Abstract
Objective
To determine whether arm circumference-to-height ratio (AHtR) predicts adolescents' cardiometabolic risk and how its predictive statistics compare to those of body mass index (BMI) percentile.
Methods
Pooled data for adolescents (N = 12,269, 12–18 years) from the National Health and Nutrition Examination Survey, U.S., 1999–2014, were analyzed. For each of the eight cardiometabolic variables, borderline-risk and high-risk were considered unhealthy, and being unhealthy on any variable was considered “unhealthy overall” in terms of cardiometabolic risk. Area under the curve and R 2 were used to compare BMI percentile and AHtR for accuracy in predicting risk.
Results
Female AHtR ≥ 0.19 and BMI percentile ≥ 94 and male AHtR ≥ 0.16 and BMI percentile ≥ 64 predicted a probability of >0.7 being unhealthy overall. AHtR predicted overall risk and unhealthy levels of six variables more accurately than BMI percentile. Significant differences were overall risk (χ 2 = 4.18; p=0.041), total cholesterol (χ 2 = 8.68; p=0.003), glycated hemoglobin (χ 2 = 5.24; p=0.022), and systolic pressure (χ 2 = 5.10; p=0.024). AHtR had higher accuracy in predicting high-density cholesterol, fasting glucose, glycated hemoglobin, and systolic/diastolic pressures plus higher specificity in predicting all variables except triglycerides. BMI percentile had higher sensitivity for all variables. Sensitivity and accuracy were higher for males. No significant race/ethnicity differences were observed.
Conclusions
Without needing adjustment for age and weight, AHtR can predict some cardiometabolic risk factors of adolescents, especially of males, more accurately than BMI percentile, thus facilitating population risk estimation and early interventions. Further research is required to validate these findings in younger children.
1. Introduction
Child and adolescent overweight and obesity significantly increase the risk of premature mortality, morbidity (e.g., diabetes, asthma, and hypertension), and related outcomes [1]. Further, obesity tracks from childhood to adulthood more strongly than any other risk factor [2]. Risk factor clustering, both biological and behavioral, is the greatest predictor of accelerated atherosclerotic processes; such clustering during childhood and adolescence can persist into adulthood [3]. Although exact criteria are unclear, the ability to detect clustering of obesity and cardiometabolic risk factors in childhood and adolescence is important for early initiation of behavioral and medical interventions [4–6], which may include simple and inexpensive lifestyle modifications, such as increased daily sleep [7].
Unfortunately, self-reported anthropometric data from adolescents are often inaccurate [8]. In the past, this presented a meaningful problem, since these self-report risk factor data for a wide variety of potentially preventable chronic health issues were insufficient to effectively inform public health interventions. Thus, there was a need to identify a measure of obesity and overweight that is accurate, simple, and cost-effective [9]. BMI has emerged as the most commonly measured parameter, worldwide, for objectively assessing general obesity plus potential-related disease risk at all ages and establishing treatment, research, and evaluation priorities. Research has revealed that BMI is correlated with fat mass and percentage of body fat at 0.90 and 0.69, respectively, so it is highly correlated with waist circumference and moderately correlated with other measures of adiposity. Thus, BMI can be used to predict adiposity-related cardiometabolic risk in situations in which a high level of precision is not required [10]. However, there are several known barriers to the use of BMI. Calculation and plotting of child and adolescent BMI have been identified by some school-based healthcare professionals as prohibitive [11], especially on a large scale, due to the difficulty of the numeric conversions and time required to perform the calculations. In pediatric primary care, some physicians have expressed concern that parents will not understand the BMI measure or even that it may be misleading for certain body types [12], and a survey of the American Academy of Pediatrics fellowship found that pediatric primary care physicians, too, are less likely to calculate BMI in the absence of an assistive tool [13]. Some concurrent research has attempted to mitigate these barriers, for example, by using color-coded BMI charts to improve metric comprehension among parents [14]. Researchers from the World Health Organization recently acknowledged some issues with BMI, but noted that in absence of an alternative viable standard, BMI-for-age is currently the sole means used to identify individuals who are overweight or obese [15].
Alternative but far less prevalent measures include waist circumference and waist-to-height ratio, which are strong predictors of cardiometabolic risk in children [16], likely because these measure visceral fat (central obesity), which releases fatty acids, hormones, and inflammatory agents, ultimately increase cardiometabolic risk. Although uncommon in epidemiological studies, mid-upper-arm circumference of pediatric populations is included in some large-scale surveys, such as National Health and Nutrition Examination Survey (NHANES), United States. Mechanisms of measurement including the Quaker arm circumference measuring stick (QUAC-stick), which was introduced in the 1960s to measure children's height at 1–9 years in order to indicate nutritional status and facilitate comparisons between countries [17]. Although the ratio of muscle mass to fat mass in mid-upper-arm circumference varies by sex and age [18], children's mid-upper-arm circumference, concurrent with triceps skinfold thickness, has been used for community-wide nutritional assessment [19]. Mid-upper-arm circumference is commonly used to quickly identify moderate to severe acute undernutrition among 6–60 month olds in resource-limited settings [20]. Moreover, in pediatric emergencies, mid-upper-arm circumference is widely used as a proxy of body weight when adjusting ventilator/equipment settings and fluid/medication doses [20, 21].
While mid-upper-arm circumference-for-age reference tables were recently developed for 1–20 year olds US population [22], little research on use of mid-upper-arm circumference for determining overweight and obesity in children and adolescents exists [23–25]. Although mid-upper-arm circumference for height reference charts are available for 0.5–10 year olds [26], only one study has utilized mid-upper-arm circumference-to-height ratio (hereafter referred to as arm-to-height ratio or AHtR) to evaluate overweight and obesity in children [25]. Further, no studies have examined the potential of mid-upper-arm circumference or AHtR to predict cardiometabolic risk of children/adolescents. Given the relative simplicity of producing these measurements compared to BMI calculation, there is a potential value in validating AHtR as an alternative to BMI in time- or resource-limited environments, or even for pediatric patients whose parents have low numeric literacy. As a proof of concept, this study used pooled NHANES data across 8 cycles to (a) determine the suitability of utilizing AHtR to predict adolescents' cardiometabolic risk and (b) compare AHtR's predictive accuracy with corresponding BMI parameters.
2. Materials and Methods
2.1. Study Setting and Dates
The analyses were based on pooled, publicly available NHANES data collected from 1999 to 2000 through 2013-2014 (8 cycles), which covered a nationally representative sample from 15 US counties. NHANES uniquely combines demographic, socioeconomic, and health-related interview information with physical examination and laboratory data.
