Abstract
Species of Diaporthe are considered important plant pathogens, saprobes, and endophytes on a wide range of plant hosts. Several species are well-known on grapevines, either as agents of pre- or post-harvest infections, including Phomopsis cane and leaf spot, cane bleaching, swelling arm and trunk cankers. In this study we explore the occurrence, diversity and pathogenicity of Diaporthe spp. associated with Vitis vinifera in major grape production areas of Europe and Israel, focusing on nurseries and vineyards. Surveys were conducted in Croatia, Czech Republic, France, Hungary, Israel, Italy, Spain and the UK. A total of 175 Diaporthe strains were isolated from asymptomatic and symptomatic shoots, branches and trunks. A multi-locus phylogeny was established based on five genomic loci (ITS, tef1, cal, his3 and tub2), and the morphological characters of the isolates were determined. Preliminary pathogenicity tests were performed on green grapevine shoots with representative isolates. The most commonly isolated species were D. eres and D. ampelina. Four new Diaporthe species described here as D. bohemiae, D. celeris, D. hispaniae and D. hungariae were found associated with affected vines. Pathogenicity tests revealed D. baccae, D. celeris, D. hispaniae and D. hungariae as pathogens of grapevines. No symptoms were caused by D. bohemiae. This study represents the first report of D. ambigua and D. baccae on grapevines in Europe. The present study improves our understanding of the species associated with several disease symptoms on V. vinifera plants, and provides useful information for effective disease management.
Keywords: canker, multi-locus sequence typing, pathogenicity, Vitis
INTRODUCTION
Diaporthe species are endophytes in asymptomatic plants, plant pathogens, or saprobes on decaying tissues of a wide range of hosts (Carroll 1986, Muralli et al. 2006, Garcia-Reyne et al. 2011, Udayanga et al. 2011). Diaporthe species are widespread, and well-known as causal agents of many important plant diseases, including root and fruit rots, dieback, stem cankers, leaf spots, leaf and pod blights and seed decay (Uecker 1988, Mostert et al. 2001a, b, Van Rensburg et al. 2006, Rehner & Uecker 1994, Santos et al. 2011, Udayanga et al. 2011, Tan et al. 2013). Species of the genus have also been used in secondary metabolite research due to their production of a large number of polyketides and a variety of unique low- and high-molecular-weight metabolites with different antibacterial, anticancer, antifungal, antimalarial, antiviral, cytotoxic and herbicidal activities (Corsaro et al. 1998, Isaka et al. 2001, Dai et al. 2005, Kumaran & Hur 2009, Yang et al. 2010, Gomes et al. 2013, Chepkirui & Stadler 2017), and for biological control of fungal pathogens (Santos et al. 2016).
Following the abolishment of dual nomenclature for fungi, the generic names Diaporthe and Phomopsis are no longer used to distinguish different morphs of this genus, and Rossman et al. (2015) proposed that the genus name Diaporthe should be retained over Phomopsis because it was introduced first, represents the majority of species, and therefore has priority.
Diaporthe was historically considered as monophyletic based on its typical sexual morph and Phomopsis asexual morph (Gomes et al. 2013). However, Gao et al. (2017) recently revealed its paraphyletic nature, showing that Mazzantia (Wehmeyer 1926), Ophiodiaporthe (Fu et al. 2013), Pustulomyces (Dai et al. 2014), Phaeocytostroma and Stenocarpella (Lamprecht et al. 2011), are embedded in Diaporthe s.lat. Furthermore, Senanayake et al. (2017) recently showed additional genera included in Diaporthe s.lat., such as Paradiaporthe and Chiangraiomyces.
The initial species concept of Diaporthe based on the assumption of host-specificity (Uecker 1988), resulted in the introduction of almost 2 000 species names available for both Diaporthe and Phomopsis (www.MycoBank.org). Most Diaporthe species can be found on diverse hosts, and can co-occur on the same host or lesion in different life modes (Rehner & Uecker 1994, Mostert et al. 2001a, Guarnaccia et al. 2016, Guarnaccia & Crous 2017). Thus, identification and description of species based on host association is not reliable within Diaporthe (Gomes et al. 2013, Udayanga et al. 2014a, b).
Before the molecular era, morphological characters such as size and shape of ascomata (Udayanga et al. 2011) and conidiomata (Rehner & Uecker 1994), were the basis on which to study the taxonomy of Diaporthe (Van der Aa et al. 1990). Recent studies demonstrated how these characters are not always informative for species level identification due to their variability under changing environmental conditions (Gomes et al. 2013).
Following the adoption of DNA sequence-based methods, the polyphasic protocols for studying the genus Diaporthe changed the taxonomy and species concepts in this genus, resulting in a rapid increase in the description of novelties. Therefore, genealogical concordance methods based on multi-gene DNA sequence data provide a much clearer approach to resolving the taxonomy for Diaporthe. Several major recent studies revealed ± 170 species supported by molecular data (Gomes et al. 2013, Lombard et al. 2014, Udayanga et al. 2014a, b, 2015, Gao et al. 2017, Dissanayake et al. 2017). Diaporthe taxonomy is actively changing, with numerous species being described each year mostly based on molecular phylogenetic approaches and morphological characterisation (Gao et al. 2017, Guarnaccia & Crous 2017).
Recent plant pathology studies confirmed Diaporthe species to be associated with several diseases on a broad range of economically significant agricultural crops such as Camellia, Citrus, Glycine, Helianthus, Persea, Vaccinium, Vitis, vegetables, fruit crops and forest plants (Van Rensburg et al. 2006, Santos & Phillips 2009, Crous et al. 2011a, b, 2016, Santos et al. 2011, Thompson et al. 2011, Grasso et al. 2012, Huang et al. 2013, Lombard et al. 2014, Gao et al. 2015, 2016, Udayanga et al. 2015, Guarnaccia et al. 2016, Guarnaccia & Crous 2017).
Diaporthe species are commonly found associated with V. vinifera and have been reported to be associated with several major diseases of grapevines. Important studies described Diaporthe species associated with grapevines using morphology, pathogenicity and molecular data (Merrin et al. 1995, Kuo & Leu 1998, Phillips 1999, Scheper et al. 2000, Mostert et al. 2001a, Van Niekerk et al. 2005, Dissanayake et al. 2015, Cinelli et al. 2016). One of the most significant studies (Van Niekerk et al. 2005) used ITS sequence data combined with morphology to examine South African strains and additional isolates obtained from worldwide collections to reveal several species associated with grapevine, such as D. ambigua, D. ampelina (as P. viticola), D. amygdali (as P. amygdali), D. australafricana, D. helianthi, D. kyushuensis (as P. vitimegaspora), D. perjuncta and D. rudis (as D. viticola). Moreover, they distinguished eight undescribed distinct species (as Phomopsis spp. 1–8) from grapevines. Schilder et al. (2005) confirmed D. ampelina (as P. viticola) to be a widespread pathogen in the Great Lakes Region of North America on the basis of DNA sequences from tef1 and cal gene regions. Diaporthe ampelina was also the most prevalent species isolated from grapevine cankers in California, where the occurrence of D. ambigua, D. eres and D. foeniculina (as D. neotheicola) was also reported in vineyards (Úrbez-Torres et al. 2013). Similarly, Baumgartner et al. (2013) identified D. ampelina and D. eres (as P. fukushii) in eastern North America. In Europe, D. eres was reported by Kaliterna et al. (2012) in Croatia and by Cinelli et al. (2016) in Italy. Four species of Diaporthe were identified after surveys in China, which included D. eres, D. hongkongensis, D. phaseolorum and D. sojae, and their pathogenicity was confirmed through artificial inoculation on detached grapevine twigs (Dissanayake et al. 2015).
Phomopsis cane and leaf spot is a major disease of grapevines, causing serious losses due to shoots breaking off at the base, stunting, dieback, loss of vigour, reduced bunch set and fruit rot (Pine 1958, 1959, Pscheidt & Pearson 1989, Pearson & Goheen 1994, Wilcox et al. 2015). Canes show brown to black necrotic irregular-shaped lesions, and clusters show rachis necrosis and brown, shrivelled berries close to harvest (Pearson & Goheen 1994). Diaporthe ampelina is historically the most common species known to cause this disease, which, together with D. amygdali, have been confirmed as severe pathogen of grapevines (Mostert et al. 2001a, Van Niekerk et al. 2005). Phomopsis cane and leaf spot is more severe in humid temperate climate regions, occurring throughout the growing season (Erincik et al. 2001). Recently, Úrbez-Torres et al. (2013) provided strong evidence for the role of P. viticola as a canker-causing organism, and suggested its addition to the fungi involved in the grapevine trunk diseases complex. Moreover, D. ampelina is the causal agent of grapevine swelling arm, induced also by D. kyushuensis (as P. vitimegaspora) (Kajitani & Kanematsu 2000, Van Niekerk et al. 2005). Cane bleaching is another grapevine symptom caused by D. perjuncta and D. ampelina (Kuo & Leu 1998, Kajitani & Kanematsu 2000, Mostert et al. 2001a, Rawnsley et al. 2004, Van Niekerk et al. 2005). Diaporthe eres was found as a weak to moderate pathogen causing wood-canker of vine (Kaliterna et al. 2012, Baumgartner et al. 2013).
Several diseases are often reported as caused by more than one Diaporthe species, or frequently, one Diaporthe species may cause various plant diseases (Santos & Phillips 2009, Diogo et al. 2010, Santos et al. 2011, Thompson et al. 2011, 2015). For example, D. caulivora, D. longicolla, D. novem and D. phaseolorum cause disease on soybean in Croatia (Santos et al. 2011). Sunflower stem blight is caused by D. gulyae, D. helianthi, D. kochmanii and D. kongii (Says-Lesage et al. 2002, Thompson et al. 2011). Devastating cankers caused by D. limonicola and D. melitensis were reported on lemon trees (Guarnaccia & Crous 2017). Moreover, D. novem has been reported as pathogen on Aspalathus linearis, Citrus spp., Glycine max, Helianthus annuus and Hydrangea macrophylla (Santos et al. 2011). Similarly, multiple Diaporthe species have been found associated with Phomopsis cane and leaf spot disease as well as cankers and swelling arm of grapevine (Phillips 1999, Kajitani & Kanematsu 2000, Mostert et al. 2001a, Rawnsley et al. 2004, Van Niekerk et al. 2005).
Only a few studies have dealt with the distribution of Diaporthe spp. on grapevine in Europe and other countries from the Mediterranean basin. Considering also the recent findings of Diaporthe species in different major grape production areas, and the changes in the species concepts, new surveys are required to study the occurrence and diversity of Diaporthe species related to grapevines and their association with diseases.
Therefore, several surveys were performed in European countries and Israel to collect grapevine specimens for Diaporthe isolations. This study was conducted in order to fully characterise these strains using morphological characters and multi-locus phylogenetic inference based on modern taxonomic concepts. In particular, the objectives of the present study were:
i. to conduct extensive surveys for sampling V. vinifera;
ii. to cultivate Diaporthe isolates;
iii. to subject those isolates to DNA sequence analyses combined with morphological characterisation;
iv. to compare the obtained results with the data from other phylogenetic studies on the genus; and
v. to evaluate the pathogenicity of the Diaporthe strains.
MATERIALS AND METHODS
Sampling and isolation
Pure cultures of Diaporthe were collected in seven European countries (Croatia, Czech Republic, France, Hungary, Italy, Spain and the UK) and Israel from asymptomatic and symptomatic Vitis vinifera plants, in both nursery and vineyard environments. Several samples showed multiple symptoms such as cane and leaf spot, cane bleaching, and additionally vascular browning and sectorial necrosis in grapevine wood. Isolations were performed from different plant organs such as canes, cordons and trunks. Isolates used in this study are maintained in the culture collection of the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, The Netherlands, and in the working collection of Pedro Crous (CPC), housed at the Westerdijk Institute (Table 1).
Table 1.
