In HIV-1-producing cells, gp41 exists in a complexed form with caveolin-1, an interaction most probably mediated by the caveolin-1 binding motif. This sequence is highly conserved in every single HIV-1 isolate, thus suggesting that there is constant selective pressure to preserve this sequence for a specific function in the HIV infectious cycle. Consequently, the CBM sequence may represent the “Achilles' heel” of HIV-1 in the development of an efficient vaccine. Our results demonstrate that macaques immunized with the CBM-based peptides displayed a delay in the onset of viral infection and CD4 depletion, as well as a significant induction of antigen-specific memory T cell response, which is essential for the control of HIV/SIV infections. Finally, as HIV-infected individuals lack anti-CBM immune responses, CBM-based vaccines could have applications as a therapeutic vaccine in AIDS patients.
KEYWORDS: AIDS, vaccine, HIV, SIV, gp41, Th1, CD4, B cell, IgG, memory T cells, TH1, human immunodeficiency virus, immunization, macaque, simian immunodeficiency virus
ABSTRACT
We have previously reported that the CBD1 peptide (SLEQIWNNMTWMQWDK), corresponding to the consensus caveolin-1 binding domain in human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein gp41, elicits peptide-specific antibodies. Here, we have investigated the cellular immune response and the protective efficacy against a simian/human immunodeficiency virus (SHIV162P3) challenge. In addition to the CBD1 peptide, peptides overlapping the caveolin-binding-motif (CBM) (622IWNNMTWMQW631 or 622IWNNMTW628) were fused to a Gag-p24 T helper epitope for vaccination. All immunized cynomolgus macaques responded to a cocktail peptide immunization by inducing specific T cells and the production of high-titer CBD1/CBM peptide-specific antibodies. Six months after the fourth vaccine boost, six control and five vaccinated animals were challenged weekly by repeated exposure to SHIV162P3 via the mucosal rectal route. All control animals were infected after 1 to 3 challenges with SHIV, while among the five vaccinated monkeys, three became infected after a delay compared to control; one was infected after the eighth viral challenge, and one remained uninfected even after the ninth SHIV challenge. Immunized animals maintained a CD4 T cell count, and their central memory CD4 T cells were less depleted than in the control group. Furthermore, SHIV challenge stimulates antigen-specific memory T cell response in vaccinated macaques. Our results indicate that peptides derived from the CBM region can be immunogenic and provide protection against SHIV infection in cynomolgus monkeys.
IMPORTANCE In HIV-1-producing cells, gp41 exists in a complexed form with caveolin-1, an interaction most probably mediated by the caveolin-1 binding motif. This sequence is highly conserved in every single HIV-1 isolate, thus suggesting that there is constant selective pressure to preserve this sequence for a specific function in the HIV infectious cycle. Consequently, the CBM sequence may represent the “Achilles' heel” of HIV-1 in the development of an efficient vaccine. Our results demonstrate that macaques immunized with the CBM-based peptides displayed a delay in the onset of viral infection and CD4 depletion, as well as a significant induction of antigen-specific memory T cell response, which is essential for the control of HIV/SIV infections. Finally, as HIV-infected individuals lack anti-CBM immune responses, CBM-based vaccines could have applications as a therapeutic vaccine in AIDS patients.
INTRODUCTION
The primary acute phase of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections is characterized by an early burst of viral replication, an exponential increase in the viral load (VL), the dissemination and seeding of the virus in all the peripheral lymphoid organs, a severe depletion of memory CD4+ T cells, and the induction of the host immune response against the virus (1). The early induction of an effective immune response against the virus plays a role in determining the levels of viral load at the end of the primary phase (i.e., the set point) (2, 3) and in discriminating pathogenic and nonpathogenic models of lentiviral infections in which the depletion of CD4 T cells is a crucial event (4–6). Furthermore, eliciting memory CD4 T cells is essential in controlling HIV/SIV infections (7–10).
Caveolin-1 is a ubiquitous cholesterol-binding protein that organizes and concentrates specific ligands within the caveola membranes (11–13). Thus, caveolin-1, in association with glycolipid-enriched membrane microdomains, is fundamental in the lateral organization of the lipid bilayer. Lipid rafts and caveolae have been reported to play a critical role in HIV entry (14–16). Thus, the HIV-1 transmembrane envelope glycoprotein gp41 interacts with caveolin-1 and exists as a stable complex in HIV-1-infected cells (17, 18). The caveolin-1 binding motif (CBM, 623WNNMTWMQW631) is localized in the ectodomain sequence of gp41. The three aromatic amino acids that are always tryptophan in the case of various HIV-1 isolates are essential for binding to caveolin-1. Furthermore, the CBD1 peptide (618SLEQIWNNMTWMQWDK633) has the capacity to bind to the caveolin-1 in solution and to penetrate plasma membranes (17). Therefore, the CBM motif in gp41 represents an interesting target for HIV vaccine strategy.
We have previously reported that the CBD1 peptide is immunogenic in rabbits, mice, and guinea pigs, as illustrated by the production of high-titer specific antibodies in immunized animals (17–20). On the other hand, the CBD1 peptide is poorly or not immunogenic in cynomolgus macaques when adjuvants that are acceptable for humans are used (21). We have also demonstrated that immunization of macaques with the CBD1 peptide requires a T helper peptide carrier to improve B cell immunogenicity. Thus, a “promiscuous” T cell epitope from the tetanus toxin (Tet830, AQYIKANSKFIGITEL) (22) was initially used to elicit the production of high-titer peptide-specific antibodies (21). These colinear T helper and B cell epitopes were synthesized using the dilysine linker (KK) that is the target sequence of the lysosomal protease cathepsin B, one of the important proteases for antigen processing in the context of major histocompatibility complex class II (MHC-II) antigen presentation (23). Interestingly, additional carrier antigens have been described to improve the immunogenicity of CBM peptides in mice, in particular, the HIV-Gag p24 sequence (Gag, 298KRWIILGLNKIVRMY312) that contains a human HLA-DR supermotif capable of binding to 13 human HLA-DR alleles (20, 24). The CBM peptides fused with the Gag peptide were more potent in inducing antibodies in mice than that fused with the Tet peptide carrier (20). Therefore, the use of such a peptide carrier could be of interest for vaccine strategies.
Whereas there is also a potential caveolin-1 binding domain in HIV-2 and SIV transmembrane proteins (19), the CBD2 peptide (SLTPDWNNMTWQEWER) did not bind to caveolin-1 in solution and did not penetrate into plasma membranes (17). The possible explanation for this is the difference in the two amino acid residues preceding the caveolin-binding motif. Thus, the isoleucine622 that is conserved in HIV-1 is replaced by either D, E, N, or R amino acids in HIV-2. The Q621 that is semiconserved in HIV-1 is replaced by a conserved proline residue in HIV-2 that could induce the formation of a β-turn and might have a dramatic effect on its function (17). Therefore, it is of importance to use virus encoding the gp41 of HIV-1 to assess protection in macaque studies.
The current study aimed at investigating the efficacy of the CBD1-based peptide vaccine formulation to elicit T cell immune responses in cynomolgus macaques and to control the replication of a competent simian/human immunodeficiency chimera virus (SHIV162P3). Macaques were vaccinated with the CBD1 peptide associated with Tet830 peptide along with CBM-based peptides (622IWNNMTWMQW631, 622IWNNMTW628) fused at their NH2 terminus with the HIV-Gag p24 human HLA-DR supermotif. Our results indicate that the CBD1/CBM-based peptide-cocktail vaccine formulation induces a T cell immune response and provides partial protection against repetitive low doses of SHIV162P3 via the mucosal rectal route in cynomolgus monkeys.
RESULTS
Humoral immune response in CBD1/CBM-vaccinated cynomolgus macaques.