2.2. Participants
In each two-year cycle, NHANES surveyed approximately 5,000 noninstitutionalized persons, using complex, multistage, stratified, clustered, and random sampling designs that oversampled Hispanics and non-Hispanic Blacks. Certain NHANES variables are limited to ages 12 and above, while some measures are obtained from only a subsample (e.g., morning fasting sample). Therefore, the age range in this study was confined to adolescence (ages 12–18) and excluded pregnant females. Individuals aged 12–18 years were included only if they had valid height records, at least one additional anthropometric variable required for calculating BMI (i.e., weight) and AHtR (i.e., mid-upper-arm circumference) plus at least one of eight cardiometabolic variables. A total of 12,269 adolescents met these standards and were included; 5,005 records contained all eight variables. Between 1999 and 2014, unweighted response rates for adolescents ranged from 76% to 89% in the interviewed sample and 74%–87% in the examined sample.
2.3.1. Demographic Variables
Age, gender, race/ethnicity, and antihypertensive, lipid-lowering, and antidiabetic medication use were self-reported.
2.3.2. Anthropometric Variables
BMI and AHtR were calculated using height, weight, and mid-upper-arm circumference measurements from NHANES physicals. Using anthropometry protocols, mid-upper-arm circumference was taken on participants aged two months and older at the right upper arm mid-point mark. The examiner faced the right side of the upright standing participant whose shoulders were relaxed with arms hanging loosely without muscle flexing. Measurement was taken to the nearest 0.1 cm using a measuring tape, ensuring it did not compress the skin yet fit snugly. Mid-upper-arm circumference was divided by height to obtain “unitless” AHtR.
BMI percentiles were computed utilizing a CDC-provided SAS syntax for z-scores and percentiles based on sex and age according to 2000 CDC growth charts [27]. For participants aged 2–19, NHANES reported age in months before 2011-2012 and, thereafter, in years [28]. If age in months was unavailable, age in years was multiplied by 12, and then 6 was added, to prevent upwards biased z-scores; prior studies yielded negligible discrepancies with this approach [29]. Per established standards, healthy-weight was defined as BMI < 85th percentile, overweight as ≥85th to <95th percentile, obesity as ≥ 95th percentile to <120% of the 95th percentile, and severe obesity as ≥120% of the 95th percentile or a BMI > 35, regardless of age [30].
2.3.3. Cardiometabolic Variables
Standard cutoffs in the Expert Panel Report 2012 of the National Heart, Lung, and Blood Institute were utilized for high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, and fasting plasma glucose; systolic and diastolic blood pressures were categorized based on age- and height-specific percentiles [2]. American Academy of Pediatrics (AAP) and American Diabetes Association (ADA) standard cutoffs were utilized for glycated hemoglobin [31]. Accordingly, healthy ranges for measures were (1) high-density lipoprotein cholesterol >45 mg/dL; (2) low-density lipoprotein cholesterol <110 mg/dL; (3) triglycerides <90 mg/dL; (4) total cholesterol <170 mg/dL; (5) fasting plasma glucose <100 mg/dL; (6) glycated hemoglobin <5.7%; (7) systolic blood pressure <90th percentile; and (8) diastolic blood pressure <90th percentile. In 18 year olds, systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg were considered healthy [32]. Borderline-risk and high-risk range measurements were considered unhealthy. Adolescents were categorized as healthy for “overall cardiometabolic risk” if their scores fell within the healthy range for all cardiometabolic variables. Being unhealthy on any one variable was considered unhealthy on “overall cardiometabolic risk.”
2.4. Statistical Analysis
To assess BMI and AHtR outliers, each variable was converted to a normal distribution based on the best Box-Cox transformation. Z-scores were calculated after adjusting for age and sex. One outlier was removed, and 66 participants reporting lipid-lowering, antidiabetic, or antihypertensive medication use were reclassified from healthy to unhealthy. Data were combined across the eight survey cycles with sample weights combined as per survey guidelines. Combined weights were calculated for participants whose data were collected in NHANES mobile examination centers; separate weights were applied for participants in the fasting subsample who provided measures of fasting plasma glucose, low-density lipoprotein cholesterol, and triglycerides.
Binary logistic regression was performed on each cardiometabolic risk outcome (0 = healthy; 1 = unhealthy) as predicted by BMI percentile or AHtR, including a main effect and interaction with sex and race/ethnicity. A receiver operating characteristic (ROC) curve was calculated for each model, with area under the curve (AUC) used to compare the ability of BMI and AHtR to accurately predict unhealthy cardiometabolic variable levels (i.e., borderline-risk and high-risk levels); an AUC of one indicates a perfect predictor and ≤0.5 indicates a worthless predictor [33]. Chi-square tests were used to evaluate the statistical significance of AUC differences between AHtR and BMI percentiles after incorporating Bonferroni's adjustment for multiple comparisons. R 2 and Max-rescaled R 2 were also used as measures of model fit for comparison [34]. Another model tested overall cardiometabolic risk by combining all eight cardiometabolic measures to identify whether individuals were in the healthy range on all measures or in the unhealthy range (i.e., borderline-risk or high-risk) on any of the eight measures.
Youden's Index, J (sensitivity + specificity − 100), identified the optimum age-invariant cutoff of AHtR separately for males and females (Figure 1), which best classified adolescents as healthy/unhealthy on overall cardiometabolic risk. Youden's Index is commonly used as a summary measure of the ROC curve because it can identify the maximum potential effectiveness of a biomarker [35]. For comparison, cardiometabolic cutoffs were identified for age-dependent BMI percentiles, separately for males and females, utilizing a similar approach. Based on these AHtR and BMI percentile cutoffs, participants were classified as high/low risk. For comparison with the current standard, individual risk level (high/low) was also determined based on whether the individual was above or below the conventional 85th percentile of BMI (i.e., overweight).
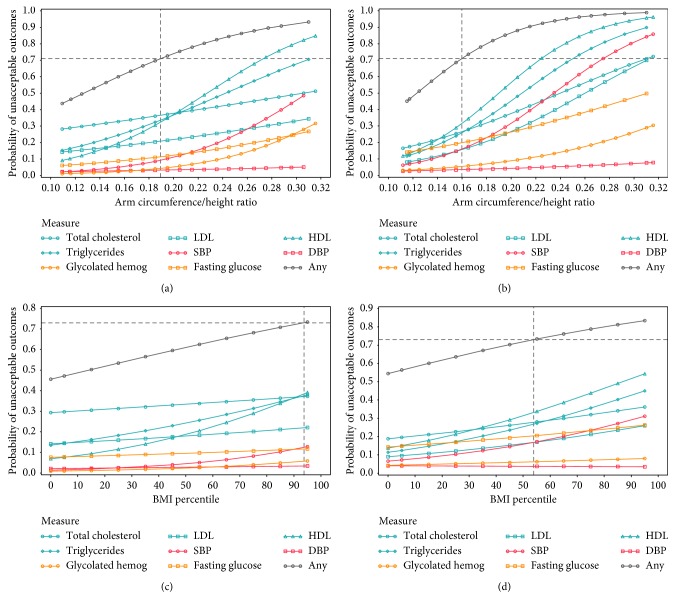
Figure 1.