Collection details and GenBank accession numbers of isolates included in this study.
| Species | Culture no.1 | Host | Country | GenBank no.2 | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | tub2 | his3 | tef1 | cal | ||||
| Diaporthe acaciigena | CBS 129521 | Acacia retinodes | Australia | KC343005 | KC343973 | KC343489 | KC343731 | KC343247 |
| D. alleghaniensis | CBS 495.72 | Betula alleghaniensis | Canada | FJ889444 | KC843228 | KC343491 | GQ250298 | KC343249 |
| D. alnea | CBS 146.46 | Alnus sp. | Netherlands | KC343008 | KC343976 | KC343492 | KC343734 | KC343250 |
| D. ambigua | CBS 187.87 | Helianthus annuus | Italy | KC343015 | KC343983 | KC343499 | KC343741 | KC343257 |
| CBS 114015 | Pyrus communis | South Africa | KC343010 | KC343978 | KC343494 | KC343736 | KC343252 | |
| CBS 117167 | Aspalathus linearis | South Africa | KC343011 | KC343979 | KC343495 | KC343737 | KC343253 | |
| CBS 143342 = CPC 29648 | Vitis vinifera | Spain | MG280968 | MG281141 | MG281314 | MG281489 | MG281662 | |
| CPC 29652 | V. vinifera | Spain | MG280969 | MG281142 | MG281315 | MG281490 | MG281663 | |
| D. ampelina | CBS 111888 | V. vinifera | USA | KC343016 | KC343984 | KC343500 | KC343742 | KC343258 |
| CBS 114016 | V. vinifera | France | AF230751 | JX275452 | – | GQ250351 | JX197443 | |
| CPC 28254 | V. vinifera | UK | MG280970 | MG281143 | MG281316 | MG281491 | MG281664 | |
| CPC 28255 | V. vinifera | UK | MG280971 | MG281144 | MG281317 | MG281492 | MG281665 | |
| CPC 28263 | V. vinifera | UK | MG280972 | MG281145 | MG281318 | MG281493 | MG281666 | |
| CPC 28269 | V. vinifera | UK | MG280973 | MG281146 | MG281319 | MG281494 | MG281667 | |
| CPC 28270 | V. vinifera | UK | MG280974 | MG281147 | MG281320 | MG281495 | MG281668 | |
| CPC 28271 | V. vinifera | UK | MG280975 | MG281148 | MG281321 | MG281496 | MG281669 | |
| CPC 28272 | V. vinifera | UK | MG280976 | MG281149 | MG281322 | MG281497 | MG281670 | |
| CPC 28273 | V. vinifera | UK | MG280977 | MG281150 | MG281323 | MG281498 | MG281671 | |
| CPC 28280 | V. vinifera | UK | MG280978 | MG281151 | MG281324 | MG281499 | MG281672 | |
| CBS 143345 = CPC 28424 | V. vinifera | Italy | MG280979 | MG281152 | MG281325 | MG281500 | MG281673 | |
| CPC 29326 | V. vinifera | France | MG280980 | MG281153 | MG281326 | MG281501 | MG281674 | |
| CPC 29328 | V. vinifera | France | MG280981 | MG281154 | MG281327 | MG281502 | MG281675 | |
| CPC 29396 | V. vinifera | Israel | MG280982 | MG281155 | MG281328 | MG281503 | MG281676 | |
| CPC 29397 | V. vinifera | Israel | MG280983 | MG281156 | MG281329 | MG281504 | MG281677 | |
| CPC 29398 | V. vinifera | Israel | MG280984 | MG281157 | MG281330 | MG281505 | MG281678 | |
| CPC 29399 | V. vinifera | Israel | MG280985 | MG281158 | MG281331 | MG281506 | MG281679 | |
| CPC 29634 | V. vinifera | Spain | MG280986 | MG281159 | MG281332 | MG281507 | MG281680 | |
| CPC 29662 | V. vinifera | Spain | MG280987 | MG281160 | MG281333 | MG281508 | MG281681 | |
| CPC 29663 | V. vinifera | Spain | MG280988 | MG281161 | MG281334 | MG281509 | MG281682 | |
| CPC 29664 | V. vinifera | Spain | MG280989 | MG281162 | MG281335 | MG281510 | MG281683 | |
| CPC 29665 | V. vinifera | Spain | MG280990 | MG281163 | MG281336 | MG281511 | MG281684 | |
| CPC 29666 | V. vinifera | Spain | MG280991 | MG281164 | MG281337 | MG281512 | MG281685 | |
| CPC 29668 | V. vinifera | Spain | MG280992 | MG281165 | MG281338 | MG281513 | MG281686 | |
| CPC 29674 | V. vinifera | Spain | MG280993 | MG281166 | MG281339 | MG281514 | MG281687 | |
| CPC 29675 | V. vinifera | Spain | MG280994 | MG281167 | MG281340 | MG281515 | MG281688 | |
| CPC 29676 | V. vinifera | Spain | MG280995 | MG281168 | MG281341 | MG281516 | MG281689 | |
| CPC 29821 | V. vinifera | Czech Republic | MG280996 | MG281169 | MG281342 | MG281517 | MG281690 | |
| CPC 29828 | V. vinifera | Croatia | MG280997 | MG281170 | MG281343 | MG281518 | MG281691 | |
| CPC 29829 | V. vinifera | Croatia | MG280998 | MG281171 | MG281344 | MG281519 | MG281692 | |
| CPC 29832 | V. vinifera | Croatia | MG280999 | MG281172 | MG281345 | MG281520 | MG281693 | |
| CPC 30076 | V. vinifera | Hungary | MG281000 | MG281173 | MG281346 | MG281521 | MG281694 | |
| D. amygdali | CBS 126679 | Prunus dulcis | Portugal | KC343022 | KC343990 | KC343506 | KC343748 | KC343264 |
| D. anacardii | CBS 720.97 | Anacardium occidentale | East Africa | KC343024 | KC343992 | KC343508 | KC343750 | KC343266 |
| D. arecae | CBS 161.64 | Areca catechu | India | KC343032 | KC344000 | KC343516 | KC343758 | KC343274 |
| D. arengae | CBS 114979 | Arenga engleri | Hong Kong | KC343034 | KC344002 | KC343518 | KC343760 | KC343276 |
| D. australafricana | CBS 111886 | V. vinifera | Australia | KC343038 | KC344006 | KC343522 | KC343764 | KC343280 |
| D. baccae | CBS 136972 | Vaccinium corymbosum | Italy | KJ160565 | MF418509 | MF418264 | KJ160597 | MG281695 |
| CBS 143343 = CPC 293303 | V. vinifera | France | MG281001 | MG281174 | MG281347 | MG281522 | MG281696 | |
| CPC 29636 | V. vinifera | Spain | MG281002 | MG281175 | MG281348 | MG281523 | MG281697 | |
| CPC 29639 | V. vinifera | Spain | MG281003 | MG281176 | MG281349 | MG281524 | MG281698 | |
| CPC 296413 | V. vinifera | Spain | MG281004 | MG281177 | MG281350 | MG281525 | MG281699 | |
| CPC 29651 | V. vinifera | Spain | MG281005 | MG281178 | MG281351 | MG281526 | MG281700 | |
| CPC 29659 | V. vinifera | Spain | MG281006 | MG281179 | MG281352 | MG281527 | MG281701 | |
| CPC 29660 | V. vinifera | Spain | MG281007 | MG281180 | MG281353 | MG281528 | MG281702 | |
| CPC 29661 | V. vinifera | Spain | MG281008 | MG281181 | MG281354 | MG281529 | MG281703 | |
| CPC 29669 | V. vinifera | Spain | MG281009 | MG281182 | MG281355 | MG281530 | MG281704 | |
| CPC 29670 | V. vinifera | Spain | MG281010 | MG281183 | MG281356 | MG281531 | MG281705 | |
| CPC 29671 | V. vinifera | Spain | MG281011 | MG281184 | MG281357 | MG281532 | MG281706 | |
| CPC 29673 | V. vinifera | Spain | MG281012 | MG281185 | MG281358 | MG281533 | MG281707 | |
| CPC 29827 | V. vinifera | Croatia | MG281013 | MG281186 | MG281359 | MG281534 | MG281708 | |
| CPC 30315 | V. vinifera | Spain | MG281014 | MG281187 | MG281360 | MG281535 | MG281709 | |
| D. bicincta | CBS 121004 | Juglans sp. | USA | KC343134 | KC344102 | KC343618 | KC343860 | KC343376 |
| D. bohemiae | CBS 143347 = CPC 282223 | Vitis spp. | Czech Republic | MG281015 | MG281188 | MG281361 | MG281536 | MG281710 |
| CBS 143348 = CPC 282233 | Vitis spp. | Czech Republic | MG281016 | MG281189 | MG281362 | MG281537 | MG281711 | |
| D. carpini | CBS 114437 | Carpinus betulus | Sweden | KC343044 | KC344012 | KC343528 | KC343770 | KC343286 |
| D. celastrina | CBS 139.27 | Celastrus sp. | USA | KC343047 | KC344015 | KC343531 | KC343773 | KC343289 |
| D. celeris | CBS 143349 = CPC 282623 | V. vinifera | UK | MG281017 | MG281190 | MG281363 | MG281538 | MG281712 |
| CBS 143350 = CPC 282663 | V. vinifera | UK | MG281018 | MG281191 | MG281364 | MG281539 | MG281713 | |
| CPC 28267 | V. vinifera | UK | MG281019 | MG281192 | MG281365 | MG281540 | MG281714 | |
| D. citri | CBS 135422 | Citrus sp. | USA | KC843311 | KC843187 | MF418281 | KC843071 | KC843157 |
| D. citrichinensis | CBS 134242 | Citrus sp. | China | JQ954648 | MF418524 | KJ420880 | JQ954666 | KC357494 |
| D. cucurbitae | DAOM42078 | Cucumis sativus | Canada | KM453210 | KP118848 | KM453212 | KM453211 | – |
| D. decedens | CBS 109772 | Corylus avellana | Austria | KC343059 | KC344027 | KC343543 | KC343785 | KC343301 |
| D. detrusa | CBS 109770 | Berberis vulgaris | Austria | KC343061 | KC344029 | KC343545 | KC343787 | KC343303 |
| D. eleagni | CBS 504.72 | Eleagnus sp. | Netherlands | KC343064 | KC344032 | KC343548 | KC343790 | KC343306 |
| D. eres | CBS 200.39 | Laurus nobilis | Germany | KC343151 | KC344119 | KC343635 | KC343877 | KC343393 |
| CBS 439.82 | Cotoneaster sp. | Scotland | KC343090 | KC344058 | KC343574 | KC343816 | KC343332 | |
| CBS 587.79 | Pinus pentaphylla | Japan | KC343153 | KC344121 | KC343637 | KC343879 | KC343395 | |
| CBS 101742 | Fraxinus sp. | Netherlands | KC343073 | KC344041 | KC343557 | KC343799 | KC343315 | |
| CBS 113470 | Castanea sativa | Australia | KC343146 | KC344114 | KC343630 | KC343872 | KC343388 | |
| CBS 116953 | Pyrus pyrifolia | New Zealand | KC343147 | KC344115 | KC343631 | KC343873 | KC343389 | |
| CBS 135428 | Juglans cinerea | USA | KC843328 | KC843229 | KJ420840 | KC843121 | KC843155 | |
| CBS 138594 | Ulmus laevis | Germany | KJ210529 | KJ420799 | KJ420850 | KJ210550 | KJ434999 | |
| CBS 138597 | V. vinifera | France | KJ210518 | KJ420783 | KJ420833 | KJ210542 | KJ434996 | |
| CBS 143344 = CPC 28217 | V. vinifera | Czech Republic | MG281020 | MG281193 | MG281366 | MG281541 | MG281715 | |
| CPC 28218 | V. vinifera | Czech Republic | MG281021 | MG281194 | MG281367 | MG281542 | MG281716 | |
| CPC 28219 | V. vinifera | Czech Republic | MG281022 | MG281195 | MG281368 | MG281543 | MG281717 | |
| CPC 28220 | V. vinifera | Czech Republic | MG281023 | MG281196 | MG281369 | MG281544 | MG281718 | |
| CPC 28221 | V. vinifera | Czech Republic | MG281024 | MG281197 | MG281370 | MG281545 | MG281719 | |
| CPC 28226 | V. vinifera | Czech Republic | MG281025 | MG281198 | MG281371 | MG281546 | MG281720 | |
| CPC 28264 | V. vinifera | UK | MG281026 | MG281199 | MG281372 | MG281547 | MG281721 | |
| CPC 28274 | V. vinifera | UK | MG281027 | MG281200 | MG281373 | MG281548 | MG281722 | |
| CPC 28275 | V. vinifera | UK | MG281028 | MG281201 | MG281374 | MG281549 | MG281723 | |
| CPC 28276 | V. vinifera | UK | MG281029 | MG281202 | MG281375 | MG281550 | MG281724 | |
| CPC 28277 | V. vinifera | UK | MG281030 | MG281203 | MG281376 | MG281551 | MG281725 | |
| CPC 28278 | V. vinifera | UK | MG281031 | MG281204 | MG281377 | MG281552 | MG281726 | |
| CPC 28279 | V. vinifera | UK | MG281032 | MG281205 | MG281378 | MG281553 | MG281727 | |
| CPC 28423 | V. vinifera | Italy | KT369109 | KT369113 | MG281379 | KT369111 | MG281728 | |
| CPC 28426 | V. vinifera | Italy | KT369110 | KT369114 | MG281380 | KT369112 | MG281729 | |
| CPC 29317 | V. vinifera | France | MG281033 | MG281206 | MG281381 | MG281554 | MG281730 | |
| CPC 29331 | V. vinifera | France | MG281034 | MG281207 | MG281382 | MG281555 | MG281731 | |
| CPC 29633 | V. vinifera | Spain | MG281035 | MG281208 | MG281383 | MG281556 | MG281732 | |
| CPC 29635 | V. vinifera | Spain | MG281036 | MG281209 | MG281384 | MG281557 | MG281733 | |
| CPC 29638 | V. vinifera | Spain | MG281037 | MG281210 | MG281385 | MG281558 | MG281734 | |
| CPC 29643 | V. vinifera | Spain | MG281038 | MG281211 | MG281386 | MG281559 | MG281735 | |
| CPC 29677 | V. vinifera | Spain | MG281039 | MG281212 | MG281387 | MG281560 | MG281736 | |
| CPC 29678 | V. vinifera | Spain | MG281040 | MG281213 | MG281388 | MG281561 | MG281737 | |
| CPC 29694 | V. vinifera | Hungary | MG281041 | MG281214 | MG281389 | MG281562 | MG281738 | |
| CPC 29695 | V. vinifera | Hungary | MG281042 | MG281215 | MG281390 | MG281563 | MG281739 | |