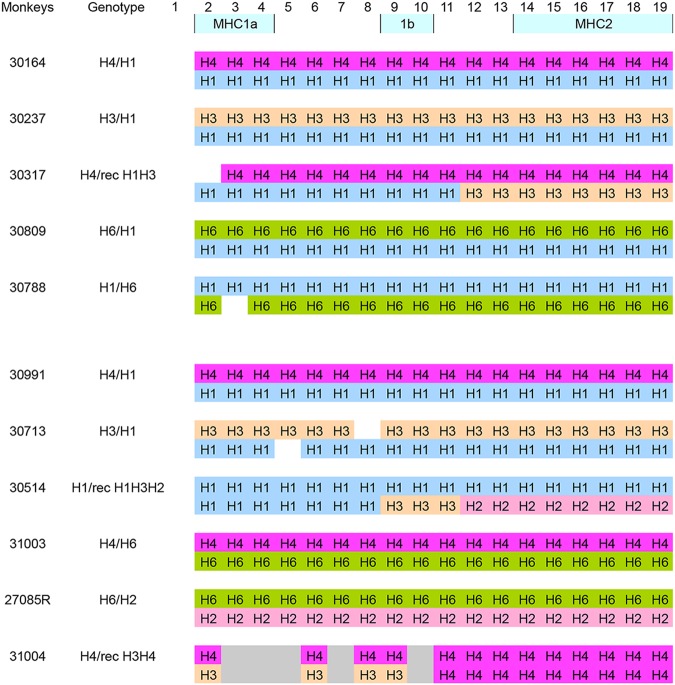
This study included 11 cynomolgus macaques (Macaca fascicularis) with MHC polymorphism, as presented in Table 1. In cynomolgus macaques, it has been proposed that H3 and H6 class IB haplotypes are associated with resistance to chimeric SHIV89.6P challenge (25, 26), while H2 and H5 class IB are associated with susceptibility to SHIV infection (27), or H4 in the context of SIVmac infection (28). In our cohort, only four animals were heterozygotes for H6, the second alleles being H2 (27085R), H4 (31003), or H1 (30809 and 30788). Two animals were H3 and had as a second allele H1 (30237 and 30713). These monkeys were distributed in both groups. Thus, six animals were kept as a control group while five monkeys were included in the immunization group. In order to trigger the induction of CBM-specific immune responses, we used conserved CBM sequences with the N-terminal conserved isoleucine residue, 622IWNNMTWMQW631 and 622IWNNMTW628, fused at their NH2 terminus with the HIV-Gag p24 peptide (Gag, 298KRWIILGLNKIVRMY312) (9). These peptides were referenced as K27W and K24W, respectively (Table 2). Immunization of macaques was then carried out using a mix of CBD1/CBM-based peptides (CBD1, K24W, and K27W) in the presence of two adjuvants, including the Montanide ISA 51, a mineral-oil-based emulsion (29), and CpG oligodeoxynucleotide, which is critical to enhance the induction of gamma interferon (IFN-γ) from CD4 T cells, compared to immunogens mixed only with Montanide ISA 51 (30). Five cynomolgus macaques were vaccinated at days 0, 42, 70, and 110, and antibody production was monitored by measuring specific IgG by enzyme-linked immunosorbent assay (ELISA) against the cocktail of peptides as well as against individual peptides used for the immunization cocktail, including CBD1, K24W, and K27W peptides. We also assessed antibodies against the HIV-1 Gag298–312 peptide that constituted the NH2 terminal of K24W and K27W peptides.
TABLE 1.
Description of the MHC haplotypes (H) in each cynomolgus macaquea
Class IA, IB, and II gene clusters were analyzed. The vaccinated group included monkeys 30164, 30237, 30317, 30809, and 30788. The control group included monkeys 30991, 30713, 30514, 31003, 27085R, and 31004.
TABLE 2.
Amino acid peptide sequences
| Peptide | Sequence |
|---|---|
| CBD1 | 618SLEQIWNNMTWMQWDK633 |
| K27W | Gag298–312-KK-622IWNNMTWMQW631 |
| K24W | Gag298–312-KK-622IWNNMTW628 |
| Gag298–312 | 298KRWIILGLNKIVRMY312 |
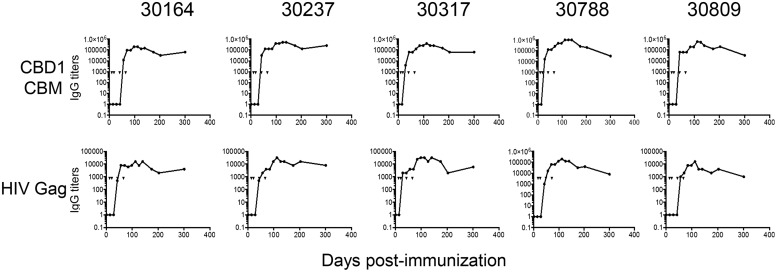
Figure 1 shows IgG antibody titers against the CBD1/CBM-based peptide cocktail vaccine formulation used for immunization from day 0 to day 300. Consistent with previous reports (18, 19, 21), we detected a CBD1/CBM-specific IgG response in vaccinated cynomolgus macaques (Fig. 1). No significant level of anti-CBD1/CBM humoral response was generated in the immunized animals until the third immunization (Fig. 1). Antibody titers plateaued thereafter; monkeys 30788 and 30809 displayed lower IgG titers at month 10 than the three other monkeys. Our results also indicated the presence of specific antibodies against the HIV Gag298–312 peptide. However, the titers were around 10- to 100-fold less than the cocktail's titers (Fig. 1). At 2 and 4 weeks after the fourth dose of vaccine (day 110), we assessed the IgG response against individual peptides (Table 3). Thus, the titers of K24W- and K27W-specific antibodies were 3- to 4-fold higher than the CBD1 titer and 10-fold higher than the Gag peptide used as a carrier. These results demonstrated that the CBD1/CBM-based peptide cocktail vaccine formulation is immunogenic in cynomolgus monkeys, generating specific B cells producing CBD1/CBM-specific antibodies.
FIG 1.
IgG responses in vaccinated cynomolgus macaques. ELISA was used to quantify specific IgG against the CBD1/CBM-based peptide-cocktail vaccine formulation. Sera were also tested against the HIV-Gag p24 peptide used as a carrier for CBM peptides. The titers correspond to the dilution of serum giving an OD value of ≥0.1. Arrowheads indicate the times of four immunizations at days 0, 42, 70, and 110.
TABLE 3.
IgG titers at weeks 2 and 4 after the last (4th) injection in macaques immunized with a CBD1/CBM cocktaila
| Macaque | Wk after last injection | IgG titer |
||||
|---|---|---|---|---|---|---|
| Cocktail | CBD1 | K27W | K24W | Gag298–312 | ||
| 30164 | 2 | 128,000 | 32,000 | 128,000 | 128,000 | 8,000 |
| 30164 | 4 | 150,000 | 32,000 | 128,000 | 128,000 | 16,000 |
| 30237 | 2 | 512,000 | 128,000 | 400,000 | 512,000 | 16,000 |
| 30237 | 4 | 512,000 | 64,000 | 300,000 | 512,000 | 16,000 |
| 30317 | 2 | 256,000 | 80,000 | 128,000 | 128,000 | 16,000 |
| 30317 | 4 | 256,000 | 64,000 | 128,000 | 150,000 | 32,000 |
| 30788 | 2 | 1,024,000 | 64,000 | 512,000 | 256,000 | 32,000 |
| 30788 | 4 | 1,024,000 | 64,000 | 512,000 | 256,000 | 40,000 |
| 30809 | 2 | 512,000 | 32,000 | 200,000 | 300,000 | 4,000 |
| 30809 | 4 | 256,000 | 30,000 | 128,000 | 256,000 | 4,000 |
Cynomolgus macaques were vaccinated at days 0, 42, 70, and 110. The titers of IgG at 2 and 4 weeks after the fourth immunization (day 110) were assessed by ELISA against the cocktail peptides used for immunization, as well as individual peptides. The titers correspond to the dilution of antipeptide antibodies giving an OD value of ≥0.1 in ELISA.
Cellular immune response in CBD1/CBM-vaccinated cynomolguses.
