Abstract
Outpatient parenteral antimicrobial therapy (OPAT) programs are becoming an increasingly popular trend in clinical practice as they offer several benefits to both patients and health-care setups. While OPAT is an established clinical practice in the Western world, the concept itself is alien to patients in India as they prefer the security of hospitals to receive antibiotics over OPAT. We evaluated the clinical response and cost comparison of ertapenem under OPAT versus inpatient settings in patients with extended-spectrum beta-lactamase (ESBL)-positive acute pyelonephritis (APN) given the increasing importance of optimizing both hospital beds and overall cost of patient care in India. APN was chosen as the indication to be studied as it is one of the common complicated urinary tract infections treated in our OPAT unit requiring 10–14 days of parenteral therapy with an agent active against various Gram-negative bacilli and multidrug-resistant organisms. One hundred patients were retrospectively studied based on whether antibiotics were administered during hospital stay alone (hospital only), during both hospital stay, and also as OPAT post discharge (hospital/OPAT) or as OPAT alone (OPAT only). Response to ertapenem and cost of treatment in inpatient versus OPAT settings were compared using Pearson's Chi-square or Fisher's exact test for categorical variables. ANOVA (or Kruskal–Wallis) was used for continuous variables. Baseline urine cultures were ESBL positive with 98% prevalence of Gram-negative bacilli (GNB). Colony counts were ≥100,000 in 74% patients. Only ertapenem, imipenem, and meropenem showed 100% sensitivity to ESBL-positive GNB in baseline urine culture and sensitivity reports. Ertapenem showed 100% sensitivity and complete clinical resolution for 96% patients with APN due to ESBL Enterobacteriaceae. It was administered as OPAT in 90% patients and significantly reduced overall treatment costs.
Keywords: Clinical resolution, cost comparison, ertapenem, extended-spectrum beta-lactamase positive acute pyelonephritis, India, outpatient parenteral antimicrobial therapy
Introduction
Outpatient parenteral antimicrobial therapy (OPAT) programs are becoming an increasingly popular trend in clinical practice as they offer several benefits to both patients and health-care setups.[1] They are also known as outpatient and home parenteral antimicrobial therapy, community-based parenteral anti-infective therapy, or “hospital in the home” services.[2] The Infectious Disease Society of America defines OPAT as the provision of parenteral antimicrobial therapy in at least two doses on different days without intervening hospitalization.[1,2,3,4] According to most reports, the advent of OPAT was in the early 1970s, more precisely in the year 1974. It was first described in North America and later on became an established clinical practice throughout the United States and also in many other countries such as Canada, Australia, New Zealand, Singapore, the UK, and Italy.[3,4,5,6,7,8,9,10,11] By 1998, the estimated number of annual OPAT treatments in the USA was approximately 2,50,000 patients.[8,10,12,13] The major utility of OPAT programs is cost-effectiveness apart from facilitating early discharge or avoiding hospital admission altogether.[1,2,10,13] While OPAT is known to reduce the cost of patient care by up to 40%–70% of inpatient costs,[5,12] early discharge facilitates lower risk of nosocomial infections, optimization of hospital beds, and greater patient physical and psychological comfort by allowing an earlier return to work or school.[1,3,5,9,10,12]
The concept of OPAT is relatively new in Asia with few publications.[9,14,15,16] Published reports are available from Singapore where OPAT services were introduced in 2001.[9,14] Since then, Singapore has maintained a collaborative prospective database across two major hospitals from 2006, has collected 2229 first OPAT episodes, and published outcomes of its OPAT practice.[2,9] One such publication compared actual costs and outcomes of care involving OPAT with conventional inpatient-only care and was reported by the authors to be the first comprehensive cost analysis of an OPAT program in Singapore.[14]
Most frequently reported infections treated as OPAT are skin and soft-tissue infections (SSTIs) such as abscess and cellulitis, osteomyelitis, late-stage Lyme disease, urinary tract infection (UTI)/pyelonephritis, septic arthritis/bursitis, bacteremia, prosthetic joint infections, endocarditis, pneumonia, and intra-abdominal and surgical wound infections.[1,4,5,12] Commonly used antibiotics in OPAT were carbapenems, daptomycin, first-, second-, and third-generation cephalosporins, penicillins, aminoglycosides, and vancomycin.[4,5,12]
