Abstract
Background
Denosumab has been shown to reduce tumor size and progression, reform mineralized bone, and increase intralesional bone density in patients with giant cell tumor of bone (GCTB); however, radiologic assessment of tumors in bone is challenging. The study objective was to assess tumor response to denosumab using three different imaging parameters in a prespecified analysis in patients with GCTB from two phase 2 studies.
Methods
The studies enrolled adults and adolescents (skeletally mature and at least 12 years of age) with radiographically measurable GCTB that were given denosumab 120 mg every 4 weeks, with additional doses on days 8 and 15 of cycle 1. The proportion of patients with an objective tumor response was assessed using either Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST), European Organisation for Research and Treatment of Cancer response criteria (positron emission tomography [PET] scan criteria), or inverse Choi density/size (ICDS) criteria. Target lesions were measured by computed tomography or magnetic resonance imaging (both studies), PET (study 2 only), or plain film radiograph (study 2 only).
Results
Most patients (71.6%) had an objective tumor response by at least one response criteria. Per RECIST, 25.1% of patients had a response; per PET scan criteria, 96.2% had a response; per ICDS, 76.1% had a response. 68.5% had an objective tumor response ≥ 24 weeks. Using any criteria, crude incidence of response ranged from 56% (vertebrae/skull) to 91% (lung/soft tissue), and 98.2% had tumor control ≥ 24 weeks. Reduced PET avidity appeared to be an early sign of response to denosumab treatment.
Conclusion
Modified PET scan criteria and ICDS criteria indicate that most patients show responses and higher benefit rates than modified RECIST, and therefore may be useful for early assessment of response to denosumab.
Trial registration
ClinicalTrials.gov Clinical Trials Registry NCT00396279 (retrospectively registered November 6, 2006) and NCT00680992 (retrospectively registered May 20, 2008).
Electronic supplementary material
The online version of this article (10.1186/s12957-018-1478-3) contains supplementary material, which is available to authorized users.
Keywords: Giant cell tumor of bone, Denosumab, RANKL, Objective tumor response
Background
Giant cell tumor of bone (GCTB) is a histologically benign bone tumor composed of mononuclear stromal and multinucleated giant cells that exhibit osteoclastic activity, typically arising in the metaphyseal/epiphyseal portions of long bones [1, 2]. GCTB causes significant bone destruction, leading to pain, pathologic fracture, and impaired joint structure and functionality [3, 4]. Surgical resection is the primary curative method for GCTB; however, aggressive interventions, such as adjuvant therapy with liquid nitrogen or phenol, are often required to decrease morbidity, avoid amputation, and ensure adequate local control [4, 5]. Effective treatment options are limited for patients with lesions in locations not amenable to surgical resection [4], and local recurrence develops after several years in approximately 10–50% and 5% of patients after intralesional treatment or wide resection, respectively [5–8].
Constitutive activation of receptor activator of nuclear factor-kappa B (RANK) ligand maintains the osteolytic phenotype in GCTB [9, 10]. Denosumab (XGEVA®, Amgen Inc., Thousand Oaks, CA, USA), a RANK ligand inhibitor, is a fully human monoclonal antibody approved for the treatment of unresectable GCTB or when resection may result in severe morbidity. Denosumab treatment of GCTB prevents further tumor progression, reduces tumor size, reforms mineralized bone, and increases intralesional bone density [10, 11].
Radiologic assessment of tumor response in bone tumors presents unique challenges, and no uniform radiographic assessment criteria to date have been advanced to specifically assess response in GCTB. To address this challenge, our analysis combined imaging assessment techniques and captured response elements from three response evaluation measures widely employed in the assessment of change in tumor burden across a variety of tumor types, with modifications to tailor the response measures specifically to the unique properties of GCTB. Imaging records from two phase 2 clinical trials that supported denosumab registration [10, 11] were analyzed with three imaging parameters to measure the changes in lesion size and density, compare available radiographic parameters, and assess treatment response to denosumab in patients with GCTB.
Methods
Study design
This analysis used data pooled from two phase 2, open-label, single-arm, international, multicenter studies of denosumab [10, 11] in skeletally mature patients (≥ 12 years of age) with histologically confirmed GCTB and radiographically measurable disease. Key exclusion criteria included current use of alternative GCTB treatments (e.g., radiation, chemotherapy, embolization, or bisphosphonates). Study 1 [10] is complete; study 2 is ongoing [11]. In both studies, patients received 120 mg denosumab subcutaneously every 4 weeks, with additional loading doses on days 8 and 15 of the first treatment cycle (i.e., month 1). Patients received denosumab until disease progression and no clinical benefit, patient decision to withdraw from the study, or until complete tumor resection. In study 2 [11], patients with complete tumor resection received an additional six doses of denosumab after resection.
Imaging assessments
Patients with ≥ 1 evaluable time point assessment were included in this analysis (Fig. 1). In study 1, computed tomography (CT) or magnetic resonance imaging (MRI) was required every 3 months [10], and in study 2, the imaging modality and frequency followed the local standard practice, which included plain film radiograph, CT, MRI, and 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography (18FDG-PET) [11]. Lesion images were retrospectively reviewed centrally by experienced bone radiologists blinded to investigator assessment. The central review was performed using a charter-specified, two-reader paradigm, with adjudication in case of interpretation discordance [11]. Key parameters and processes of the integrated, independent analysis of objective tumor response were agreed upon following consultation with regulatory authorities.