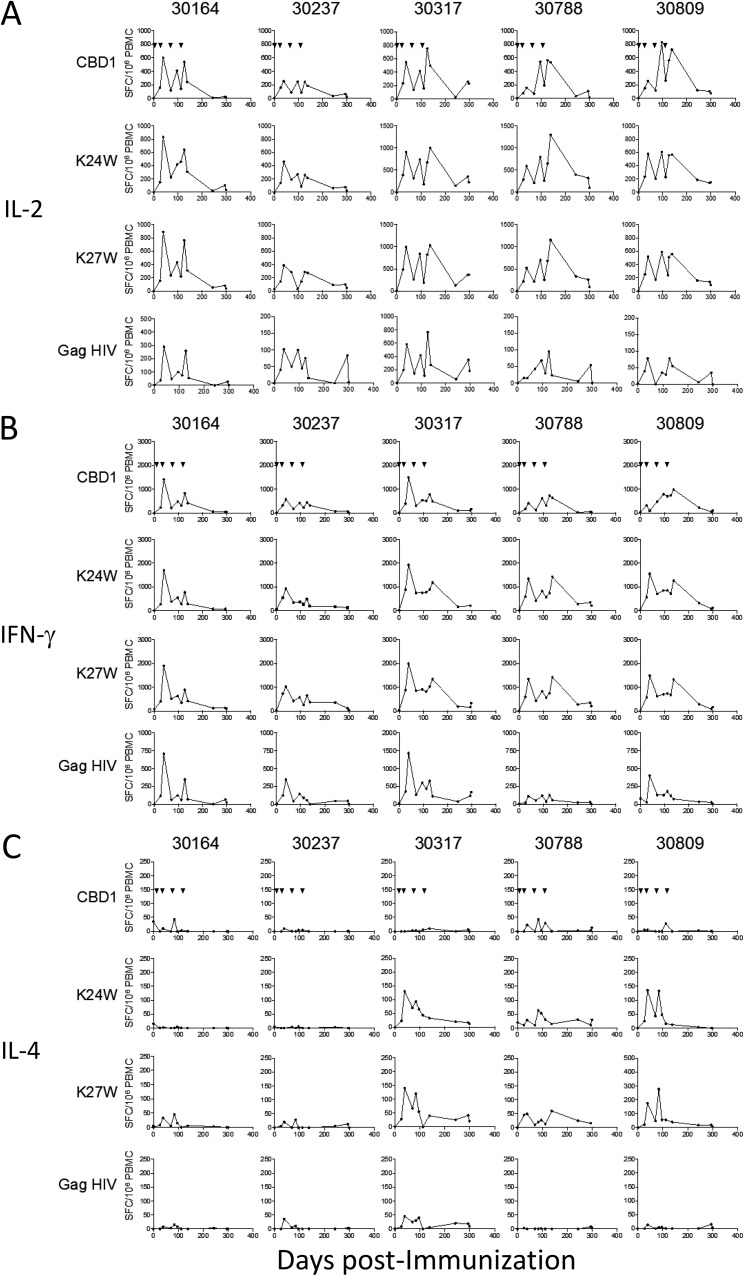
We next investigated the magnitude of specific T cells in vaccinated monkeys by measuring the numbers of IFN-γ-, interleukin-2 (IL-2)-, and IL-4-producing cells by enzyme-linked immunosorbent spot assays (ELISpot assays). Peripheral blood mononuclear cells (PBMCs) are stimulated in vitro with either CBD1, K24W, K27W, or HIV Gag298–312 peptides, the last being used as a carrier for K24W and K27W peptides. All vaccinated monkeys displayed specific IL-2- (Fig. 2A) and IFN-γ-producing T cells (Fig. 2B), which are characteristics of the T helper 1 (Th1) profile. These cells are specific to K24W, K27W, and CBD1 peptides. The dynamics of specific T cells were quite similar after in vitro stimulation with either the CBD1 peptide (which does not contain the gag sequence) or the K24W and K27W peptides, but the magnitudes were 2-fold higher in the latter. The dynamics of the immune response revealed higher levels of specific T cells 1 week after the second boost, which progressively declined thereafter. The third and fourth boosts did not show higher numbers of effector T cells and did not reach the values observed after the second boost. We also detected specific HIV Gag298–312 T cells, but the range of producing cells was lower than that observed with K24W and K27W peptides. Thus, by analyzing the different time points and the five monkeys, we found that in comparison to HIV Gag298–312, the numbers of IL-2-producing cells after K24W and K27W stimulation are 6.5- and 6.2-fold higher, respectively. These results indicated a common T cell epitope between CBD1 and K24W/K27W peptides, with the minimal sequence being 622IWNNMTW628. In contrast, we observed few IL-4-producing T cells (Fig. 2C), which concerned only 3 of the 5 vaccinated animals (30317, 30788, and 30809). These results show that the CBD1/CBM cocktail formulation is immunogenic in cynomolgus macaques, generating specific Th1 effector cells. The finding of the minimal T cell epitope sequence, 622IWNNMTW628, is of great interest due to its conservation in each of the HIV-1 isolates.
FIG 2.
T cell responses in individual vaccinated monkeys. PBMCs were stimulated with CBD1, K24W, K27W, and HIV-Gag p24 peptides. IL-2 (A), IFN-γ (B), and IL-4 (C) ELISpot assays were performed at different time points. Data are expressed as spot-forming cells (SFC) per million PBMCs minus the background (medium alone), which did not exceed 80 SFC per million cells. Arrowheads indicate immunizations.
Delay in the peak of viral load in CBD1/CBM-vaccinated cynomolgus macaques.
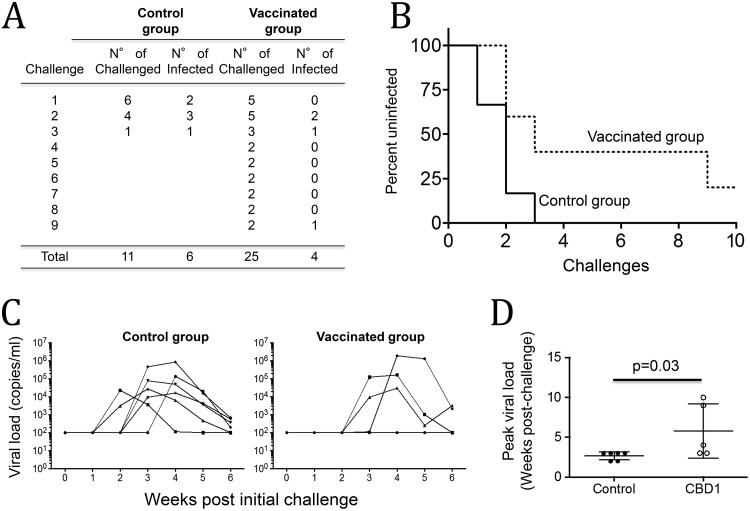
The efficacy of the CBD1/CBM-based peptide-cocktail vaccine formulation was evaluated by challenging vaccinated animals with SHIV that expresses the HIV-1 envelope glycoprotein. Six months after the fourth vaccine boost, animals were challenged with repetitive low doses of SHIV162P3 (0.33 50% animal infectious dose [AID50]; NIH) via the mucosal rectal route. All the six animals from the control group were infected after 1 to 3 challenges with SHIV162P3. Among the five vaccinated cynomolgus macaques, three became infected and one was protected until the eighth viral challenge (macaque 30788), one remaining protected even after the ninth challenge (macaque 30237). Thus, in the vaccinated group, the numbers of effective challenges to infect animals (n = 25) were higher than in the control group (n = 11). The difference between vaccinated and control groups was significant (P = 0.039, Fisher's exact test) (Fig. 3A), and the percentage of uninfected animals was also significant (P = 0.05, as measured by the Mantel-Cox test) (Fig. 3B). Although the levels of viral load were not different in infected monkeys from the vaccinated group compared to the control group (Fig. 3C), we observed a delay in viral load (Fig. 3D). Indeed, in the control group, we detected SHIV162P3 replication in the blood 2 weeks after the first challenge in two monkeys, at week 3 in three monkeys, and in only one monkey at week 4. In contrast, in the vaccinated group, only two animals displayed the virus at week 3, and one at week 4 after the first challenge. We arbitrarily considered the peak of viral load to be at week 10 for monkey 30237, which remained uninfected.
FIG 3.
Protective effect of CBD1/CBM vaccination. Six months after the fourth vaccine boost, animals were challenged with a repetitive low dose of SHIV162P3 (0.33 AID50; NIH) via the mucosal rectal route. (A) Acquisition rates in both control and CBD1/CBM-vaccinated monkeys. All animals from the control group were infected after 1 to 3 challenges with SHIV162P3. The difference between vaccinated and control groups was evaluated using Fisher's exact test (P = 0.03). (B) Survival curves of CBD1/CBM-vaccinated macaques versus controls. Statistical significance was evaluated using the Mantel-Cox test (P = 0.05). (C) Plasma viral loads of SHIV162P3-infected monkeys were evaluated by quantitative RT-PCR. (D) Peak viral load in control (closed symbols) and CBD1/CBM-vaccinated (open symbols) macaques. We arbitrarily considered the peak of viral load for the uninfected monkey at week 10. Statistical significance was evaluated using the Mann-Whitney test.
Altogether, our results demonstrated that a strategy based on CBD1/CBM-based peptide formulation is partially protective in controlling SHIV162P3 infection in a model of repetitive low-dose challenges.
CD4 depletion is reduced in CBD1/CBM-vaccinated cynomolgus macaques.
