG682060 |
| CFCC 52101* | China | Machilus leptophylla | MG682081 | MG682021 | MG682041 | MG682061 | |
| Disculoides eucalypti | CPC 17650 | Australia | Eucalyptus sp. | JQ685517 | JQ685523 | NA | NA |
| Disculoides eucalyptorum | CBS 132184 = CPC 17648 | Australia | Eucalyptus viminalis | NR120090 | JQ685524 | NA | NA |
| Ditopella ditopa | AR 3423 = CBS 109748 | Austria | Alnus glutinosa | EU199187 | EU199126 | EU199145 | NA |
| Erythrogloeum hymenaeae | CPC 18819 | Brazil | Hymenaea courbaril | JQ685519 | JQ685525 | NA | NA |
| Gnomonia gnomon | CBS 199.53 | Italy | Corylus avellana | AY818956 | AF408361 | EU219295 | EU221885 |
| Harknessia eucalypti | CBS 342.97 | Australia | Eucalyptus regnans | AY720745 | AF408363 | NA | NA |
| Harknessia molokaiensis | AR 3578 = CBS 109779 | USA | Eucalyptus robusta | NA | AF408390 | NA | NA |
| Hercospora tiliae | AR 3526 | Austria | Tilia tomentosa | NA | AF408365 | NA | NA |
| Hyaliappendispora galii | MFLUCC 16-1208 | Italy | Galium sp. | MF190149 | MF190095 | NA | NA |
| Juglanconis appendiculata | D96 | Austria | Juglans nigra | KY427139 | KY427139 | KY427189 | KY427208 |
| Juglanconis juglandina | ME23 | Austria | Juglans nigra | KY427150 | KY427150 | KY427200 | KY427219 |
| Juglanconis oblonga | ME14 | USA | Juglans cinerea | KY427151 | KY427151 | KY427201 | KY427220 |
| Juglanconis pterocaryae | ME20 | Japan | Pterocarya rhoifolia | KY427155 | KY427155 | KY427205 | KY427224 |
| Lamproconium desmazieri | MFLUCC 14-1047 | Russia | Tilia cordata | KX430132 | KX430133 | NA | MF377592 |
| MFLUCC 15-0870 | Russia | Tilia tomentosa | KX430134 | KX430135 | MF377605 | MF377591 | |
| Lasmenia sp. | CBS 124123 | Puerto Rico | Nephelium lappaceum | GU797406 | JF838338 | NA | NA |
| CBS 124124 | Puerto Rico | Nephelium lappaceum | JF838336 | JF838341 | NA | NA | |
| Luteocirrhus shearii | CBS 130776 | Australia | Banksia baxteri | NR120254 | NG042770 | NA | NA |
| Macrohilum eucalypti | CPC 10945 | New Zealand | Eucalyptus sp. | DQ195781 | DQ195793 | NA | NA |
| CPC 19421 | Australia | Eucalyptus piperita | KR873244 | KR873275 | NA | NA | |
| Melanconiella ellisii | BPI 878343 | USA | Carpinus caroliniana | JQ926271 | JQ926271 | JQ926339 | JQ926406 |
| Melanconiella hyperopta | AR 3832 = CBS 131492 | Austria | Carpinus betulus | JQ926278 | JQ926278 | NA | NA |
| Melanconiella spodiaea | MSH | Austria | Carpinus betulus | JQ926298 | JQ926298 | JQ926364 | JQ926431 |
| Melanconis alni | AR 3748 | Austria | Alnus viridis | EU199195 | EU199130 | EU199153 | NA |
| Melanconis betulae | CFCC 50471 | China | Betula albosinensis | KT732952 | KT732971 | KT732984 | KT733001 |
| Melanconis itoana | CFCC 50474 | China | Betula albosinensis | KT732955 | KT732974 | KT732987 | KT733004 |
| Melanconis marginalis | AR 3442 = CBS 109744 | Canada | Alnus rubra | EU199197 | AF408373 | EU219301 | EU221991 |
| Melanconis stilbostoma | CFCC 50475 | China | Betula platyphylla | KT732956 | KT732975 | KT732988 | KT733005 |
| Nakataea oryzae | CBS 243.76 | NA | NA | KM484861 | DQ341498 | NA | NA |
| Ophiodiaporthe cyatheae | YMJ1364 | China | Cyathea lepifera | JX570889 | JX570891 | JX570893 | NA |
| Pachytrype princeps | Rogers S | USA | NA | NA | FJ532382 | NA | NA |
| Pachytrype rimosa | FF1066 | Costa Rica | NA | NA | FJ532381 | NA | NA |
| Paradiaporthe artemisiae | MFLUCC 14-0850 | Italy | Artemisia sp. | MF190155 | MF190100 | NA | NA |
| MFLUCC 17-1663 | Italy | Artemisia sp. | MF190156 | MF190101 | NA | NA | |
| Phaeoappendispora thailandensis | MFLUCC 13-0161 | Thailand | Quercus sp. | MF190157 | MF190102 | MF377613 | NA |
| Phaeodiaporthe appendiculata | CBS 123821 = D77 | Austria | Acer campestre | KF570156 | KF570156 | NA | NA |
| CBS 123809 = D76 | Austria | Acer campestre | KF570155 | KF570155 | NA | NA | |
| Phragmoporthe conformis | AR 3632 = CBS 109783 | Canada | Alnus rubra | DQ323527 | AF408377 | NA | NA |
| Plagiostoma euphorbiae | CBS 340.78 | Netherlands | Euphorbia palustris | EU199198 | AF408382 | DQ368643 | NA |
| Plagiostoma salicellum | AR 3455 = CBS 109775 | Austria | Salix sp. | DQ323529 | AF408345 | EU199141 | EU221916 |
| Prosopidicola mexicana | CBS 113530 | USA | Prosopis glandulosa | AY720710 | NA | NA | NA |
| CBS 113529 | USA | Prosopis glandulosa | AY720709 | KX228354 | NA | NA | |
| Pseudomelanconis caryae | CFCC 52110* | China | Carya cathayensis | MG682082 | MG682022 | MG682042 | MG682062 |
| CFCC 52111* | China | Carya cathayensis | MG682083 | MG682023 | MG682043 | MG682063 | |
| CFCC 52112* | China | Carya cathayensis | MG682084 | MG682024 | MG682044 | MG682064 | |
| CFCC 52113* | China | Carya cathayensis | MG682085 | MG682025 | MG682045 | MG682065 | |
| Pseudoplagiostoma eucalypti | CBS 124807 | Venezuela | Eucalyptus urophylla | GU973512 | GU973606 | NA | NA |
| CBS 116382 | Thailand | Eucalyptus camaldulensis | GU973514 | GU973608 | NA | NA | |
| Pseudoplagiostoma oldii | CBS 115722 | Australia | Eucalyptus camaldulensis | GU973535 | GU973610 | NA | NA |
| Pseudoplagiostoma variabile | CBS 113067 | Uruguay | Eucalyptus globulus | GU973536 | GU973611 | NA | NA |
| Pyricularia grisea | Ina168 | NA | NA | AB026819 | AB026819 | NA | NA |
| Rossmania ukurunduensis | AR 3484 | Russia | Acer ukurunduense | NA | EU683075 | NA | NA |
| Sillia ferruginea | AR 3440 = CBS 126567 | Austria | Corylus avellana | JF681959 | EU683076 | NA | NA |
| Stegonsporium protopyriforme | CBS 117041 | Austria | Acer pseudoplatanus | NR126119 | EU039992 | NA | NA |
| Stegonsporium pyriforme | CBS 124487 | UK | Acer heldreichii | KF570160 | KF570160 | KF570190 | NA |
| Stilbospora macrosperma | CBS 121883 | Austria | Carpinus betulus | JX517290 | JX517299 | KF570196 | NA |
| CBS 121695 | Netherlands | Carpinus betulus | JX517288 | JX517297 | NA | NA | |
| Sydowiella depressula | CBS 813.79 | Switzerland | Rubus sp. | NA | EU683077 | NA | NA |
| Sydowiella fenestrans | AR 3777 = CBS 125530 | Russia | Chamerion angustifolium | JF681956 | EU683078 | NA | NA |
| Synnemasporella aculeans | CFCC 52094* | China | Rhus chinensis | MG682086 | MG682026 | MG682046 | MG682066 |
| CFCC 52095* | China | Rhus chinensis | MG682087 | MG682027 | MG682047 | MG682067 | |
| CFCC 52096* | China | Rhus chinensis | MG682088 | MG682028 | MG682048 | MG682068 | |
| AR 3878 = CBS 126566 | USA | Rhus glabra | NA | EU255134 | NA | NA | |
| Synnemasporella toxicodendri | CFCC 52097* | China | Toxicodendron sylvestre | MG682089 | MG682029 | MG682049 | MG682069 |
| CFCC 52098* | China | Toxicodendron sylvestre | MG682090 | MG682030 | MG682050 | MG682070 | |
| CFCC 52099* | China | Toxicodendron sylvestre | MG682091 | MG682031 | MG682051 | MG682071 | |
Note: CBS: Westerdijk Fungal Biodiversity Institute (CBS-KNAW Fungal Biodiversity Centre), Utrecht, The Netherlands; CFCC: China Forestry Culture Collection Center, Beijing, China; CPC: Culture collection of Pedro Crous, The Netherlands; MFLU: Mae Fah Luang University herbarium, Thailand; MFLUCC: Mae Fah Luang University Culture Collection, Thailand; NA: not applicable. All the new isolates used in this study are marked by an asterisk (*) and the strains from generic type species are in bold.