Fig. 1.
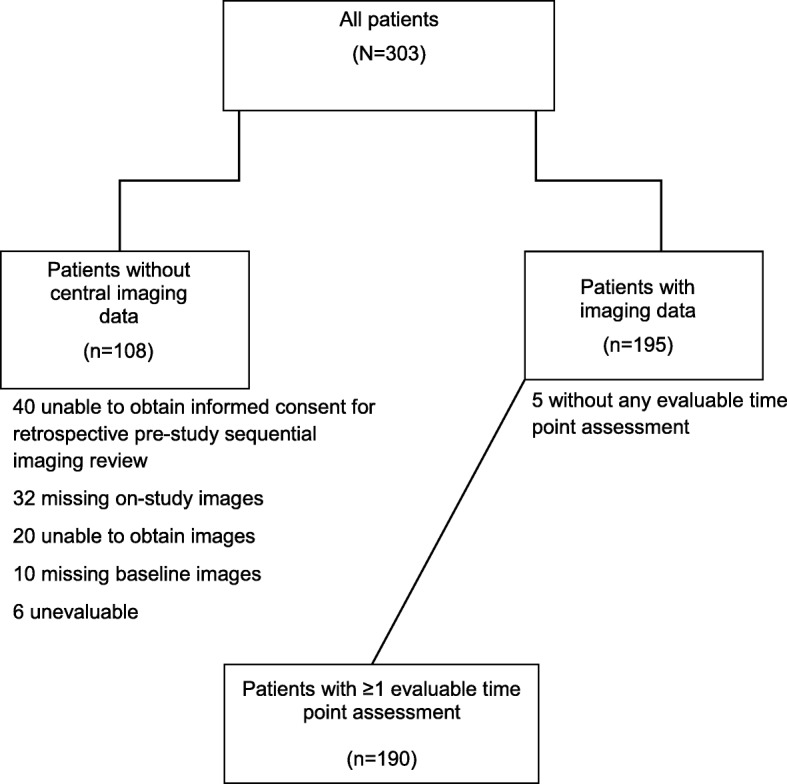
CONSORT diagram
All available CT, MRI, and whole-body 18FDG-PET images were provided for the assessment of tumor response and disease progression using prespecified criteria (Table 1). Up to three response evaluation parameters were used to capture the unique anatomic and radiologic features of each lesion and response to treatment. These included criteria for modified Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST), European Organisation for Research and Treatment of Cancer (EORTC; referred to as PET scan criteria), and inverse Choi density/size (ICDS) as outlined in Table 1 [12–14]. Postbaseline time points for assessment of tumor response, including the length of therapy by the patient, are summarized in Additional file 1: Figure S1.
Table 1.
Response criteria
| Modified RECIST 1.1[13] | Modified EORTC [12] | ICDS [14] | |
|---|---|---|---|
| CR | Disappearance of all target lesions; all target lymph nodes are < 10 mm in the short axis | Complete resolution of abnormal 18FDG uptake within the tumor volume of all target lesions to a level that is indistinguishable from surrounding normal tissue | Disappearance of all disease |
| PR | At least a 30% decrease in SLD using baseline SLD as a reference | Reduction of the sum of the SUVmax by ≥ 15–25% after 1 cycle and a decrease of ≥ 25% compared with baseline after > 1 treatment cycle | A decrease in size (%Δ Choi SLD) ≥ 10% or an increase in CT density > 15% compared with baseline, no new lesions, and no obvious progression of nonmeasurable disease |
| SD | Neither sufficient shrinkage of target lesions to qualify for PR nor sufficient increase to qualify for PD, taking as reference the nadir SLD | %ΔΣ SUVmax increased by < 25% or decreased by < 15% compared with baseline and no visible extent of 18FDG tumor uptake (> 20% in the longest dimension) | Does not meet the criteria for CR, PR, or PD; no symptomatic deterioration attributed to tumor progression |
| PD | At least a 20% increase in the SLD of target lesions, taking as reference the nadir SLD; in addition to the relative increase of 20% in SLD, the SLD must also demonstrate an absolute increase of ≥ 5 mm | %ΔΣ SUVmax increased by ≥ 25% compared with baseline scan, visible increase in the extent of 18FDG uptake (> 20% in the longest dimension) or the appearance of new 18FDG uptake in metastatic lesions | An increase in unidimensional tumor size (Choi SLD) ≥ 10% and does not meet the criteria for PR using CT density; any new lesions identified by CT/MRI; new intratumoral nodules or increase in the size of existing intratumoral nodules |
| UE | A target lesion present at baseline that subsequently became UE | 18FDG-PET exam was unavailable or deemed UE;a response will be UE unless unequivocal PD is determined on the basis of the evaluable target lesion | The CT/MRI exam is unavailable or deemed UE; if a target lesion is deemed UE by density and size measurement and the rules for PD do not apply, a response of CR, PR, or SD cannot be assigned for the time point and the response will be UE |
RECIST Response Evaluation Criteria in Solid Tumors, EORTC European Organisation for Research and Treatment of Cancer, ICDS inverse Choi density/size, CR complete response, 18FDG-PET 2-deoxy-2- [18F]-fluorodeoxyglucose positron emission tomography, PR partial response, SLD sum of longest diameter, SUVmax maximum standardized uptake value, SD stable disease, PD progressive disease CT computed tomography, MRI magnetic resonance imaging, UE unevaluable
aThe UE rate for this study was essentially 0
Statistics
Statistical analyses were descriptive in nature, and only summary statistics were presented. The analyses included the proportion of patients with an objective tumor response, time to first objective tumor response, duration of objective tumor response, and the proportions of patients with sustained (≥ 4, 12, and 24 weeks) objective tumor response and tumor control (complete response [CR], partial response [PR], or stable disease [SD]). Objective tumor response was defined as either CR or PR using any of the three tumor response evaluation criteria. The proportion of patients with an objective tumor response by baseline target lesion location and the percentage changes from baseline for lesion diameter and density were also summarized.