Changes in general obesity measures and corresponding cardiometabolic risk probabilities, ages 12–18 by sex, N = 12,268. (a) Probability of unacceptable outcomes, for females, (b) probability of unacceptable outcomes, for males, (c) BMI percentile and unhealthy level of each variable, for females, and (d) BMI percentile and unhealthy level of each variable, for females. CR = cardiometabolic risk; TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; HbA1c = glycated hemoglobin; FPG = fasting plasma glucose; “Any” = unhealthy level on any of the eight CR variables (indicates “overall” CR). Unhealthy level of each CR variable includes both borderline-risk and high-risk levels, defined according to National Heart, Lung, and Blood Institute Expert Panel Report 2012, American Academy of Pediatrics, and American Diabetes Association. Arm-to-height ratio ≥ 0.19 in females and ≥ 0.16 in males regardless of age were identified as the best CR cutoffs, indicating ≥0.71 probability of having unhealthy level in any of the eight CR variables. BMI ≥ 94th percentile in females and ≥64th percentile in males at a given age were identified as the best CR cutoffs, indicating ≥0.73 probability of having unhealthy level in any of the eight CR variables.
SAS 9.4 SURVEYFREQ was used to calculate weighted frequencies based on survey weights, accounting for NHANES design effects of clustering and stratification with percentages based on weighted frequencies. Frequency and percentages for individuals aged 12–18 with healthy and unhealthy levels of each cardiometabolic variable within each BMI and AHtR level were cross-tabulated. Analyses included all adolescents plus males and females separately.
Logistic regression was repeated with each binary anthropometric indicator (high/low risk) to predict binary cardiometabolic outcomes (healthy/unhealthy). Altogether and separately for sex and race/ethnicity, classification was characterized and compared using sensitivity, specificity, positive predictive value, negative predictive value, accuracy ((true positives + true negatives)%), and J-statistic.
3. Results
Among 12,268 adolescents with calculated BMI, 62.7% (females 62.4%, males 63.0%; Hispanics 59.0%, Blacks 60.8%, Whites 68.1%, and other races 69.7%), were below the 85th percentile of the reference population reported by the CDC in 2000 [27]. Further, 16.43%, 12.71%, and 8.18% met criteria for overweight, obesity, and severe obesity, respectively. Table 1 provides AUC, R 2, and Max-rescaled R 2 as evaluation metrics for classifying cardiometabolic variables (healthy/unhealthy levels) based on AHtR and BMI percentiles. For all three parameters, AHtR predicted overall cardiometabolic risk (i.e., unhealthy level of any cardiometabolic variable) and unhealthy levels of six cardiometabolic variables (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, glycated hemoglobin, systolic blood pressure, and diastolic blood pressure) more accurately than BMI percentiles. Four AUC differences between AHtR and BMI percentile were significant: overall cardiometabolic risk (χ 2 = 4.18; p=0.041), total cholesterol (χ 2 = 8.68; p=0.003), glycated hemoglobin (χ 2 = 5.24; p=0.022), and systolic blood pressure (χ 2 = 5.10; p=0.024). However, only total cholesterol remained significant after adjustment for multiple comparisons (p=0.038). With triglycerides and fasting plasma glucose, R 2 and Max-rescaled R 2 were better for AHtR, but AUC was better for BMI percentile, with a statistically nonsignificant difference.
Table 1.
Evaluation metrics for ability of general obesity measures to predict cardiometabolic risk, ages 12–18, N = 12,268.
| Cardiometabolic risk variables | General obesity measures | AUC difference between arm-to-height ratio and BMI percentile | |||||
|---|---|---|---|---|---|---|---|
| Arm-to-height ratio (sex-specific) | BMI percentile (age- and sex-specific) | ||||||
| R 2 | Max-rescaled R 2 | AUC | R 2 | Max-rescaled R 2 | AUC | ||
| Overall risk | 0.058 | 0.083 | 0.647 | 0.044 | 0.063 | 0.638 | χ 2 = 4.18; p=0.041 |
| TC | 0.018 | 0.025 | 0.585 | 0.012 | 0.017 | 0.577 | χ 2 = 8.68; p=0.003 |
| LDL-C | 0.021 | 0.033 | 0.607 | 0.014 | 0.022 | 0.595 | χ 2 = 3.62; p=0.057 |
| HDL-C | 0.098 | 0.136 | 0.691 | 0.088 | 0.122 | 0.689 | χ 2 = 1.18; p=0.278 |
| TG | 0.053 | 0.075 | 0.646 | 0.049 | 0.069 | 0.648 | χ 2 = 0.24; p=0.621 |
| SBP | 0.082 | 0.143 | 0.728 | 0.072 | 0.126 | 0.722 | χ 2 = 5.10; p=0.024 |
| DBP | 0.001 | 0.004 | 0.545 | 0.001 | 0.003 | 0.538 | χ 2 = 0.35; p=0.556 |
| HbA1C | 0.016 | 0.048 | 0.646 | 0.010 | 0.029 | 0.634 | χ 2 = 5.24; p=0.022 |
| FPG | 0.034 | 0.058 | 0.640 | 0.031 | 0.052 | 0.642 | χ 2 = 0.70; p=0.403 |
AUC = area under the curve; TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; HbA1c = glycated hemoglobin; FPG = fasting plasma glucose; unhealthy level of each cardiometabolic risk variable includes both borderline-risk and high-risk levels, defined according to National Heart, Lung, and Blood Institute Expert Panel Report 2012, American Academy of Pediatrics, and American Diabetes Association. Overall risk indicates unhealthy level on any of the eight CR variables.
The effect of AHtR on cardiometabolic risk did not depend on age, i.e., the interaction was not statistically significant. The AHtR age-invariant cutoff was ≥0.19 for females and ≥0.16 for males, with a ≥0.71 probability of being unhealthy on any of the eight cardiometabolic variables (Figure 1). Among 12,140 adolescents with calculated AHtR, 54.88% were below cutoff (males 36.52%, females 74.23%; Hispanics 52.08%, Whites 58.91%, Blacks 53.01%, and other races 62.64%). Comparable cardiometabolic cutoffs of BMI percentiles were ≥94th for females and ≥64th for males, with a ≥0.73 probability of being unhealthy on any of the eight cardiometabolic variables. Among 12,268 adolescents with calculated BMI percentiles, 54.99% were below cutoff (males 34.01%, females 77.05%; Hispanics 52.84%, Whites 59.07%, Blacks 52.14%, and other races 63.05%). Figure 1 plots changes in each cardiometabolic variable and overall cardiometabolic risk against changes in AHtR and BMI percentile by sex. Compared to BMI percentile, greater AHtR slopes were observed, with more curve around the cutoff, especially in males. Table 2 demonstrates weighted percentages of adolescents, in aggregate and by sex, with unhealthy levels of each cardiometabolic variable within each level of anthropometric variable. Of all cardiometabolic variables, unhealthy levels of lipid-panel variables (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) contributed most to the increased overall cardiometabolic risk.