| CPC 29820 | V. vinifera | Czech Republic | MG281043 | MG281216 | MG281391 | MG281564 | MG281740 | |
| CPC 29822 | V. vinifera | Czech Republic | MG281044 | MG281217 | MG281392 | MG281565 | MG281741 | |
| CPC 29823 | V. vinifera | Czech Republic | MG281045 | MG281218 | MG281393 | MG281566 | MG281742 | |
| CPC 29824 | V. vinifera | Czech Republic | MG281046 | MG281219 | MG281394 | MG281567 | MG281743 | |
| CPC 29825 | V. vinifera | Czech Republic | MG281047 | MG281220 | MG281395 | MG281568 | MG281744 | |
| CPC 29826 | V. vinifera | Croatia | MG281048 | MG281221 | MG281396 | MG281569 | MG281745 | |
| CPC 30055 | V. vinifera | Croatia | MG281049 | MG281222 | MG281397 | MG281570 | MG281746 | |
| CPC 30070 | V. vinifera | Hungary | MG281050 | MG281223 | MG281398 | MG281571 | MG281747 | |
| CPC 30072 | V. vinifera | Hungary | MG281051 | MG281224 | MG281399 | MG281572 | MG281748 | |
| CPC 30073 | V. vinifera | Hungary | MG281052 | MG281225 | MG281400 | MG281573 | MG281749 | |
| CPC 30074 | V. vinifera | Hungary | MG281053 | MG281226 | MG281401 | MG281574 | MG281750 | |
| CPC 30075 | V. vinifera | Hungary | MG281054 | MG281227 | MG281402 | MG281575 | MG281751 | |
| CPC 30077 | V. vinifera | Hungary | MG281055 | MG281228 | MG281403 | MG281576 | MG281752 | |
| CPC 30078 | V. vinifera | Hungary | MG281056 | MG281229 | MG281404 | MG281577 | MG281753 | |
| CPC 30080 | V. vinifera | Hungary | MG281057 | MG281230 | MG281405 | MG281578 | MG281754 | |
| CPC 30081 | V. vinifera | Hungary | MG281058 | MG281231 | MG281406 | MG281579 | MG281755 | |
| CPC 30082 | V. vinifera | Hungary | MG281059 | MG281232 | MG281407 | MG281580 | MG281756 | |
| CPC 30083 | V. vinifera | Hungary | MG281060 | MG281233 | MG281408 | MG281581 | MG281757 | |
| CPC 30084 | V. vinifera | Hungary | MG281061 | MG281234 | MG281409 | MG281582 | MG281758 | |
| CPC 30085 | V. vinifera | Hungary | MG281062 | MG281235 | MG281410 | MG281583 | MG281759 | |
| CPC 30087 | V. vinifera | Hungary | MG281063 | MG281236 | MG281411 | MG281584 | MG281760 | |
| CPC 30088 | V. vinifera | Hungary | MG281064 | MG281237 | MG281412 | MG281585 | MG281761 | |
| CPC 30089 | V. vinifera | Hungary | MG281065 | MG281238 | MG281413 | MG281586 | MG281762 | |
| CPC 30090 | V. vinifera | Hungary | MG281066 | MG281239 | MG281414 | MG281587 | MG281763 | |
| CPC 30091 | V. vinifera | Hungary | MG281067 | MG281240 | MG281415 | MG281588 | MG281764 | |
| CPC 30092 | V. vinifera | Hungary | MG281068 | MG281241 | MG281416 | MG281589 | MG281765 | |
| CPC 30093 | V. vinifera | Hungary | MG281069 | MG281242 | MG281417 | MG281590 | MG281766 | |
| CPC 30094 | V. vinifera | Hungary | MG281070 | MG281243 | MG281418 | MG281591 | MG281767 | |
| CPC 30095 | V. vinifera | Hungary | MG281071 | MG281244 | MG281419 | MG281592 | MG281768 | |
| CPC 30096 | V. vinifera | Hungary | MG281072 | MG281245 | MG281420 | MG281593 | MG281769 | |
| CPC 30098 | V. vinifera | Hungary | MG281073 | MG281246 | MG281421 | MG281594 | MG281770 | |
| CPC 30101 | V. vinifera | Hungary | MG281074 | MG281247 | MG281422 | MG281595 | MG281771 | |
| CPC 30102 | V. vinifera | Hungary | MG281075 | MG281248 | MG281423 | MG281596 | MG281772 | |
| CPC 30103 | V. vinifera | Hungary | MG281076 | MG281249 | MG281424 | MG281597 | MG281773 | |
| CPC 30104 | V. vinifera | Hungary | MG281077 | MG281250 | MG281425 | MG281598 | MG281774 | |
| CPC 30105 | V. vinifera | Hungary | MG281078 | MG281251 | MG281426 | MG281599 | MG281775 | |
| CPC 30106 | V. vinifera | Hungary | MG281079 | MG281252 | MG281427 | MG281600 | MG281776 | |
| CPC 30107 | V. vinifera | Hungary | MG281080 | MG281253 | MG281428 | MG281601 | MG281777 | |
| CPC 30108 | V. vinifera | Hungary | MG281081 | MG281254 | MG281429 | MG281602 | MG281778 | |
| CPC 30109 | V. vinifera | Hungary | MG281082 | MG281255 | MG281430 | MG281603 | MG281779 | |
| CPC 30111 | V. vinifera | Hungary | MG281083 | MG281256 | MG281431 | MG281604 | MG281780 | |
| CPC 30112 | V. vinifera | Hungary | MG281084 | MG281257 | MG281432 | MG281605 | MG281781 | |
| CPC 30113 | V. vinifera | Hungary | MG281085 | MG281258 | MG281433 | MG281606 | MG281782 | |
| CPC 30114 | V. vinifera | Hungary | MG281086 | MG281259 | MG281434 | MG281607 | MG281783 | |
| CPC 30115 | V. vinifera | Hungary | MG281087 | MG281260 | MG281435 | MG281608 | MG281784 | |
| CPC 30116 | V. vinifera | Hungary | MG281088 | MG281261 | MG281436 | MG281609 | MG281785 | |
| CPC 30119 | V. vinifera | Hungary | MG281089 | MG281262 | MG281437 | MG281610 | MG281786 | |
| CPC 30120 | V. vinifera | Hungary | MG281090 | MG281263 | MG281438 | MG281611 | MG281787 | |
| CPC 30121 | V. vinifera | Hungary | MG281091 | MG281264 | MG281439 | MG281612 | MG281788 | |
| CPC 30122 | V. vinifera | Hungary | MG281092 | MG281265 | MG281440 | MG281613 | MG281789 | |
| CPC 30123 | V. vinifera | Hungary | MG281093 | MG281266 | MG281441 | MG281614 | MG281790 | |
| CPC 30124 | V. vinifera | Hungary | MG281094 | MG281267 | MG281442 | MG281615 | MG281791 | |
| CPC 30125 | V. vinifera | Hungary | MG281095 | MG281268 | MG281443 | MG281616 | MG281792 | |
| CPC 30126 | V. vinifera | Hungary | MG281096 | MG281269 | MG281444 | MG281617 | MG281793 | |
| CPC 30127 | V. vinifera | Hungary | MG281097 | MG281270 | MG281445 | MG281618 | MG281794 | |
| CPC 30128 | V. vinifera | Hungary | MG281098 | MG281271 | MG281446 | MG281619 | MG281795 | |
| CPC 30131 | V. vinifera | Hungary | MG281099 | MG281272 | MG281447 | MG281620 | MG281796 | |
| CPC 30132 | V. vinifera | Hungary | MG281100 | MG281273 | MG281448 | MG281621 | MG281797 | |
| CPC 30133 | V. vinifera | Hungary | MG281101 | MG281274 | MG281449 | MG281622 | MG281798 | |
| CPC 30134 | V. vinifera | Hungary | MG281102 | MG281275 | MG281450 | MG281623 | MG281799 | |
| CPC 30135 | V. vinifera | Hungary | MG281103 | MG281276 | MG281451 | MG281624 | MG281800 | |
| CPC 30136 | V. vinifera | Hungary | MG281104 | MG281277 | MG281452 | MG281625 | MG281801 | |
| CPC 30137 | V. vinifera | Hungary | MG281105 | MG281278 | MG281453 | MG281626 | MG281802 | |
| CPC 30138 | V. vinifera | Hungary | MG281106 | MG281279 | MG281454 | MG281627 | MG281803 | |
| CPC 30139 | V. vinifera | Hungary | MG281107 | MG281280 | MG281455 | MG281628 | MG281804 | |
| CPC 30140 | V. vinifera | Hungary | MG281108 | MG281281 | MG281456 | MG281629 | MG281805 | |
| CPC 30141 | V. vinifera | Hungary | MG281109 | MG281282 | MG281457 | MG281630 | MG281806 | |
| CPC 30143 | V. vinifera | Hungary | MG281110 | MG281283 | MG281458 | MG281631 | MG281807 | |
| CPC 30144 | V. vinifera | Hungary | MG281111 | MG281284 | MG281459 | MG281632 | MG281808 | |
| CPC 30145 | V. vinifera | Hungary | MG281112 | MG281285 | MG281460 | MG281633 | MG281809 | |
| CPC 30146 | V. vinifera | Hungary | MG281113 | MG281286 | MG281461 | MG281634 | MG281810 | |
| CPC 30147 | V. vinifera | Hungary | MG281114 | MG281287 | MG281462 | MG281635 | MG281811 | |
| CPC 30148 | V. vinifera | Hungary | MG281115 | MG281288 | MG281463 | MG281636 | MG281812 | |
| CPC 30149 | V. vinifera | Hungary | MG281116 | MG281289 | MG281464 | MG281637 | MG281813 | |
| CPC 30150 | V. vinifera | Hungary | MG281117 | MG281290 | MG281465 | MG281638 | MG281814 | |
| CPC 30151 | V. vinifera | Hungary | MG281118 | MG281291 | MG281466 | MG281639 | MG281815 | |
| CPC 30152 | V. vinifera | Hungary | MG281119 | MG281292 | MG281467 | MG281640 | MG281816 | |
| CPC 30317 | V. vinifera | Spain | MG281120 | MG281293 | MG281468 | MG281641 | MG281817 | |
| CPC 30318 | V. vinifera | Spain | MG281121 | MG281294 | MG281469 | MG281642 | MG281818 | |
| CPC 30319 | V. vinifera | Spain | MG281122 | MG281295 | MG281470 | MG281643 | MG281819 | |
| D. fibrosa | CBS 109751 | Rhamnus cathartica | Austria | KC343099 | KC344067 | KC343583 | KC343825 | KC343341 |
| D. foeniculina | CBS 187.27 | Camellia sinensis | Italy | KC343107 | KC344075 | KC343591 | KC343833 | KC343349 |
| CBS 111553 | Foeniculum vulgare | Spain | KC343101 | KC344069 | KC343585 | KC343827 | KC343343 | |
| CBS 123209 | Foeniculum vulgare | Portugal | KC343105 | KC344073 | KC343589 | KC343831 | KC343347 | |
| D. helianthi | CBS 592.81 | Helianthus annuus | Serbia | KC343115 | KC344083 | KC343599 | KC343841 | JX197454 |
| D. helicis | CBS 138596 | Hedera helix | France | KJ210538 | KJ420828 | KJ420875 | KJ210559 | KJ435043 |
| D. hispaniae | CBS 143351 = CPC 303213 | V. vinifera | Spain | MG281123 | MG281296 | MG281471 | MG281644 | MG281820 |
| CBS 143352 = CPC 303233 | V. vinifera | Spain | MG281124 | MG281297 | MG281472 | MG281645 | MG281821 | |
| D. hongkongensis | CBS 115448 | Dichroa febrifuga | China | KC343119 | KC344087 | KC343603 | KC343845 | KC343361 |
| D. hungariae | CPC 30129 | V. vinifera | Hungary | MG281125 | MG281298 | MG281473 | MG281646 | MG281822 |
| CBS 143353 = CPC 301303 | V. vinifera | Hungary | MG281126 | MG281299 | MG281474 | MG281647 | MG281823 | |
| CBS 143354 = CPC 301423 | V. vinifera | Hungary | MG281127 | MG281300 | MG281475 | MG281648 | MG281824 | |
| CPC 30316 | V. vinifera | Spain | MG281128 | MG281301 | MG281476 | MG281649 | MG281825 | |
| CPC 30320 | V. vinifera | Spain | MG281129 | MG281302 | MG281477 | MG281650 | MG281826 | |
| CPC 30322 | V. vinifera | Spain | MG281130 | MG281303 | MG281478 | MG281651 | MG281827 | |
| D. impulsa | CBS 114434 | Sorbus aucuparia | Sweden | KC343121 | KC344089 | KC343605 | KC343847 | KC343363 |
| D. inconspicua | CBS 133813 | Maytenus ilicifolia | Brazil | KC343123 | KC344091 | KC343607 | KC343849 | KC343365 |
| D. infecunda | CBS 133812 | Schinus terebinthifolius | Brazil | KC343126 | KC344094 | KC343610 | KC343852 | KC343368 |
| D. neilliae | CBS 144.27 | Spiraea sp. | USA | KC343144 | KC344112 | KC343628 | KC343870 | KC343386 |
| D. nothofagi | BRIP 54801 | Nothofagus cunninghamii | Australia | JX862530 | KF170922 | – | JX862536 | – |
| D. novem | CBS 127271 | Glycine max | Croatia | KC343157 | KC344125 | KC343641 | KC343883 | KC343399 |
| D. oncostoma | CBS 589.78 | Robinia pseudoacacia | France | KC343162 | KC344130 | KC343646 | KC343888 | KC343404 |
| D. perjuncta | CBS 109745 | Ulmus glabra | Austria | KC343172 | KC344140 | KC343656 | KC343898 | KC343414 |
| D. perseae | CBS 151.73 | Persea gratissima | Netherlands | KC343173 | KC344141 | KC343657 | KC343899 | KC343415 |
| D. phaseolorum | CBS 113425 | Olearia cf. rani | New Zealand | KC343174 | KC344142 | KC343658 | KC343900 | KC343416 |
| CBS 127465 | Actinidia chinensis | New Zealand | KC343177 | KC344145 | KC343661 | KC343903 | KC343419 | |
| D. pseudomangiferae | CBS 101339 | Mangifera indica | Dominican Republic | KC343181 | KC344149 | KC343665 | KC343907 | KC343423 |
| D. pseudophoenicicola | CBS 462.69 | Phoenix dactylifera | Spain | KC343184 | KC344152 | KC343668 | KC343910 | KC343426 |
| D. pulla | CBS 338.89 | Hedera helix | Yugoslavia | KC343152 | KC344120 | KC343636 | KC343878 | KC343394 |
| D. rudis | CBS 266.85 | Rosa rugosa | Netherlands | KC343237 | KC344205 | KC343721 | KC343963 | KC343479 |