Among the carbapenems, there are several publications where ertapenem has been used as OPAT since 2010 across various clinical indications.[10,17,18,19,20] The earliest publication describing ertapenem as a new opportunity in OPAT was published in 2004 by Tice.[21] Data from the Glasgow OPAT service from May 2007 to 2012 listed ertapenem as the most frequently used first-line antimicrobial agent for UTI followed by abdominal abscess.[7,10]
In India, due to a high prevalence of extended-spectrum beta-lactamase (ESBLs) in Gram-negative bacteria (GNB) of 60%–70% reaching up to 80% in Klebsiella,[22,23] beta-lactam-beta-lactamase inhibitors (BL-BLis) and Group 1 carbapenem (ertapenem) are preferred choices due to economic reasons to treat ESBL infections in high-risk patients. Such patients are elderly, not seriously ill, have had minimal exposure to hospitals and antibiotic therapies over the previous 90 days and have no risk of infections with Gram-negative nonfermenters such as Pseudomonas and Acinetobacter. The most common indications are complicated UTI (cUTI), complicated intra-abdominal infection, and SSTIs. However, in spite of the increasing clinical usage of ertapenem, it is mostly prescribed in hospitalized patients, as OPAT is neither an established nor widely accepted concept in our country. Furthermore, Indians prefer the security of hospitals to receive antibiotics over OPAT as the concept of OPAT itself is alien. This was further substantiated by our literature search, which did not reveal any publications or national guidelines on OPAT from India to the best of our knowledge.
Due to this scenario and the increasing importance of optimizing both hospital beds and overall cost of patient care in a developing country like ours, we evaluated the clinical response and cost comparison of ertapenem under OPAT versus inpatient settings in patients diagnosed with ESBL-positive acute pyelonephritis (APN). APN has often been reported as the most common clinical manifestation of cUTI,[18,24] and with no suitable oral antibiotic therapy for patients with cUTIs caused by ESBL-producing organisms, they can be treated effectively through OPAT programs with once-daily parenteral ertapenem in clinically stable patients.[20]
Subjects and Methods
A retrospective analysis was conducted at two tertiary care centers (Apollo, Chennai, Tamil Nadu and KIMS, Thiruvananthapuram, Kerala, India) for ESBL-positive APN from 2010 to 2014. Patient demographics, clinical response, and actual treatment costs were collected from hospital records and billing systems. A total of 100 patients across the two centers (70 at Apollo and 30 at KIMS) were found to have received ertapenem for the treatment of APN during this period. Institutional ethical clearance was obtained, and the study was also registered in clinical trials registry, India (CTRI REF/2014/01/006325).
Patient demographics recorded were age, gender, details of hospital stay, and clinical history. Microbiology details included results of urine routine examination and microscopy, ESBL status at the beginning and end of treatment, and urine culture and sensitivity (C/S) pattern. Treatment details of APN for each patient included duration, dose, empiric versus definitive usage of ertapenem, and detailed history of antibiotics used during escalation or de-escalation. In case antibiotics other than ertapenem were used as the first-line treatment, reason for switch to ertapenem during the course of therapy was also captured. Finally, details of clinical resolution and evidence of microbiological cure (negative ESBL in urine C/S reports) at the end of treatment were also recorded. Actual costs incurred toward all outpatient and inpatient billing charges were captured to analyze cost comparison of OPAT versus inpatient hospitalization.
Objectives
The primary objective was to evaluate the clinical response of ertapenem in ESBL-positive APN. Secondary objective was to evaluate the cost comparison of ertapenem under OPAT versus inpatient settings.
Inclusion/exclusion criteria
Data of all adult patients over 18 years of age with a diagnosis of APN and who received >3 doses of Ertapenem therapy were included for analysis. APN was defined as patients with fever, flank pain or costovertebral angle tenderness, pyuria (≥5 WBCs/HPF) or urinary symptoms, and confirmed ESBL Enterobacteriaceae in urine culture. Patients who required concomitant antimicrobials in addition to ertapenem were excluded from the study.
Statistical analysis
Response to ertapenem and cost of treatment in inpatient versus OPAT settings were compared using Pearson's Chi-square or Fisher's exact test for categorical variables. ANOVA (or Kruskal–Wallis) was used for continuous variables.