Table 2.
Weighted percent∗ for unhealthy levels of cardiometabolic risk variables within levels of general obesity measures, N = 12,268.
| Gender | Predictor | Categories | Overall CR | TC | LDL-C | HDL-C | TG | SBP | DBP | HbA1C | FPG |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All adolescents | Conventional cutoffs of BMI percentile | Healthy | 63.7 | 27.6 | 15.6 | 25.6 | 24.8 | 9.3 | 3.0 | 3.5 | 14.5 |
| Overweight | 75.2 | 34.6 | 23.2 | 43.6 | 36.5 | 17.0 | 2.8 | 3.5 | 16.9 | ||
| Obese | 84.8 | 40.4 | 26.7 | 56.1 | 49.3 | 22.9 | 5.3 | 4.5 | 21.9 | ||
| Severe obese | 89.3 | 41.7 | 35.2 | 71.3 | 59.8 | 31.7 | 4.4 | 12.9 | 30.2 | ||
| CR cutoffs of BMI percentile | Low risk | 61.7 | 29.9 | 17.1 | 23.8 | 24.8 | 7.5 | 3.0 | 3.2 | 11.7 | |
| High risk | 80.8 | 33.2 | 22.7 | 50.5 | 41.5 | 21.9 | 3.7 | 5.6 | 24.1 | ||
| CR cutoffs of arm-to-height ratio | Low risk | 61.5 | 28.8 | 16.0 | 24.2 | 25.1 | 7.4 | 2.9 | 3.3 | 13.1 | |
| High risk | 81.0 | 34.7 | 24.2 | 50.2 | 41.1 | 22.3 | 3.9 | 5.6 | 22.2 | ||
|
| |||||||||||
| Females | Conventional cutoffs of BMI percentile | Healthy | 59.4 | 32.6 | 17.2 | 19.6 | 24.9 | 4.5 | 3.0 | 2.6 | 9.2 |
| Overweight | 67.3 | 35.7 | 21.9 | 35.9 | 33.3 | 8.7 | 2.3 | 3.4 | 8.0 | ||
| Obese | 80.1 | 41.7 | 25.1 | 45.0 | 42.0 | 10.0 | 5.1 | 4.8 | 13.8 | ||
| Severe obese | 86.2 | 39.5 | 30.1 | 63.3 | 49.8 | 23.5 | 3.0 | 10.5 | 20.4 | ||
| CR cutoffs of BMI percentile | Low risk | 60.8 | 33.2 | 18.5 | 22.6 | 26.2 | 5.3 | 2.8 | 2.8 | 9.3 | |
| High risk | 80.6 | 40.3 | 25.2 | 49.8 | 43.8 | 14.3 | 4.3 | 6.5 | 15.1 | ||
| CR cutoffs of arm-to-height ratio | Low risk | 60.7 | 32.8 | 18.3 | 22.2 | 26.8 | 5.2 | 2.9 | 2.7 | 9.7 | |
| High risk | 78.7 | 40.6 | 25.2 | 47.5 | 39.7 | 13.3 | 3.8 | 6.0 | 13.0 | ||
|
| |||||||||||
| Males | Conventional cutoffs of BMI percentile | Healthy | 67.9 | 22.9 | 14.0 | 31.4 | 24.8 | 14.0 | 3.0 | 4.4 | 19.7 |
| Overweight | 82.8 | 33.5 | 24.5 | 50.7 | 39.6 | 24.8 | 3.3 | 3.5 | 25.3 | ||
| Obese | 89.1 | 39.3 | 28.3 | 65.9 | 56.0 | 33.9 | 5.5 | 4.3 | 29.4 | ||
| Severe obese | 91.9 | 43.7 | 39.6 | 78.2 | 68.1 | 38.8 | 5.5 | 14.9 | 38.4 | ||
| CR cutoffs of BMI percentile | Low risk | 63.5 | 22.6 | 14.1 | 26.3 | 21.9 | 12.3 | 3.5 | 4.3 | 16.6 | |
| High risk | 80.9 | 31.1 | 22.0 | 50.7 | 40.8 | 24.1 | 3.6 | 5.4 | 26.7 | ||
| CR cutoffs of arm-to-height ratio | Low risk | 63.2 | 21.1 | 11.8 | 28.0 | 22.1 | 11.5 | 3.0 | 4.2 | 19.3 | |
| High risk | 81.8 | 32.5 | 23.9 | 51.1 | 41.6 | 25.4 | 3.9 | 5.5 | 25.4 | ||
CR = cardiometabolic risk; TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; HbA1c = glycated hemoglobin; FPG = fasting plasma glucose. Overall CR indicates unhealthy level on any of the eight CR variables. Unhealthy level of each CR variable includes both borderline-risk and high-risk levels, defined according to National Heart, Lung, and Blood Institute Expert Panel Report 2012, American Academy of Pediatrics, and American Diabetes Association. Conventionally, BMI < 85th percentile was considered healthy; ≥85th to <95th percentile, overweight; ≥95th percentile to <120% of 95th percentile, obese; ≥120% of 95th percentile or BMI > 35 regardless of age was severe obese. For CR, BMI ≥ 94th percentile in females and BMI ≥ 64th percentile in males, at a given age, as well as arm-to-height ≥ 0.19 in females and arm-to-height ≥ 0.16 in males, regardless of age, were considered high-risk. ∗Sample weights were utilized across the eight survey cycles as per survey guidelines. Combined weights were calculated for participants whose data were collected in mobile examination centers; separate weights were applied for participants in the fasting subsample who provided measures of FPG, LDL-C, and TG.
Table 3 presents sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and J-statistic for overall cardiometabolic risk classification (healthy/unhealthy), using each binary anthropometric indicator (high/low risk), in all adolescents and separately by sex. For cardiometabolic cutoffs of AHtR and BMI percentile, male sensitivity and accuracy were higher, while female specificity was higher. For all adolescents and separately for males, sensitivity, negative predictive value, accuracy, and J-statistic were highest with use of the age-invariant AHtR cutoff. For females, sensitivity, negative predictive value, accuracy, and J-statistic were highest with the use of BMI 85th percentile, while specificity and positive predictive value were highest with BMI 94th percentile (cardiometabolic cutoff). For all individuals and separately for males, specificity and positive predictive values were highest with the use of BMI 85th percentile.