| CBS 109292 | Laburnum anagyroides | Austria | KC843331 | KC843177 | – | KC843090 | KC843146 | |
| CBS 113201 | V. vinifera | Portugal | KC343234 | KC344202 | KC343718 | KC343960 | KC343476 | |
| CBS 114011 | V. vinifera | Portugal | KC343235 | KC344203 | KC343719 | KC343961 | KC343477 | |
| CBS 114436 | Sambucus cf. racemosa | Sweden | KC343236 | KC344204 | KC343720 | KC343962 | KC343478 | |
| CBS 143346 = CPC 28224 | V. vinifera | Czech Republic | MG281131 | MG281304 | MG281479 | MG281652 | MG281828 | |
| CPC 28225 | V. vinifera | Czech Republic | MG281132 | MG281305 | MG281480 | MG281653 | MG281829 | |
| CPC 28252 | V. vinifera | UK | MG281133 | MG281306 | MG281481 | MG281654 | MG281830 | |
| CPC 28253 | V. vinifera | UK | MG281134 | MG281307 | MG281482 | MG281655 | MG281831 | |
| CPC 28265 | V. vinifera | UK | MG281135 | MG281308 | MG281483 | MG281656 | MG281832 | |
| CPC 28268 | V. vinifera | UK | MG281136 | MG281309 | MG281484 | MG281657 | MG281833 | |
| CPC 28425 | V. vinifera | Italy | MG281137 | MG281310 | MG281485 | MG281658 | MG281834 | |
| CPC 29320 | V. vinifera | France | MG281138 | MG281311 | MG281486 | MG281659 | MG281835 | |
| CPC 29649 | V. vinifera | Spain | MG281139 | MG281312 | MG281487 | MG281660 | MG281836 | |
| CPC 29658 | V. vinifera | Spain | MG281140 | MG281313 | MG281488 | MG281661 | MG281837 | |
| D. saccarata | CBS 116311 | Protea repens | South Africa | KC343190 | KC344158 | KC343674 | KC343916 | KC343432 |
| D. schini | CBS 133181 | Schinus terebinthifolius | Brazil | KC343191 | KC344159 | KC343675 | KC343917 | KC343433 |
| D. sojae | CBS 116019 | Caperonia palustris | USA | KC343175 | KC344143 | KC343659 | KC343901 | KC343417 |
| CBS 139282 | Glycine max | USA | KJ590719 | KJ610875 | KJ659208 | KJ590762 | KJ612116 | |
| D. sterilis | CBS 136969 | Vaccinium corymbosum | Italy | KJ160579 | KJ160528 | MF418350 | KJ160611 | KJ160548 |
| D. subclavata | ICMP20663 | Citrus unshiu | China | KJ490630 | KJ490451 | KJ490572 | KJ490509 | – |
| D. terebinthifolii | CBS 133180 | Schinus terebinthifolius | Brazil | KC343216 | KC344184 | KC343700 | KC343942 | KC343458 |
| D. toxica | CBS 534.93 | Lupinus angustifolius | Western Australia | KC343220 | KC344188 | KC343704 | KC343946 | KC343462 |
| D. vaccinii | CBS 160.32 | Vaccinium macrocarpon | USA | AF317578 | KC344196 | KC343712 | GQ250326 | KC343470 |
| CBS 118571 | Va. corymbosum | USA | KC343223 | KC344191 | KC343718 | KC343949 | KC343465 | |
| CBS 122114 | Va. corymbosum | USA | KC343225 | KC344193 | KC343709 | KC343951 | KC343467 | |
| CBS 135436 | Va. corymbosum | USA | AF317570 | KC843225 | KJ420877 | JQ807380 | KC849457 | |
| Diaporthella corylina | CBS 121124 | Corylus sp. | China | KC343004 | KC343972 | KC343488 | KC343730 | KC343246 |
1 BRIP: Plant Pathology Herbarium, Department of Primary Industries, Dutton Park, Queensland, Australia; CPC: Culture collection of P.W. Crous, housed at Westerdijk Fungal Biodiversity Institute; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; DAOM: Canadian Collection of Fungal Cultures or the National Mycological Herbarium, Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; ICMP: International Collection of Microorganisms from Plants, Landcare Research, Auckland, New Zealand. Ex-type and ex-epitype cultures are indicated in bold.
2 ITS: internal transcribed spacers 1 and 2 together with 5.8S nrDNA; tub2: partial beta-tubulin gene; his3: partial histone H3 gene; tef1: partial translation elongation factor 1-α gene; cal: partial calmodulin gene. Sequences generated in this study are indicated in italics.
3 Isolates used for pathogenicity test.
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted using a Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following manufacturer’s instructions. Partial regions of five loci were amplified. The primers ITS5 and ITS4 (White et al. 1990) were used to amplify the internal transcribed spacer region (ITS) of the nuclear ribosomal RNA operon, including the 3’ end of the 18S nrRNA, the first internal transcribed spacer region, the 5.8S nrRNA gene; the second internal transcribed spacer region and the 5’ end of the 28S nrRNA gene. The primers EF1-728F and EF1-986R (Carbone & Kohn 1999) were used to amplify part of the translation elongation factor 1-α gene (tef1). The primers CAL-228F and CAL-737R (Carbone & Kohn 1999) or CL1/CL2A (O’Donnell et al. 2000) were used to amplify part of the calmodulin (cal) gene. The partial histone H3 (his3) region was amplified using the CYLH3F and H3-1b primer set (Glass & Donaldson 1995, Crous et al. 2004a) and the beta-tubulin (tub2) region was amplified using the Bt2a and Bt2b primer set (Glass & Donaldson 1995) or Tub2FD (Aveskamp et al. 2009) and T22 (O’Donnell & Cigelnik 1997). The PCR products were sequenced in both directions using the BigDye® Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems Life Technologies, Carlsbad, CA, USA), after which amplicons were purified through Sephadex G-50 Fine columns (GE Healthcare, Freiburg, Germany) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analyzed on an Applied Biosystems 3730xl DNA Analyser (Life Technologies, Carlsbad, CA, USA). The DNA sequences generated were analysed and consensus sequences were computed using the program SeqMan Pro (DNASTAR, Madison, WI, USA).
Phylogenetic analyses
Novel sequences generated in this study were blasted against the NCBIs GenBank nucleotide database to determine the closest relatives for a taxonomic framework of the studied isolates. Alignments of different gene regions, including sequences obtained from this study and sequences downloaded from GenBank, were initially performed by using the MAFFT v. 7 online server (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh & Standley 2013), and then manually adjusted in MEGA v. 7 (Kumar et al. 2016).
To establish the identity of the isolates at species level, phylogenetic analyses were conducted first individually for each locus (data not shown) and then as combined analyses of five loci. Two separate analyses were conducted for the D. eres species complex and the remainder of the Diaporthe spp. included in this study, as similarly performed in a recent study about Colletotrichum taxonomy (Guarnaccia et al. 2017). Additional reference sequences were selected based on recent studies on Diaporthe species (Gomes et al. 2013, Udayanga et al. 2014a, b). Phylogenetic analyses were based on Maximum Parsimony (MP) for all the individual loci and on both MP and Bayesian Inference (BI) for the multi-locus analyses. For BI, the best evolutionary model for each partition was determined using MrModeltest v. 2.3 (Nylander 2004) and incorporated into the analyses. MrBayes v. 3.2.5 (Ronquist et al. 2012) was used to generate phylogenetic trees under optimal criteria per partition. The Markov Chain Monte Carlo (MCMC) analysis used four chains and started from a random tree topology. The heating parameter was set to 0.2 and trees were sampled every 1 000 generations. Analyses stopped once the average standard deviation of split frequencies was below 0.01. The MP analyses were performed using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10; Swofford 2003). Phylogenetic relationships were estimated by heuristic searches with 100 random addition sequences. Tree bisection-reconnection was used, with the branch swapping option set on ‘best trees’ only with all characters weighted equally and alignment gaps treated as fifth state. Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistence index (RC) were calculated for parsimony and the bootstrap analyses (Hillis & Bull 1993) were based on 1 000 replications. Sequences generated in this study are deposited in GenBank (Table 1) and alignments and phylogenetic trees in TreeBASE (www.treebase.org).
Taxonomy
Agar plugs (6-mm-diam) were taken from the edge of actively growing cultures on MEA and transferred onto the centre of 9-cm-diam Petri dishes containing 2 % tap water agar supplemented with sterile pine needles (PNA; Smith et al. 1996), potato dextrose agar (PDA), oatmeal agar (OA) and malt extract agar (MEA) (Crous et al. 2009), and incubated at 21–22 °C under a 12 h near-ultraviolet light/12 h dark cycle to induce sporulation as described in recent studies (Gomes et al. 2013, Lombard et al. 2014). Colony characters and pigment production on MEA, OA and PDA were noted after 15 d. Colony colours were rated according to Rayner (1970). Cultures were examined periodically for the development of ascomata and conidiomata. Colony diameters were measured after 7 and 10 d. The morphological characteristics were examined by mounting fungal structures in clear lactic acid and 30 measurements at ×1 000 magnification were determined for each isolate using a Zeiss Axioscope 2 microscope with interference contrast (DIC) optics. Descriptions, nomenclature and illustrations of taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004b).
Pathogenicity
Pathogenicity testing was conducted using a proven inoculation method for Diaporthe (Mostert et al. 2001a, Úrbez-Torres et al. 2009, Dissanayake et al. 2015). Green shoots (6–8 mm diam, 15–30 cm long), cut from healthy mature grapevine cv. ‘Riesling’, were artificially inoculated to determine the pathogenicity of the five Diaporthe species not previously reported to be associated with Vitis spp.
Ten different isolates representing D. baccae, D. bohemiae, D. celeris, D. hispaniae and D. hungariae, were selected (Table 1). Green canes were collected in July 2017 and were brought to the laboratory. All the leaves, lateral branches, and tendrils were removed. Canes were inoculated the same day they were sampled. Canes were surface-sterilized in 10 % sodium hypochlorite for 10 min. After air drying, five canes were inoculated with each Diaporthe isolate. Canes were superficially wounded in between two nodes forming a slit using a sterile blade. Inoculations were conducted by placing a 1-wk-old, 6 mm diam agar plug from each fungal culture on a wound. Wounds were then wrapped with Parafilm® (American National Can, Chicago, IL, USA). Ten shoots were inoculated as described above with 6-mm-diam non-colonised MEA plugs as negative controls. Inoculated canes were immediately placed in 6 L transparent plastic containers with a tight-fitting lid containing wet paper towels with 400 mL distilled water to maintain a humid environment. Five canes per plastic container including controls were arranged in a completely randomized design. Inoculated canes were collected after 21 d of incubation at room temperature and inspected for lesion development. Each cane was cut longitudinally through the inoculation point to evaluate the type of symptom developed. In order to demonstrate pathogenicity, the inoculated fungi were re-isolated from canes showing lesions, and the identity of the re-isolated fungi was confirmed by sequencing the tef1 and tub2 loci as described above.