Results
Patients
Of the 303 patients, 190 (study 1 [n = 27] and study 2 [n = 163]) were included in this analysis. Of these, 187 had measurable anatomic lesion size evaluable by CT, 26 had functional imaging by 18FDG-PET, and 176 had CT-evaluable lesions, assessed for Hounsfield unit (HU) density and size, and were included in the RECIST, PET scan criteria, and ICDS evaluations, respectively.
Study 1 patients primarily had axial skeleton lesions not amenable to surgery with curative intent. Study 2 patients were divided into resectable lesions for which surgery could lead to significant morbidity (cohort 1) and unresectable tumors (cohort 2). All patients had radiographic evidence of active primary or recurrent GCTB within the previous year, with target lesions distributed across the disease spectrum; pelvis/sacrum (n = 61; 32%), lower extremities (n = 39; 21%), and lung (n = 38; 20%) were common target lesion sites. Most patients (70%) had prior GCTB resection/surgery, 20% had received prior bisphosphonates, and 20% had received prior radiotherapy (Table 2). Median (range) of time of patient participation was 13.4 months (1.7–48.9); patients received a median (range) of 16 doses (4–54) of denosumab. Baseline demographics and disease characteristics for patients without evaluable imaging analysis were similar to the population included in this analysis (Amgen Inc., data on file).
Table 2.
Baseline demographics and disease characteristics
| Overall (N = 190) | |
|---|---|
| Sex, n (%) | |
| Female | 105 (55) |
| Male | 85 (45) |
| Age, median (Q1, Q3), years | 33 (26, 43) |
| ECOG performance statusa, n (%) | |
| 0 | 106 (56) |
| 1 | 76 (40) |
| 2 | 6 (3) |
| Previous treatment | |
| Resection/surgery | 132 (70) |
| Bisphosphonates | 38 (20) |
| Radiotherapy | 37 (20) |
| Chemotherapy | 21 (11) |
| GCTB disease type, n (%) | |
| Recurrent unresectable | 92 (48) |
| Primary unresectable | 43 (23) |
| Recurrent resectable | 29 (15) |
| Primary resectable | 26 (14) |
| Location of target lesionb, n (%) | |
| Pelvis/sacrum | 61 (32) |
| Lower extremities | 39 (21) |
| Lung | 38 (20) |
| Spine | 18 (10) |
| Upper extremities | 17 (9) |
| Otherc | 11 (6) |
| Skull/neck | 5 (3) |
| Missing | 1 (1) |
Q quartile, ECOG Eastern Cooperative Oncology Group, GCTB giant cell tumor of bone
aECOG missing for two patients
bBased on case report form
cIncludes other soft tissue and bone sites
Overall, 136/190 patients (71.6% [95% CI, 64.6–77.9%]) had an objective tumor response (CR or PR) by at least one response criteria. Per RECIST, 47/187 patients (25.1% [95% CI, 19.1–32.0%]) had a response; per PET scan criteria, 25/26 patients (96.2% [95% CI, 80.4–99.9%]) had a response; per ICDS, 134/176 patients (76.1% [95% CI, 69.1–82.2%]) had a response (Table 3). Using any response criteria, the median time to first objective tumor response (Kaplan-Meier estimate) was about 3 months per PET scan and ICDS criteria and was not estimable per RECIST. Overall, tumor responses were sustained; most patients (68.5%) had an objective tumor response for ≥ 24 weeks (Table 3). When analyzed by study and cohort, response rates were similar for PET scan criteria and ICDS (Table 3). Variations were observed when using RECIST, which showed a lower rate of response for study 1 (11%) than study 2 (28%). Within study 2, the response rates per RECIST were 32% and 17% for cohort 1 and cohort 2, respectively (Table 3). Similar results were observed for sustained objective tumor responses at weeks 4, 12, and 24 (Table 3).
Table 3.