Morphology
Species identification was based on morphological features of the ascomata or conidiomata produced on infected plant tissues and micromorphology, supplemented by cultural characteristics. Cross-sections were prepared by hand using a double-edge blade under a dissecting microscope. At least 10 conidiomata/ascomata, 10 asci and 30 conidia/ascospores were measured to calculate the mean size and standard deviation (SD). Microscopic photographs were captured with a Nikon Eclipse 80i microscope equipped with a Nikon digital sight DS-Ri2 high definition colour camera, using differential interference contrast (DIC) illumination and the Nikon software NIS-Elements D Package v. 3.00. Adobe Bridge CS v. 6 and Adobe Photoshop CS v. 5 were used for the manual editing. Nomenclatural novelties and descriptions were deposited in MycoBank (Crous et al. 2004). Colony diameters were measured, and the colony colours described after 3 wk according to the colour charts of Rayner (1970).
DNA extraction, amplification and sequencing
Genomic DNA was extracted using a modified CTAB method, with fungal mycelium harvested from PDA plates with cellophane (Doyle & Doyle 1990). The ITS region was amplified with the primers ITS1 and ITS4 (White et al. 1990), the LSU region with the primers LR0R and LR5 (Vilgalys & Hester 1990), the rpb2 region with primers fRPB2-5F and fRPB2-7cR (Liu et al. 1999), and the tef1-α gene with the primers EF1-728F and EF1-986R (Carbone & Kohn 1999). The PCR mixture for all regions consisted of 1 μL genomic DNA, 3 mM MgCl2, 20 μM of each dNTP, 0.2 μM of each primer and 0.25 U BIOTAQ DNA polymerase (Bioline). Conditions for PCR of ITS and LSU genes constituted an initial denaturation step of 2 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 45 s at 51 °C and 1 min at 72 °C, and a final denaturation step of 8 min at 72 °C, while the tef1-α gene was performed using an initial denaturation step of 2 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 45 s at 56 °C and 1 min at 72 °C, and a final denaturation step of 8 min at 72 °C. For the rpb2 amplification, conditions consisted of five cycles of 45 s at 95 °C, 45 s at 56 °C and 2 min at 72 °C, then five cycles with a 53 °C annealing temperature and 30 cycles with a 50 °C annealing temperature. The DNA sequencing was performed using an ABI PRISM® 3730XL DNA Analyzer with BigDye® Terminater Kit v. 3.1 (Invitrogen) at the Shanghai Invitrogen Biological Technology Company Limited (Beijing, China).
Molecular data analyses
DNA sequences generated by each primer combination were used to obtain consensus sequences using SeqMan v. 7.1.0 in the DNASTAR Lasergene Core Suite software (DNASTAR Inc., Madison, WI, USA). Reference sequences were selected based on ex-type or ex-epitype sequences available from relevant published literature (Rossman et al. 2007, Cheewangkoon et al. 2010, Crous et al. 2012, 2015, Suetrong et al. 2015, Norphanphoun et al. 2016, Hongsanan et al. 2017, Senanayake et al. 2017, Voglmayr et al. 2017) (Table 1). All sequences were aligned using MAFFT v. 6 (Katoh & Toh 2010) and edited manually using MEGA v. 6 (Tamura et al. 2013). Phylogenetic analyses were performed using PAUP v. 4.0b10 for maximum parsimony (MP) analysis (Swofford 2003), MrBayes v. 3.1.2 for Bayesian Inference (BI) analysis (Ronquist & Huelsenbeck 2003), and PhyML v. 7.2.8 for Maximum Likelihood (ML) analysis (Guindon et al. 2010). The first analyses were performed on the combined multi-gene dataset (ITS, LSU, rpb2, tef1-α) to compare isolates of Diaporthales species to ex-type sequence data from recent studies (Table 1).
A partition homogeneity test (PHT) with heuristic search and 1 000 replicates was performed using PAUP v. 4.0b10 to test the discrepancy among the ITS, LSU, rpb2 and tef1-α sequence dataset in reconstructing phylogenetic trees. Maximum parsimony (MP) analysis was run using a heuristic search option of 1 000 search replicates with random-additions of sequences with a tree bisection and reconnection (TBR) algorithm. Maxtrees were set to 5 000, branches of zero length were collapsed and all equally parsimonious trees were saved. Other calculated parsimony scores were tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency (RC). Maximum likelihood (ML) analysis was performed with a GTR site substitution model, including a gamma-distributed rate heterogeneity and a proportion of invariant sites (Guindon et al. 2010). The branch support was evaluated with a bootstrapping (BS) method of 1 000 replicates (Hillis & Bull 1993).
MrModeltest v. 2.3 was used to estimate the best nucleotide substitution model settings for each gene (Posada & Crandall 1998). Bayesian inference (BI) was performed based on the DNA dataset from the results of the MrModeltest, using a Markov Chain Monte Carlo (MCMC) algorithm in MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). Two MCMC chains were run from random trees for 1 000 M generations and stopped when average standard deviation of split frequencies fell below 0.01. Trees were saved each 1 000 generations. The first 25 % of trees were discarded as the burn-in phase of each analysis, and the posterior probabilities (BPP) were calculated from the remaining trees (Rannala & Yang 1996). Nakataea oryzae (CBS 243.76) and Pyricularia grisea (Ina168) were selected as outgroups in all analyses (Fan et al. 2016b). Phylograms were shown using FigTree v. 1.3.1 (Rambaut & Drummond 2010). Novel sequences generated in the current study were deposited in GenBank (Table 1) and the aligned matrices used for phylogenetic analyses in TreeBASE (www.treebase.org; accession number: S22175).
RESULTS
Molecular phylogenetic analyses
The alignment based on the sequence dataset (ITS, LSU, rpb2 and tef1-α) included 122 ingroup taxa, comprising 3 261 characters in the aligned matrix. Of these, 1 562 characters were constant, 184 variable characters were parsimony-uninformative and 1 515 characters were parsimony informative. The MP analysis resulted in 119 equally most parsimonious trees (TL = 8 082, CI = 0.385, RI = 0.761, RC = 0.293) and the first tree is shown in Fig. 1. For BI analyses, the general time reversible model, additionally assuming a proportion of invariant sites with gamma-distributed substitution rates of the remaining sites (GTR + I + G) was determined to be the best for the ITS, LSU and tef1-α loci by MrModeltest, while the most appropriate model for the rpb2 locus was the Tamura-Nei model, additionally assuming a proportion of invariant sites with gamma-distributed substitution rates of the remaining sites (TrN + I + G). The MP and ML bootstrap support values above 70 % are shown at the first and second position, respectively. Branches with significant Bayesian posterior probability (≥ 0.95) in Bayesian analyses were thickened in the phylogenetic tree. The phylogram based on four genes indicated 24 known lineages, representing 22 known families and two unknown taxa lacking typification studies, namely Diaporthella and Phaeoappendispora. Four new lineages belonging to the Diaporthales, distinct from all known taxa, are herein described as three new families and a new genus in Erythrogloeaceae (Fig. 1).
Fig. 1.
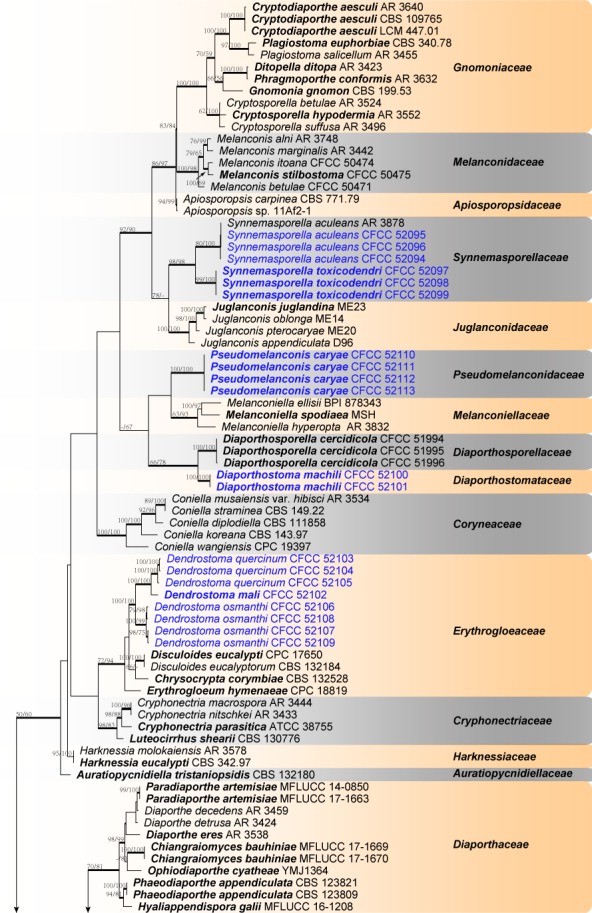

Phylogram of Diaporthales resulting from MP analysis based on combined ITS, LSU, rpb2 and tef1-α. MP and ML bootstrap support values above 70 % are shown at the first and second position. Thickened branches represent posterior probabilities above 0.95 from BI. Type species are in bold. Strains obtained in the current study are in blue. — Scale bar = 200 changes.
Taxonomy
Diaporthostomataceae X.L. Fan & C.M. Tian, fam. nov. — MycoBank MB823983
Etymology. Name derived from the type genus, Diaporthostoma.
Type genus. Diaporthostoma.