RESULTS
Sampling and isolation
Symptoms caused by Diaporthe spp. were frequently observed on Vitis spp., including Phomopsis cane and leaf spot, cane bleaching, and additionally vascular internal browning, sectorial necrosis, and other necrotic lesions on grapevine wood. Symptoms were observed on rootstock and scion grapevine plants. A total of 175 monosporic isolates resembling those of the genus Diaporthe were collected. The Diaporthe isolates were recovered from multiple locations of all the countries investigated (Table 1, 2). Based on preliminary ITS sequencing, all 175 isolates were selected (Table 1) for phylogenetic analyses and further taxonomic study.
Table 2.
Number of isolates collected for each Diaporthe sp. identified and country investigated.
| Croatia | Czech Republic | France | Hungary | Israel | Italy | Spain | UK | Total | |
|---|---|---|---|---|---|---|---|---|---|
| D. ambigua | – | – | – | – | – | – | 2 | – | 2 |
| D. ampelina | 3 | 1 | 2 | 1 | 4 | 1 | 10 | 9 | 31 |
| D. baccae | 1 | – | 1 | – | – | – | 12 | – | 14 |
| D. bohemiae | – | 2 | – | – | – | – | – | – | 2 |
| D. celeris | – | – | – | – | – | – | – | 3 | 3 |
| D. eres | 2 | 11 | 2 | 72 | – | 2 | 9 | 7 | 105 |
| D. hispaniae | – | – | – | – | – | – | 2 | – | 2 |
| D. hungariae | – | – | – | 3 | – | – | 3 | – | 6 |
| D. rudis | – | 2 | 1 | – | – | 1 | 2 | 4 | 10 |
| Total | 6 | 16 | 6 | 76 | 4 | 4 | 40 | 23 | 175 |
Phylogenetic analyses
The 10 MP trees derived from the single gene sequence alignments (ITS, tef1, cal, his3 and tub2) for both the D. eres species complex and the remaining Diaporthe spp. produced topologically similar trees, and confirmed that 108 isolates recovered in this study belong to the D. eres species complex. The remaining 67 isolates were identified as various Diaporthe species. The combined species phylogeny of the D. eres species complex (TreeBASE: S21957) consisted of 129 sequences, including the outgroup sequences of D. toxica (culture CBS 534.93). The remaining species were included in a combined phylogeny (TreeBASE: S21958) consisting of 117 sequences, including the outgroup sequences of Diaporthella corylina (CBS 121124). A total of 3 805 characters (ITS: 1–583, tef: 590–1 232, tub2: 1 239–2 574, cal: 2 581–3 305, his3: 3 312–3 805) were included in the D. eres complex phylogenetic analyses, of which 423 characters were parsimony-informative, 543 were variable and parsimony-uninformative and 2 815 characters were constant. A maximum of 1 000 equally most parsimonious trees were saved (Tree length = 1 858, CI = 0.625, RI = 0.840 and RC = 0.525). Regarding the remainder of Diaporthe species, a total of 4 220 characters were included in the phylogenetic analyses (ITS: 1–640, tef: 647–1 360, tub2: 1 367–2 807, cal: 2 814–3 625, his3: 3 632–4 220), of which 1 524 characters were parsimony-informative, 909 were variable and parsimony-uninformative and 1 763 characters were constant. A maximum of 1 000 equally most parsimonious trees were saved (Tree length = 8 303, CI = 0.530, RI = 0.877 and RC = 0.465). Bootstrap support values from the parsimony analysis were plotted on the Bayesian phylogenies presented in Fig. 1 and 2. For both of the Bayesian analyses, MrModeltest suggested that all partitions should be analysed with dirichlet state frequency distributions, except for the ITS partition in the D. eres species complex analysis, which was analysed with a fixed state frequency distribution. The following models were recommended by MrModeltest and used in the Bayesian analysis of the D. eres species complex: SYM+I+G for ITS, HKY+G for tef1, tub2 and his3 and GTR+G for cal. The ITS partition had 90 unique site patterns, the tef1 partition 164, the tub2 partition 256, the cal partition 182, the his3 partition 147, and the analysis ran for 43 040 000 generations, resulting in 86 082 trees of which 64 562 trees were used to calculate the posterior probabilities. Regarding the Bayesian analysis of the remaining Diaporthe species, the following models were used according to MrModeltest: GTR+I+G for ITS, tef1 and cal, HKY+I+G for tub2 and GTR+I+G for cal. The ITS partition had 217 unique site patterns, the tef1 partition 501, the tub2 partition 560, the cal partition 510, the his3 partition 259, and the analysis ran for 1 930 000 generations, resulting in 3 862 trees of which 2 898 trees were used to calculate the posterior probabilities.
Fig. 1.
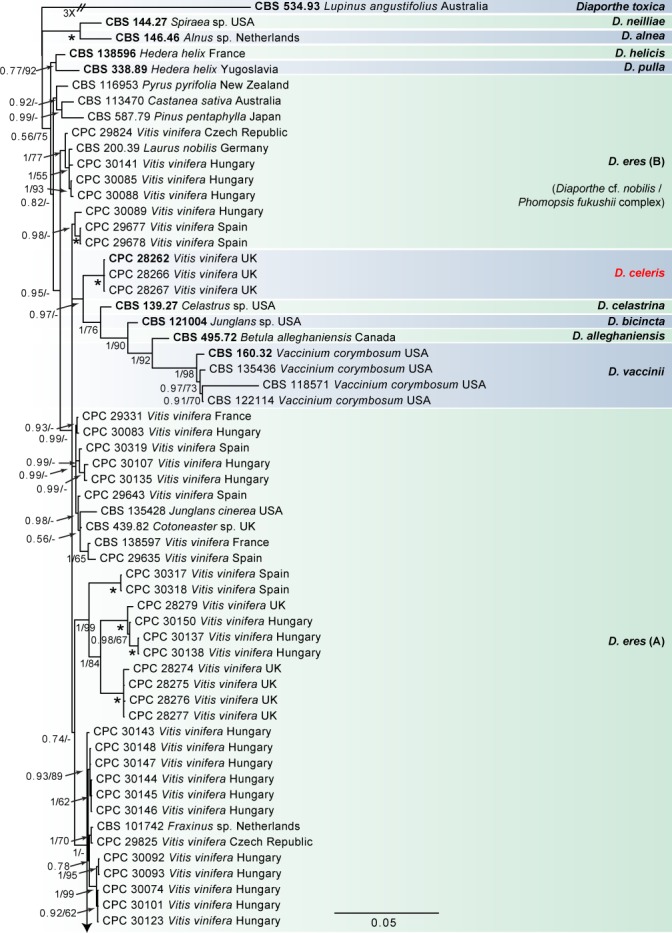
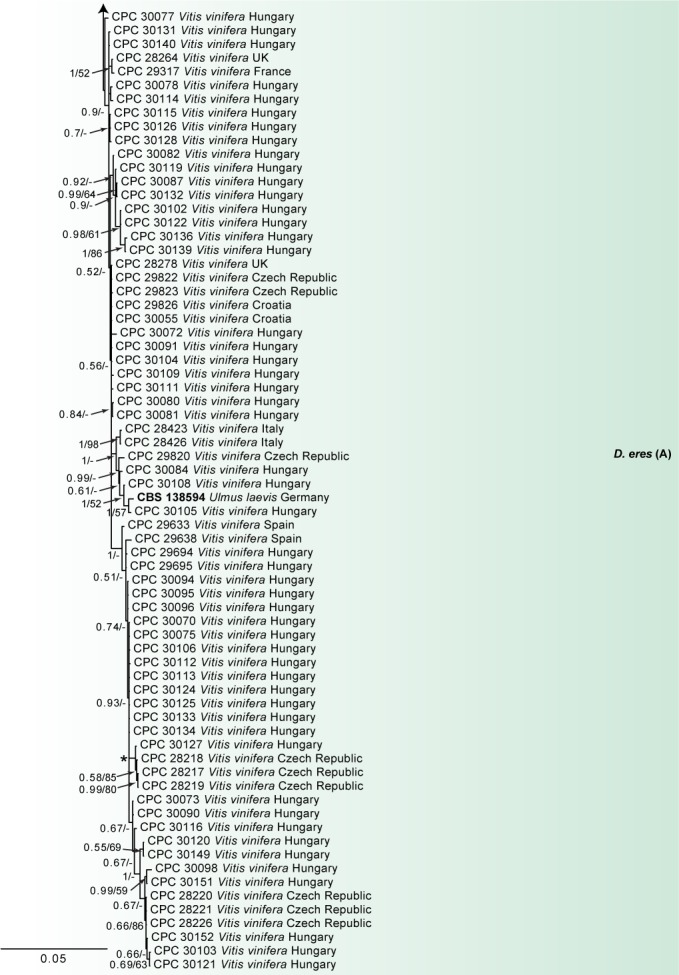
Consensus phylogram of 86 082 trees resulting from a Bayesian analysis of the combined ITS, tub2, his3, tef1 and cal sequence alignments of the D. eres complex. Bootstrap support values and Bayesian posterior probability values are indicated at the nodes. The asterisk symbol (*) represents full support (1/100). Substrate and country of origin are listed next to the strain numbers. Ex-type isolates are indicated in bold. The novel species are shown in red text. The tree was rooted to Diaporthe toxica (CBS 534.93).
Fig. 2.
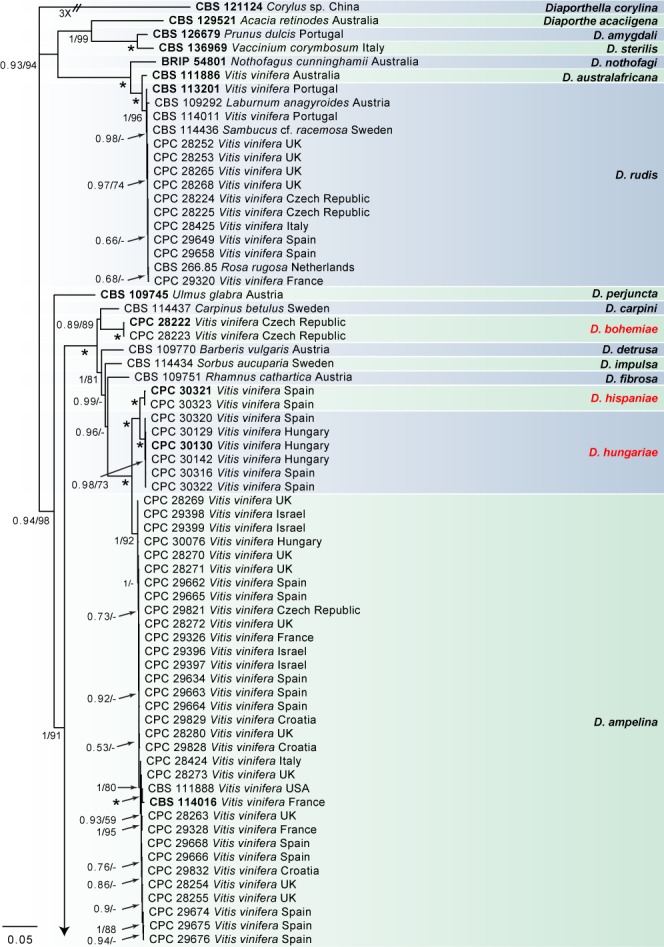
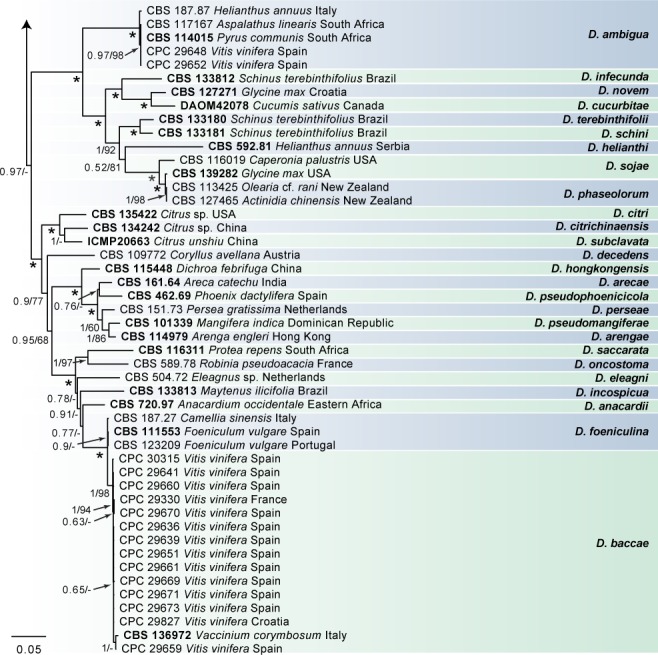
Consensus phylogram of 3 862 trees resulting from a Bayesian analysis of the combined ITS, tub2, his3, tef1 and cal sequence alignments of Diaporthe spp. Bootstrap support values and Bayesian posterior probability values are indicated at the nodes. The asterisk symbol (*) represents full support (1/100). Substrate and country of origin are listed next to the strain numbers. Ex-type isolates are indicated in bold. The novel species are shown in red text. The tree was rooted to Diaporthella corylina (CBS 121124).