Objective tumor response resultsa
| Overall best response | RECIST 1.1 | EORTC | ICDS | |
|---|---|---|---|---|
| Proportion of responders, n/N (%) | ||||
| Overall | 136/190 (71.6) | 47/187 (25.1) | 25/26 (96.2) | 134/176 (76.1) |
| Study 1 | 20/27 (74.1) | 3/27 (11.1) | 15/16 (93.8) | 18/23 (78.3) |
| Study 2 | 116/163 (71.2) | 44/160 (27.5) | 10/10 (100.0) | 116/153 (75.8) |
| Cohort 1 | 76/114 (66.7) | 36/113 (31.9) | 4/4 (100.0) | 76/105 (72.4) |
| Cohort 2 | 40/49 (81.6) | 8/47 (17.0) | 6/6 (100.0) | 40/48 (83.3) |
| Median time to first OTR, months (95% CI)b | 3.1 (2.89–3.65) | NE (20.9–NE) | 2.7 (1.64–2.79) | 3.0 (2.79–3.48) |
| Patients with sustained OTR, n/N (%) | ||||
| Overall | ||||
| ≥ 4 weeks | 102/153 (66.7) | 32/150 (21.3) | 18/20 (90.0) | 101/143 (70.6) |
| ≥ 12 weeks | 98/144 (68.1) | 32/141 (22.7) | 16/17 (94.1) | 97/135 (71.9) |
| ≥ 24 weeks | 76/111 (68.5) | 26/109 (23.9) | 11/12 (91.7) | 76/102 (74.5) |
| Study 1 | ||||
| ≥ 4 weeks | 15/24 (62.5) | 2/24 (8.3) | 11/13 (84.6) | 13/20 (65.0) |
| ≥ 12 weeks | 14/20 (70.0) | 2/20 (10.0) | 10/11 (90.9) | 13/17 (76.5) |
| ≥ 24 weeks | 12/17 (70.6) | 2/17 (11.8) | 8/9 (88.9) | 12/14 (85.7) |
| Study 2 | ||||
| ≥ 4 weeks | 87/129 (67.4) | 30/126 (23.8) | 7/7 (100.0) | 88/123 (71.5) |
| ≥ 12 weeks | 84/124 (67.7) | 30/121 (24.8) | 6/6 (100.0) | 84/118 (71.2) |
| ≥ 24 weeks | 64/94 (68.1) | 24/92 (26.1) | 3/3 (100.0) | 64/88 (72.7) |
| Cohort 1 | ||||
| ≥ 4 weeks | 59/91 (64.8) | 25/90 (27.8) | 3/3 (100.0) | 60/85 (70.6) |
| ≥ 12 weeks | 56/87 (64.4) | 25/86 (29.1) | 3/3 (100.0) | 57/81 (70.4) |
| ≥ 24 weeks | 49/73 (67.1) | 22/73 (30.1) | 2/2 (100.0) | 50/67 (74.6) |
| Cohort 2 | ||||
| ≥ 4 weeks | 28/38 (73.7) | 5/36 (13.9) | 4/4 (100.0) | 28/38 (73.7) |
| ≥ 12 weeks | 28/37 (75.7) | 5/35 (14.3) | 3/3 (100.0) | 27/37 (73.0) |
| ≥ 24 weeks | 15/21 (71.4) | 2/19 (10.5) | 1/1 (100.0) | 14/21 (66.7) |
| Patients with tumor controlc, % | ||||
| ≥ 4 weeks | 148/153 (96.7) | 145/150 (96.7) | 19/20 (95.0) | 139/143 (97.2) |
| ≥ 12 weeks | 139/144 (96.5) | 137/141 (97.2) | 17/17 (100.0) | 131/135 (97.0) |
| ≥ 24 weeks | 109/111 (98.2) | 108/109 (99.1) | 12/12 (100.0) | 101/102 (99.0) |
RECIST Response Evaluation Criteria in Solid Tumors, EORTC European Organisation for Research and Treatment of Cancer, ICDS inverse Choi density/size; NE not estimable, OTR objective tumor response
aPatients with at least one evaluable time point assessment
bKaplan-Meier estimate
cDefined as CR + PR + SD
Objective tumor response by target lesion location showed that the crude incidences of response (95% CI) using any criteria were 14/24 (58.3% [36.6–77.9%]) for pelvis, 22/37 (59.5% [42.1–75.2%]) for sacrum, 32/40 (80.0% [64.4–90.9%]) for lower extremity, 39/43 (90.7% [77.9–97.4%]) for lung/soft tissue, 15/20 (75.0% [50.9–91.3%]) for upper extremity, and 14/25 (56.0% [34.9–75.6%]) for vertebrae/skull. Figure 2 shows CT images before and after denosumab treatment in a patient with sacral GCTB. Tumor control ≥ 24 weeks was observed in 98.2% of patients using any criteria; similar rates were observed for the other response criteria (Table 3).
Fig. 2.