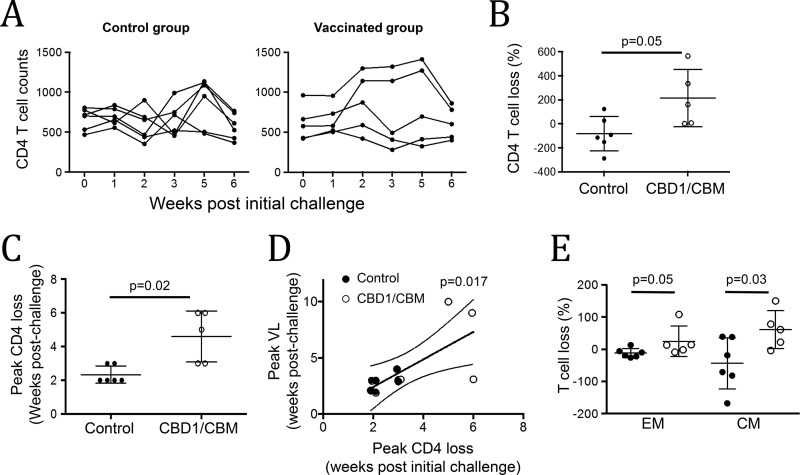
It is well known that after infection, early events are predictive of AIDS outcome, particularly the depletion of memory CD4 T cells (3, 6, 31–33). Monitoring CD4 T cell dynamics may provide a concomitant readout of protection. In peripheral blood, we observed that in the control group, 4 animals of 6 displayed lower CD4 T cell counts 2 weeks postchallenge, while only 1 animal in the vaccinated group showed a decrease at the same time (Fig. 4A). No depletion was observed in the two uninfected monkeys (30237 and 30788). The magnitude of CD4 T cell loss, at week 2 compared to the baseline, was significantly higher in the control group than in the vaccinated group (−82 ± 58.6 versus 213.6 ± 107, P = 0.05) (Fig. 4B). The peak of CD4 depletion occurs earlier in the control group (week 2.3) than that observed in the CBD1/CBM-vaccinated group (week 4.6, P = 0.02) (Fig. 4C). This delay of CD4 T cell depletion is consistent with the delay in the detection of the viral load observed in vaccinated cynomolgus macaques (Fig. 4D). Because early depletion of memory CD4 T cells is associated with further disease progression to AIDS (3, 6, 34), we then analyzed by flow cytometry the central (CD95+ CD28+) and effector memory (CD95+ CD28−) CD4 T cells. Our results showed that the depletion of memory CD4 T cells at week 2 postchallenge, particularly of central memory, is higher in the control group than in the vaccinated group (−43.8 ± 32 versus 61.4 ± 26 T cells, P = 0.03) (Fig. 4E).
FIG 4.
CBD1/CBM vaccination prevents memory CD4 T cell depletion. (A) CD4 T cell counts from control and CBD1/CBM-vaccinated monkeys at different weeks postchallenge. (B) CD4 T cell depletion at week 2 in control (closed circles) and CBD1/CBM-vaccinated (open circles) animals. (C) Peak of CD4 T depletion (in weeks postchallenge) in control (closed circles) and CBD1/CBM-vaccinated (open circles) animals. (D) Correlation between the peak of CD4 T cell depletion and the peak of viral load (VL). We arbitrarily considered the peak of viral load for the uninfected monkey at week 10. (E) Proportion of effector (EM) and central memory CD4 T cells depleted at week 2 from control (closed circles) and CBD1/CBM-vaccinated (open circles) monkeys. Statistical significance was evaluated using the Mann-Whitney test.
Thus, CBD1/CBM-derived peptide vaccination preserved the pool of memory CD4 T cells early after SHIV162P3 infection in cynomolgus macaques.
T cell immune response in SHIV162P3-challenged cynomolgus monkeys.
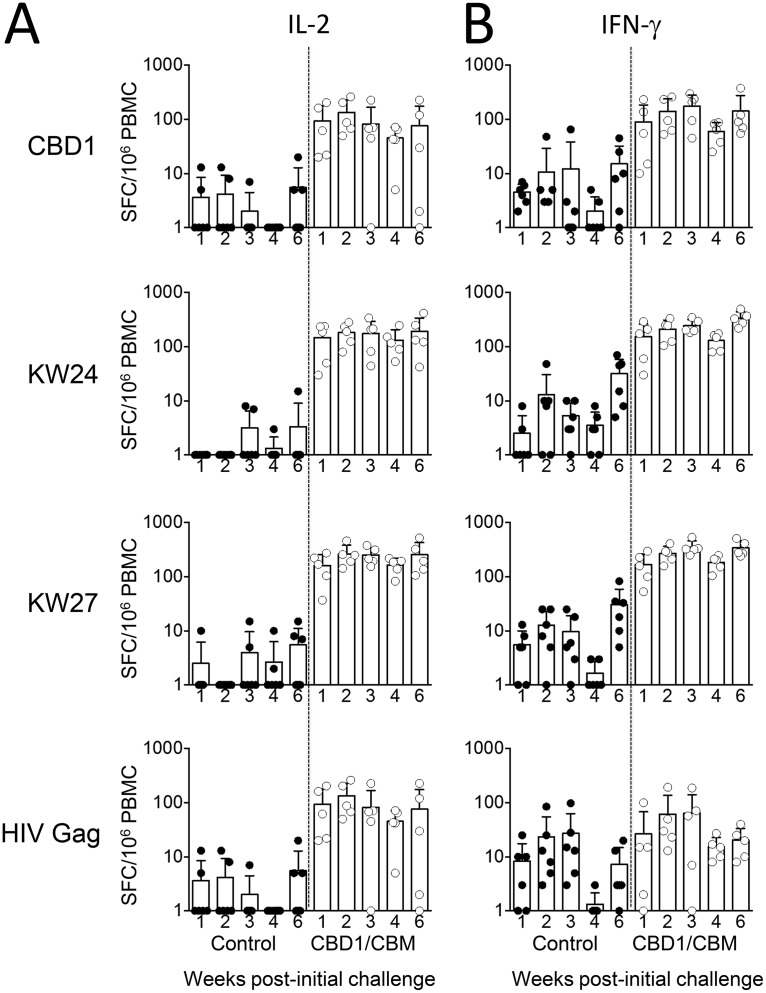
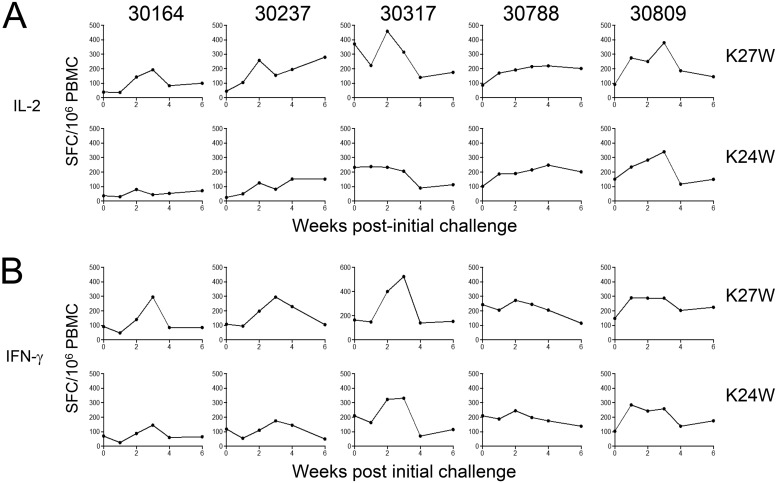
We then assessed the specificity and the dynamics of effector T cells by determining the number of IL-2-, IFN-γ-, and IL-4-producing cells by ELISpot assays in both control and vaccinated groups. PBMCs were stimulated with either K24W, K27W, or CBD1 peptides. In the control group, the numbers of specific T cells producing either IL-2 or IFN-γ were extremely low (mostly fewer than 20 spots) at different weeks postchallenge (Fig. 5A and B). In the vaccinated group, the numbers of specific IL-2- and IFN-γ-producing cells detected after in vitro stimulation with either K24W, K27W, or CBD1 peptides were mostly higher than 80 spots (Fig. 5A and B). Our results demonstrated that the numbers of IL-2- and IFN-γ-producing cells after an SHIV162P3 challenge of the vaccinated macaques were significantly higher than those detected on the day of the challenge (Fig. 6A and B). Thus, after CBD1 stimulation, the numbers of IL-2-producing cells were 82 ± 23 before and 204 ± 48 after SHIV162P3 challenge (P = 0.04). Similarly, K24W induced 140 ± 22 versus 322 ± 52 IL-2-producing cells (P = 0.009), before and after infection, respectively; and K27W induced 136 ± 34 versus 385 ± 41 IL-2-producing cells (P = 0.002). Whereas the immune response was transient in three monkeys, IL-2-producing T cells persisted in animals that controlled infection (30237 and 30788). These results highlighted the fact that SHIV162P3 infection amplifies the pool of T cells specific for K27W, even in SHIV-negative animals. In a similar manner, numbers of IFN-γ-producing cells are higher after infection than those observed before the SHIV162P3 challenge (K24W, 134 ± 27 versus 232 ± 33, P = 0.01; K27W, 151 ± 24 versus 329 ± 49, P = 0.03). While the two peptides have been coupled with the same carrier (HIV Gag298–312 peptide), the magnitude of immunosorbent spots was higher for K27W than for the K24W peptide, which contains the whole amino acid sequence of the CBM, 622IWNNMTWMQW631. Because it cannot be excluded that K27W/K24W immune responses are related to specific T cells directed against the HIV Gag298–312 peptide sequence after SHIV162P3 challenge, we assessed the in vitro HIV gag response in the control and vaccinated groups. The ranges of IL-2- and IFN-γ-producing cells after HIV Gag stimulation were lower than those of either the CBD1, KW24, or KW27 responses (Fig. 5A and B). No IL-4 was detected in either of the two groups (not shown). In contrast to the increase in antigen-specific memory T cell response after the SHIV162P3 challenge, there was no apparent increase in the antibody titers against CBD1/CBM-based peptides (Table 4).