In the D. eres complex analysis (Fig. 1), 98 V. vinifera isolates clustered with five reference strains of D. eres (A), whilst seven isolates clustered with four reference strains of D. eres (B), the clade previously known as the Diaporthe cf. nobilis/Phomopsis fukushii complex (Gomes et al. 2013). Moreover, three isolates were identified as D. celeris, forming a highly-supported subclade (1.00/100) in the complex. In the other analyses, 10 isolates clustered with the ex-type strain of D. rudis, 31 isolates with the ex-type strain and other reference strains of D. ampelina, 2 with the ex-type and other reference strains of D. ambigua and 14 isolates with the ex-type strain of D. baccae (Fig. 2). Furthermore, two isolates were identified as D. bohemiae (closely related to D. carpini), two isolates as D. hispaniae and six as D. hungariae (close to D. ampelina). The individual alignments and resulting trees of the five single genes in both analyses were compared with respect to their performance in species recognition. In the D. eres complex analysis, D. celeris was differentiated with tef1, his3 and cal, whilst in the other analysis D. bohemiae was differentiated by every single gene used. Moreover, the single locus tub2, was informative enough to distinguish D. hispaniae, D. hungariae and D. ampelina.
Taxonomy
Morphological observations, supported by phylogenetic inference, were used to identify five known species (D. ambigua, D. ampelina, D. baccae, D. eres and D. rudis), and to describe four new species (Table 3). Culture characteristics were assessed, and the colour of upper and lower surfaces on different media determined as shown in Fig. 3, 4, 5, 6. Based on the results of both the phylogenetic and morphological analyses, the four distinct novel species are described below.
Table 3.
Diaporthe spp. associated with grapevines and their morphological characteristics.
| Species | Conidiomata (μm) | Conidiophores (μm) | Alpha conidia (μm) | Beta conidia (μm) | References |
|---|---|---|---|---|---|
| D. ambigua | – | 15–45 × 2–3 | 6–8 × 2–3 | – | Van Rensburg et al. (2006) |
| D. ampelina | up to 430 | 5–35 × 1–3 | 9.5–10.5 × 2–3 | 20–25 × 0.5–1 | Gomes et al. (2013) |
| D. amygdali | up to 800 | 6–25 × 1–2 | 4.5–8 × 1–2 | 12–20 × 0.5–1 | Mostert et al. (2001a) |
| D. australafricana | – | – | 5–6 × 1.5–2 | – | Van Niekerk et al. (2005) |
| D. baccae | up to 650 | 20–57 × 2–3 | 7–9 × 2–3 | 20–24 × 1–2 | Lombard et al. (2014) |
| D. bohemiae | up to 400 | 5–20 × 1.5–4 | 7.5–8.5 × 1.5–3 | – | This study |
| D. celeris | up to 650 | 5–18 × 1–3 | 5.5–7.5 × 2–3 | 16–22.5 × 1–2 | This study |
| D. eres | 200–250 | 10–15 × 2–3 | 6.5–8.5 × 3–4 | 22–28 × 1–1.5 | Udayanga et al. (2014a) |
| D. foeniculina | 400–700 | 9–15(–18) × 1–2 | 8.5–9 × 2.3–2.5 | 22–28 × 1.4–1.6 | Udayanga et al. (2014b) |
| D. helianthi | up to 380 | 11.5–23.5 × 1.8–3.5 | – | 11.5–32 × 0.5–2 | Gao et al. (2017) |
| D. hispaniae | up to 400 | 5–30 × 1–4 | 9–14.5 × 2–4 | 18–24 × 1–2 | This study |
| D. hongkongensis | up to 200 | 5–12 × 2–4 | 6–7 × 2.5 | 18–22 × 1.5–2 | Gomes et al. (2013) |
| D. hungariae | up to 650 | 5–25 × 1–3.5 | 9.5–16 × 2–3.5 | – | This study |
| D. kyushuensis | up to 860 | – | 15.5–24 × 4.5–8 | 25–55 × 1–2 | Kajitani & Kanematsu (2000) |
| D. perjuncta | – | 17–23 × 1.5–2.5 | 5–7 × 2–2.5 | 12–20 × 0.5–1 | Mostert et al. (2001a) |
| D. phaseolorum | up to 300 | 7–12 × 2–3 | 7.3–10.3 × 2.8–3.5 | – | Udayanga et al. (2015) |
| D. rudis | up to 500 | 20–45 × 2–2.4 | 6.3–8.7 × 2–2.5 | 27–35.2 × 3–4.2 | Udayanga et al. (2014b) |
| D. sojae | 200–250 | 12–16 × 2–4 | 5.3–7.3 × 2–3 | – | Udayanga et al. (2015) |
Fig. 3.
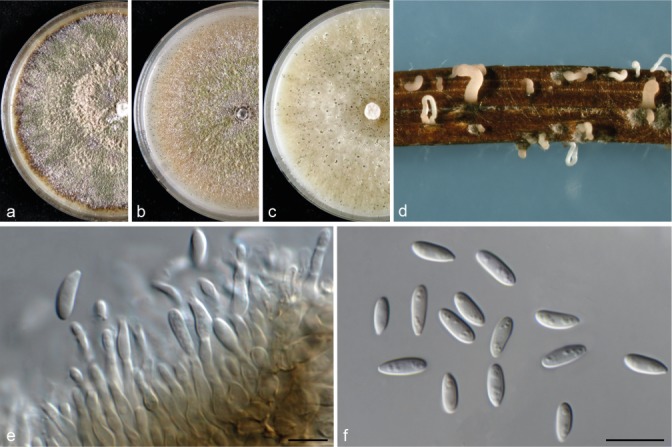
Diaporthe bohemiae (CBS 143347). a–c. Colonies on MEA, PDA and OA, respectively; d. conidiomata sporulating on PNA; e. conidiogenous cells; f. alpha conidia. — Scale bars = 10 μm.
Fig. 4.
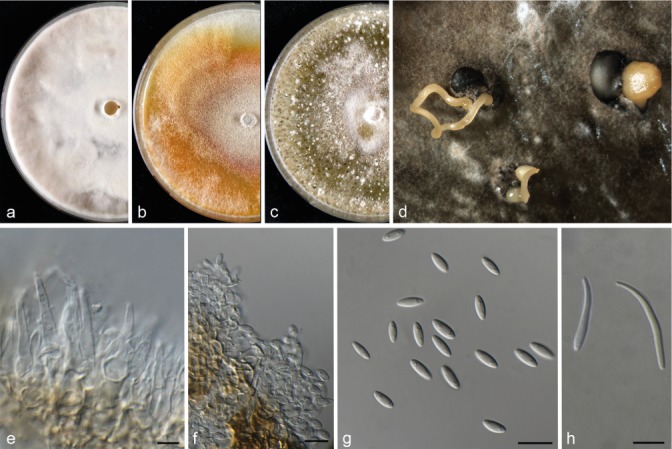
Diaporthe celeris (CBS 143349). a–c. Colonies on MEA, PDA and OA, respectively; d. conidiomata sporulating on OA; e. conidiophores; f. conidiogenous cells; g. alpha conidia; h. beta conidia. — Scale bars = 10 μm.
Fig. 5.
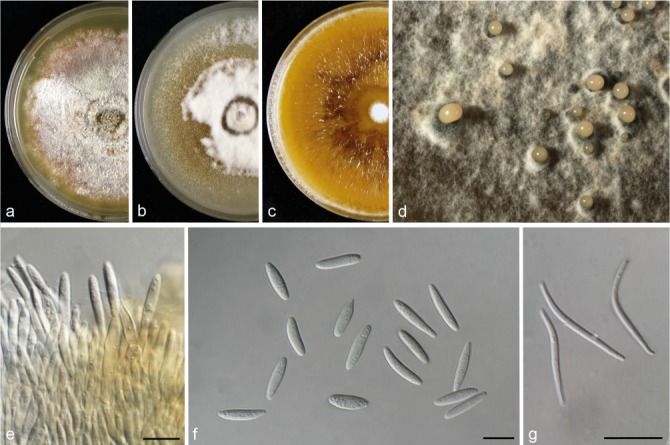
Diaporthe hispaniae (CBS 143351). a–c. Colonies on MEA, PDA and OA, respectively; d. conidiomata sporulating on PDA; e. conidiogenous cells; f. alpha conidia; g. beta conidia. — Scale bars = 10 μm.
Fig. 6.
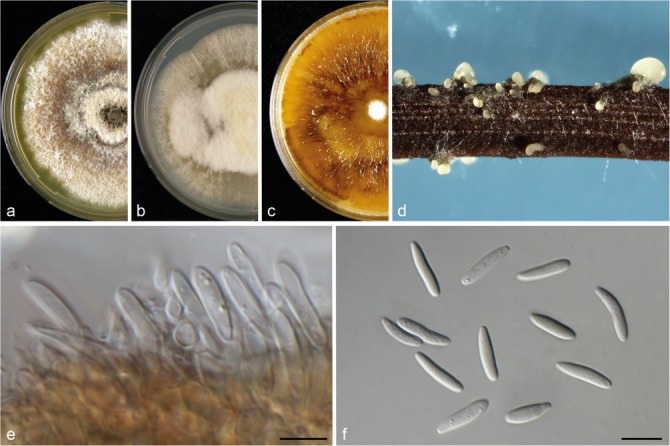
Diaporthe hungariae (CBS 143353). a–c. Colonies on MEA, PDA and OA, respectively; d. conidiomata sporulating on PNA; e. conidiogenous cells; f. alpha conidia. — Scale bars = 10 μm.
Diaporthe bohemiae Guarnaccia, Eichmeier & Crous, sp. nov. — MycoBank MB823244; Fig. 3
Etymology. Named after the country where it was collected, Czech Republic (ancient Latin name, Bohemia).
Conidiomata pycnidial on PNA, globose or irregular, solitary, deeply embedded in PDA, erumpent, dark brown to black, 250–400 μm diam, whitish translucent to yellow conidial drops exuded from the ostioles. Conidiophores hyaline, smooth, 1-septate, densely aggregated, cylindrical, straight, 5–20 × 1.5–4 μm. Conidiogenous cells phialidic, hyaline, terminal, cylindrical, 6–8 × 1–2 μm, tapered towards the apex. Paraphyses intermingled among conidiophores, hyaline, smooth, 1–3-septate, up to 70 μm long, apex 1–2 μm diam. Alpha conidia produced on all the tested media, aseptate, fusiform, hyaline, multi-guttulate and acute at both ends, 7.5–8.5 × 1.5–3 μm, mean ± SD = 7.6 ± 0.6 × 2.3 ± 0.3 μm, L/W ratio = 3.3. Beta conidia and gamma conidia not observed.
Culture characteristics — Colonies covering the medium within 9 d at 21 °C, with surface mycelium flattened, dense and felty. Colony on MEA, PDA and OA at first white, becoming cream to yellowish, flat on PDA and OA, and dark brown on MEA, with dense and felted mycelium. Reverse pale brown with brownish dots with age, with visible solitary conidiomata at maturity on MEA and PDA. On OA visible solitary conidiomata within 10 d.
Materials examined. Czech Republic, Znojmo, Dyjákovičky, from root of Vitis spp., 30 Mar. 2015, A. Eichmeier (CBS H-23236 – holotype; CBS 143347 = CPC 28222 – culture ex-type); from root of Vitis spp., 30 Mar. 2015, A. Eichmeier (culture CBS 143348 = CPC 28223).
Notes — Diaporthe bohemiae was collected from roots of Vitis spp. used as rootstock, in the Czech Republic. This species is phylogenetically close but clearly differentiated from D. carpini based on ITS, tef1, tub2, his3 and cal sequence similarity (98 % in ITS, 91 % in tef1, 96 % in tub2, 94 % in his3, and 94 % in cal). Morphologically, D. bohemiae differs from D. carpini in its shorter alpha conidia (5.5–8.5 vs 7–9 μm) (Gomes et al. 2013) and the shape of its alpha conidia having acute ends, not observed in D. carpini which has conidia with rounded ends (Wehmeyer 1933).
Diaporthe celeris Guarnaccia, Woodhall & Crous, sp. nov. — MycoBank MB823245; Fig. 4
Etymology. From Latin celere ‘fast’, referring to the fast growth rate on different media.
Conidiomata pycnidial on PNA, globose or irregular, solitary, deeply embedded in OA, erumpent, dark brown to black, 350–650 μm diam, yellowish translucent to brown conidial cirrus or drops exuded from the ostioles. Conidiophores hyaline, smooth, 1-septate, unbranched, ampulliform, cylindrical, straight, 5–18 × 1–3 μm. Conidiogenous cells phialidic, hyaline, terminal, cylindrical, 5–8 × 1–2 μm, tapered towards the apex. Paraphyses not observed. Alpha conidia aseptate, fusiform, hyaline, mono- to biguttulate and acutely rounded at both ends, 5.5–7.5 × 2–3 μm, mean ± SD = 6.6 ± 0.5 × 2.5 ± 0.3 μm, L/W ratio = 2.6. Beta conidia hyaline, aseptate, eguttulate, filiform, curved, tapering towards both ends, 16–22.5 × 1–2 μm, mean ± SD = 19.7 ± 2.1 × 1.4 ± 0.3 μm, L/W ratio = 14. Gamma conidia not observed.