Sexual morph: Pseudostromata immersed in host bark and slightly erumpent from host tissues. Ectostromatic disc ovoid to ellipsoid, yellowish to dark grey. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia conical, surrounding the ectostromatic disc. Ostioles single, dark grey to black. Paraphyses deliquescent. Asci 8-spored, with an apical ring. Ascospores hyaline, fusoid, bicellular. Asexual morph: not observed.
Notes — The current phylogenetic analyses placed the new family Diaporthostomataceae in a highly supported clade (MP/ML/BI = 100/100/1) closely related to Diaporthosporellaceae. Diaporthostomataceae is morphologically distinct from Diaporthosporellaceae (Yang et al. 2018) by discrete perithecia and fusoid, straight to curved ascospores with a median septum.
Diaporthostoma X.L. Fan & C.M. Tian, gen. nov. — MycoBank MB823984
Etymology. Name derived from the morphological similarity with the genus Diaporthe.
Type species. Diaporthostoma machili X.L. Fan & C.M. Tian.
Sexual morph: Pseudostromata immersed in host bark, slightly erumpent from the bark surface. Ectostromatic disc yellowish to dark grey, nearly flat, ovoid to ellipsoid. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia conical, surrounding the ectostromatic disc, regularly scattered. Ostioles single, dark grey to black. Paraphyses deliquescent. Asci oblong to cylindrical-clavate, 8-spored, 2–3-seriate, with a more or less distinct apical ring. Ascospores hyaline, smooth, fusoid, multiguttulate, straight to curved, bicellular, with an inconspicuous median septum. Asexual morph: not observed.
Diaporthostoma machili X.L. Fan & C.M. Tian, sp. nov. — MycoBank MB823985; Fig. 2
Fig. 2.

Morphology of Diaporthostoma machili from Machilus leptophylla. a, b. Habit of pseudostromata on branches; c. transverse section through pseudostroma; d. longitudinal section through pseudostroma; e, f. asci and ascospores; g. ascospores. — Scale bars: a = 1 mm; b–d = 100 μm; e–g = 10 μm.
Etymology. Name derived from the host genus, Machilus.
Sexual morph: Pseudostromata immersed in host bark, slightly erumpent, 400–700 μm diam. Ectostromatic disc yellowish to dark grey, nearly flat, ovoid to ellipsoid, 120–140 μm diam. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia conical, surrounding the ectostromatic disc, regularly scattered, 380–420 μm diam. Ostioles single, dark grey to black, 65–85 μm diam. Paraphyses deliquescent. Asci oblong to cylindrical-clavate, 8-spored, 2–3-seriate, with a more or less distinct apical ring, (26–)30–38(–40) × 6–8 μm (x̄ = 33.5 ± 1.5 × 7 ± 0.5 μm, n = 20). Ascospores hyaline, smooth, fusoid, multiguttulate, straight to slight curved, bicellular, with a median septum, not constricted at the septum, 11–14 × 2–2.5 (x̄ = 12 ± 1.1 × 2.3 ± 0.2 μm, n = 30) μm. Asexual morph: not observed.
On PDA, cultures are initially white, becoming dark vinaceous after 3 wk. The colonies are flat with an irregular edge, producing white, sparse aerial mycelium; texture initially uniform, producing a dark brick-coloured circular ring on the margin after 3 d; sterile.
Host & Distribution — On Machilus leptophylla in China.
Materials examined (all on twigs and branches of Machilus leptophylla). China, Zhejiang Province, Hangzhou City, Linan, Tianmu Mountain, N30°19′18.21″ E119°26′18.21″, 354 m asl, 20 Apr. 2017, Q. Yang & Z. Du (holo-type CF 2017475; living ex-type culture CFCC 52100); Zhejiang Province, Hangzhou City, Linan, Tianmu Mountain, N30°19′17.33″ E119°26′15.60″, 350 m asl, 20 Apr. 2017, Q. Yang & Z. Du (CF 2017479; living culture CFCC 52101).
Notes — Diaporthostoma machili is the type species of Diaporthostoma, and is thus far only known to occur on Machilus leptophylla. Morphologically, it is characterised by the scattered, conical perithecia and fusoid, straight to curved ascospores with a median septum, which differs with other species in Diaporthales.
Erythrogloeaceae Senan. et al., Stud. Mycol. 86: 258. 2017. emend.
Type genus. Erythrogloeum Petr.
Sexual morph: Pseudostromata small to large, erumpent, consisting of an inconspicuous ectostromatic disc generally with orange colour, semi-immersed to superficial, causing a more or less pustulate bark surface. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia umber to fuscous black, covered with orange to umber pseudostromatic tissue, surrounding the ectostromatic disc, with small to long ostioles that emerge within the ectostromatic disc. Paraphyses deliquescent. Asci 8-spored, with an apical ring, grouped at the base with other asci, becoming detached from the perithecial wall. Ascospores hyaline, fusoid to cylindrical, bicellular. Asexual morph: foliicolous, associated with leaf spots. Conidiomata epiphyllous, subepidermal, sometimes eustromatic, acervular or subglobose, brown to black or yellow-orange, amphigenous, opening by irregular rupture, wall of 2–6 layers of orange-brown textura angularis, exuding slimy orange masses of conidia. Conidiophores hyaline to amber. Conidiogenous cells lining the inner cavity of conidioma, hyaline to olivaceous, smooth, subcylindrical to ampulliform, tapering to a long, thin neck, at times apical part elongated into a long neck, proliferating several times percurrently near apex, with flaring collarettes, or apex truncate, with minute periclinal thickening. Conidia hyaline to olivaceous, smooth, guttulate or not, thin-walled, ellipsoid, fusoid, ovoid to somewhat obclavate, straight to curved, apex subobtuse, obtusely rounded, base truncate, with prominent marginal frill, or dimorphic, intermixed in same conidiomata. Macroconidia broadly ellipsoid to obovoid, hyaline, smooth, granular to guttulate, thick-walled, apex obtuse, base flattened. Microconidia hyaline, smooth, guttulate, fusoid-ellipsoid, acutely rounded at apex, truncate at base (emended from Senanayake et al. 2017).
Notes — The family Erythrogloeaceae was recently introduced by Senanayake et al. (2017) to accommodate Chrysocrypta, Disculoides and Erythrogloeum species having epiphyllous acervuli, and subcylindrical to ampulliform conidiogenous cells. These authors did not report any sexual morph associated with these genera. During our investigation, phylogenetic inferences using DNA sequences from some materials with a sexual morph placed these samples in a highly supported clade (MP/ML/BI = 100/100/1) in the Erythrogloeaceae. The family Erythrogloeaceae is emended here to include the morphological features of the new sexual morphs observed during our study. These fungi have typical diaporthalean perithecia with clavate asci, and fusoid to cylindrical, bicellular ascospores.
Dendrostoma X.L. Fan & C.M. Tian, gen. nov. — MycoBank MB823986
Etymology. Name derived from pseudostromata emerging from woody host tissue.
Type species. Dendrostoma mali X.L. Fan & C.M. Tian.
Sexual morph: Pseudostromata small to large, distinct, circular, erumpent, consisting of an inconspicuous, usually orange ectostromatic disc, semi-immersed to superficial, causing a pustulate bark surface. Ectostromatic disc flat or concave, orange, surrounded by bark flaps. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia conspicuous, umber to fuscous black, embedded in orange to umber pseudostromatic tissue, regularly scattered, surrounding the ectostromatic disc, with small to long ostioles that emerge within the ectostromatic disc. Ostioles flat in the disc or sometimes slightly projecting, cylindrical, sometimes obscuring the disc, covered by an orange, umber to fuscous black crust. Paraphyses deliquescent. Asci fusoid, 8-spored, 2–3-seriate, with an apical ring, becoming detached from the perithecial wall. Ascospores hyaline, fusoid to cylindrical, symmetrical to asymmetrical, straight to curved, bicellular, with a median septum, constricted at the septum, smooth, multiguttulate. Asexual morph: observed on PDA. Conidiomata pycnidial, hemispherical, somewhat erumpent, coated with aerial mycelium. Conidiophores hyaline to amber. Conidiogenous cells enteroblastic, polyphialidic, hyaline, verruculose, ampulliform to doliiform. Conidia hyaline, aseptate, ovoid to ellipsoid, or fusoid.
Notes — The current phylogenetic analyses placed the new genus Dendrostoma in a highly supported clade (MP/ML/BI = 100/100/1) closely related to other genera in Erythrogloeaceae (Senanayake et al. 2017). Dendrostoma is described based on the typical diaporthalean perithecia with clavate asci and fusoid to cylindrical, bicellular ascospores. This study describes one genus and four species from China, and the host association appears to provide an important character for reliable identification. However, further collections are needed to confirm the host ranges and geographical distributions.
Dendrostoma mali X.L. Fan & C.M. Tian, sp. nov. — MycoBank MB823987; Fig. 3
Fig. 3.

Morphology of Dendrostoma mali from Malus spectabilis. a–c. Habit of pseudostromata on branches; d. transverse section of perithecia; e. longitudinal section through perithecia; f–i. asci and ascospores; j, k. ascospores; l, m. conidiomata on PDA; n. conidiophores and conidiogenous cells; o. conidia. — Scale bars: a = 1 mm; b–e, l, m = 500 μm; f–k, n, o = 10 μm.
Etymology. Name derived from the host genus, Malus.