Table 3.
Characteristics of overall and specific cardiometabolic risk classifications, using measures of general obesity, ages 12–18, N = 12,268.
| CR | Predictor | Sensitivity | Specificity | PPV | NPV | True + | False + | True − | False − | Total | Accuracy (%) | J |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall all adolescents | BMI ≥ 85th percentile | 42.78 | 77.73 | 82.16 | 36.17 | 1511 | 328 | 1145 | 2021 | 5005 | 53.07 | 20.51 |
| CR cutoffs, BMI percentile | 51.08 | 70.67 | 80.68 | 37.59 | 1774 | 432 | 1041 | 1758 | 5005 | 56.24 | 21.75 | |
| CR cutoffs, arm-to-height | 52.01 | 70.67 | 80.96 | 38.05 | 1837 | 432 | 1041 | 1695 | 5005 | 57.50 | 22.68 | |
|
| ||||||||||||
| Overall female | BMI ≥ 85th percentile | 43.88 | 74.85 | 76.97 | 41.05 | 685 | 205 | 610 | 876 | 2376 | 54.50 | 18.73 |
| CR cutoffs, BMI percentile | 27.80 | 88.22 | 81.89 | 38.95 | 434 | 96 | 719 | 1127 | 2376 | 48.53 | 16.02 | |
| CR cutoffs, arm-to-height | 30.17 | 84.05 | 78.37 | 38.59 | 471 | 130 | 685 | 1090 | 2376 | 48.65 | 14.22 | |
|
| ||||||||||||
| Overall male | BMI ≥ 85th percentile | 41.91 | 81.31 | 87.04 | 31.85 | 826 | 123 | 535 | 1145 | 2629 | 51.77 | 23.22 |
| CR cutoffs, BMI percentile | 67.99 | 48.94 | 79.95 | 33.79 | 1340 | 336 | 322 | 631 | 2629 | 63.22 | 16.93 | |
| CR cutoffs, arm-to-height | 69.30 | 54.1 | 81.89 | 37.04 | 1366 | 302 | 356 | 605 | 2629 | 65.50 | 23.40 | |
|
| ||||||||||||
| TC | BMI ≥ 85th percentile | 45.33 | 66.02 | 39.15 | 71.46 | 1646 | 2558 | 4969 | 1985 | 11158 | 59.28 | 11.35 |
| CR cutoffs, BMI percentile | 45.47 | 61.68 | 36.41 | 70.10 | 1651 | 2884 | 4643 | 1980 | 11158 | 56.41 | 7.15 | |
| CR cutoffs, arm-to-height | 41.86 | 66.60 | 37.68 | 70.37 | 1520 | 2514 | 5013 | 2111 | 11158 | 58.55 | 8.46 | |
|
| ||||||||||||
| LDL-C | BMI ≥ 85th percentile | 49.85 | 66.59 | 26.69 | 84.47 | 505 | 1387 | 2764 | 508 | 5164 | 63.30 | 16.44 |
| CR cutoffs, BMI percentile | 49.85 | 62.25 | 24.37 | 83.57 | 505 | 1567 | 2584 | 508 | 5164 | 59.82 | 12.10 | |
| CR cutoffs, arm-to-height | 46.69 | 66.59 | 25.43 | 83.66 | 473 | 1387 | 2764 | 540 | 5164 | 62.68 | 13.28 | |
|
| ||||||||||||
| HDL-C | BMI ≥ 85th percentile | 55.62 | 71.54 | 50.12 | 75.82 | 2107 | 2097 | 5272 | 1681 | 11157 | 66.14 | 27.17 |
| CR cutoffs, BMI percentile | 59.64 | 69.13 | 49.82 | 76.91 | 2259 | 2275 | 5094 | 1529 | 11157 | 65.90 | 28.76 | |
| CR cutoffs, arm-to-height | 54.80 | 73.43 | 51.46 | 75.97 | 2076 | 1958 | 5411 | 1712 | 11157 | 67.11 | 28.23 | |
|
| ||||||||||||
| TG | BMI ≥ 85th percentile | 53.16 | 70.74 | 45.12 | 76.95 | 882 | 1073 | 2594 | 777 | 5326 | 65.26 | 23.90 |
| CR cutoffs, BMI percentile | 54.97 | 66.65 | 42.72 | 76.59 | 912 | 1223 | 2444 | 747 | 5326 | 63.01 | 21.62 | |
| CR cutoffs, arm-to-height | 50.63 | 70.55 | 43.75 | 75.95 | 840 | 1080 | 2587 | 819 | 5326 | 64.34 | 21.18 | |
|
| ||||||||||||
| SBP | BMI ≥ 85th percentile | 58.18 | 66.48 | 23.48 | 89.99 | 1046 | 3409 | 6760 | 752 | 11967 | 65.23 | 24.65 |
| CR cutoffs, BMI percentile | 67.19 | 64.54 | 25.09 | 91.75 | 1208 | 3606 | 6563 | 590 | 11967 | 64.94 | 31.73 | |
| CR cutoffs, arm-to-height | 62.24 | 69.01 | 26.21 | 91.18 | 1119 | 3151 | 7018 | 679 | 11967 | 68.00 | 31.25 | |
|
| ||||||||||||
| DBP | BMI ≥ 85th percentile | 39.49 | 62.95 | 3.53 | 96.81 | 156 | 4266 | 7247 | 239 | 11908 | 62.17 | 2.44 |
| CR cutoffs, BMI percentile | 43.80 | 60.04 | 3.62 | 96.89 | 173 | 4601 | 6912 | 222 | 11908 | 59.50 | 3.83 | |
| CR cutoffs, arm-to-height | 41.77 | 64.65 | 3.90 | 97.00 | 165 | 4070 | 7443 | 230 | 11908 | 63.89 | 6.42 | |
|
| ||||||||||||
| HbA1C | BMI ≥ 85th percentile | 51.32 | 63.22 | 7.30 | 95.83 | 310 | 3934 | 6762 | 294 | 11300 | 62.58 | 14.54 |
| CR cutoffs, BMI percentile | 57.45 | 60.42 | 7.58 | 96.18 | 347 | 4233 | 6463 | 257 | 11300 | 60.27 | 17.87 | |
| CR cutoffs, arm-to-height | 51.82 | 64.86 | 7.69 | 95.97 | 313 | 3759 | 6937 | 291 | 11300 | 64.16 | 16.68 | |
|
| ||||||||||||
| FPG | BMI ≥ 85th percentile | 45.45 | 65.18 | 20.54 | 85.79 | 405 | 1567 | 2933 | 486 | 5391 | 61.92 | 10.63 |
| CR cutoffs, BMI percentile | 56.00 | 63.31 | 23.21 | 87.90 | 499 | 1651 | 2849 | 392 | 5391 | 62.10 | 19.32 | |
| CR cutoffs, arm-to-height | 49.16 | 66.76 | 22.65 | 86.90 | 438 | 1496 | 3004 | 453 | 5391 | 63.85 | 15.91 | |
CR = cardiometabolic risk; TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; HbA1c = glycated hemoglobin; FPG = fasting plasma glucose. Unhealthy level of each CR variable includes both borderline-risk and high-risk levels, defined according to National Heart, Lung, and Blood Institute Expert Panel Report 2012, American Academy of Pediatrics, and American Diabetes Association. PPV = positive predictive value; NPV = negative predictive value; true + = number of true positives; false + = number of false positives; true − = number of true negatives; false − = number of false negatives; accuracy = percent of (true positives + true negatives); J = sensitivity + specificity − 100 (i.e., Youden's Index). Conventionally, BMI ≥ 85th percentile (overweight) was considered high risk. For CR, BMI ≥ 94th percentile in females and BMI ≥ 64th percentile in males, at a given age, as well as arm-to-height ≥ 0.19 in females and arm-to-height ≥ 0.16 in males, regardless of age, were considered high risk.