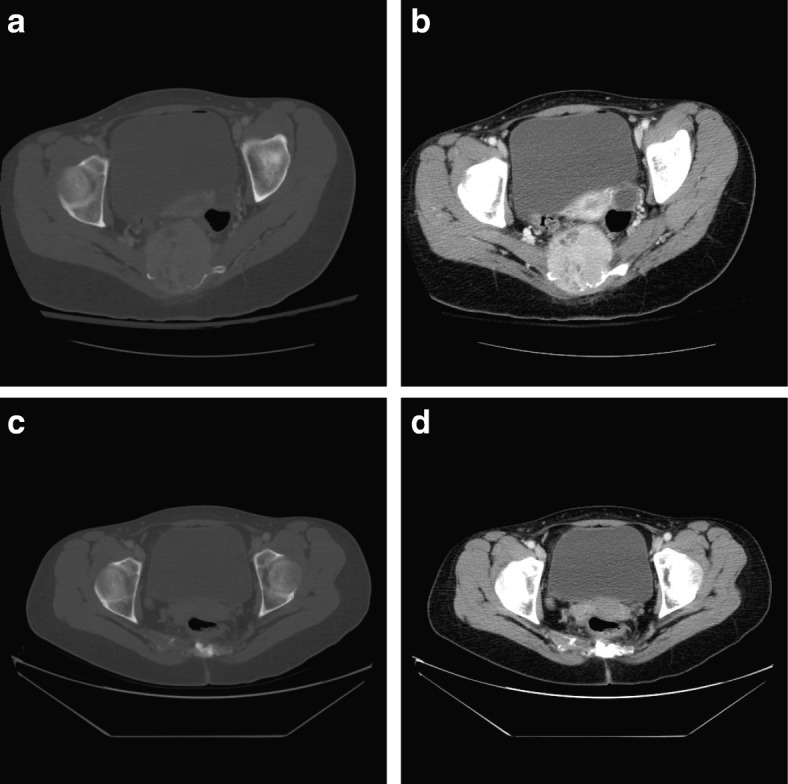
Sacral GCTB before and after treatment with denosumab. a Bone window and b soft tissue window pretreatment CT scan from August 14, 2009, through the level of the upper hip joints. There is extensive bone destruction and a large soft tissue mass that displaces the rectum. c Bone window and d soft tissue window CT repeat scan on December 12, 2013 (about 4 years and 4 months later) following treatment with denosumab (about 3 years and 10 months; first dose on January 21, 2010, and last dose on November 21, 2013). The soft tissue mass is now negligible, and the bone is reconstituting. CT computed tomography; GCTB giant cell tumor of bone
Median (range) lesion size was 62.5 mm (10–283), consistent with the advanced disease in the study population (Table 4). Anatomic extent, measured by longest diameter (LD), demonstrated that the greatest percentage decreases in size occurred ≤ 3 months on-study and were consistent and sustained. Considering the best percentage change in LD, arrangement in increasing order of degree of response per the ICDS evaluation (Additional file 1: Figure S2a) revealed a group of patients that did not respond to therapy, with an LD increase ≥ 10% (n = 4, 2%); a second group of patients with SD and LD changes ± 10% (n = 76, 43%); and a third group of patients with an LD decreases ≥ 10% (n = 95, 54%). For responders (defined by ≥ 10% reduction in tumor size; Table 1) with a measurable decrease in LD, there was an evenly graded distribution of best LD reduction ranging from 11 to > 70%.
Table 4.
Baseline LD and SUVmax summary in patients with ≥ 1 evaluable time point assessment of 18FDG-PET avidity
| n | Mean | SD | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| LD, mm | 174 | 68.4 | 40.8 | 10.0 | 38.0 | 62.5 | 91.0 | 283.0 |
| SUVmax | 26 | 11.1 | 4.7 | 3.8 | 7.9 | 10.6 | 13.6 | 21.6 |
18FDG-PET 2-deoxy-2-[18F]-fluorodeoxyglucose positron emission tomography, LD longest diameter, max maximum, min minimum, Q quartile, SUVmax maximum standardized uptake value
Using HU density as a response parameter, the best percentage change in density for target lesions showed that 99 of 124 patients (80%) had ≥ 15% increase and 25 patients (20%) had < 15% increase (Additional file 1: Figure S2b); 15% is the density cutoff for the response per Choi gastrointestinal stromal tumor (GIST) criteria [14]. HU evaluation showed that percentage increases in tumor density ≤ 6 months on-study were consistent and sustained; mean HU values rarely decreased once increases were observed, with medians of 93 and 108 at postbaseline time point assessments 1 and 2, respectively. Time point assessments were ≥ 24 weeks apart [11].
At baseline, the mean (SD) maximum standardized uptake value (SUVmax) of 18FDG-PET in 26 patients using PET scan criteria was 11.1 (4.7), indicative of high metabolic activity in GCTB lesions before denosumab treatment (Table 4). Almost 100% of lesions showed a rapid reduction in 18FDG-PET avidity at the earliest time point assessment (Table 3). PET responsiveness did not appear to vary with lesion location. Reduction in 18FDG-PET avidity therefore appeared to be an early and universal sign of response to denosumab treatment.