Results
One hundred patients were evaluated for response to ertapenem in APN, which included 43 males and 57 females. They were divided into three groups based on whether antibiotics were administered during hospital stay alone (hospital only), during both hospital stay, and also as OPAT postdischarge (hospital/OPAT) or as OPAT alone (OPAT only) for all further analysis. The number of patients in each group was hospital only (10), hospital/OPAT (63), and OPAT only (27). Since both hospitals had a well-established OPAT practice, majority patients fell into the hospital/OPAT group (Apollo 41/70 and KIMS 22/30).
Diabetes, hypertension, and renal and cardiac disorders were the top four comorbidities, followed by thyroid disorders, malignancies, respiratory disorders, liver disorders, and rheumatoid arthritis. Fever, abdominal pain, dysuria, vomiting, and chills were main presenting features. Others were back pain, increased frequency of urination, oliguria, recurrent UTI, dyspnea, loose stools, groin pain, hematuria, weight loss, and renal calculi [Table 1]. Baseline urine cultures were ESBL positive with 98% prevalence of GNB (Escherichia coli 89% and Klebsiella 9%). Colony counts were ≥100,000 in 74% patients. Only ertapenem, imipenem, and meropenem showed 100% sensitivity to ESBL-positive GNB in baseline urine C/S reports. Sensitivity of other antibiotics is listed in Figure 1.
Table 1.
Baseline patient characteristics
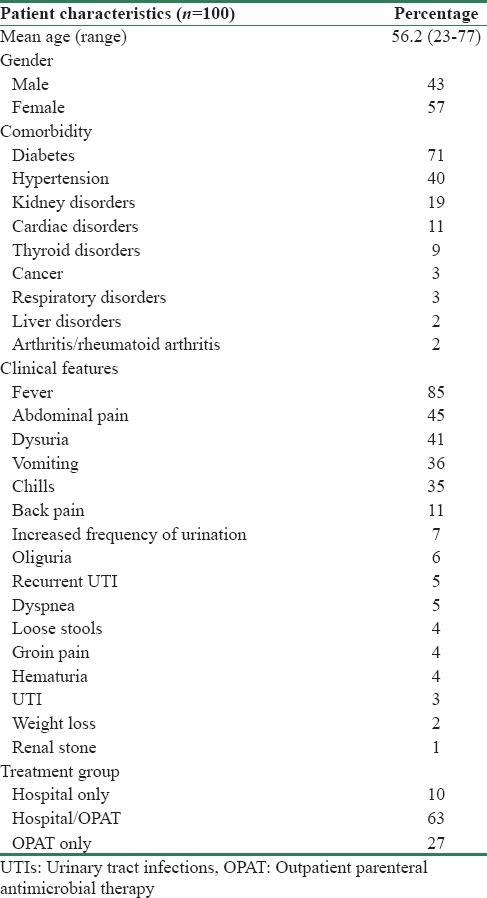
Figure 1.
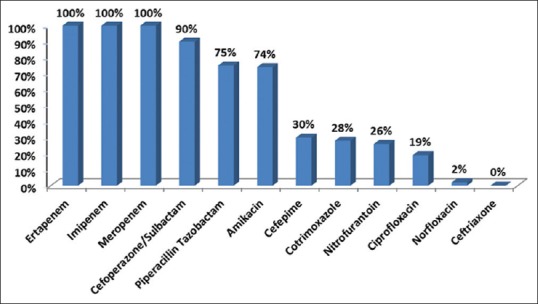
Antimicrobial sensitivity patterns as per baseline urine culture and sensitivity reports
Complete clinical resolution with ertapenem was seen in 96 patients, no resolution in two, and information was unavailable for two. Among patients showing complete resolution, 82 received definitive and 14 received empirical treatment with ertapenem. Baseline colony counts were ≥100,000 in 74 (77%) patients among those who showed complete clinical cure. Sixty patients received ertapenem both during IP and OPAT, 26 only as OPAT, and 10 only as inpatients.