Table 3 also demonstrates the sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and J-statistic for classification of each binary cardiometabolic variable in all adolescents, using each binary anthropometric indicator. While AHtR had the highest accuracy for high-density lipoprotein cholesterol, fasting plasma glucose, glycated hemoglobin, systolic blood pressure, and diastolic blood pressure as well as highest specificity for all cardiometabolic variables except for triglycerides, BMI 85th percentile had the highest accuracy for total cholesterol, low-density lipoprotein cholesterol, and triglycerides. The cardiometabolic cutoff of BMI percentile had the highest sensitivity for all cardiometabolic variables. Appendices 1 and 2 provide corresponding results for females and males, respectively, by race/ethnicity.
4. Discussion
The current study comparing AHtR to BMI percentile is a “proof of concept” for AHtR's ability to predict adolescent cardiometabolic risk. In a prior Chinese study, AHtR ≥ 0.15 in both sexes was associated with elevated BMI, with sensitivities of 86.0% and 85.4% plus specificities of 91.5% and 87.8% for males and females, respectively [25]. In the current study, AHtR ≥ 0.19 in males and AHtR ≥ 0.16 in females predicted a ≥0.71 probability of being unhealthy on overall cardiometabolic risk, with higher sensitivity and classification accuracy for males. BMI ≥ 94th percentile in females and BMI ≥ 64th percentile in males predicted comparable risk. The observed sex difference is in agreement with the prior nationally representative studies. May et al. found that adolescent males were more likely than females to have one (36% vs 28%), two (10% vs 7%), or more than two (4% vs 0.8%) cardiometabolic risk factors, whereas a consistent dose-response increase in the prevalence of cardiometabolic risk factors was observed by weight categories [36]. A sex difference was also observed consistently across racial/ethnic groups. For example, Hispanic/Latino males had a greater prevalence of diabetes (21% vs 12%) and hypertension (9% vs 3%) and were more likely to have prediabetes (OR = 2) than their female counterparts [37]. Although the current study did not find considerable difference by race/ethnicity, cross-national validation of these results is warranted.
One meaningful impetus for this study was the research literature suggesting that BMI is not a perfect measure of general obesity [38]—specifically, the findings that healthcare providers indicated that the complexity of BMI was a barrier to its regular use [11–13]. BMI involves commonly understood body weight and height, but is somewhat complicated to calculate. For children and adolescents, assessors interpret age-sex specific BMI percentile charts, requiring additional numeric skills. Importantly, BMI has moved to the forefront of recommended metrics due to its accuracy, simplicity, and low cost [7]. Thus, the finding that AHtR performed similarly to BMI in calculating cardiometabolic risk factors in adolescents in this sample may suggest its potential use as a complementary measure of general obesity in situations where one or more barriers to using BMI are present. In contrast, many other measures of total adiposity, such as dual-energy x-ray absorptiometry, are more difficult than BMI to obtain in ordinary survey and clinical settings, and most importantly, in homes, precluding self-monitoring. Further, measuring central obesity using waist circumference per guidelines by minimizing the effects of clothing, respiration, and stomach content and palpating iliac crest and costal margin accurately may prove difficult [39]. It may also raise ethical and cultural concerns due to intrusiveness, especially in the context of surveillance programs.
Compared to BMI percentile, use of AHtR for cardiometabolic risk prediction may be advantageous in some situations because it is (1) not age-dependent—does not require percentile charts; (2) not weight-dependent—does not require weighing scales, does not require daily scale calibration [40], and is not sensitive to short-term weight fluctuations; (3) a ratio of two lengths, hence, interpretable without unit conversion; and (4) easier to calculate—requires less time and effort. As a measure, AHtR also has limitations. Its prediction accuracy is lower than BMI for females. Additionally, as with other circumference measures [41], mid-upper-arm circumference has no uniformly accepted measurement approach and is susceptible to measurement errors. While prior studies revealed significant correlations between mid-upper-arm circumference and height [25, 42], this study considered adolescents' height as an empirically established alternative to age for evaluating obesity [16].
AHtR also has advantages over mid-upper-arm circumference. For US children and adolescents aged 1–20 years, mid-upper-arm circumference-for-age reference charts, including arm muscle area (AMA) and arm fat area (AFA), are available [22]. However, height status had complex independent relationships with arm measures, irrespective of the ranking by age and sex [22]. Mei et al. indicated that it is difficult to assess a child's age in some circumstances [26]. Even if the age is known, use of age-based mid-upper-arm circumference percentile charts may be complicated, due to lack of resources, knowledge, and skills. AHtR may be a good alternative in such circumstances. Recent studies demonstrated a steady increase of adolescent mid-upper-arm circumference with age, although increases in mid-upper-arm circumference components had age- and sex-related differences [22]. Upper-arm fat mass gains were slower in males from 12 to 15 years of age, compared to more-intense increases among females throughout adolescence. However, upper-arm muscle mass had sharper gains with age in males than in females [22]. Accordingly, the identification of sex-specific AHtR cutoffs in this study was consistent with prior research.