Discussion
We observed impressive tumor control rates, with nearly all patients with GCTB showing sustained tumor control for ≥ 24 weeks, using any of the response criteria. Increases in lesion density by HU likely reflected the pharmacodynamic response to denosumab treatment (i.e., suppression of osteolysis and increased formation of dense fibro-osseous tissue and/or woven bone [9]). This clinical benefit allows patients to defer or downstage their planned surgical procedure when surgical resection is likely to result in severe morbidity [15]. In contrast, a purely size-based evaluation using RECIST is potentially insensitive in assessing response in bone lesions with a mixed osteolytic and expanding soft tissue component; the size of GCTB tumors changes little with targeted therapies. Therefore, an inverse modification of the ICDS was used to evaluate both GCTB density and size; either a decrease in size or an increase in density was considered a response to treatment. In GCTB, decreases in tumor size per LD are believed to reflect cytoreduction, in alignment with RECIST principles for solid tumor assessment. The kinetics of GCTB responses to denosumab therapy showed rapid cytoreduction that peaked by 3 months and was maintained thereafter, with responses of ≥ 24 weeks in nearly all patients. The Choi criteria were developed to monitor response in a soft tissue sarcoma undergoing targeted therapy where tumor cell viability and radiological size reduction may be uncoupled during the response to treatment [14]. Analogous to GIST, in the setting of GCTB, we believe that the ICDS criteria used in the present study perform as pharmacodynamic markers of effect and may offer an advantage to conventional RECIST.
In our study, patients had unresectable tumors or tumors requiring highly invasive or disabling surgery in an attempt to achieve surgical cure; therefore, there was a large number of pelvic, spine, and pulmonary lesions that complicated radiographic evaluation of response. Using ICDS, four patients had a ≥ 10% increase in LD, two of whom sustained increases in tumor size after study enrollment but before administration of denosumab. These two patients experienced sustained disease control lasting several months while receiving denosumab continuously, and for one patient, 12 additional months of disease control following discontinuation of denosumab. The remaining two patients had atypical GCTB. One had multiostotic and metastatic GCTB with lesions in the pelvis, rib, and lung at study entry and received denosumab for 8 months before being lost to follow-up. Multiostotic GCTB accounts for < 1% of all GCTB and has a different clinical presentation than solitary lesions; typically, patients are younger, suggesting a germ-line component that confers susceptibility to the disease [16–21]. The other patient with atypical GCTB with an increased tumor size had a clinically aggressive disease with ten previous attempts at surgical resection before enrollment. While these patients met all histological entry criteria and had pathologically confirmed GCTB, it remains unclear whether their atypical courses before and during denosumab treatment suggest an aggressive clinical variant of classical GCTB or an alternative diagnosis. Because true nonresponse to denosumab in GCTB is rare, patients with nonresponse may deserve more comprehensive sampling for histological disease assessment. The best percentage change in density for target lesions in the ICDS evaluation showed that 80% of the 124 patients evaluable for density had a ≥ 15% increase in density, reflecting the desired outcome of denosumab therapy.
Our results confirm and extend findings reported in a smaller study [22] where 88% patients (n = 17) had an objective tumor response using any response criteria after denosumab treatment (median duration of 13.1 months). In the present study, the proportions of patients with an objective tumor response were 35% per RECIST, 82% per PET scan criteria, and 71% per ICDS criteria (size/density). The median time to objective tumor response using any of the response criteria was 3.0 months (95% CI, 2.9–3.1). The benefit of denosumab in GCTB has already been established [10, 11]; our results provide clinicians with additional information on imaging and monitoring patients with GCTB treated with denosumab.
The single-arm study design limits our analysis; however, the central independent review of images was conducted to minimize this limitation. Furthermore, this study had a large number of unevaluable patients, there was no protocol-defined imaging schedule or methodology (which is standard for this type of study), and only a few PET scans were done, as PET was optional. We also limited our definition of sustained tumor control to a time frame of 24 weeks, which may be considered short by some clinicians; however, there are no well-established tumor response criteria for patients with GCTB [22]. We also did not examine any association between response and extent of prior treatment or other factors. The retrospective nature of this analysis made obtaining historical images difficult.
There are inherent limitations associated with using RECIST alone for assessment of denosumab response in GCTB because of the sometimes modest reduction in tumor size despite clinical benefit. Reduction in 18FDG-PET avidity predicted a favorable tumor response and sustained tumor control with denosumab treatment. Given the rarity of denosumab refractoriness in typical GCTB, new or continued high SUVmax levels while on denosumab should alert clinicians to the possibility of an aggressive clinical variant or an alternate diagnosis such as sarcoma.
Our data do not suggest an increase in the risk of osteosarcoma following denosumab treatment. There are recent case studies of patients with GCTB treated with denosumab who have developed osteosarcoma [23, 24]; three patients were diagnosed with osteosarcoma during denosumab treatment in primary reports of the studies used for our analysis [10, 11]. Patients with GCTB are at higher risk for developing osteosarcoma than the general population, with approximately 2–5% of patients developing secondary sarcoma following radiotherapy or surgical resection [25–27]. There also remains the previously reported, equally difficult task of identifying patients with small foci of sarcomatous change within the large field of otherwise benign-appearing GCTB [28]. The incidence of pathologic fracture is up to 30% in patients with GCTB; data to date do not indicate an increased rate with denosumab [8, 29, 30].
Conclusions
Modified PET scan criteria and ICDS criteria showed responses in most patients in our analysis, indicating a substantially higher benefit rate compared to that assessed by modified RECIST. PET or CT with ICDS provided an early indication of treatment response. Moreover, all response criteria indicated tumor control ≥ 24 weeks to denosumab. Loss of 18FDG-PET avidity may have a dual role in both predicting long-term disease control and offering clinicians some reassurance that there is not a focus of sarcoma with the GCTB lesion, which would likely remain 18FDG-PET avid despite denosumab treatment. Further research is required to determine the appropriate imaging technique to be used longitudinally in a given patient, although many practitioners favor a combination of plain radiographs and CT. Regardless of the modality used, careful evaluation of nonresponders is necessary.