FIG 5.
T cell responses in control and CBD1/CBM-vaccinated monkeys after SHIV challenge. PBMCs from controls (n = 6) and CBD1/CBM-vaccinated macaques (n = 5) were stimulated with CBD1, K24W, K27W, and HIV Gag peptides. IL-2 (A) and IFN-γ (B) ELISpots were used to quantify specific T cell responses at different weeks postchallenge. Data are expressed as spot-forming cells (SFC) per million PBMCs minus the background (medium alone).
FIG 6.
Individual responses of CBD1/CBM-vaccinated monkeys. Numbers of IL-2 (A) and IFN-γ (B) spot-forming cells (SFC) per million PBMCs from CBD1/CBM-vaccinated monkeys after in vitro K27W or K24W peptide stimulations at different weeks postchallenge. Data are expressed as spot-forming cells (SFC) per million PBMCs minus the background (medium alone).
TABLE 4.
IgG titers before and after the SHIV challenge in macaques immunized with a CBD1/CBM cocktaila
| Macaque | Time (wk) before or after challenge | IgG titer |
||||
|---|---|---|---|---|---|---|
| Cocktail | CBD1 | K27W | K24W | Gag298–312 | ||
| 30164 | −2 | 32,000 | 4,000 | 16,000 | 16,000 | 1,000 |
| 30164 | +3 | 16,000 | 4,000 | 8,000 | 8,000 | 1,000 |
| 30164 | +8 | 16,000 | 4,000 | 8,000 | 16,000 | 1,500 |
| 30237 | −2 | 64,000 | 3,000 | 20,000 | 32,000 | 1,000 |
| 30237 | +3 | 64,000 | 3,000 | 16,000 | 32,000 | 1,000 |
| 30237 | +8 | 64,000 | 2,000 | 16,000 | 32,000 | 1,000 |
| 30317 | −2 | 16,000 | 4,000 | 16,000 | 16,000 | 2,000 |
| 30317 | +3 | 12,000 | 4,000 | 8,000 | 16,000 | 2,000 |
| 30317 | +8 | 8,000 | 2,000 | 8,000 | 16,000 | 2,000 |
| 30788 | −2 | 32,000 | 2,000 | 16,000 | 8,000 | 1,500 |
| 30788 | +3 | 16,000 | 2,000 | 16,000 | 4,000 | 1,000 |
| 30788 | +8 | 16,000 | 2,000 | 16,000 | 8,000 | 1,000 |
| 30809 | −2 | 16,000 | 32,000 | 16,000 | 6,000 | 1,500 |
| 30809 | +3 | 16,000 | 30,000 | 16,000 | 6,000 | 2,000 |
| 30809 | +8 | 16,000 | 30,000 | 8,000 | 6,000 | 1,500 |
Cynomolgus macaques were challenged weekly by repeated exposure to low doses of SHIV. The titers of IgG, 2 weeks before (−2) and after (weeks +3 and +8) SHIV challenges, were assessed by ELISA against the cocktail peptides used for immunization as well as individual peptides. The titers correspond to the dilution of antipeptide antibodies giving an OD value of ≥0.1 in ELISA.
Altogether, these results indicate that SHIV162P3 challenge expands immune cells, which are directed against the CBM sequence.
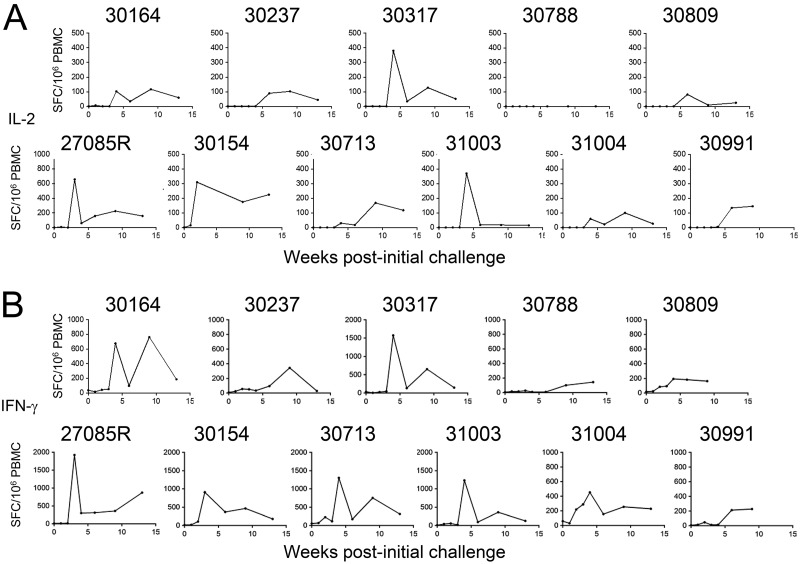
Furthermore, the range of ELISpot responses also provides the opportunity to examine the relationships between T cell responses induced by vaccination and the acquisition of specific T cells after the SHIV162P3 challenge. Thus, we assessed the SIV gag response in control and vaccinated groups. It should be noted that no apparent detectable SIV Gag immune T cell response was found prior to SHIV infection (Fig. 7, point 0 of the weeks postchallenge). In vaccinated monkeys challenged with SHIV162P3, the peak of T cells expressing IL-2 and IFN-γ after SIV Gag stimulation was observed between weeks 4 and 6 (Fig. 7A and B). Our results indicate that the magnitude of IL-2-expressing cells at the peak tends to be lower in vaccinated monkeys (135 ± 63) than in the control group (290 ± 85), but the difference was not statistically significant (P = 0.06). Regarding the number of IFN-γ-producing cells, four monkeys (27085R, 30154, 30713, and 31003) of six in the control group demonstrated more than 800 specific immunosorbent spots, whereas only one animal (30317) reached this level in the vaccinated group. This animal in the vaccinated group has the highest level of viral load (1.9 × 106 copies/ml at week 4). Interestingly, the two monkeys (27085R and 30154) that displayed the earliest VL (Fig. 3C) also had the early peak of IFN-γ-producing cells (week 3) (Fig. 7B). Thus, the range of SIV gag responses could be an indirect marker of the extent of viral replication (35). On the other hand, both in vaccinated and nonvaccinated animals, the numbers of IL-4-producing T cells were extremely low (less than 20 spots in 9 monkeys of the 11) (data not shown).
FIG 7.
Magnitude of SIV Gag response in control and CBD1/CBM-vaccinated monkeys after SHIV challenge. PBMCs from CBD1/CBM-vaccinated macaques (30164, 30237, 30317, 30788, and 30809) and control macaques (27085R, 30154, 30713, 31003, 31004, 30991) were stimulated with SIV Gag antigen, and the numbers of IL-2 (A) and IFN-γ (B) ELISpots were assessed at different weeks postchallenge. Data are shown as spot-forming cells (SFC) per million PBMCs minus the background (medium alone).
Thus, consistent with the delay in the emergence of viral detection, we found that the magnitude of SIV Gag immune response is low and delayed in CBD1/CBM-vaccinated monkeys, compared to the nonvaccinated animals.