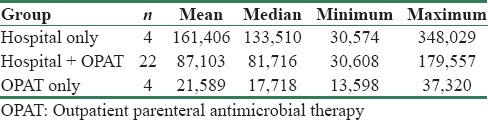
A significant reduction in treatment cost was seen in patients who received ertapenem as OPAT (P = 0.001) at Apollo [Table 2]. Median charges at Apollo were hospital only (INR 264,302), hospital/OPAT (INR 189,554), and OPAT only (INR 41,380), showing a decreasing trend [Table 3]. All charges were inflated to 2014 using consumer price index (base year = 2010). Median charges at KIMS were hospital only (INR 133,510), hospital/OPAT (INR 81,716), and OPAT only (INR 17,718). Although a decrease in treatment costs was also seen in patients who received ertapenem as OPAT at KIMS, statistical significance could not be derived for this center because of the small sample size in the hospital only and OPAT only groups [Table 4].
Table 2.
Cost comparison of median per patient charges at Apollo by treatment group (INR 2014)

Table 3.
Per patient charges by treatment group (INR 2014) - Apollo
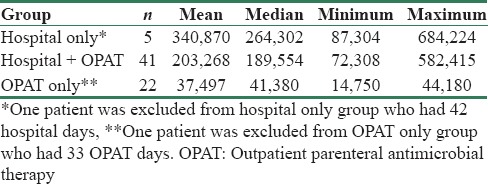
Table 4.
Per patient charges by treatment group (INR 2014) - KIMS
Discussion
This study was initially planned across four centers but was later executed in two due to the limited OPAT practice in Indian health-care setups. Reasons for this are lack of an organized network of multidisciplinary teams comprising infectious disease physicians, specialized nurses, clinical pharmacists, and microbiologists who are trained to deliver OPAT services. Added to this, the potential for unexpected risks and adverse events in unsupervised settings and lack of national guidelines on criteria for patient and antimicrobial selection, laboratory monitoring, follow-up, and measurement of clinical outcomes deter many physicians from implementing this practice.[5] However, many tertiary care hospitals are now establishing their own home health services which are equipped to deliver OPAT either through the visiting nurse or infusion center model. The third commonly cited OPAT model of self-administration by the patient himself or his family is still in its nascent stage in India.[1,7,21]
Patient selection criteria followed for OPAT at both participating centers in our study were as per international guidelines and published literature, where care was taken to see that every selected patient had an established diagnosis based on clinical and microbiological criteria with the absence of oral treatment options.[1] APN was chosen as the indication to be studied as it is one of the common cUTIs treated worldwide and also in our OPAT unit requiring 10–14 days of parenteral therapy with an agent active against various Gram-negative bacilli and multidrug-resistant organisms.[1,18,20,24,25] In addition, treatment of cUTI is usually initiated empirically, before identification of the causative organism(s).[26] This emphasizes the need for selecting the right antimicrobial based on knowledge of local epidemiology and in vitro sensitivity patterns.
Ertapenem is the most appropriate empiric antibiotic for ESBL APN in our health-care setting because of its broad antimicrobial spectrum, good in vitro activity against cephalosporin-resistant organisms producing ESBLs or AmpC b-lactamases, and restricted activity against nosocomial pathogens such as Pseudomonas aeruginosa, Acinetobacter species, and methicillin-resistant staphylococci and enterococci.[20,24,27,28] This was reflected in our baseline urine cultures where ESBL-producing E. coli and Klebsiella accounted for 98% of causative pathogens and was similar to reports by other authors.[24,25] Ertapenem, imipenem, and meropenem were the only antibiotics which showed 100% in vitro sensitivity to ESBL-positive GNB in our study cohort, followed by cefoperazone sulbactam (90%), piperacillin-tazobactam (75%), amikacin (74%), cefepime (30%), co-trimoxazole (28%), nitrofurantoin (26%), ciprofloxacin (19%), and norfloxacin (2%). All cultures were completely resistant to ceftriaxone [Figure 1]. In our analysis, ertapenem also demonstrated complete clinical resolution in 96 out of 100 patients. No resolution was seen in two patients, and information about a clinical cure was unavailable in another two. Moreover, even though cefoperazone-sulbactam has shown good sensitivity of 90% in our setup, ertapenem with a once-daily dosing schedule is a more convenient option than twice a day of cefoperazone-sulbactam when OPAT is considered.