Additional file
Figure S1. Postbaseline time point assessments for tumor response by study for patients with ≥ 1 evaluable time point assessment. Per protocol, the sites were instructed to perform CT or MRI scans of the lesion at baseline and quarterly during the treatment period. 18FDG-PET scans were performed at the discretion of the investigator. Because this was a retrospective, independent image review, no specific acquisition parameters were provided. Sites were instructed to use their standard acquisition parameters for CT, MRI, and 18FDG-PET. Consistent use of the imaging modalities, parameters, and contrast was recommended for reproducibility. CT computed tomography, 18FDG‑PET 2-deoxy-2-[18F] fluoro-D-glucose positron emission tomography; MRI magnetic resonance imaging. Figure S2. (a) Best percentage change in SLD for target lesions in the ICDS evaluation and (b) best percentage change in density for target lesions in the ICDS evaluation. ICDS inverse Choi density/size; LD longest diameter; SLD sum of longest diameter. (DOCX 233 kb)
Acknowledgements
We thank the study staff, patients, and their families for their invaluable contributions to the studies. Lori Gorton (Amgen Inc.), James Ziobro (funded by Amgen Inc.), and Rick Davis (Complete Healthcare Communications, LLC, North Wales, PA, funded by Amgen Inc.) assisted in preparing the manuscript.
Funding
This study was funded by Amgen, Inc.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- 18FDG-PET
2-Deoxy-2-[18F] fluoro-d-glucose positron emission tomography
- CR
Complete response
- CT
Computed tomography
- EORTC
European Organisation for Research and Treatment of Cancer
- GCTB
Giant cell tumor of bone
- GIST
Gastrointestinal stromal tumor
- HU
Hounsfield unit
- ICDS
Inverse Choi density/size
- LD
Longest diameter
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- PR
Partial response
- RANK
Receptor activator of nuclear factor-kappa B
- RECIST
Response Evaluation Criteria in Solid Tumors version 1.1
- SD
Stable disease
- SUVmax
Maximum standardized uptake value
Authors’ contributions
JE, RG, RH, HG, EC, SC, and PR participated in the conception and design of the study, data collection and acquisition, and the analysis and interpretation of the data. IJ and ZJR participated in the conception and design of the study and analysis and interpretation of the data. LS, MO, CF, AB, and BAB participated in the analysis and interpretation of data. All authors participated in the writing and editing of this manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the institutional review board or ethics committee for each site, and all patients provided written informed consent.
Consent for publication
By giving written informed consent, all patients gave their approval for publication of the results of the study. There are no individually identifiable patient-level data.
Competing interests
JE has received fees for lectures on GCTB and participation on scientific advisory boards regarding denosumab for Amgen Inc. He is a member of the board of directors for Genovis Inc. LS has served as consulting radiologist for Amgen, Inc. for this trial. RG has served on the scientific advisory board for Amgen, Inc. for this trial. RH has served as a primary investigator for Amgen, Inc. and Novartis and has served as a board member of the nonprofit Mattie Miracle Cancer Foundation. HG’s institution has received grants and consultancy fees from Amgen, Inc. EC has served as a consultant on advisory boards for Amgen, Inc., EMD Serono, and Bayer. SC has served as an advisor and consultant for Amgen, Inc. PR has served on advisory boards for Novartis; Pfizer; Bayer; PharmaMar; Ariad; Amgen, Inc.; GlaxoSmithKline; AstraZeneca; Clinigen; and Lilly and has received honoraria from Novartis; Pfizer; Bayer; PharmaMar; Amgen, Inc.; GlaxoSmithKline; and Lilly. MO is an employee of CoreLab Partners (now Bioclinica). AF, IJ, ZJR, AB, and BAB were employees of Amgen, Inc. at the time of the study; they are currently employees of Atara Biotherapeutics, Inc.; Pfizer; Kite Pharma, Inc.; Arog Pharmaceuticals, Inc.; and AbbVie, respectively.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jacob Engellau, Phone: +46 46 17 76 48, Email: jacob.engellau@med.lu.se.
Leanne Seeger, Email: lseeger@mednet.ucla.edu.
Robert Grimer, Email: rob.grimer@btopenworld.com.
Robert Henshaw, Email: Robert.M.Henshaw@medstar.net.
Hans Gelderblom, Email: A.J.Gelderblom@lumc.nl.
Edwin Choy, Email: ECHOY@mgh.harvard.edu.
Sant Chawla, Email: santchawla@sarcomaoncology.com.
Peter Reichardt, Email: peter.reichardt@helios-klinken.de.
Michael O’Neal, Email: Michael.ONeal@bioclinica.com.
Amy Feng, Email: chunyao_feng@yahoo.com.
Ira Jacobs, Email: iraallenjacobs@aol.com.
Zachary J. Roberts, Email: zjroberts@gmail.com
Ada Braun, Email: adabraun@yahoo.de.