Pseudostromata erumpent, consisting of an inconspicuous orange ectostromatic disc, semi-immersed to superficial, causing a pustulate bark surface, 1300–2100 μm diam. Ectostromatic disc flat or concave, orange, or brown to black, sometimes concealed by ostioles, surrounded by bark flaps, 350–800 μm diam. Central column yellowish to brownish. Stromatic zones lacking. Perithecia conspicuous, umber to fuscous black, regularly scattered, surrounding the ectostromatic disc, 300–500 μm diam. Ostioles 2–6 per disc, flat in the disc or sometimes slightly projecting, cylindrical, covered by an orange, umber to fuscous black crust, 70–100 μm diam. Paraphyses deliquescent. Asci fusoid, 8-spored, biseriate, with an apical ring, 40–60(–65) × 7–10(–11) μm (x̄ = 47 ± 5.3 × 8.5 ± 1.1 μm, n = 10). Ascospores hyaline, fusoid to cylindrical, smooth, multiguttulate, often containing two guttules per cell, symmetrical to asymmetrical, straight to slightly curved, bicellular, with a median septum distinctly constricted, 12–14 × 3–4 μm (x̄ = 13 ± 1 × 3.4 ± 0.3 μm, n = 30). Conidiomata pycnidial, hemispherical, somewhat erumpent, coated with white aerial mycelium, 1200–2500 μm, conidial masses extruding from the ostioles. Conidiophores hyaline, occasionally amber at the base, aseptate. Conidiogenous cells enteroblastic, polyphialidic, with 1–2 integrated loci, hyaline, verruculose, ampulliform to doliiform. Conidia hyaline, aseptate, ovoid to ellipsoid, apex obtuse, 3–4.5 × 2–2.5 μm (x̄ = 3.6 ± 0.5 × 2.2 ± 0.2 μm, n = 30).
On PDA, cultures are white. Colonies are flat with regular edge; texture initially uniform, producing concentric circles after 3 wk with sparse conidiomata irregularly distributed on the agar surface.
Host & Distribution — On Malus spectabilis in China.
Material examined. China, Zhejiang Province, Hangzhou City, Linan, Tianmu Mountain, N30°19′02.62″ E119°26′34.33″, 320 m asl, on twigs and branches of Malus spectabilis, 21 Apr. 2017, Q. Yang & Z. Du (holotype CF 2017445; living ex-type culture CFCC 52102).
Notes — Dendrostoma mali is the type species of Dendrostoma, and presently is only known on Malus spectabilis. It can be distinguished from other known Dendrostoma spp. by the fusoid to cylindrical ascospores, and the ovoid to ellipsoid conidia with obtuse apices. Dendrostoma mali is assumed to be host specific which needs to be confirmed by additional studies.
Dendrostoma quercinum X.L. Fan & C.M. Tian, sp. nov. — MycoBank MB823989; Fig. 4
Fig. 4.
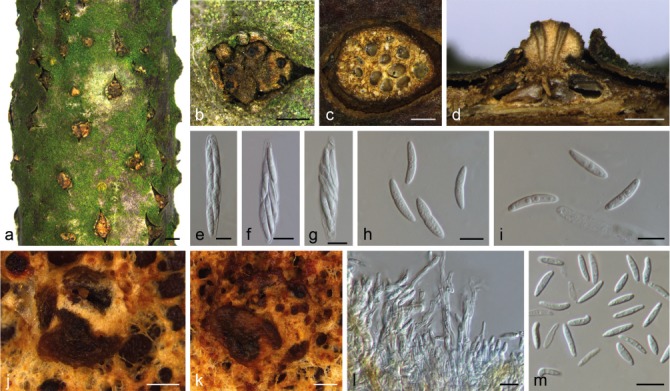
Morphology of Dendrostoma quercina from Quercus acutissima. a, b. Habit of pseudostromata on branches; c. transverse section of pseudostroma; d. longitudinal section through pseudostroma; e–g. asci and ascospores; h, i. ascospores; j, k. conidiomata on PDA; l. conidiophores and conidiogenous cells; m. conidia. — Scale bars: a = 1 mm; b–d, j, k = 500 μm; e–i, l, m = 10 μm.
Etymology. Name derived from the host genus, Quercus.
Pseudostromata erumpent, consisting of an inconspicuous orange ectostromatic disc, semi-immersed to erumpent, causing a pustulate bark surface, 800–1500 μm diam. Ectostromatic disc flat or concave, orange, or brown to black, sometimes concealed by ostioles, surrounded by bark flaps, 500–1100 μm diam. Central column yellowish to brownish. Stromatic zones lacking. Perithecia conspicuous, umber to fuscous black, regularly scattered, surrounding the ectostromatic disc, 250–500 μm. Ostioles 3–8 per disc, flat in disc or sometimes slightly projecting, cylindrical, covered by an orange, umber to fuscous black crust, 100–150 μm diam. Paraphyses deliquescent. Asci fusoid, 8-spored, 2–3-seriate, with an apical ring, 53–70 × 9.5–10 μm (x̄ = 60.5 ± 4 × 9.8 ± 0.3 μm, n = 10). Ascospores hyaline, fusoid to cylindrical, smooth, multi-guttulate, often containing two guttules per cell, symmetrical to asymmetrical, straight to slightly curved, bicellular, with a median septum distinctly constricted, 16–22(–24) × 3–4 μm (x̄ = 20 ± 1.7 × 3.5 ± 0.4 μm, n = 30). Conidiomata pycnidial, hemispherical, somewhat erumpent, covered with cinnamon aerial mycelium, 900–2200 μm diam; conidial masses extruding from ostioles. Conidiophores hyaline, occasionally amber at the base, aseptate. Conidiogenous cells enteroblastic, polyphialidic, with 1–2 integrated loci, hyaline, verruculose, ampulliform to doliiform. Conidia hyaline, aseptate, fusoid, acute at each end, 10.5–14 × 2.5(–3) μm (x̄ = 12 ± 1 × 2.5 ± 0.2 μm, n = 30).
On PDA, cultures are white, becoming hazel in the centre after 2 wk. The colonies are flat with regular edge; texture initially uniform, becoming dense in the centre after 2 wk, producing circular conidiomata at the margin of the compact centre.
Host & Distribution — On Quercus acutissima in China.
Materials examined (all on twigs and branches of Quercus acutissima). China, Zhejiang Province, Hangzhou City, Hangzhou Botanical Garden, N30°15′13.25″ E120°06′56.33″, 49 m asl, 17 Apr. 2017, Q. Yang & Z. Du (holotype CF 2017461; living ex-type culture CFCC 52103); Zhejiang Province, Hangzhou City, Hangzhou Botanical Garden, N30°15′12.52″, E120°06′57.02″, 50 m asl, 17 Apr. 2017, Q. Yang & Z. Du (CF 2017462; living culture CFCC 52104); Hangzhou City, Hangzhou Botanical Garden, N30°15′13.77″ E120°06′59.93″, 46 m asl, 17 Apr. 2017, Q. Yang & Z. Du (CF 2017470; living culture CFCC 52105).
Notes — Dendrostoma quercinum can be distinguished from D. mali and D. osmanthi by its larger ascospores (16–24 × 3–4 μm), and DNA sequence data.
Dendrostoma osmanthi X.L. Fan & C.M. Tian, sp. nov. — MycoBank MB823990; Fig. 5
Fig. 5.

Morphology of Dendrostoma osmanthi from Osmanthus fragrans. a, b. Habit of pseudostromata on branches; c. transverse section of pseudostroma; d. longitudinal section through pseudostroma; e–g. asci and ascospores; h. ascospores; i. conidiomata on PDA; j. conidiophores and conidiogenous cells; k. conidia. — Scale bars: a = 1 mm; b–d, i = 500 μm; e–h, j, k = 10 μm.
Etymology. Name derived from the host genus, Osmanthus.
Pseudostromata erumpent, consisting of an inconspicuous orange ectostromatic disc, semi-immersed to superficial, causing a pustulate bark surface, 1200–1400 μm diam. Ectostromatic disc flat or concave, orange, brown to black, sometimes concealed by ostioles, surrounded by bark flaps, 500–1100 μm diam. Central column yellowish to brownish. Stromatic zones lacking. Perithecia conspicuous, umber to fuscous black, regularly scattered, surrounding the ectostromatic disc, 300–400 μm diam. Ostioles 4–16 per disc, flat in the disc or sometimes slightly projecting, cylindrical, covered by an orange, umber to fuscous black crust, 80–130 μm diam. Paraphyses deliquescent. Asci fusoid, 8-spored, biseriate, with an apical ring, 55–65 × 7.5–9.5(–10) μm (x̄ = 59 ± 2.1 × 8.7 ± 0.7 μm, n = 10). Ascospores hyaline, fusoid to cylindrical, smooth, often containing two guttules per cell to multiguttulate, symmetrical to asymmetrical, straight to slightly curved, bicellular, with a median septum distinctly constricted, 11.5–14.5 × 3.5–4 μm (x̄ = 13 ± 1.2 × 3.7 ± 0.2 μm, n = 30). Conidiomata pycnidial, hemispherical, somewhat erumpent, coated with cinnamon aerial mycelium, 900–2200 μm diam, with translucent conidial droplets emerging from ostioles. Conidiophores hyaline, aseptate. Conidiogenous cells enteroblastic, polyphialidic, with 1–2 integrated loci, hyaline, ampulliform to doliiform. Conidia hyaline, aseptate, fusoid, acute at each end, 7.5–10.5(–12) × 2–2.5 μm (x̄ = 9.5 ± 1.2 × 2.3 ± 0.2 μm, n = 30).
On PDA, cultures are white, becoming slight isabelline after 2 wk. The colonies are flat with irregular edge; texture initially uniform, producing concentric circles after 2 wk with sparse conidiomata irregularly distributed on the agar surface.