DISCUSSION
Here, we demonstrated that the CBD1/CBM-based vaccine cocktail preparation is immunogenic in cynomolgus macaques, inducing cell-mediated Th1 immunity as well as a previously shown specific antibody response (19, 20). As a proof of concept, we demonstrated that such a formulation delays infection by SHIV162P3, a pathogenic CCR5-tropic virus, and is associated with a lower depletion of memory CD4 T cells than in nonvaccinated monkeys. The early detection of antigen-specific memory T cell response in vaccinated macaques following the SHIV challenge is of great interest, since memory T cells are associated with the control of HIV/SIV infection (7–10, 36). The finding that the minimal T cell epitope in the CBD1/CBM vaccine formulation is the sequence 622IWNNMTW628, which is immunogenic and conserved in the caveolin-1 binding motif of HIV-2/SIV, also supports the observation that the SHIV162P3 challenge expands this specific Th1 effector cell.
Accordingly, the potent immunogenicity of CBD1, K24W, and K27W peptides in the CBD1/CBM vaccine formulation indicates that each peptide is immunogenic. Indeed, T cells from vaccinated monkeys responded to in vitro stimulation, using either the CBD1 peptide or the K24W and K27W peptides, as demonstrated by the secretion of both IL-2 and IFN-γ. In the context of this Gag carrier, the CBD1/CBM vaccine cocktail preparation also induces specific IgG antibody. We have previously reported that CBD1 peptide is immunogenic for B cells when fused with the tetanus T helper cell epitope, whereas no response was observed when coupled to keyhole limpet hemocyanin (KLH) (19, 20). Thus, the CBM fusion with the HIV Gag sequence can induce both efficient cellular and humoral Th1 immunity in cynomolgus macaques and may be an advantage in the context of human immunization, because it binds 13 human HLA-DR alleles (24).
Our results indicated that the B and T cell immune responses measured in the blood of immunized macaques are not parallel, since cellular immunity was induced after the first and second immunizations but declined thereafter while the specific IgG response increased and persisted at this time. This may be indicative that T cells do not recirculate in peripheral blood but are probably sequestrated in the lymphoid tissues, such as peripheral lymph nodes (LNs), the spleen, and even in mucosal tissues. Recently, there has been much interest in a new T cell subset, namely, T follicular helper (Tfh) cells, which have strong support for B cell immunity (37–40). Therefore, we cannot exclude that CBD1/CBM-vaccinated animals develop specific Tfh cells, which may participate in the production of mucosal antibodies. Indeed, the protection afforded by the CBD1/CBM-based vaccine cocktail preparation is in the context of repeated rectal SHIV162P3 challenges.
Our data also indicated that certain qualitative and quantitative aspects of T cell responses are associated with a lower risk of infection. Specifically, our results demonstrated that IFN-γ immunosorbent spots against SIV Gag can be a surrogate marker of protection in infected monkeys. As shown here, monkeys that are infected have higher numbers of IFN-γ-secreting cells than protected monkeys. Therefore, there is an inverse correlation with the viral infection. The detection of these circulating cells is certainly indicative of the extent of antigen present in the tissues following SHIV replication. Furthermore, a persistent IL-2 response against K27W is associated with a protective benefit against repeated challenges with SHIV162P3. We, as well as others, have previously demonstrated that loss of memory CD4 T cells during the acute phase is a predictive marker of further disease progression (3, 6, 31–33). In this context, the rhesus cytomegalovirus (RhCMV) vector, by establishing memory T cell responses at potential sites of SIV infection, is protective (41). Therefore, our report provides evidence that a CBD1/CBM vaccine cocktail preparation, by preserving the pool of memory T cells, can be associated with attenuated rectal infection. Although we recognize that correlation does not establish causality, it is possible that the measured cell-mediated immunity is a surrogate for some other effector functions that mediate protection against the acquisition of SHIV. Thus, in inducing effector memory T cells, the CBD1/CBM vaccine cocktail preparation can promote and help CD8 T cells control SHIV infection, and this merits to be addressed. Furthermore, we recently demonstrated that blocking caspase activation, which preserved the pool of memory, is associated with the presence of effector cytotoxic CD4 T cells (42). Therefore, whether a vaccine based on CBD1/CBM peptides induces effector cytotoxic T cells remains to be assessed. Regarding the B cell response, our data indicate that titers of specific antibody are not increased after a SHIV challenge, and the titer of specific IgG was lower in the monkey (30788) that completely controlled the SHIV infection. Furthermore, CBD1/CBM-vaccinated monkeys did not display blood-neutralizing antibody responses against SHIV162P3 (data not shown). In the context of an RV144 trial, in which the canarypox vector (ALVAC) expressing the gag/pol/nef, boosted with gp120, had a modest vaccine efficacy (43), there is no link with neutralizing antibodies. Although blood Ig neutralization seems to not be the main linked mechanism, we cannot exclude that additional antibody-mediated protection is involved in the control of the repeated rectal SHIV162P3 challenges, such as antibody-dependent cellular cytotoxicity (ADCC), or even in inducing defective viruses, as previously observed using antibodies directed against the CBD1/CBM epitope (18). Therefore, whether cell- or Ig-mediated immune mechanisms are associated with protection in CBD1/CBM-vaccinated monkeys remains to be clarified.
The SHIV162P3 challenge of CBD1/CBM-vaccinated macaques took place 6 months after the last immunization. Thus, the rapid generation of antigen (CBD1, K24W, and K27W)-specific memory T cell response following the SHIV challenge in vaccinated macaques provides the potentiality of our vaccine strategy, since it reveals that there is a recall memory T cell response induced by the native CBM epitope of gp41 present in SHIV162P3. CBM-based formulations could also provide therapeutic vaccine strategies in HIV-infected individuals who lack any natural B and T cell immune responses against the CBM epitope, 622IWNNMTWMQW631 (18, 19). It was hypothesized that the absence of anti-CBD1 epitope immune response in HIV-1-infected individuals was due to the N-linked glycosylation of the asparagine N625 (25). However, the generation of specific memory T cell immune responses in CBD1/CBM-vaccinated macaques following a SHIV challenge indicates that such unglycosylated synthetic peptides indeed have the capacity to generate T cell memory to the CBM peptide moiety in the native gp41.
The progression of SIV-induced AIDS, associated with a low-set-point viral load and prolonged survival time, varies greatly from one animal to another and can be influenced by MHC polymorphism (such as Mamu-A*0010, Mamu-A3*1303, and Mamu-B*08 in Indian rhesus macaques [44, 45]). In cynomolgus macaques, H3 and H6 class IB haplotypes have been proposed to be associated with resistance to a chimeric SHIV89.6P challenge (25, 26), while H2 and H5 class IB are associated with susceptibility to infection (27). H1 was also previously reported to be enriched in, but not exclusive to, monkeys that control SIV (46, 47). In the cohort, only four animals were heterozygotes for H6, the second allele being H2 (27085R), H4 (31003), or H1 (30809 and 30788). Two animals were H3 and had as a second allele H1 (30237 and 30713). The two animals that showed strong protection against SHIV infection were 30237 (H3/H1) and 30788 (H1/H6). In the control group, three monkeys, 30713 (H3/H1), 31003 (H4/H6), and 27085R (H6/H2), which share H3 and H6 genotypes, were infected. Although we cannot exclude an impact of MHC alleles in reducing the viral load, it is noteworthy that, despite the two monkeys having the same H6 allele in common, only the vaccinated macaque 30809 was protected, in contrast to the control macaque 31003. Similarly, despite the same H3 allele in common, only the vaccinated macaque 30237 was protected, in contrast to the control monkey 30713.
Finally, this study demonstrates that the K27W peptide should be the CBM peptide choice for future studies. The CBM epitope in gp41 of infectious virus particles remains consistently conserved in HIV-1-infected individuals. This conserved unique feature of the CBM epitope, associated with its strong immunogenicity, capable of inducing by systemic immunization a protection against repeated low-dose rectal SHIV162P3 infections, provides promising perspectives for the use of the K27W peptide as an efficient epitope vaccine candidate for HIV/AIDS.
MATERIALS AND METHODS
Animals.