In India, the overall prevalence of ESBL GNB is approximately 60%–80%, with AmpC and metallo-beta-lactamases coexisting in 25%–43% cases.[22,23,29] These organisms are known to delay clinical and microbiological cure as they confer resistance to third-generation cephalosporins and monobactams apart from reducing sensitivities to fluoroquinolones, aminoglycosides, BL-BLis, and trimethoprim-sulfamethoxazole.[1] This warrants parenteral carbapenems and in turn leads to prolonged hospital stays and increased overall treatment costs.[24] A similar C/S pattern was elicited in our study cohort as described previously. Carbapenems are the drug of choice for severe ESBL/AmpC infections, and ertapenem becomes the first-line empiric choice where Pseudomonas-sparing coverage is mandated due to its narrower activity compared to other carbapenems. In cases where there is a combined risk of both ESBLs and Gram-negative nonfermenters, imipenem/meropenem become the first-line empiric choice with a de-escalation to ertapenem during definitive therapy post-C/S reports.[18,25,26] In our hospital too, the treatment protocol for APN is to use carbapenems such as imipenem or meropenem for critically ill patients requiring ICU admission and subsequently de-escalate to cefoperazone-sulbactam or ertapenem if sensitive. For stable patients with APN, BL-BLI like cefoperazone-sulbactam or ertapenem is started upfront as carbapenem sparers empirically.
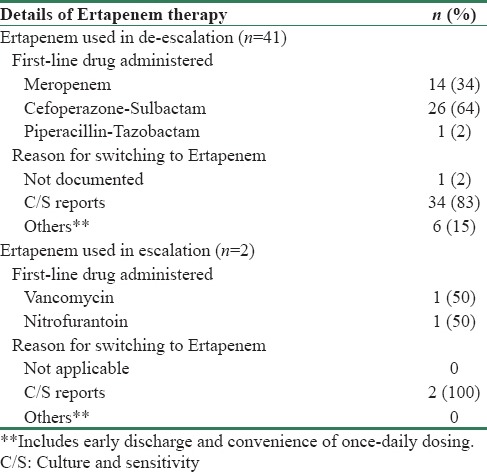
Choosing the correct empirical therapy (>80% sensitivity) for high-risk patients, on the basis of type of pathogens responsible for cUTIs and their resistance patterns, is important as it ensures timely cure, reduced risk of complications, and minimal recurrence.[25] This is especially important in our country where microbiological tests are delayed due to economic reasons, and antibiotic abuse is rampant. Moreover, early automated culture diagnostics and advanced rapid diagnostic tests such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry are unavailable even in most tertiary care hospitals, and conventional C/S reports become available only in 48–72 h. However, this is often not followed in clinical practice majorly due to lack of adequate knowledge on latest trends in local microbiology and in vitro sensitivity patterns. This was reflected in our analysis where in spite of an initial diagnosis of APN, ertapenem was started empirically only in 17 patients and was later prescribed post urine C/S reports to 83 patients. Ertapenem, however, was the most commonly chosen first-line drug for treatment of ESBL APN in 57 patients across both centers [Table 5]. Remainder 41 patients were de-escalated to ertapenem post-C/S reports where first-line drugs were meropenem (14 patients), cefoperazone sulbactam (26 patients), and piperacillin-tazobactam (1 patient). Two patients, who were initially prescribed vancomycin and nitrofurantoin, were also switched over to ertapenem post sensitivity reports [Table 6]. Apart from C/S results, early discharge and convenience of once-daily dosing were other reasons documented for switching over to ertapenem. In such a scenario, perhaps an adequate knowledge of the regional microbiology data by all prescribing physicians would have resulted in a larger number of patients receiving the right empiric antimicrobial as a first-line therapy. This would have in turn minimized need to switch antibiotics post-C/S reports and could have perhaps translated into an early recovery and discharge for the patient.
Table 5.
Pattern of ertapenem prescription in treatment of acute pyelonephritis
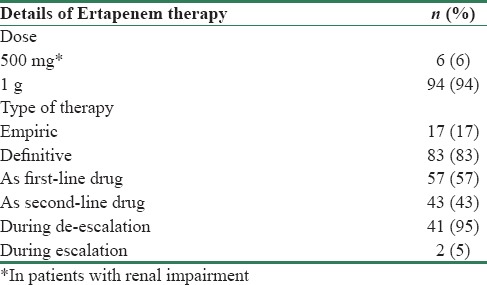
Table 6.