Host & Distribution — On Osmanthus fragrans in China.
Materials examined (all on twigs and branches of Osmanthus fragrans). China, Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°17′00.77″ E119°43′28.70″, 139 m asl, 23 Apr. 2017, Q. Yang & Z. Du (holo-type CF 2017473; living ex-type culture CFCC 52106); Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N33°17′00.35″ E119°43′26.70″, 154 m asl, 23 Apr. 2017, Q. Yang & Z. Du (CF 2017476; living culture CFCC 52108); Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°17′30.67″ E119°43′20.30″, 139 m asl, 23 Apr. 2017, Q. Yang & Z. Du (CF 2017474; living culture CFCC 52107); Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N33°17′32.33″ E119°43′22.15″, 154 m asl, 23 Apr. 2017, Q. Yang & Z. Du (CF 2017477; living culture CFCC 52109).
Notes — The bark surface of Osmanthus fragrans is pustulate, with the fungus appearing to be pathogenic to this host. Dendrostoma osmanthi is similar to D. mali but differs by having fusoid to cylindrical ascospores that are distinctly constricted at the median septum. The phylogenetic inferences indicated this species as an individual well-supported clade (MP/ML/BI = 100/99/1) in the genus Dendrostoma.
Pseudomelanconidaceae C.M. Tian & X.L. Fan, fam. nov. — MycoBank MB823991
Etymology. Name derived from the type genus, Pseudomelanconis.
Type genus. Pseudomelanconis C.M. Tian & X.L. Fan.
Asexual morph: melanconium-like. Conidiomata in bark, acervular, with an inconspicuous ectostromatic disc causing a more or less pustulate bark surface. Central column beneath the disc more or less conical and becoming pale brown or olive at maturity. The marginal part of ectostroma comprises conidiophores and their basal cell layers. Conidiophores aseptate, unbranched, cylindrical, hyaline to pale brown, smooth-walled. Conidiogenous cells annellidic. Conidia ellipsoid to elongate pyriform, brown at maturity with hyaline sheath. Sexual morph: not observed.
Notes — The asexual morph of the new family Pseudomelanconidaceae is similar to members of Melanconiellaceae, Melanconidaceae and Juglanconidaceae (Fan et al. 2016b, Voglmayr et al. 2017), but differs mainly by having conidiogenous cells with discreet annellations and an inconspicuous hyaline conidial sheath when mature. The phylogenetic inferences resolved this family as an individual group with well-supported value (MP/ML/BI = 100/100/1) from other families of Diaporthales.
Pseudomelanconis C.M. Tian & X.L. Fan, gen. nov. — MycoBank MB823992
Etymology. Name derived from pseudo- (false-, in Greek) and the genus name Melanconis.
Type species. Pseudomelanconis caryae C.M. Tian & X.L. Fan.
Asexual morph: melanconium-like. Conidiomata in bark, acervular, immersed in host bark to erumpent. Ectostromatic disc inconspicuous, causing a more or less pustulate bark surface. Central column beneath the disc more or less conical. The marginal part of the central column comprises conidiophores and their basal cell layers. Conidiophores unbranched, aseptate, cylindrical, hyaline to pale brown, smooth-walled, sometimes reduced to conidiogenous cells. Conidiogenous cells annellidic, sometimes with apical collarette. Conidia hyaline when immature, becoming brown at maturity, ellipsoid to oblong, aseptate, multiguttulate, with distinct hyaline sheath, becoming inconspicuous when mature. Conidial wall smooth on the outer surface, with inconspicuous to distinct, sometimes confluent irregular verrucae on the inner surface. Sexual morph: not observed.
Pseudomelanconis caryae C.M. Tian & X.L. Fan, sp. nov. — MycoBank MB823993; Fig. 6
Fig. 6.
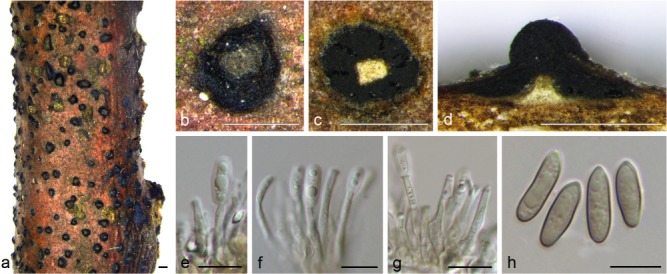
Morphology of Pseudomelanconis caryae from Carya cathayensis. a, b. Habit of conidiomata on branches; c. transverse section of conidioma; d. longitudinal section through conidioma; e–g. conidiophores and conidiogenous cells; h. conidia. — Scale bars: a = 1 mm; b–d = 500 μm; e–h = 10 μm.
Etymology. Named after the host genus from which it was isolated, Carya.
Asexual morph: melanconium-like. Conidiomata acervular, 500–800 μm diam, immersed in host bark to erumpent, covered by brown to blackish exuding conidial masses at maturity. Central column beneath the disc more or less conical. Conidiophores unbranched, aseptate, cylindrical, hyaline to pale brown, smooth-walled, 14–30 μm. Conidiogenous cells annellidic, occasionally with distinct annellations and collarettes. Conidia hyaline when immature, becoming greyish sepia to olivaceous, ellipsoid to oblong, multiguttulate, aseptate, (12.5–)13–15(–16) × 4–5 μm (x̄ = 14 ± 1.1 × 4.5 ± 0.3 μm, n = 30), with distinct hyaline sheath, 0.5–1 μm diam, becoming inconspicuous when mature. Conidial wall smooth on the outer surface. Sexual morph: not observed.
On PDA, cultures are initially white, becoming grey olivaceous. The colonies are flat, with irregular margins; texture initially uniform, becoming compact in centre after 3 wk. Conidiomata sparse, producing black conidial droplets, irregularly distributed over the agar surface.
Host & Distribution — On Carya cathayensis in China.
Materials examined (all on twigs and branches of Carya cathayensis). China, Zhejiang Province, Hangzhou City, Linan, Tianmu Mountain, N30°18′48.85″ E119°26′36.99″, 288 m asl, 21 Apr. 2017, Q. Yang & Z. Du (holotype CF 2017466; living ex-type culture CFCC 52110); Zhejiang Province, Hangzhou City, Linan, Tianmu Mountain, N30°18′49.19″ E119°26′37.24″, 281 m asl, 21 Apr. 2017, Q. Yang & Z. Du (CF 2017467; living culture CFCC 52111); Hangzhou City, Linan, Tianmu Mountain, N30°18′48.77″ E119°26′36.56″, 287 m asl, 21 Apr. 2017, Q. Yang & Z. Du (CF 2017468; living culture CFCC 52112); Hangzhou City, Linan, Tianmu Mountain, N30°18′49.14″ E119°26′30.44″, 285 m asl, 21 Apr. 2017, Q. Yang & Z. Du (CF 2017469; living culture CFCC 52113).
Notes — Pseudomelanconis caryae is the type species of Pseudomelanconis, and only occurs on Carya cathayensis in China. Isolates were identified as P. caryae based on their characteristic morphology, host, and DNA phylogeny (MP/ML/BI = 100/100/1). Juglanconis oblonga is similar to P. caryae, but it can be distinguished by larger brown to blackish conidia (18–22.7 × 9.2–12), and distinctly integrated annellations, as well as DNA sequence data (Voglmayr et al. 2017). Melanconis juglandis var. caryae was recorded from Carya cathayensis, which was considered as a distinct species by Wehmeyer (1941). However, it differs from P. caryae primarily by hyaline alpha (10.5–14 × 5–7 μm) and beta conidia (2–2.5 × 0.8–1 μm; Wehmeyer 1941). Wehmeyer (1937) also transferred Melanconiella pallida from Carya spp. to Melanconis, which differs from P. caryae in dark brown, subspherical to ovoid or oblong-cylindrical conidia (18–26.5 × 13.3–16.5 μm). Although Pseudomelanconis has acervular conidiomata covered by a pustulate conidial mass on the bark surface similar to Melanconis and Juglanconis, DNA sequence data confirmed them to represent a distinct phylogenetic lineage. Results of recent molecular phylogenetic investigations revealed a remarkably high diversity of corticolous melanconium-like fungi in Diaporthales (Fan et al. 2016b, Voglmayr et al. 2012, 2017).
Synnemasporellaceae X.L. Fan & J.D.P. Bezerra, fam. nov. — MycoBank MB823994
Etymology. Name derived from the type genus, Synnemasporella.
Type genus. Synnemasporella X.L. Fan & J.D.P. Bezerra.
Pseudostromata appearing upon the bark surface as pustules containing small groups of a few ostioles emergent through the adherent periderm, covered by a whitish pulverulence. Stromatic zones lacking. Perithecia spherical or flattened, with long necks, thickly clustered beneath the ectostromatic disks. Asci clavate. Ascospores biseriate, fusoid-ellipsoid, two-celled, hyaline, usually with a short, hyaline, bristle-like appendage at each end. Conidiomata synnematal or pycnidial. Synnemata determinate, parallel, consisting of slender, cylindrical black stalks and a spherical, capitate, shiny black mass of conidia which was cut off from the ends of the numerous entwined hyphae of the stalk; conidiogenous cells zone concave. Pycnidia with a central circular ostiole, hemispherical, immersed, somewhat erumpent. Conidiophores aggregated, straight to curved. Conidiogenous cells aggregated, hyaline, straight to curved, cylindrical. Conidia cylindrical to clavate, with a discrete hilum, smooth, pale brown.