Eleven adult cynomolgus macaques (female Macaca fascicularis) were imported from Mauritius and housed in the facilities of the Commissariat à l'Énergie Atomique et aux Énergies Alternatives (CEA, Fontenay-aux-Roses, France). Nonhuman primates (NHPs) are used at the CEA in accordance with French national regulations (CEA Permit Number A 92-032-02). The CEA follows the standards for human care and use of laboratory of the Office for Laboratory Animal Welfare (OLAW, USA) under OLAW assurance number A5826-01. The use of NHPs at the CEA is also in accordance with the recommendation of the newly published European Directives (2010/63, recommendation no. 9). The number of animals included in the present study represents the minimal number of monkeys used as a proof of concept regarding the 3R rule. All animals used in this study were tested and selected if negative for SIV, simian T-lymphotropic virus (STLV), herpes B virus, filovirus, simian retrovirus 1 (SRV-1), SRV-2, and measles. The animals were sedated with ketamine chlorhydrate (10 to 15 mg/kg of body weight; Rhone-Mérieux, Lyon, France) before immunization, blood sample collection, and virus injection. The MHC genotype was determined with 20 microsatellites scattered across the MHC region, as previously described (28) and summarized in Table 1.
Peptides and immunizations.
Peptides were synthesized by NeoMPS (Strasbourg, France). They included the CBD1 peptide (618SLEQIWNNMTWMQWDK633) (21) and the two overlapping CBM peptides (622IWNNMTWMQW631 and 622IWNNMTW628), which were fused at their NH2 terminus with the HIV-Gag p24 human HLA-DR supermotif (298KRWIILGLNKIVRMY312) using the dilysine linker. The CBM peptides are referred to as K27W (K-R-W-I-I-L-G-L-N-K-I-V-R-M-Y-K-K-I-W-N-N-M-T-W-M-Q-W) and K24W (K-R-W-I-I-L-G-L-N-K-I-V-R-M-Y-K-K-I-W-N-N-M-T-W) as previously described (20). Peptides (CBD1, K24W, and K27W; 150 μg of each peptide per animal) were mixed with 1,800 μl of Montanide ISA 51 VG (SOP P/2857/GB/02; provided by Seppic S.A., France) and 125 μl of CpG ODN 10103 (stock solution at 24 mg/ml) as an adjuvant, purchased from Coley Pharmaceutical Group, USA (21). The final mix (500 μl) was injected subcutaneously at the back. Animals were injected at days 0, 28, 70, and 110. The SIV gag peptide pool was provided by the AIDS Research and Reference Program/NIAID, NIH (AIDS/NIH).
SIV infection.
SHIV162P3 stock (Harvest 2 lot 4 2.21.07; NIH AIDS Research & Reference Reagent Program) was titrated by the intrarectal (IR) route in cynomolgus macaques; the IR titer is 20 AID50/ml. Six months after the last vaccination (week 35), animals were challenged with repeated IR low doses (0.33 AID50). Each animal was challenged until a plasma viral load above or equal to 104 copies/ml was detected.
Blood samples and flow cytometric analysis.
Baseline blood drawings were performed for sera and peripheral blood mononuclear cell (PBMC) measurements of antibody production by ELISA (20, 21) and T cell responses. T cell subsets were analyzed by flow cytometry using the BD LSRII analyzer. The following antibodies were used: anti-CD45 PerCP (clone D058-1283; BD Biosciences), anti-CD3 Alexa Fluor 700 (clone SP34-2; BD Biosciences), anti-CD4 phycoerythrin (PE) (clone L200; BD Biosciences), anti-CD8 V450 (clone RPA-T8; BD Biosciences), anti-CD95 allophycocyanin (APC) (clone DX2; BD Biosciences), and anti-CD28 PE Texas Red (clone 28.2; eBiosciences). Data were analyzed using FlowJo software.
ELISA.
The immune sera were titrated by ELISA using 96-well plates (MaxiSorp; Dynatech) coated with either a cocktail of CBD1/CBM peptides (1 μg/ml) used for immunization or HIV-1 Gag298–312 peptide (at 0.5 μg/ml) in coating buffer (100 mM carbonate/bicarbonate buffer, pH 9.6). Plates were then washed, first with phosphate-buffered saline (PBS) and then with the saturation buffer (PBS containing detergent Tween at 0.05%). Sera at 1/1,000 dilution in PBS containing 10% fetal calf serum (FCS) were then serially diluted in the plates and incubated for 90 min at 37°C. After washing with PBS-Tween buffer, a sheep anti-human immunoglobulin conjugated with horseradish peroxidase (HRP; AbD Serotec, UK) was added at 1/4,000 dilution (in PBS-FCS) and incubated for 45 min at 37°C. Indeed, compared to secondary anti-human antibodies, anti-monkey antibodies give higher background levels. Following incubation and washing, o-phenylenediamine dihydrochloride (OPD) substrate in phosphate-citrate buffer at pH 5 was added to the wells. Following color development, the reaction mixture was quantitated at 450 nm as described previously (18, 19). The antibody titers correspond to the reciprocal dilution of the respective macaque serum giving an optical density (OD) value equal to 0.1 (measured at 450 nm). Under such experimental conditions, no reactivity was observed in sera from control macaques or in the vaccinated group before immunization.
ELISpots.
Briefly, multiScreen 96-well filtration plates (Millipore, Guyancourt, France) were saturated with 35% ethanol, washed, and coated by incubation overnight with monoclonal antibody against either monkey IFN-γ (clone GZ-4; Mabtech, Nacka, Sweden), human IL-2 (U-cytech, Utrecht, Netherlands), or human IL-4 (Mabtech) at the concentration of 10 μg/ml in PBS (Laboratoires Eurobio, Les Ulis, France) at 4°C. Plates were washed 5 times with PBS and then blocked by incubation for 1 h at 37°C with culture medium, consisting of RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% heat-inactivated fetal calf serum (Laboratoires Eurobio). PBMCs were recovered by density gradient centrifugation, and 2 × 105 cells were added to each well. Peptides were then added in duplicate to a final concentration of 2 μg/ml of each peptide in the culture medium. Plates were incubated for 18 h at 37°C in an atmosphere containing 5% CO2. They were then washed 5 times with PBS. Biotinylated anti-IFN-γ antibody (clone 7-B6-1; Mabtech), anti-IL-2 antibody (U-cytech), or anti-IL-4 antibody was then added at a concentration of 1 μg/ml in 0.5% FCS in PBS. The plates were incubated overnight at 4°C or 2 h at 37°C and then washed 5 times with PBS. Plates were incubated with 0.25 μg/ml alkaline phosphatase-streptavidin conjugate (Sigma-Aldrich, St-Quentin Fallavier, France) for 1 h at 37°C and then washed 5 times with PBS. Spots were developed by adding NBT-BCIP (nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate substrate; Sigma-Aldrich) and counted with an automated ELISpot reader, ELR04 XL (Autoimmun Diagnostika GmbH, Strassberg, Germany). The background was calculated as twice the mean number of IFN-γ spot-forming cells (SFC) per 106 PBMC (IFN-γ, IL-2, or IL-4 SFC/million PBMCs) in nonstimulated samples. Samples yielding more than 50 IFN-γ, IL-2, or IL-4 SFC/million PBMCs after removal of the background were scored as positive.
Viral load.
Replication of SHIV162P3 was quantified using quantitative reverse transcription (RT)-PCR for the measurement of the viral RNA copy numbers, with primer pairs used for gag as previously described (48). The detection limit of this method is 60 copies/ml, and the quantification limit is 300 copies/ml.
Statistical analysis.
Statistics were performed using GraphPad Prism 5 software. Fisher's exact test and the nonparametric Mann-Whitney test were employed for comparisons, as indicated in the figures. P values of <0.05 indicate a significant difference. The Mantel-Cox test was used to establish the efficacy of the vaccine compared with the control group.
ACKNOWLEDGMENTS
This work was supported by grants from the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS). J.E. acknowledges support from the Canada Research Chair Program.