As children with overweight or obesity are more likely to have unhealthy levels of cardiometabolic risk [43, 44], inclusion of varying degrees of obesity may increase the accuracy of cardiometabolic risk prediction [43]. Numerous studies have evaluated the association between obesity and other biological risk factors [2], with some suggesting that BMI is imperfect for predicting cardiometabolic risk, considering metabolically obese healthy-weight subjects [45] and metabolically healthy subjects with obesity [46]. Conversely, some researchers have argued that BMI performed better than body fat mass in predicting cardiometabolic risk [47]. Furthermore, central obesity had a stronger association with cardiometabolic risk in children than general obesity [16]. While predictive accuracy is a major concern in determining the suitability of anthropometric indicators for cardiometabolic risk prediction in survey and clinical settings, low-cost and simplicity also can be crucial. This study revealed that, compared to BMI percentile, AHtR classified overall cardiometabolic risk and several cardiometabolic variables better in males. These results were consistent with prior studies which revealed that mid-upper-arm circumference correlates more with systolic blood pressure than with diastolic blood pressure [48], although empirical evidence is unavailable for other risk factors.
This study had several overarching limitations. Children under 12 years were excluded because some cardiometabolic variables were unavailable for them, limiting the conclusions that can be drawn about the appropriateness of AHtR for that population. This study did not compare measures of central obesity or body fat mass with AHtR and BMI percentile, because the focus was general obesity; however, numerous studies have compared those measures with BMI percentile [43, 44]. Also, this study did not analyze correlations of AHtR with other anthropometric parameters and age, as it was designed to identify a meaningful sex-specific AHtR cutoff that predicts a higher cardiometabolic risk, a concept which is similar to widely used cutoff value 0.5 for waist-to-height ratio. The optimal AHtR and BMI percentile cutoffs for the cardiometabolic risk assessment were not cross-validated in an independent sample. Due to the cross-sectional design, this study was unable to examine changes in anthropometric variables and cardiometabolic risks over the 16-year-period, establish causality between obesity and cardiometabolic risk, or determine associated morbidity and mortality. In addition, NHANES deliberately oversampled Black and Hispanic adolescents. Only Whites, Blacks, and Hispanics were included in the analysis because the number of other racial/ethnic group participants was insufficient. Underweight adolescents (BMI < 5th percentile; n=726) were merged with healthy range for BMI, considering that the focus of this study was obesity. Further, for multifactorial conditions, such as obesity and cardiometabolic abnormalities, acceptance of any given parameter solely as “unhealthy” may not describe the whole picture, as there can be several interparameter relations. Similarly, the coexistence of multiple cardiometabolic risk factors should be treated as worse than having just one, and ideally, different levels of risk (e.g., one risk factor and two risk factors) should be assessed. Finally, some individuals had missing values for one or more cardiometabolic or anthropometric variables.
Behavioral interventions that effectively reduce the weight of children with overweight demonstrate gradually smaller effects at greater obesity levels [5], with interventions displaying an inverse correlation between effectiveness and age [6]. Furthermore, children with obesity have greater risk of developing cardiometabolic abnormalities early in life [4]. This study indicated that, as a simple and low-cost measure of general obesity, AHtR can reasonably predict cardiometabolic risk in adolescents and has a higher predictive accuracy than BMI percentile in males, thus allowing for early interventions. This may be especially pertinent in contexts where one or more barriers to BMI computation exist. Further research is required to validate these findings in younger children. Attention should be paid to include AHtR (or mid-upper-arm circumference in addition to height) in adolescent anthropometric surveys, school-based surveillance programs, and clinical evaluations, whenever cardiometabolic risk prediction is considered worthwhile, but invasive procedures are not feasible or permissible.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Supplementary Materials
Appendix 1 provides the sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and J-statistic for classification of each binary cardiometabolic variable for females by race/ethnicity, using each binary anthropometric indicator. Appendix 2 provides corresponding data for males.
References
- 1.Reilly J. J., Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International Journal of Obesity. 2010;35:p. 891. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services NH, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Bethesda, MD, USA: National Heart, Lung, and Blood Institute; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avellone G., Digarbo V., Cordova R., et al. Evaluation of cardiovascular risk-factors in overweight and obese subjects. International Angiology. 1994;13(1):25–29. [PubMed] [Google Scholar]
- 4.Shashaj B., Bedogni G., Graziani M. P., et al. Origin of cardiovascular risk in overweight preschool children a cohort study of cardiometabolic risk factors at the onset of obesity. Jama Pediatrics. 2014;168(10):917–924. doi: 10.1001/jamapediatrics.2014.900. [DOI] [PubMed] [Google Scholar]
- 5.Johnston C. A., Tyler C., Palcic J. L., Stansberry S. A., Gallagher M. R., Foreyt J. P. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. Journal of Pediatrics. 2011;158(4):624–627. doi: 10.1016/j.jpeds.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 6.Danielsson P., Kowalski J., Ekblom O., Marcus C. Response of severely obese children and adolescents to behavioral treatment. Archives of Pediatrics & Adolescent Medicine. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 7.Countryman A. J., Saab P. G., Llabre M. M., Penedo F. J., McCalla J. R., Schneiderman N. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Annals of Behavioral Medicine. 2013;45(1):121–131. doi: 10.1007/s12160-012-9428-8. [DOI] [PubMed] [Google Scholar]
- 8.Jayawardene W., Lohrmann D., YoussefAgha A. Discrepant body mass index: behaviors associated with height and weight misreporting among US Adolescents from the National Youth Physical Activity and Nutrition Study. Childhood Obesity. 2014;10(3):225–233. doi: 10.1089/chi.2014.0002. [DOI] [PubMed] [Google Scholar]
- 9.Stevens J., McClain J. E., Truesdale K. P. Selection of measures in epidemiologic studies of the consequences of obesity. International Journal of Obesity. 2008;32:S60–S66. doi: 10.1038/ijo.2008.88. [DOI] [PubMed] [Google Scholar]
- 10.Abbate L. M., Stevens J., Schwartz T. A., Renner J. B., Helmick C. G., Jordan J. M. Anthropometric measures, body composition, body fat distribution, and knee osteoarthritis in women. Obesity. 2006;14(7):1274–1281. doi: 10.1038/oby.2006.145. [DOI] [PubMed] [Google Scholar]
- 11.Stalter A. M., Chaudry R. V., Polivka B. J. Facilitating factors and barriers to BMI screening in schools. Journal of School Nursing. 2010;26(4):320–330. doi: 10.1177/1059840510368524. [DOI] [PubMed] [Google Scholar]