Notes — The new family Synnemasporellaceae is proposed to accommodate fungi without the typical characters of any of the two-celled, hyaline-spored, stromatic genera. Also, the synnematal and pycnidial conidiomata differ widely from melanconium-like fungi, having pale brown conidia with a distinct hilum (Wehmeyer 1933). The phylogenetic inferences resolved this family as a well-supported clade (MP/ML/BI = 98/98/1) between the families Juglanconidaceae and Apiosporopsidaceae. Members of the new family differ from Juglanconidaceae and Apiosporopsidaceae (Senanayake et al. 2017, Voglmayr et al. 2017) mainly in the type of the host plant association, disease symptoms, ascomatal and/or conidiomatal characters, shape of ascospores, conidiogenous cells and conidia, and distinct synnemata.
Synnemasporella X.L. Fan & J.D.P. Bezerra, gen. nov. — MycoBank MB823995
Etymology. Name derived from the synnematous conidiomata.
Type species. Synnemasporella toxicodendri X.L. Fan & J.D.P. Bezerra.
Sexual morph (based on Wehmeyer 1933): Pseudostromata appearing upon the surface as pustules containing small groups of a few ostioles emergent through the adherent periderm, or as larger dense fascicles of elongate-cylindrical ostioles, erumpent through a whitish pulverulent disk. Ectostromatic disc often obliterated by the erumpent ostioles, many of which are covered by a whitish pulverulence. Perithecia spherical or somewhat flattened, with long slender necks, thickly clustered beneath the ectostromatic disks. Asci clavate. Ascospores biseriate, long fusoid-ellipsoid, two-celled, hyaline, constricted at the septum, usually with a short, hyaline, bristle-like appendage at each end. Asexual morph: Conidiomata synnematal or pycnidial. Synnemata long and determinate, erumpent, growing from the host tissue, pale brown, straight to curved, parallel, conidiogenous cells zone concave and dark, with some host tissue at the base of synnema. Pycnidia with a central circular ostiole, hemispherical, immersed, somewhat erumpent. Conidiophores aggregated, aseptate, straight to curved. Conidiogenous cells aggregated, hyaline, straight to curved, cylindrical, arranged alongside one another, each producing one conidium. Conidia cylindrical to oblong-cylindrical, with a discrete hilum, smooth, multiguttulate, pale brown.
Synnemasporella aculeans (Schwein.) X.L. Fan & J.D.P. Bezerra, comb. nov. — MycoBank MB823996; Fig. 7
Fig. 7.

Morphology of Synnemasporella aculeans from Rhus chinensis. a–c. Habit of synnemata on branches; d, e. longitudinal section through synnema; f, m, n. conidiophores and conidiogenous cells; g, h, o. conidia; i, j. habit of pycnidia on branches; k. transverse section of pycnidium; l. longitudinal section through pycnidium. — Scale bars: a–c = 1 mm; d, j–l = 500 μm; e = 100 μm; f–h, m–o = 10 μm; i = 5 mm.
Basionym. Sphaeria aculeans Schwein., Trans. Amer. Philos. Soc. 4: 204. 1832.
Synonym. Cryptodiaporthe aculeans (Schwein.) Wehm., Monogr. Gen. Diaporthe Nitschke & Segreg., Univ. Michigan Stud., Sci. Ser. 9: 212. 1933.
Sexual morph (based on Wehmeyer 1933): Pseudostromata appearing upon the surface as pustules containing small groups of a few ostioles emergent through the adherent periderm, or as larger dense fascicles of elongate-cylindrical ostioles, erumpent through a whitish pulverulent disk, 0.3–1 mm diam, covered by a whitish pulverulence. Stromatic zones lacking. Perithecia spherical or flattened, with long slender necks, thickly clustered beneath the ectostromatic disks, 260–480 × 250–400 μm. Asci clavate, 47–65 × 5–8 μm. Ascospores biseriate, long fusoid-ellipsoid, two-celled, hyaline, constricted at the septum, 12–18 × 2.5–3 μm, and usually with a short, hyaline, bristle-like appendage at each end, 2–2.5 μm length. Asexual morph: Conidiomata synnematal or pycnidial. Synnemata long and determinated, growing from the host tissue, pale to brown, straight to curved, parallel, with convex and dark conidiogenous cells zone, and some host tissue at the base of synnema, 1100–1500 μm high, 200–400 μm diam. Conidiophores aggregated, aseptate, straight to curved, 20–30 μm. Conidiogenous cells aggregated, hyaline, straight to curved, cylindrical, arranged alongside one another at the end of the synnemata, each producing one conidium. Conidia oblong-cylindrical, with a distinct hilum, smooth, multiguttulate, hyaline when young and becoming pale brown at maturity, 8–10(–11) × 3–3.5 μm (x̄ = 9.3 ± 0.9 × 3.2 ± 0.3 μm, n = 30). Pycnidia with a central circular ostiole, hemispherical, immersed, somewhat erumpent, containing an irregular one-chambered locule with black conidial mass, 700–1000 μm. Conidiophores aggregated, aseptate, straight to curved, 20–35 μm. Conidiogenous cells aggregated, hyaline, straight to curved, cylindrical, arranged alongside one another at the base of the pycnidia, each producing one conidium. Conidia ovoid to oblong-fusoid, one-celled, hyaline, multiguttulate, (6.5–)7–8.5(–9) × 2.5–3(–3.5) μm (x̄ = 7.6 ± 0.6 × 3 ± 0.3 μm, n = 30).
On PDA, cultures are initially white, becoming straw on the margin after 3 wk. The colonies are felty with regular edge; texture initially uniform, producing concentric circle on the margin after 3 d; sterile.
Host & Distribution — On Rhus copallina, R. diversiloba, R. glabra, R. javanica, R. typhina and R. vernix in Japan and USA (Wehmeyer 1933, Kobayashi 1970, Mejía et al. 2011), and on R. chinensis in China.
Materials examined (all on twigs and branches of Rhus chinensis). China, Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°15′32.83″ E119°43′30.73″, 47 m asl, 22 Apr. 2017, Q. Yang & Z. Du (CF 2017464; living culture CFCC 52094); Jiangxi Province, Dexing City, Phoenix Lake, N28°56′15.20″ E117°35′32.12″, 40 m asl, 8 Apr. 2017, B. Cao (CF 2017401; living culture CFCC 52096); Jiangxi Province, Dexing City, Phoenix Lake, N28°56′14.11″ E117°35′32.84″, 41 m asl, 8 Apr. 2017, B. Cao (CF 2017400; living culture CFCC 52095).
Notes — Synnemasporella aculeans is proposed as a new combination in the new genus Synnemasporella based on the description of Cryptodiaporthe aculeans, which was introduced producing perithecial ascomata, and an asexual morph producing sporodochial and/or pycnidial conidiomata (Wehmeyer 1933). Wehmeyer (1933) placed C. aculeans provisionally in Cryptodiaporthe and suggested this genus as a ‘heterogeneous group of species which will probably be segregated into several genera when the relationships of its species are better known’, highlighting that C. aculeans could be proposed as a new genus based on its atypical morphological features (see notes of Synnemasporellaceae). Sogonov et al. (2008) treated Cryptodiaporthe (type C. aesculi) as a synonym of Plagiostoma, which was followed by Mejía et al. (2011) and Voglmayr et al. (2017). Recently, Senanayake et al. (2017) separated the genera Plagiostoma and Cryptodiaporthe in their phylogenetically inferences, and pointed out that Gnomoniaceae comprised 24 genera, including Plagiostoma and Cryptodiaporthe. In the current study, the phylogram indicated that our isolates clustered into the same clade (MP/ML/BI = 80/100/1) with the only available culture of C. aculeans (AR3878). Based on the phylogenetic inferences and morphological features, we transferred C. aculeans to the new genus Synnemasporella, as a new combination S. aculeans. Synnemasporella aculeans is similar to S. toxicodendri, but differs from it in having shorter synnemata (1100–1500 μm vs 1200–1800 μm), a convex conidiogenous cells zone on top of synnemata, and larger, oblong-cylindrical conidia (8–11 × 3–3.5 μm).
This species also represents two types of conidiomata in Diaporthales, namely pycnidia and synnemata. This uncommon phenomenon was also recorded by Wehmeyer (1933), who observed the production of sporodochia on twigs of Rhus. Wehmeyer (1933) also reported the production of conidiomata when cultures were grown on agar, ‘showing all intergradations between true pycnidia and true sporodochia’.
Synnemasporella toxicodendri X.L. Fan & J.D.P. Bezerra, sp. nov. — MycoBank MB823997; Fig. 8
Fig. 8.
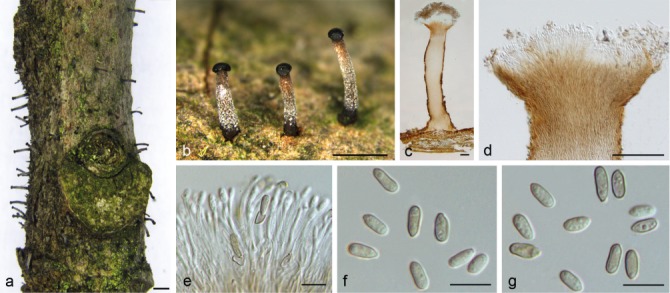
Morphology of Synnemasporella toxicodendri from Toxicodendron sylvestre. a, b. Habit of synnemata on branches; c, d. longitudinal section through synnema; e. conidiophores and conidiogenous cells; f, g. conidia. — Scale bars: a, b = 1 mm; c, d = 100 μm; e–g = 10 μm.