Culture characteristics — Colonies covering the medium within 6 d at 21 °C, with surface mycelium flattened, dense and felty. Colony on MEA with white floccose mycelium. On PDA and OA at first white, becoming cream to brown and grey, respectively, flat on PDA and OA, and dark brown on MEA, with abundant production of conidiomata only on OA. Reverse pale brown on MEA and whitish to cream on PDA and OA.
Materials examined. UK, Sussex, from trunk of Vitis vinifera, 12 Nov. 2013, J. Woodhall (CBS H-23237 – holotype; CBS 143349 = CPC 28262 – culture ex-type); from trunk of Vitis vinifera, 12 Nov. 2013, J. Woodhall (culture CBS 143350 = CPC 28266).
Notes — Diaporthe celeris was isolated from V. vinifera in the UK. Three strains representing this species cluster in a well-supported clade embedded in the D. eres species complex. This species is phylogenetically close but clearly differentiated from D. celastrina based on tef1, his3 and cal sequence similarity (96 % in tef1, 96 % in his3, and 98 % in cal) and from D. eres based on tef1 sequence similarity (97 %). Morphologically, D. celeris differs from D. celastrina in the production of beta conidia not observed in D. celastrina, and from D. eres in its fast growth rate in culture and shorter alpha conidia (Udayanga et al. 2014a).
Diaporthe hispaniae Guarnaccia, Armengol & Crous, sp. nov. — MycoBank MB823246; Fig. 5
Etymology. Named after the country where it was collected, Spain (ancient Latin name, Hispania).
Conidiomata pycnidial in culture on PNA, globose or irregular, scattered or solitary, deeply embedded in MEA and PDA, erumpent, dark brown to black, 150–400 μm diam, cream translucent to orange conidial drops exuded from the ostioles. Conidiophores hyaline, some filiform, smooth, aseptate, densely aggregated, cylindrical, straight, 5–30 × 1–4 μm. Conidiogenous cells phialidic, hyaline, terminal, cylindrical, 6–10 × 1–2 μm, tapered towards the apex. Paraphyses not observed. Alpha conidia common, fusiform, hyaline, rarely curved, apex acutely rounded, base obtuse to subtruncate, multi-guttulate, aseptate, 9–14.5 × 2–4 μm, mean ± SD = 11.4 ± 1.3 × 2.7 ± 0.4 μm, L/W ratio = 4.2. Beta conidia less common, straight or curved, 18–24 × 1–2 μm, mean ± SD = 22.7 ± 2.3 × 1.6 ± 0.3 μm, L/W ratio = 14.2. Gamma conidia not observed.
Culture characteristics — Colonies covering the medium within 12 d at 21 °C, with surface mycelium flattened, dense and felty. Colony on MEA and PDA at first white becoming pale brown to grey with abundant production of sporulating conidiomata. On OA cream to dark brown. Reverse pale brown to cream on MEA and PDA, dark brown on OA.
Materials examined. Spain, Valencia, Aielo de Malferit, from necrotic scion of Vitis vinifera, 2016, J. Armengol (CBS H-23238 – holotype; CBS 143351 = CPC 30321 – culture ex-type); from necrotic wood of Vitis vinifera, 2016, J. Armengol (culture CBS 143352 = CPC 30323).
Notes — Diaporthe hispaniae was isolated from V. vinifera samples collected in Spain. Two strains representing this species cluster separately in a well-supported clade, and appear most closely related to D. ampelina based on the tub2 sequence similarity (93 %). This species is phylogenetically close but clearly differentiated from D. hungariae (described below) by 53 unique fixed alleles in tub2. Morphologically, D. hispaniae differs from D. ampelina in its longer alpha conidia and larger beta conidia (Gomes et al. 2013). This species differs from D. hungariae in the production of beta conidia.
Diaporthe hungariae Guarnaccia, Armengol & K.Z. Váczy, sp. nov. — MycoBank MB823247; Fig. 6
Etymology. Named after the country where the ex-type strain was collected, Hungary (ancient Latin name, Hungaria).
Conidiomata pycnidial in culture on PNA, globose or irregular, solitary, aggregated or solitary, deeply embedded in MEA, PDA and OA, erumpent, dark brown to black, 150–650 μm diam, white translucent to cream conidial cirrus or drops exuded from the ostioles. Conidiophores hyaline, acute, smooth, aseptate, densely aggregated, cylindrical, straight, 5–25 × 1–3.5 μm. Conidiogenous cells phialidic, hyaline, terminal, cylindrical, 6–9 × 1–2 μm, tapered towards the apex. Paraphyses not observed. Alpha conidia commonly found, fusiform, hyaline, rarely curved, apex acutely rounded, base obtuse to subtruncate, mono- to multi-guttulate, aseptate, 9.5–16 × 2–3.5 μm, mean ± SD = 11.7 ± 1.4 × 2.6 ± 0.4 μm, L/W ratio = 4.5. Beta and gamma conidia not observed.
Culture characteristics — Colonies covering the medium within 15 d at 21 °C, with surface mycelium flattened, dense and felty. Colony on MEA and PDA at first white becoming pale brown to grey. On OA cream to dark brown showing sectorial areas with abundant production of sporulating conidiomata. Reverse pale brown to cream on MEA and PDA, dark brown on OA.
Materials examined. Hungary, Pécs, from trunk of Vitis vinifera, 28 Aug. 2014, K.Z. Váczy (CBS H-23239 – holotype; CBS 143353 = CPC 30130 – culture ex-type); from trunk of Vitis vinifera, 28 Aug. 2014, K.Z. Váczy (culture CBS 143354 = CPC 30142).
Notes — Diaporthe hungariae was isolated from V. vinifera samples collected in Hungary and Spain. Two isolates from Hungary were used for the species description. Six strains representing this species cluster separately in a well-supported clade, and appear most closely related to D. ampelina based on tub2 sequence similarity (93 %). This species is phylogenetically close but clearly differentiated from D. hispaniae (described above) by 53 unique fixed alleles in tub2. Morphologically, D. hungariae differs from D. ampelina in its larger conidiomata, longer alpha conidia and the absence of beta conidia, normally observed in D. ampelina and also in D. hispaniae (Gomes et al. 2013).
Pathogenicity
After 21 d, all the Diaporthe isolates induced necrotic lesions on the inoculated grapevines shoots except for the isolates of D. bohemiae, and the fungi were successfully re-isolated (Fig. 7f, g). Cankers and internal discolourations were observed in correspondence to inoculation points. No symptoms were observed on the control shoots. Preliminary differences in aggressiveness among the isolates and susceptibility of V. vinifera were observed: D. hispaniae and D. hungariae caused larger cankers and necrotic lesions than D. baccae and D. celeris, whilst D. bohemiae caused no symptoms.
Fig. 7.

a–e. Natural and f–g. artificial symptoms on V. vinifera with associated Diaporthe species. a–c. Lesions of Phomopsis cane and leaf spot on shoot: a. initial symptoms (courtesy Alessandro Vitale); b. severe symptoms on green; c. dead shoot (courtesy José Luis Ramos Sáez de Ojer). — d–e. Cane bleaching (courtesy José Luis Ramos Sáez de Ojer). — f–g. External and internal discoloration of shoot inoculated with D. hispaniae (CPC 30323).
DISCUSSION
We collected 175 Diaporthe strains from eight countries. Single gene and multilocus DNA sequence analyses were performed using five loci (ITS, tef1, tub2, his3, and cal) commonly used in previous phylogenetic studies of Diaporthe species (Gomes et al. 2013, Udayanga et al. 2014a, b, Santos et al. 2017). Only the closest taxa to the nine Diaporthe species recovered in this study, were selected based on BLAST searches of NCBIs GenBank nucleotide database and included in the phylogenetic analyses. The final phylogenetic trees clearly distinguished four species newly described here (D. bohemiae, D. celeris, D. hispaniae and D. hungariae) and five known species (D. ambigua, D. ampelina, D. baccae, D. eres and D. rudis).
After sampling grapevine plants in several European countries and in Israel, molecular phylogenetic and morphological analyses were used to evaluate the diversity of Diaporthe species associated with this host. Several Diaporthe species are well-established in Europe in association with important diseases affecting agricultural crops such as peach, soybean, blueberry, citrus and avocado (Santos et al. 2011, Lombard et al. 2014, Guarnaccia et al. 2016, Prencipe et al. 2017, Guarnaccia & Crous 2017).
Diaporthe spp. are also frequently associated with grapevine diseases worldwide (Mostert et al. 2001a, Van Niekerk et al. 2005), such as Phomopsis cane and leaf spot, consisting of shoots breaking off, stunting, dieback and fruit rot. Moreover, cankers, swelling arms, and cane bleaching are serious diseases caused by Diaporthe spp. (Rawnsley et al. 2004, Úrbez-Torres et al. 2013). Diaporthe ampelina (= Phomopsis viticola) is known to affect all green parts of grapevines and is the main Diaporthe species causing Phomopsis cane and leaf spot. This species has been studied since 1958 (Pine 1958, 1959, Pscheidt & Pearson 1989), and recently, its ability to also cause wood cankers was demonstrated (Úrbez-Torres et al. 2013). Diaporthe kyushuensis and D. perjuncta are respectively known for causing swelling arm and dormant cane bleaching (Kajitani & Kanematsu 2000). Diaporthe ambigua, D. eres and D. foeniculina occurred in Californian vineyards (Úrbez-Torres et al. 2013). Diaporthe eres was also reported as causing diseases in Croatia and Italy (Kaliterna et al. 2012, Cinelli et al. 2016), whilst D. eres, D. hongkongensis, D. phaseolorum and D. sojae were reported as pathogens in China (Dissanayake et al. 2015).
DNA sequence data are essential in resolving taxonomic questions, redefining species boundaries, and accurate naming of species as required for the effective communication about plant pathogens. Regarding Diaporthe, Santos et al. (2017) showed that species separation is better when five loci (ITS, tef1, tub2, his3 and cal) are simultaneously used to build the resulting phylogenies. Recent phylogenetic analyses of the genus Diaporthe studied more than 170 species, and grouped some of those in species complexes, such as D. arecae, D. eres and D. sojae, which include important plant pathogenic species (Huang et al. 2013, Udayanga et al. 2014a, 2015). Moreover, a polyphasic approach has substantially reshaped the taxonomy of Diaporthe species involved with grapevine diseases (Mostert et al. 2001a, Van Niekerk et al. 2005, Dissanayake et al. 2015).
Although several studies on the presence of Diaporthe in major grapevine production areas were conducted in the past, this was never the case in Europe, and thus a large-scale investigation of Diaporthe spp. associated with grapevine was needed. This study provides the first molecular characterisation of Diaporthe diversity related to Vitis spp. in Europe and Israel, combined with morphological characterisation.
A combined alignment of seven genes (act, Apn2, cal, tef1, his3, FG1093 and tub2) was incorporated in a recent revision of the D. eres complex, among which the tef1, Apn2 and his3 genes were considered as the most informative loci for defining species in this complex (Udayanga et al. 2014a). The ITS region was excluded from their phylogenetic analysis and the authors stated that a poorly supported non-monophyletic grouping was observed when ITS sequences were included in the combined analysis. This problem was detected in our phylogenetic analysis of the D. eres complex and in other studies (Gomes et al. 2013, Dissanayake et al. 2017, Gao et al. 2017) where two separate clades of D. eres are observed (D. eres (A) and D. eres (B), Fig. 1). The D. eres (A) clade included the ex-epitype culture CBS 138594, several other known taxa in the D. eres complex and 98 strains collected from grapevines in the present (Fig. 1), and a previous study (Cinelli et al. 2016). Several highly-supported subclades clustered with D. eres (A). However, they were not clearly differentiated based on both single-locus and morphological similarity. Thus, they are not considered as new species. The D. eres (B) clade, previously known as the Diaporthe cf. nobilis/Phomopsis fukushii complex (Gomes et al. 2013), grouped four reference strains of D. eres, according to the seven-gene analysis from Udayanga et al. (2014b), and seven isolates from grapevines. Diaporthe eres was recovered from grapevines in all the countries investigated except Israel. A further three strains collected in the UK was revealed to represent a new species (D. celeris) in the D. eres complex, clearly differentiated from the closest species (D. celastrina and D. eres) based on multi-locus phylogenetic analyses and morphology.
Another two new species, D. hungariae (reported from Hungary and Spain) and D. hispaniae (from Spain), were closely related, but clearly separated based on morphological and molecular characteristics from D. ampelina, historically known as the most aggressive Diaporthe species of grapevine and found in all the countries sampled in this study. The final species described in this study as new is D. bohemiae, that was collected in the Czech Republic. Diaporthe rudis was isolated from samples collected in Czech Republic, France, Italy, Spain and UK, confirming its role as key pathogen of grapevine. Two isolates of D. ambigua were recovered in Spain, and for the first time after its description by Lombard et al. (2014), D. baccae was found in Croatia, France and Spain. Diaporthe baccae was previously found in Croatia by Kaliterna et al. (2012) but wrongly identified as closely related D. foeniculina (as D. neotheicola).
Preliminary pathogenicity tests of the species found associated with grapevine for the first time in the current study focused on green shoots (Phillips 1999, Mostert et al. 2001a, Van Niekerk et al. 2005). Inoculation of green shoots in growth chambers with D. celeris and D. baccae resulted in the development of lesions. The most severe symptoms were detected on stems inoculated with D. hispaniae and D. hungariae. Therefore, this study provides results about the ability from these species to cause disease of grapevines, together with the well-known key pathogen D. ampelina. The other inoculated species, D. bohemiae, was not able to induce lesions, appearing to be an endophyte in grapevines.