- 12.Flower K. B., Perrin E. M., Viadro C. I., Ammerman A. S. Using body mass index to identify overweight children: barriers and facilitators in primary care. Ambulatory Pediatrics. 2007;7(1):38–44. doi: 10.1016/j.ambp.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein J. D., Sesselberg T. S., Johnson M. S., et al. Adoption of body mass index guidelines for screening and counselling in pediatric practice. Pediatrics. 2010;125(2):265–272. doi: 10.1542/peds.2008-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oettinger M. D., Finkle J. P., Esserman D., et al. Color-coding improves parental understanding of body mass index charting. Academic Pediatrics. 2009;9(5):330–338. doi: 10.1016/j.acap.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Onis M., Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? International Journal of Pediatric Obesity. 2010;5(6):458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A. K., Metzger D. L., Daymont C., Hadjiyannakiss S., Rodd C. J. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: association with cardio-metabolic risks. Pediatric Research. 2015;78(6):723–729. doi: 10.1038/pr.2015.160. [DOI] [PubMed] [Google Scholar]
- 17.Brown R. C. Nutrition surveillance by QUAC stick. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81(6):1038–1039. doi: 10.1016/0035-9203(87)90391-9. [DOI] [PubMed] [Google Scholar]
- 18.Frisancho A. R. New norms of upper limb fat and muscle areas for assessment of nutritional-status. American Journal of Clinical Nutrition. 1981;34(11):2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 19.McDowell I. Interpretation of arm circumference as an indicator of nutritional status. Archives of Disease in Childhood. 1982;57(4):292–296. doi: 10.1136/adc.57.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. WHO Guideline, Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 21.Bern C., Nathanail L. Is mid-upper-arm circumference a useful tool for screening in emergency settings. The Lancet. 1995;345(8950):631–633. doi: 10.1016/s0140-6736(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 22.Addo O. Y., Himes J. H., Zemel B. S. Reference ranges for midupper arm circumference, upper arm muscle area, and upper arm fat area in US children and adolescents aged 1–20 y. American Journal of Clinical Nutrition. 2017;105(1):111–120. doi: 10.3945/ajcn.116.142190. [DOI] [PubMed] [Google Scholar]
- 23.Craig E., Bland R., Ndirangu J., Reilly J. J. Use of mid-upper arm circumference for determining overweight and overfatness in children and adolescents. Archives of Disease in Childhood. 2014;99(8):763–766. doi: 10.1136/archdischild-2013-305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazıcıoğlu M. M., Hatipoğlu N., Öztürk A., Çiçek B., Üstünbaş H. B., Kurtoğlu S. Waist circumference and mid−upper arm circumference in evaluation of obesity in children aged between 6 and 17 years. Journal of Clinical Research in Pediatric Endocrinology. 2010;2(4):144–150. doi: 10.4274/jcrpe.v2i4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Q., Wang R., Lou D.-H., Ma C.-M., Liu X.-L., Yin F.-Z. Mid-upper-arm circumference and arm-to-height ratio in evaluation of overweight and obesity in han children. Pediatrics & Neonatology. 2014;55(1):14–19. doi: 10.1016/j.pedneo.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Mei Z., Grummer-Strawn L. M., De Onis M., Yip R. The development of a MUAC-for-height reference, including a comparison to other nutritional status screening indicators. Bulletin of the World Health Organization. 1997;75(4):333–341. [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden C. L., Kuczmarski R. J., Flegal K. M., et al. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics Version. Pediatrics. 2002;109(1):p. 45. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey. Hyattsville, MD, USA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 29.Skinner A., Skelton J. A. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatrics. 2014;168(6):561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 30.Kelly A. S., Barlow S. E., Rao G., et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/cir.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 31.Copeland K. C., Silverstein J., Moore K. R., et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 32.Falkner B., Daniels S. R., Flynn J. T., et al. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. [PubMed] [Google Scholar]
- 33.Zweig M. H., Campbell G. Receiver-operating characteristic (roc) plots—a fundamental evaluation tool in clinical medicine. Clinical Chemistry. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 34.Cox D. R., Snell E. J. The Analysis of Binary Data. 2nd. London, UK: Chapman & Hall; 1989. [Google Scholar]
- 35.Ruopp M. D., Perkins N. J., Whitcomb B. W., Schisterman E. F. Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biometrical journal Biometrische Zeitschrift. 2008;50(3):419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May A. L., Kuklina E. V., Yoon P. W. Prevalence of cardiovascular disease risk factors among US adolescents, 1999-2008. Pediatrics. 2012;129(6):1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 37.Isasi C. R., Parrinello C. M., Ayala G. X., et al. Sex differences in cardiometabolic risk factors among hispanic/latino youth. Journal of Pediatrics. 2016;176:p. 121. doi: 10.1016/j.jpeds.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman K. J. BMI-related errors in the measurement of obesity. International Journal of Obesity. 2008;32(S3):S56–S59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 39.Barrios P., Martin-Biggers J., Quick V., Byrd-Bredbenner C. Reliability and criterion validity of self-measured waist, hip, and neck circumferences. BMC Medical Research Methodology. 2016;16:p. 49. doi: 10.1186/s12874-016-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohman T., Roche A., Martorell R. Anthropometric Standardization Reference Manual, Abridgededition. Champaign, IL, USA: Human Kinetics Books; 1992. [Google Scholar]
- 41.Klein S., Allison D. B., Heymsfield S. B., et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. American Journal of Clinical Nutrition. 2007;85(5):1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 42.Yabanci N., Kilic S., Simsek I. The relationship between height and arm span, mid-upper arm and waist circumferences in children. Annals of Human Biology. 2010;37(1):70–75. doi: 10.3109/03014460903198517. [DOI] [PubMed] [Google Scholar]
- 43.Skinner A. C., Perrin E. M., Moss L. A., Skelton J. A. Cardiometabolic risks and severity of obesity in children and young adults. New England Journal of Medicine. 2015;373(14):1307–1317. doi: 10.1056/nejmoa1502821. [DOI] [PubMed] [Google Scholar]
- 44.Jayawardene W. P., Lohrmann D. K., Dickinson S., Talagala S., Torabi M. R. Clinical measures of obesity and cumulative cardiometabolic risk in adolescents. Clinical Obesity. 2016;7(1):11–21. doi: 10.1111/cob.12171. [DOI] [PubMed] [Google Scholar]
- 45.Conus F., Rabasa-Lhoret R., Peronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Applied Physiology Nutrition and Metabolism-Physiologie Appliquee Nutrition Et Metabolisme. 2007;32(1):4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 46.Primeau V., Coderre L., Karelis A. D., et al. Characterizing the profile of obese patients who are metabolically healthy. International Journal of Obesity. 2011;35(7):971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 47.Bosy-Westphal A., Geisler C., Onur S., et al. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. International Journal of Obesity. 2006;30(3):475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 48.Ledwaba K. R., Nkalanga F., Monyeki K. D., Van Staden M. The relationship between mid-upper arm circumference and blood pressure of private school children aged 6-13 years, in Polokwane, South Africa. Annals of Pediatrics & Child Health. 2014;2(4):p. 1026. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1 provides the sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and J-statistic for classification of each binary cardiometabolic variable for females by race/ethnicity, using each binary anthropometric indicator. Appendix 2 provides corresponding data for males.