Etymology. Name derived from the host genus, Toxicodendron.
Conidiomata synnematal. Synnemata long and determinate, growing from host tissue, pale to brown, straight to curved, parallel, with flat to slightly concave and dark conidiogenous cells zone and some host tissue at the base of synnema, 1200–1800 μm high, 150–300 μm diam. Conidiophores aggregated, aseptate, straight to curved, reduced to conidiogenous cells, 20–30 μm. Conidiogenous cells aggregated, hyaline, straight to curved, cylindrical, arranged adjacent to one another at the end of the synnema, producing one conidium each. Conidia cylindrical to oblong-cylindrical, with a discrete hilum, smooth, multiguttulate, pale brown, 6–8 × 2.5–4 μm (x̄ = 7.3 ± 0.6 × 3.1 ± 0.3 μm, n = 30). Sexual morph: not observed.
On PDA, cultures are initially white, becoming sepia on the bottom after 3 d. The colonies are felty with an irregular edge; texture initially uniform, producing concentric circles after 3 wk; sterile.
Host & Distribution — From Toxicodendron sylvestre in China.
Materials examined (all on twigs and branches of Toxicodendron sylvestre). China, Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°15′32.84″ E119°43′31.21″, 54 m asl, 22 Apr. 2017, Q. Yang & Z. Du (holotype CF 2017481; living ex-type culture CFCC 52097); Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°15′32.21″ E119°43′31.55″, 51 m asl, 22 Apr. 2017, Q. Yang & Z. Du (CF 2017483; living culture CFCC 52099); Zhejiang Province, Hangzhou City, Linan, Xijing Mountain, N30°15′31.12″ E119°43′30.77″, 52 m asl, 22 Apr. 2017, Q. Yang & Z. Du (CF 2017482; living culture CFCC 52098).
Notes — Structures of S. toxicodendri were observed growing on diseased wood of Toxicodendron sylvestre in China, and so far, only occurs on T. sylvestre. Morphologically, it can be distinguished from S. aculeans and other genera in Diaporthales because it is characterised by the higher synnemata (1200–1800 μm) growing from the host tissue, with flat to slightly concave and dark conidiogenous cells zone on the top. The sexual morph of this species is not known and further collections are required to resolve its life cycle.
DISCUSSION
In this study we propose three new families, namely Diaporthostomataceae, Pseudomelanconidaceae, Synnemasporellaceae, and a new genus, Dendrostoma (Erythrogloeaceae), including three new species. The new materials studied here were collected in Zhejiang Province, China. This province was chiefly selected due to Tianmu Mountain, which is considered as a biodiversity hotspot with a high diversity for forest species (Zhou 1995). In the current study, all specimens were collected from symptomatic branches and twigs associated with canker or dieback disease of Anacardiaceae (Rhus chinensis, Toxicodendron sylvestre), Fagaceae (Quercus acutissima), Juglandaceae (Carya cathayensis), Lauraceae (Machilus leptophylla), Oleaceae (Osmanthus fragrans) and Rosaceae (Malus spectabilis), suggesting that many additional undiscovered species of diaporthalean fungi exist in China.
The classification of Diaporthales presented here integrates results from prior analyses (Castlebury et al. 2002, Rossman et al. 2007) and discoveries of new taxa from many other research groups (Cheewangkoon et al. 2010, Crous et al. 2012, 2015, Suetrong et al. 2015, Norphanphoun et al. 2016, Senanayake et al. 2017, Voglmayr et al. 2017). Diaporthales are mostly characterised by diaporthalean perithecia with an elongate beak and unitunicate asci with a characteristic refractive apical annulus when mature (Rossman et al. 2007). However, these characters are difficult to fully study in vivo due to the absence of various morphological morphs. As a result, the family concepts have thus been unstable, with many species being transferred from one genus or family to another (Rossman et al. 2007, Hongsanan et al. 2017, Senanayake et al. 2017). Based on newly generated molecular data, the current systematic framework provides good support for this order and related families (Castlebury et al. 2002, Rossman et al. 2007, Crous et al. 2015, Senanayake et al. 2017, Voglmayr et al. 2017). The current study revised the Diaporthales and accepted 25 families in the order (Table 2). However, some nodes remain weakly supported, and genera such as Diaporthella and Phaeoappendispora require further collection and study (Senanayake et al. 2017).
Table 2.
Placement of families in Diaporthales by different authors.
| Nannfeldt (1932) | Von Arx & Müller (1954) | Wehmeyer & Hanlin (1975) | Barr (1978) | Rossman et al. (2007) | Senanayake et al. (2017) | This paper (2018) |
|---|---|---|---|---|---|---|
| Diaportheaceae | Diaportheaceae | Diaportheaceae | Diaportheaceae | Cryphonectriaceae | Apiosporopsidaceae | Apiosporopsidaceae |
| Valsaceae | Gnomoniaceae | Gnomoniaceae | Diaportheaceae | Apoharknessiaceae | Apoharknessiaceae | |
| Valsaceae | Melanconidaceae | Gnomoniaceae | Asterosporiaceae | Asterosporiaceae | ||
| Valsaceae | Melanconidaceae | Auratiopycnidiellaceae | Auratiopycnidiellaceae | |||
| Pseudovalsaceae | Coryneaceae | Coryneaceae | ||||
| Schizoparmeaceae | Cryphonectriaceae | Cryphonectriaceae | ||||
| Sydowiellaceae | Cytosporaceae | Cytosporaceae | ||||
| Togniniaceae | Diaporthaceae | Diaporthaceae | ||||
| Valsaceae | Erythrogloeaceae | Diaporthosporellaceae | ||||
| Gnomoniaceae | Diaporthostomataceae | |||||
| Harknessiaceae | Erythrogloeaceae | |||||
| Juglanconidaceae | Gnomoniaceae | |||||
| Lamproconiaceae | Harknessiaceae | |||||
| Macrohilaceae | Juglanconidaceae | |||||
| Melanconidaceae | Lamproconiaceae | |||||
| Melanconiellaceae | Macrohilaceae | |||||
| Prosopidicolaceae | Melanconidaceae | |||||
| Pseudoplagiostomataceae | Melanconiellaceae | |||||
| Schizoparmeaceae | Prosopidicolaceae | |||||
| Stilbosporaceae | Pseudomelanconidaceae | |||||
| Sydowiellaceae | Pseudoplagiostomataceae | |||||
| Schizoparmeaceae | ||||||
| Stilbosporaceae | ||||||
| Sydowiellaceae | ||||||
| Synnemasporellaceae |
As the morphological features in Diaporthales are highly diverse, phylogenetic studies have been useful to elucidate the diversity in this group, and the inclusion or exclusion of taxa in this order. In this study we proposed a new genus in Erythrogloeaceae, namely Dendrostoma, which is characterised by typical diaporthalean perithecia with clavate asci, and fusoid to cylindrical, bicellular ascospores. The family Erythrogloeaceae was recently proposed by Senanayake et al. (2017) to accommodate Chrysocrypta, Disculoides and Erythrogloeum based on morphological features and phylogenetic analyses. Members of this family are mainly characterised by acervular conidiomata, hyaline to olivaceous conidia, and the presence of macro- and microconidia. Although Senanayake et al. (2017) did not observe any sexual morph in this family, we did collect a sexual morph in the present study, and thus emended the family description accordingly.
The new family Diaporthostomataceae is introduced here based on phylogenetic inferences and morphology of its members, which are mainly characterised by conical and discrete perithecia, and bicellular, fusoid ascospores. The new family is morphologically distinct from its sister family Diaporthosporellaceae, which is also distinguished from other diaporthalean families by irregularly uniseriate, allantoid or subreniform ascospores, phialidic conidiophores, and cylindrical to ellipsoidal, aseptate conidia (Yang et al. 2018). Members of Diaporthosporellaceae are known from twigs and branches of Cercis chinensis (Yang et al. 2018), and representatives of Diaporthostomataceae occur on Machilus leptophylla, both occurring in China.
Pseudomelanconidaceae is described here (on Carya cathayensis in China) based on phylogenetic inferences, and on few morphological features which distinguish it from members of Melanconium-related Melanconiellaceae, Melanconidaceae and Juglanconidaceae. Senanayake et al. (2017) introduced Melanconiellaceae to accommodate the previous unresolved Melanconiella clade in Diaporthales. Melanconiella species were previously observed to be highly host-specific to the host family Betulaceae, and confined to the north temperate zone, namely Europe and North America (Voglmayr et al. 2012). Du et al. (2017) extended the host and geographic range to include Cornus controversa and Juglans regia in China. The Melanconidaceae was recently revised by Senanayake et al. (2017), and included a single genus, Melanconis, which is characterised by perithecial ascomata having 8-spored asci, hyaline, ellipsoid, 1-septate ascospores, and acervular conidiomata with hyaline to brown, ellipsoid or subglobose conidia. Members of this family include saprobic and plant pathogenic species in North America and Europe (Senanayake et al. 2017). Juglanconidaceae was introduced by Voglmayr et al. (2017) and it comprises a single genus, Juglanconis, which occurs on dead, corticated twigs and branches of Juglandaceae species. Morphologically, members of this family have perithecial ascomata, octosporous asci with an apical ring, hyaline, bicellular ascospores with or without gelatinous appendages, and acervular conidiomata with brown conidia with gelatinous sheaths (Senanayake et al. 2017, Voglmayr et al. 2017).