Antimicrobial escalation and de.escalation pattern in patients treated for acute pyelonephritis as per hospital records
Hence, results of our study corroborate reports by earlier authors that ertapenem is the most appropriate first-line empirical treatment for patients with a complicated upper UTI like APN, when ESBL-producing E. coli is suspected.[24] Ertapenem is also one of the most frequently used antimicrobials to treat cUTIs in OPAT settings due to its long elimination half-life which allows for once-daily dosing, stability for 6 h at 25°C, and moderate risk for phlebitis. Dose administered in our study cohort was 1 g OD in 94 patients and an adjusted dose of 500 mg OD in 6 patients with renal impairment.[1,4,5,6,10,18,19,21,26,30,31]
Treatment guidelines for antimicrobial use in common syndromes published by the Indian Council of Medical Research (ICMR) in 2017 mention ertapenem and piperacillin-tazobactam as preferred empiric antimicrobial agents in the treatment of APN. The guidelines explain that this is because most Enterobacteriaceae isolates are ESBL producing organisms curbing the utility of third- and fourth-generation cephalosporins and most beta-lactam antibiotics, due to which carbapenems have to be resorted to most often. Data from ICMR's Antimicrobial Resistance Surveillance Network have shown that the percentage of resistant isolates to cefoperazone-sulbactam is approximately 33%, 62%, and 39% for E. coli, Klebsiella sp., and Enterobacter sp. Similarly, the approximate percentage of resistant isolates to piperacillin-tazobactam was seen to be 43%, 68%, and 57% for E. coli, Klebsiella sp., and Enterobacter sp. at a national level. This makes ertapenem the most appropriate empiric choice for APN. The guidelines also mention that a carbapenem is preferred if blood culture is positive.[32]
Guidelines on urological infections published by the European Association of Urology in 2015 also mentions ertapenem as one of the recommended initial empirical parenteral antimicrobial therapies in severe acute uncomplicated pyelonephritis.[33]
Although OPAT offers many advantages to patients, economic benefit is the major reason for its increasing utilization globally. Cost analyses of OPAT programs in the USA, Canada, and several European countries have reported savings in the range of 18%–87% over inpatient hospitalization costs. A similar trend was also reported in Asia from Singapore.[1,14,34] Our analysis too showed similar results where median costs were significantly lower for patients who received treatment as OPAT alone or post early discharge compared to those who were completely treated as inpatients during their course of illness. As actual costs were considered in our analysis, savings in overall treatment cost to patients reflect the economic benefits of OPAT in Indian health care. This is important in a country where national health-care reimbursement programs are lacking, provisions for employee health insurance are not mandatory, and awareness about the importance of private health cover is limited. As a result, most Indians end up paying for health-care costs on their own which often disrupts personal finances in cases of unplanned health expenditure.
Limitations
Although the major strength of our study is that it is the first to evaluate OPAT practice using a carbapenem in India, there were several limitations. Apart from being a retrospective study, only two centers could participate as OPAT practice is a niche concept and OPAT using an antimicrobial which is usually classified as “restricted” by most hospital formularies is rare. These were also reasons why patient data analyzed across the two centers were unequal. As any cost-effectiveness analysis is incomplete without measuring patient outcomes, though our study measured clinical resolution and evidence of microbiological cure as documented in case records, frequently reported outcome measures such as cost per quality-adjusted life year, adverse events, and re-admission rates were not captured.[1,2,13,17]
Conclusions
OPAT programs, despite the many benefits that they offer patients and service providers, are considered successful only if they maintain clinical standards similar to inpatient care.[14] Hence, major drivers for success of such programs are robust standard operating procedures, effective communication among the multidisciplinary team personnel, and well-defined criteria for monitoring clinical outcomes. Ertapenem though widely prescribed in hospitalized patients also has a distinct place in treating complex infections such as APN as OPAT not only due to its microbiological and pharmacological profile but also because of its unique place in antimicrobial stewardship programs as a broad-spectrum Pseudomonas-sparing antimicrobial with the potential to cover Gram-negative, mixed, aerobic, and anaerobic infections.[25] This has been reemphasized by recent treatment guidelines for urological infections from the ICMR in 2017 and European Association of Urology in 2015, both of which mention ertapenem as one of the recommended empiric antimicrobial agents in the management of APN.[32,33]
Ertapenem in our analysis also showed 100% sensitivity and complete clinical resolution for 96% patients with APN due to ESBL Enterobacteriaceae. It was administered as OPAT in 90% patients and significantly reduced overall treatment costs. Additional studies monitoring adverse events and patient satisfaction at a larger number of centers with an established OPAT practice are needed to better define when in hospital treatment is required.