Bruce A. Bach, Email: bbach@post.harvard.edu
References
- 1.Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006;37:35–51. doi: 10.1016/j.ocl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Futamura N, Urakawa H, Tsukushi S, Arai E, Kozawa E, Ishiguro N, Nishida Y. Giant cell tumor of bone arising in long bones possibly originates from the metaphyseal region. Oncol Lett. 2016;11:2629–2634. doi: 10.3892/ol.2016.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;209:9–24. [PubMed]
- 4.Szendrői Miklós. European Surgical Orthopaedics and Traumatology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. Giant-Cell Tumour of Bone (GCT) pp. 4037–4054. [Google Scholar]
- 5.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469:1181–1187. doi: 10.1007/s11999-010-1560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szendroi M. Giant-cell tumour of bone. J Bone Joint Surg Br. 2004;86:5–12. doi: 10.1302/0301-620X.86B1.14053. [DOI] [PubMed] [Google Scholar]
- 7.Arbeitsgemeinschaft K, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendroi M, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. doi: 10.2106/00004623-198769010-00018. [DOI] [PubMed] [Google Scholar]
- 9.Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, Jun S, Jacobs I. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18:4415–4424. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 10.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 11.Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 12.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22:2860–2868. doi: 10.1245/s10434-015-4634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu KK, Ross PM, Mitchell DC, Sprague HH. Evolution of a case of multicentric giant cell tumor over a 23-year period. Clin Orthop Relat Res. 1986;213:279–88. [PubMed]
- 17.Sim FH, Dahlin DC, Beabout JW. Multicentric giant-cell tumor of bone. J Bone Joint Surg Am. 1977;59:1052–1060. doi: 10.2106/00004623-197759080-00009. [DOI] [PubMed] [Google Scholar]
- 18.Tornberg DN, Dick HM, Johnston AD. Multicentric giant-cell tumors in the long bones. A case report. J Bone Joint Surg Am. 1975;57:420–422. doi: 10.2106/00004623-197557030-00026. [DOI] [PubMed] [Google Scholar]
- 19.Hindman BW, Seeger LL, Stanley P, Forrester DM, Schwinn CP, Tan SZ. Multicentric giant cell tumor: report of five new cases. Skelet Radiol. 1994;23:187–190. doi: 10.1007/BF00197457. [DOI] [PubMed] [Google Scholar]
- 20.Hoch B, Inwards C, Sundaram M, Rosenberg AE. Multicentric giant cell tumor of bone. Clinicopathologic analysis of thirty cases. J Bone Joint Surg Am. 2006;88:1998–2008. doi: 10.2106/JBJS.E.01111. [DOI] [PubMed] [Google Scholar]
- 21.Wirbel R, Blumler F, Lommel D, Syre G, Krenn V. Multicentric giant cell tumor of bone: synchronous and metachronous presentation. Case Rep Orthop. 2013;2013:756723. doi: 10.1155/2013/756723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda T, Morioka H, Nishida Y, Kakunaga S, Tsuchiya H, Matsumoto Y, Asami Y, Inoue T, Yoneda T. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol. 2015;26:2149–2154. doi: 10.1093/annonc/mdv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–3055. doi: 10.1007/s11999-015-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broehm CJ, Garbrecht EL, Wood J, Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015;2015:767198. doi: 10.1155/2015/767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97:2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 26.Sanerkin NG. Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer. 1980;46:1641–1649. doi: 10.1002/1097-0142(19801001)46:7<1641::AID-CNCR2820460725>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.McGrath PJ. Giant-cell tumour of bone: an analysis of fifty-two cases. J Bone Joint Surg Br. 1972;54:216–229. doi: 10.1302/0301-620X.54B2.216. [DOI] [PubMed] [Google Scholar]
- 28.Hefti FL, Gachter A, Remagen W, Nidecker A. Recurrent giant-cell tumor with metaplasia and malignant change, not associated with radiotherapy. A case report. J Bone Joint Surg Am. 1992;74:930–934. doi: 10.2106/00004623-199274060-00015. [DOI] [PubMed] [Google Scholar]
- 29.Turcotte Robert E., Wunder Jay S., Isler Marc H., Bell Robert S., Schachar Norman, Masri Bassam A., Moreau Guy, Davis Aileen M. Giant Cell Tumor of Long Bone: A Canadian Sarcoma Group Study. Clinical Orthopaedics and Related Research. 2002;397:248–258. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64:755–761. doi: 10.2106/00004623-198264050-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Postbaseline time point assessments for tumor response by study for patients with ≥ 1 evaluable time point assessment. Per protocol, the sites were instructed to perform CT or MRI scans of the lesion at baseline and quarterly during the treatment period. 18FDG-PET scans were performed at the discretion of the investigator. Because this was a retrospective, independent image review, no specific acquisition parameters were provided. Sites were instructed to use their standard acquisition parameters for CT, MRI, and 18FDG-PET. Consistent use of the imaging modalities, parameters, and contrast was recommended for reproducibility. CT computed tomography, 18FDG‑PET 2-deoxy-2-[18F] fluoro-D-glucose positron emission tomography; MRI magnetic resonance imaging. Figure S2. (a) Best percentage change in SLD for target lesions in the ICDS evaluation and (b) best percentage change in density for target lesions in the ICDS evaluation. ICDS inverse Choi density/size; LD longest diameter; SLD sum of longest diameter. (DOCX 233 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.