The molecular phylogenetic analyses also revealed another well-supported family, Synnemasporellaceae, which is closely related to Juglanconidaceae (Fig. 1). We identified one species of this clade as Cryptodiaporthe aculeans based on the ambiguous asexual features of this species, which can produce synnemata and pycnidia. Interestingly, C. aculeans was ‘arbitrarily placed in the genus Cryptodiaporthe’ by Wehmeyer (1933), who also suggested that this species could be described as a new genus ‘since it does not show the typical characters of any of the two-celled, hyaline-spored, stromata genera’. Recent studies, however, did not include Cryptodiaporthe aculeans in their phylogenetic analyses (Sogonov et al. 2008, Mejía et al. 2011, Senanayake et al. 2017). In the present study, this fungus was transferred to Synnemasporella as a new combination, based on the original description of Wehmeyer (1933), and the only sequenced culture (AR3878) available in GenBank. Synnemasporellaceae comprises fungi distinguished by the presence of spherical or flattened perithecia with long necks, clavate asci, fusoid-ellipsoid, two-celled, hyaline ascospores, usually with a short, hyaline, bristle-like appendage at each end, and synnematal and/or pycnidial conidiomata producing cylindrical to clavate, smooth, pale brown conidia.
As shown in this study, future studies addressing the family-level organization of the Diaporthales should routinely include data for protein-coding genes, especially rpb2 and tef1-α. It is hoped that the classification proposed here will also provide an updated phylogenetic framework that will facilitate further revision of the Diaporthales.
Acknowledgments
This study is financed by National Natural Science Foundation of China (Project No.: 31670647) and National Key R&D Program of China (Project No.: 2017YFD0600105). JDP Bezerra thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) of Brazil for scholarships.
REFERENCES
- Alvarez LV, Groenewald JZ, Crous PW. 2016. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Studies in Mycology 85: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostakis SL. 1987. Chestnut blight: the classical problem of an introduced pathogen. Mycologia 79: 23–37. [Google Scholar]
- Barr ME. 1978. The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycologia Memoir 7: 1–232. [Google Scholar]
- Barr ME. 1990. Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39: 43–184. [Google Scholar]
- Berbee ML, Taylor JW. 1992. Two ascomycete classes based on fruiting-body characters and ribosomal DNA sequence. Molecular Biology and Evolution 9: 278–284. [DOI] [PubMed] [Google Scholar]
- Cannon PF. 1988. Proposal to merge the Phyllachorales with the Diaporthales, with a new family structure. Systema Ascomycetum 7: 23–43. [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, et al. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Verkley GJM, et al. 2010. Re-evaluation of Cryptosporiopsis eucalypti and Cryptosporiopsis-like species occurring on Eucalyptus leaves. Fungal Diversity 44: 89–105. [Google Scholar]
- Crous PW, Carris LM, Giraldo A, et al. 2015. The genera of fungi-fixing the application of the type species of generic names–G2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6: 163–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch Mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012. A re-appraisal of Harknessia (Diaporthales), and the introduction of Harknessiaceae fam. nov. Persoonia 28: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Du Z, Fan XL, Yang Q, et al. 2017. Host and geographic range extensions of Melanconiella, with a new species M. cornuta in China. Phytotaxa 327: 252–260. [Google Scholar]
- Fan XL, Du Z, Hyde KD, et al. 2016a. Cryptosporella platyphylla, a new species associated with Betula platyphylla in China. Phytotaxa 253: 285–292. [Google Scholar]
- Fan XL, Du Z, Liang YM, et al. 2016b. Melanconis (Melanconidaceae) associated with Betula spp. in China. Mycological Progress 15: 40. [Google Scholar]
- Fan XL, Hyde KD, Liu M, et al. 2015a. Cytospora species associated with walnut canker disease in China, with description of a new species C. gigalocus. Fungal Biology 119: 310–319. [DOI] [PubMed] [Google Scholar]
- Fan XL, Hyde KD, Udayanga D, et al. 2015b. Diaporthe rostrata, a novel ascomycete from Juglans mandshurica associated with walnut dieback. Mycological Progress 14: 1–8. [Google Scholar]
- Fan XL, Liang YM, Ma R, et al. 2014. Morphological and phylogenetic studies of Cytospora (Valsaceae, Diaporthales) isolates from Chinese scholar tree, with description of a new species. Mycoscience 55: 252–259. [Google Scholar]
- Gramaje D, Mostert L, Groenewald JZ, et al. 2015. Phaeoacremonium: from esca disease to phaeohyphomycosis. Fungal Biology 119: 759–783. [DOI] [PubMed] [Google Scholar]
- Gryzenhout M, Myburg H, Wingfield BD, et al. 2006. Cryphonectriaceae (Diaporthales), a new family including Cryphonectria, Chrysoporthe, Endothia and allied genera. Mycologia 98: 239–249. [DOI] [PubMed] [Google Scholar]
- Guarnaccia V, Crous PW. 2017. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 8: 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, et al. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Kirk PM, Sutton BC, et al. 1995. Ainsworth & Bisby’s dictionary of the fungi. 8th edition. CAB International, Wallingford, USA. [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192. [Google Scholar]
- Hongsanan S, Maharachchikumbura SSN, Hyde KD, et al. 2017. An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Diversity 84: 1–17. [Google Scholar]
- Huang F, Hou X, Dewdney MM, et al. 2013. Diaporthe species occurring on citrus in China. Fungal Diversity 61: 237–250. [Google Scholar]
- Huang F, Udayanga D, Wang X, et al. 2015. Endophytic Diaporthe associated with citrus: A phylogenetic reassessment with seven new species from China. Fungal Biology 119: 331–347. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2012. Phylogenetic relationships of five genera of Xylariales and Rosasphaeria gen. nov. (Hypocreales). Fungal Diversity 52: 75–98. [Google Scholar]
- Jones EBG, Suetrong S, Sakayaroj J, et al. 2015. Classification of marine ascomycota, basidiomycota, blastocladiomycota and chytridiomycota. Fungal Diversity 73: 1–72. [Google Scholar]
- Katoh K, Toh H. 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26: 1899–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Canoon PF, Minter DW, et al. 2008. Ainsworth & Bisby’s dictionary of the fungi, 10nd edn, Wallingford, UK. [Google Scholar]
- Kobayashi T. 1970. Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bulletin of the Government Forest Experiment Station 226: 1–242. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y, Groenewald JZ, Cai L, et al. 2017. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology 86: 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, et al. 2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68: 211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M. 1983. Monographie taxonomique des Gnomoniaceae. Beihefte zur Sydowia 9: 1–315. [Google Scholar]
- Nannfeldt JA. 1932. Studien über die Morphologie und Systematik der nicht-lichenisierten inoperculaten Discomyceten. Nova Acta Regiae Societatis Scientiarum Upsaliensis, series 4, 8: 1–368. [Google Scholar]
- Norphanphoun C, Hongsanan S, Doilom M, et al. 2016. Lamproconiaceae fam. nov. to accommodate Lamproconium desmazieri. Phytotaxa 270: 89–102. [Google Scholar]
- Posada D, Crandall KA. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond A. 2010. FigTree v.1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, UK. [Google Scholar]
- Rannala B, Yang Z. 1996. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. [DOI] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, London, UK. [Google Scholar]
- Redlin SC, Stack RW. 1988. Gnomoniella fraxini sp. nov., teleomorph of the ash anthracnose fungus and its connection to Discula fraxinea comb. nov. Mycotaxon 32: 175–198. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA. 2007. A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, et al. 2017. Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. 2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetrong S, Klaysuban A, Sakayaroj J, et al. 2015. Tirisporellaceae, a new family in the order Diaporthales (Sordariomycetes, Ascomycota). Cryptogamie Mycologie 36: 319–330. [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (* and other methods). Version 4.0b10. Sunderland, England, UK. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Castlebury LA, Jaklitsch WM. 2017. Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales). Persoonia 38: 136–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2014. Stilbosporaceae resurrected: generic reclassification and speciation. Persoonia 33: 61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Von Arx JA, Müller E. 1954. Die Gattungen der amerosporen Pyrenomyceten. Beiträge Kryptogamenflora der Schweiz 11: 1–434. [Google Scholar]
- Von Höhnel F. 1917. System der Diaportheen. Berichte der Deutschen Botanischen Gesellschaft 35: 631–638. [Google Scholar]
- Wehmeyer LE. 1933. The genus Diaporthe Nitschke and its segregates. University of Michigan, USA. [Google Scholar]
- Wehmeyer LE. 1937. Studies of certain species of Melanconis on Carpinus, Ostrya and Corylus. Mycologia 29: 599–617. [Google Scholar]
- Wehmeyer LE. 1941. A revision of Melanconis, Pseudovalsa, Prosthecium and Titania. University of Michigan Studies, Scientific Series 14: 1–161. [Google Scholar]
- Wehmeyer LE, Hanlin RT. 1975. The pyrenomycetous fungi. New York, USA. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a guide to methods and applications 18: 315–322. [Google Scholar]
- Yang Q, Du Z, Fan XL, et al. 2018. Diaporthosporellaceae, a novel family of Diaporthales (Sordariomycetes, Ascomycota). Mycoscience. doi: https://doi.org/10.1016/j.myc.2017.11.005. In press. [Google Scholar]
- Zhang N, Blackwell M. 2001. Molecular phylogeny of dogwood anthracnose fungus (Discula destructiva) and the Diaporthales. Mycologia 93: 355–365. [Google Scholar]
- Zhou C. 1995. The ecological characteristics of forest biodiversity of Tianmu Mountain and its sustainability. Journal of Zhejiang Forestry Science and Technology 16: 1–7. [Google Scholar]