Financial support and sponsorship
This study was supported by funding from MSD Pharmaceuticals Pvt. Ltd.
Conflicts of interest
S. Puthran and T. Petigara are employees of MSD Pharmaceuticals Pvt. Ltd., Mumbai or Merck and Co., Inc., Kenilworth, NJ, USA and are involved in data analysis, interpretation, and report writing.
References
- 1.Candel FJ, Julián-Jiménez A, González-Del Castillo J. Current status in outpatient parenteral antimicrobial therapy: A practical view. Rev Esp Quimioter. 2016;29:55–68. [PubMed] [Google Scholar]
- 2.MacKenzie M, Rae N, Nathwani D. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: A review of the last decade. Int J Antimicrob Agents. 2014;43:7–16. doi: 10.1016/j.ijantimicag.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Ravelingien T, Buyle F, Deryckere S, Sermijn E, Debrauwere M, Verplancke K, et al. Optimization of a model of out-of-hospital antibiotic therapy (OPAT) in a Belgian university hospital resulting in a proposal for national implementation. Acta Clin Belg. 2016 May;19:1–6. doi: 10.1080/17843286.2016.1183285. [DOI] [PubMed] [Google Scholar]
- 4.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 5.Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT) Int J Antimicrob Agents. 2015;46:307–12. doi: 10.1016/j.ijantimicag.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: Challenges and checklists. J Antimicrob Chemother. 2015;70:965–70. doi: 10.1093/jac/dku517. [DOI] [PubMed] [Google Scholar]
- 7.Seaton RA, Barr DA. Outpatient parenteral antibiotic therapy: Principles and practice. Eur J Intern Med. 2013;24:617–23. doi: 10.1016/j.ejim.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Esposito S, Noviello S, Leone S, Tice A, Seibold G, Nathwani D, et al. Outpatient parenteral antibiotic therapy (OPAT) in different countries: A comparison. Int J Antimicrob Agents. 2004;24:473–8. doi: 10.1016/j.ijantimicag.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Seetoh T, Lye DC, Cook AR, Archuleta S, Chan M, Sulaiman Z, et al. An outcomes analysis of outpatient parenteral antibiotic therapy (OPAT) in a large Asian cohort. Int J Antimicrob Agents. 2013;41:569–73. doi: 10.1016/j.ijantimicag.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Tam I, Weigel B, 4th, Breeze JL, Paulus JK, Nelson J, et al. Comparative outcomes of β-lactam antibiotics in outpatient parenteral antibiotic therapy: Treatment success, readmissions and antibiotic switches. J Antimicrob Chemother. 2015;70:2389–96. doi: 10.1093/jac/dkv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BSAC OPAT. [Last accessed on 2016 Jun 27]. Available from: http://www.e-opat.com .