The present study is the first evaluation of Diaporthe species associated with grapevines in Europe and Israel, combining morphology and molecular data, providing useful information for evaluating pathogenicity of the various species. To our knowledge, this study represents also the first report of D. baccae associated with grapevines, and of D. ambigua on grapevines in Europe.
Acknowledgments
This research was supported by the European COST Action FA1303 on Sustainable control of grapevine trunk diseases (ManaGTD). Surveys and fungal collection performed in Hungary were supported by Szechenyi 2020 programme, the European Regional Development Fund, the Hungarian Government (GINOP-2.3.2-15-2016-00061) and EU H2020 project no. 652601. We are grateful to Arien van Iperen (cultures), Mieke Starink-Willemse and Elias Jonk (DNA isolation, amplification, and sequencing) for their technical assistance.
REFERENCES
- Aveskamp MM, Verkley GJM, De Gruyter J, et al. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 363–382. [DOI] [PubMed] [Google Scholar]
- Baumgartner K, Fujiyoshi F, Travadon R, et al. 2013. Characterization of species of Diaporthe from wood cankers of grape in Eastern North American vineyards. Plant Disease 97: 912–920. [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Carroll GC. 1986. The biology of endophytism in plants with particular reference to woody perennials. In: Fokkema NJ, Van den Heuvel J. (eds), Microbiology of the phyllosphere: 205–222. Cambridge University Press, Cambridge. [Google Scholar]
- Chepkirui C, Stadler M. 2017. The genus Diaporthe: a rich source of diverse and bioactive metabolites. Mycological Progress 16: 477–494. [Google Scholar]
- Cinelli T, Mondello V, Marchi G, et al. 2016. First report of Diaporthe eres associated with cane blight of grapevine (Vitis vinifera) in Italy. Plant Disease 100: 532. [Google Scholar]
- Corsaro MM, De Castro C, Evidente A, et al. 1998. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydrate Research 308: 349–357. [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004b. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ, Risede JM, et al. 2004a. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, et al. 2011a. Fungal Planet description sheets: 69–91. Persoonia 26: 108–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Swart L, et al. 2011b. Fungal pathogens of Proteaceae. Persoonia 27: 20–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. 2009. Fungal Biodiversity. [CBS Laboratory Manual Series 1]. Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DQ, Wijayawardene NN, Bhat DJ, et al. 2014. Pustulomyces gen. nov. accommodated in Diaporthaceae, Diaporthales, as revealed by morphology and molecular analyses. Cryptogamie, Mycologie 35: 63–72. [Google Scholar]
- Dai J, Krohn K, Flörke U, et al. 2005. Novel highly substituted biraryl ethers, phomopsines D-G, isolated from endophytic fungus Phomopsis sp. from Adenocarpus foliolosus. European Journal of Organic Chemistry 23: 5100–5105. [Google Scholar]
- Diogo ELF, Santos JM, Phillips AJL. 2010. Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Diversity 44: 107–115. [Google Scholar]
- Dissanayake AJ, Liu M, Zhang W, et al. 2015. Morphological and molecular characterisation of Diaporthe species associated with grapevine trunk disease in China. Fungal Biology 119: 283–294. [DOI] [PubMed] [Google Scholar]
- Dissanayake AJ, Phillips AJL, Hyde KD, et al. 2017. The current status of species in Diaporthe. Mycosphere 8: 1106–1156. [Google Scholar]
- Erincik O, Madden LV, Ferree DC, et al. 2001. Effect of growth stage on susceptibility of grape berry and rachis tissues to infection by Phomopsis viticola. Plant Disease 85: 517–520. [DOI] [PubMed] [Google Scholar]
- Fu CH, Hsieh HM, Chen CY, et al. 2013. Ophiodiaporthe cyatheae gen. et sp. nov., a diaporthalean pathogen causing a devastating wilt disease of Cyathea lepifera in Taiwan. Mycologia 105: 861–872. [DOI] [PubMed] [Google Scholar]
- Gao YH, Liu F, Cai L. 2016. Unravelling Diaporthe species associated with Camellia. Systematics and Biodiversity 14: 102–117. [Google Scholar]
- Gao YH, Lui F, Duan W, et al. 2017. Diaporthe is paraphyletic. IMA Fungus 8: 153–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YH, Su YY, Sun W, et al. 2015. Diaporthe species occurring on Lithocarpus glabra in China, with descriptions of five new species. Fungal Biology 119: 295–309. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyne A, López-Medrano F, Morales JM, et al. 2011. Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: first report of human infection by this fungus. Transplant Infectious Disease 13: 204–207. [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, et al. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso FM, Marini M, Vitale A, et al. 2012. Canker and dieback on Platanus × acerifolia caused by Diaporthe scabra. Forest Pathology 42: 510–513. [Google Scholar]
- Guarnaccia V, Crous PW. 2017. Emerging citrus diseases in Europe caused by Diaporthe spp. IMA Fungus 8: 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia V, Groenewald JZ, Polizzi G, et al. 2017. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 39: 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia V, Vitale A, Cirvilleri G, et al. 2016. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. European Journal of Plant Pathology 146: 963–976. [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192. [Google Scholar]
- Huang F, Hou X, Dewdney MM, et al. 2013. Diaporthe species occurring on citrus in China. Fungal Diversity 61: 237–250. [Google Scholar]
- Isaka M, Jaturapat A, Rukseree K, et al. 2001. Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species. Journal of Natural Products 64: 1015–1018. [DOI] [PubMed] [Google Scholar]
- Kajitani Y, Kanematsu S. 2000. Diaporthe kyushuensis sp. nov. the teleomorph of the causal fungus of grapevine swelling arm in Japan, and its anamorph Phomopsis vitimegaspora. Mycoscience 41: 111–114. [Google Scholar]
- Kaliterna J, Milicevic T, Cvjetkovic B. 2012. Grapevine trunk diseases associated with fungi from the Diaporthaceae family in Croatian vineyards. Archives of Industrial Hygiene and Toxicology 63: 471–478. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran RS, Hur BK. 2009. Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol. Biotechnology and Applied Biochemistry 54: 21–30. [DOI] [PubMed] [Google Scholar]
- Kuo KC, Leu LS. 1998. Phomopsis vitimegaspora: a pathogenic Phomopsis from vines. Mycotaxon 66: 497–499. [Google Scholar]
- Lamprecht SC, Crous PW, Groenewald JZ, et al. 2011. Diaporthaceae associated with root and crown rot of maize. IMA Fungus 2: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Van Leeuwen GCM, Guarnaccia V, et al. 2014. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathologia Mediterranea 53: 287–299. [Google Scholar]
- Merrin SJ, Nair NG, Tarran J. 1995. Variation in Phomopsis recorded on grapevine in Australia and its taxonomic and biological implications. Australasian Plant Pathology 24: 44–56. [Google Scholar]
- Mostert L, Crous PW, Kang JC, et al. 2001a. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 146–167. [Google Scholar]
- Mostert L, Kang JC, Crous PW, et al. 2001b. Phomopsis saccharata sp. nov., causing a canker and die-back disease of Protea repens in South Africa. Sydowia 53: 227–235. [Google Scholar]
- Muralli TS, Suryanarayanan TS, Geeta R. 2006. Endophytic Phomopsis species: host range and implications for diversity estimates. Canadian Journal of Microbiology 52: 673–680. [DOI] [PubMed] [Google Scholar]
- Nylander JAA. 2004. MrModeltest v. 2. Program distributed by the author. Uppsala, Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Nirenberg HI, Aoki T, et al. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41: 61–78. [Google Scholar]
- Pearson RC, Goheen C. 1994. Phomopsis cane and leaf spot. In: Hewitt WB, Pearson RC. (eds), Compendium of grape diseases: 17–18. APS Press, St Paul, MI. [Google Scholar]
- Phillips AJL. 1999. The relationship between Diaporthe perjuncta and Phomopsis viticola on grapevines. Mycologia 91: 1001–1007. [Google Scholar]
- Pine TS. 1958. Etiology of the dead-arm of grapevines. Phytopathology 48: 192–197. [Google Scholar]
- Pine TS. 1959. Development of the grape dead-arm disease. Phytopathology 49: 738–743. [Google Scholar]
- Prencipe S, Nari L, Vittone G, et al. 2017. First report of Diaporthe eres causing stem canker on peach (Prunus persica) in Italy. Plant Disease 101: 1052. [Google Scholar]
- Pscheidt JW, Pearson RC. 1989. Effect of grapevine training systems and pruning practices on occurrence of Phomopsis cane and leaf spot. Plant Disease 73: 825–828. [Google Scholar]
- Rawnsley B, Wicks TJ, Scott ES, et al. 2004. Diaporthe perjuncta does not cause Phomopsis cane and leaf spot disease of grapevine in Australia. Plant Disease 88: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, UK. [Google Scholar]
- Rehner SA, Uecker FA. 1994. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Canadian Journal of Botany 72: 1666–1674. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Adams GC, Cannon PF, et al. 2015. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Phillips AJL. 2009. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Diversity 34: 111–125. [Google Scholar]
- Santos JM, Vrandečić K, Cìosić J, et al. 2011. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 27: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos L, Alves A, Alves R. 2017. Evaluating multi-locus phylogenies for species boundaries determination in the genus Diaporthe. PeerJ 5:e3120; doi: https://doi.org/10.7717/peerj.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos PJC, Savi DC, Gomes RR, et al. 2016. Diaporthe endophytica and D. terebinthifolii from medicinal plants for biological control of Phyllosticta citricarpa. Microbiological Research 186–187: 153–160. [DOI] [PubMed] [Google Scholar]
- Says-Lesage V, Roeckel-Drevet P, Viguié A, et al. 2002. Molecular variability within Diaporthe/Phomopsis helianthi from France. Phytopathology 92: 308–313. [DOI] [PubMed] [Google Scholar]
- Scheper RWA, Crane DC, Whisson DL, et al. 2000. The Diaporthe teleomorph of Phomopsis Taxon 1 on grapevine. Mycological Research 104: 226–231. [Google Scholar]
- Schilder AMC, Erincik O, Castlebury L, et al. 2005. Characterization of Phomopsis spp. infecting grapevines in the Great Lakes region of North America. Plant Disease 89: 755–762. [DOI] [PubMed] [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, et al. 2017. Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, et al. 1996. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4.0b10. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tan YP, Edwards J, Grice KRE, et al. 2013. Molecular phylogenetic analysis reveals six new species of Diaporthe from Australia. Fungal Diversity 61: 251–260. [Google Scholar]
- Thompson SM, Tan YP, Shivas RG, et al. 2015. Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia. Persoonia 35: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Tan YP, Young AJ, et al. 2011. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia 27: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2014a. Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Diversity 67: 203–229. [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2014b. Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 32: 83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2015. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biology 119: 383–407. [DOI] [PubMed] [Google Scholar]
- Udayanga D, Liu X, McKenzie EHC, et al. 2011. The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Diversity 50: 189–225. [Google Scholar]
- Uecker FA. 1988. A world list of Phomopsis names with notes on nomenclature, morphology and biology. Mycological Memoirs 13: 1–231. [Google Scholar]
- Úrbez-Torres JR, Adams P, Kamas J, et al. 2009. Identification, incidence and pathogenicity of fungal species associated with grapevine dieback in Texas. American Journal of Enology and Viticulture 60: 497–507. [Google Scholar]
- Úrbez-Torres JR, Peduto F, Smith RJ, et al. 2013. Phomopsis dieback: a grapevine trunk disease caused by Phomopsis viticola in California. Plant Disease 97: 1571–1579. [DOI] [PubMed] [Google Scholar]
- Van der Aa HA, Noordeloos ME, De Gruyter J. 1990. Species concepts in some larger genera of the Coelomycetes. Studies in Mycology 32: 3–19. [Google Scholar]
- Van Niekerk JM, Groenewald JZ, Farr DF, et al. 2005. Reassessment of Phomopsis species on grapevines. Australasian Plant Pathology 34: 27–39. [Google Scholar]
- Van Rensburg JCJ, Lamprecht SC, Groenewald JZ, et al. 2006. Characterization of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Studies in Mycology 55: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer LE. 1926. A biologic and phylogenetic study of stromatic Sphaeriales. American Journal of Botany 13: 575–645. [Google Scholar]
- Wehmeyer LE. 1933. The genus Diaporthe Nitschke and its segregates. University of Michigan Press, Ann Arbon. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR Protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, California. [Google Scholar]
- Wilcox WA, Ellis MA, Ramsley B, et al. 2015. Phomopsis cane and leaf spot. In: Wilcox WF, Gubler WD, Uyemoto JK. (eds), Compendium of grape diseases, disorders, and pests, second ed. APS, S. Paul, MN, USA. [Google Scholar]
- Yang J, Xu F, Huang C, et al. 2010. Metabolites from the mangrove endophytic fungus Phomopsis sp. (#zsu-H76). European Journal of Organic Chemistry 19: 3692–3695. [Google Scholar]