REFERENCES
- 1.Hurtrel B, Petit F, Arnoult D, Müller-Trutwin M, Silvestri G, Estaquier J. 2005. Apoptosis in SIV infection. Cell Death Differ 12:979–990. doi: 10.1038/sj.cdd.4401600. [DOI] [PubMed] [Google Scholar]
- 2.Lifson JD, Nowak MA, Goldstein S, Rossio JL, Kinter A, Vasquez G, Wiltrout TA, Brown C, Schneider D, Wahl L. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol 71:9508–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monceaux V, Estaquier J, Février M, Cumont M-C, Rivière Y, Aubertin A-M, Ameisen JC, Hurtrel B. 2003. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. AIDS 17:1585–1596. doi: 10.1097/00002030-200307250-00002. [DOI] [PubMed] [Google Scholar]
- 4.Estaquier JM, Idziorek T, De Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin A-M, Venet A, Mehtali M, Muchmore E, Michel P. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci U S A 91:9431–9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441–452. doi: 10.1016/S1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 6.Viollet L, Monceaux V, Petit F, Fang RHT, Cumont M-C, Hurtrel B, Estaquier J. 2006. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J Immunol 177:6685–6694. doi: 10.4049/jimmunol.177.10.6685. [DOI] [PubMed] [Google Scholar]
- 7.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. 1998. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 12.van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. 2003. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol 13:92–100. doi: 10.1016/S0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Rudick M, Anderson RG. 2002. Multiple functions of caveolin-1. J Biol Chem 277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 14.Mañes S, del Real G, Lacalle RA, Lucas P, Gómez-Moutón C, Sánchez-Palomino S, Delgado R, Alcamí J, Mira E, Martínez-A C. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep 1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen DH, Hildreth JE. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol 74:3264–3272. doi: 10.1128/JVI.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono A, Freed EO. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A 98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benferhat R, Sanchez-Martinez S, Nieva JL, Briand JP, Hovanessian AG. 2008. The immunogenic CBD1 peptide corresponding to the caveolin-1 binding domain in HIV-1 envelope gp41 has the capacity to penetrate the cell membrane and bind caveolin-1. Mol Immunol 45:1963–1975. doi: 10.1016/j.molimm.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Hovanessian AG, Briand J-P, Said EA, Svab J, Ferris S, Dali H, Muller S, Desgranges C, Krust B. 2004. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 is an efficient B cell epitope vaccine candidate against virus infection. Immunity 21:617–627. doi: 10.1016/j.immuni.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Rey-Cuillé MA, Svab J, Benferhat R, Krust B, Briand JP, Muller S, Hovanessian AG. 2006. HIV-1 neutralizing antibodies elicited by the candidate CBD1 epitope vaccine react with the conserved caveolin-1 binding motif of viral glycoprotein gp41. J Pharm Pharmacol 58:759–767. doi: 10.1211/jpp.58.6.0006. [DOI] [PubMed] [Google Scholar]
- 20.Benferhat R, Krust B, Rey-Cuille MA, Hovanessian AG. 2009. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 (CBD1) contains several overlapping neutralizing epitopes. Vaccine 27:3620–3630. doi: 10.1016/j.vaccine.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 21.Benferhat R, Martinon F, Krust B, Le Grand R, Hovanessian AG. 2009. The CBD1 peptide corresponding to the caveolin-1 binding domain of HIV-1 glycoprotein gp41 elicits neutralizing antibodies in cynomolgus macaques when administered with the tetanus T helper epitope. Mol Immunol 46:705–712. doi: 10.1016/j.molimm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, Kittlesen D, Deacon D, Hibbitts S, Grosh WW. 2001. Phase I trial of a melanoma vaccine with gp100280–288 peptide and tetanus helper peptide in adjuvant. Clin Cancer Res 7:3012–3024. [PubMed] [Google Scholar]
- 23.Lennon-Dumenil AM, Bakker AH, Wolf-Bryant P, Ploegh HL, Lagaudriere-Gesbert C. 2002. A closer look at proteolysis and MHC-class-II-restricted antigen presentation. Curr Opin Immunol 14:15–21. doi: 10.1016/S0952-7915(01)00293-X. [DOI] [PubMed] [Google Scholar]
- 24.Wilson CC, Palmer B, Southwood S, Sidney J, Higashimoto Y, Appella E, Chesnut R, Sette A, Livingston BD. 2001. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. J Virol 75:4195–4207. doi: 10.1128/JVI.75.9.4195-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson WE, Sauvron JM, Desrosiers RC. 2001. Conserved, N-linked carbohydrates of human immunodeficiency virus type 1 gp41 are largely dispensable for viral replication. J Virol 75:11426–11436. doi: 10.1128/JVI.75.23.11426-11436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mee ET, Berry N, Ham C, Sauermann U, Maggiorella MT, Martinon F, Verschoor EJ, Heeney JL, Le Grand R, Titti F. 2009. MHC haplotype H6 is associated with sustained control of SIVmac251 infection in Mauritian cynomolgus macaques. Immunogenetics 61:327–339. doi: 10.1007/s00251-009-0369-8. [DOI] [PubMed] [Google Scholar]
- 27.Florese RH, Wiseman RW, Venzon D, Karl JA, Demberg T, Larsen K, Flanary L, Kalyanaraman V, Pal R, Titti F. 2008. Comparative study of Tat vaccine regimens in Mauritian cynomolgus and Indian rhesus macaques: influence of Mauritian MHC haplotypes on susceptibility/resistance to SHIV 89.6 P infection. Vaccine 26:3312–3321. doi: 10.1016/j.vaccine.2008.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarnink A, Dereuddre-Bosquet N, Vaslin B, Le Grand R, Winterton P, Apoil P-A, Blancher A. 2011. Influence of the MHC genotype on the progression of experimental SIV infection in the Mauritian cynomolgus macaque. Immunogenetics 63:267–274. doi: 10.1007/s00251-010-0504-6. [DOI] [PubMed] [Google Scholar]
- 29.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. 2002. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines 1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Duan L, Estes JD, Ma Z-M, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 32.Cumont M-C, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Le Grand R, Müller-Trutwin M. 2008. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol 82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 34.Arnoult D, Petit F, Lelievie J, Lecossier D, Hance A, Monceaux V, Fang RHT, Huntrel B, Ameisen J, Estaquier J. 2003. Caspase-dependent and-independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ 10:1240–1252. doi: 10.1038/sj.cdd.4401289. [DOI] [PubMed] [Google Scholar]
- 35.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Banchereau J, Vinuesa CG. 2015. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues V, Laforge M, Campillo-Gimenez L, Soundaramourty C, Correia-de-Oliveira A, Dinis-Oliveira RJ, Ouaissi A, Cordeiro-da-Silva A, Silvestre R, Estaquier J. 2014. Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog 10:e1004096. doi: 10.1371/journal.ppat.1004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moukambi F, Rabezanahary H, Rodrigues V, Racine G, Robitaille L, Krust B, Andreani G, Soundaramourty C, Silvestre R, Laforge M. 2015. Early loss of splenic Tfh cells in SIV-infected rhesus macaques. PLoS Pathog 11:e1005287. doi: 10.1371/journal.ppat.1005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moukambi F, Rodrigues V, Fortier Y, Rabezanahary H, Borde C, Krust B, Andreani G, Silvestre R, Petrovas C, Laforge M, Estaquier J. 2017. CD4 T follicular helper cells and HIV infection: friends or enemies? Front Immunol 8:135. doi: 10.3389/fimmu.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M Jr, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laforge M, Silvestre R, Rodrigues V, Garibal J, Campillo-Gimenez L, Mouhamad S, Monceaux V, Cumont MC, Rabezanahary H, Pruvost A, Cordeiro-da-Silva A, Hurtrel B, Silvestri G, Senik A, Estaquier J. 2018. The anti-caspase inhibitor Q-VD-OPH prevents AIDS disease progression in SIV-infected rhesus macaques. J Clin Invest 128:1627–1640. doi: 10.1172/JCI95127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mühl T, Krawczak M, ten Haaft P, Hunsmann G, Sauermann U. 2002. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J Immunol 169:3438–3446. doi: 10.4049/jimmunol.169.6.3438. [DOI] [PubMed] [Google Scholar]
- 45.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM. 2007. Mamu-B* 08-positive macaques control simian immunodeficiency virus replication. J Virol 81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budde ML, Greene JM, Chin EN, Ericsen AJ, Scarlotta M, Cain BT, Pham NH, Becker EA, Harris M, Weinfurter JT, O'Connor SL, Piatak M Jr, Lifson JD, Gostick E, Price DA, Friedrich TC, O'Connor DH. 2012. Specific CD8+ T cell responses correlate with control of simian immunodeficiency virus replication in Mauritian cynomolgus macaques. J Virol 86:7596–7604. doi: 10.1128/JVI.00716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ericsen AJ, Starrett GJ, Greene JM, Lauck M, Raveendran M, Deiros DR, Mohns MS, Vince N, Cain BT, Pham NH, Weinfurter JT, Bailey AL, Budde ML, Wiseman RW, Gibbs R, Muzny D, Friedrich TC, Rogers J, O'Connor DH. 2014. Whole genome sequencing of SIV-infected macaques identifies candidate loci that may contribute to host control of virus replication. Genome Biol 15:478. doi: 10.1186/s13059-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannioui A, Bourry O, Sellier P, Delache B, Brochard P, Andrieu T, Vaslin B, Karlsson I, Roques P, Le Grand R. 2009. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology 6:106. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]