- 12.Muldoon EG, Switkowski K, Tice A, Snydman DR, Allison GM. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT) Infect Dis (Lond) 2015;47:39–45. doi: 10.3109/00365548.2014.967290. [DOI] [PubMed] [Google Scholar]
- 13.Czoski Murray C, Twiddy M, Meads D, Hess S, Wright J, Mitchell ED, et al. Community IntraVenous Antibiotic Study (CIVAS): Protocol for an evaluation of patient preferences for and cost-effectiveness of community intravenous antibiotic services. BMJ Open. 2015;5:e008965. doi: 10.1136/bmjopen-2015-008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of outpatient parenteral antibiotic therapy (OPAT): An Asian perspective. Int J Antimicrob Agents. 2009;33:46–51. doi: 10.1016/j.ijantimicag.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Esamai F, Tshefu AK, Ayede AI, Adejuyigbe EA, Wammanda RD, Baqui AH, et al. Ongoing trials of simplified antibiotic regimens for the treatment of serious infections in young infants in South Asia and Sub-Saharan Africa: Implications for policy. Pediatr Infect Dis J. 2013;32(Suppl 1):S46–9. doi: 10.1097/INF.0b013e31829ff941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidi AK, Tikmani SS, Warraich HJ, Darmstadt GL, Bhutta ZA, Sultana S, et al. Community-based treatment of serious bacterial infections in newborns and young infants: A randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–72. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira PR, Felix Cda S, Carvalho VC, Giovani AM, Reis RS, Beraldo M, et al. Outpatient parenteral antimicrobial therapy for orthopedic infections – A successful public healthcare experience in Brazil. Braz J Infect Dis. 2016;20:272–5. doi: 10.1016/j.bjid.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trad MA, Zhong LH, Llorin RM, Tan SY, Chan M, Archuleta S, et al. Ertapenem in outpatient parenteral antimicrobial therapy for complicated urinary tract infections. J Chemother. 2017;29:25–9. doi: 10.1080/1120009X.2016.1158937. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi ZA, Syed A, Doi Y. Safety and efficacy of long-term outpatient ertapenem therapy. Antimicrob Agents Chemother. 2014;58:3437–40. doi: 10.1128/AAC.02721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazaz R, Chapman AL, Winstanley TG. Ertapenem administered as outpatient parenteral antibiotic therapy for urinary tract infections caused by extended-spectrum-beta-lactamase-producing gram-negative organisms. J Antimicrob Chemother. 2010;65:1510–3. doi: 10.1093/jac/dkq152. [DOI] [PubMed] [Google Scholar]
- 21.Tice AD. Ertapenem: A new opportunity for outpatient parenteral antimicrobial therapy. J Antimicrob Chemother. 2004;53(Suppl 2):ii83–6. doi: 10.1093/jac/dkh210. [DOI] [PubMed] [Google Scholar]
- 22.Dalela G. Prevalence of extended spectrum beta lactamase (ESBL) producers among gram negative bacilli from various clinical isolates in a tertiary care hospital at Jhalawar, Rajasthan, India. J Clin Diagn Res. 2012;6:182–7. [Google Scholar]
- 23.Gupta V. An update on newer B-lactamases. Indian J Med Res. 2007;126:417–27. [PubMed] [Google Scholar]
- 24.Saltoglu N, Karali R, Yemisen M, Ozaras R, Balkan II, Mete B, et al. Comparison of community-onset healthcare-associated and hospital-acquired urinary infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and antimicrobial activities. Int J Clin Pract. 2015;69:766–70. doi: 10.1111/ijcp.12608. [DOI] [PubMed] [Google Scholar]
- 25.Park DW, Peck KR, Chung MH, Lee JS, Park YS, Kim HY, et al. Comparison of ertapenem and ceftriaxone therapy for acute pyelonephritis and other complicated urinary tract infections in Korean adults: A randomized, double-blind, multicenter trial. J Korean Med Sci. 2012;27:476–83. doi: 10.3346/jkms.2012.27.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: Combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004;53(Suppl 2):ii67–74. doi: 10.1093/jac/dkh208. [DOI] [PubMed] [Google Scholar]
- 27.Cunha BA. Ertapenem. A review of its microbiologic, pharmacokinetic and clinical aspects. Drugs Today (Barc) 2002;38:195–213. doi: 10.1358/dot.2002.38.3.820127. [DOI] [PubMed] [Google Scholar]
- 28.Parakh A, Krishnamurthy S, Bhattacharya M. Ertapenem. Kathmandu Univ Med J (KUMJ) 2009;7:454–60. [PubMed] [Google Scholar]
- 29.Gupta V, Garg R, Garg S, Chander J, Attri AK. Coexistence of extended spectrum beta-lactamases, AMPC beta-lactamases and metallo beta-lactamases in Acinetobacter baumannii from burns patients: A Report from a Tertiary Care Center in India. Ann Burns Fire Disasters. 2013;26:189–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Tomera KM, Burdmann EA, Reyna OG, Jiang Q, Wimmer WM, Woods GL, et al. Ertapenem versus ceftriaxone followed by appropriate oral therapy for treatment of complicated urinary tract infections in adults: Results of a prospective, randomized, double-blind multicenter study. Antimicrob Agents Chemother. 2002;46:2895–900. doi: 10.1128/AAC.46.9.2895-2900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez-Cruz F, Jasovich A, Cajigas J, Jiang Q, Imbeault D, Woods GL, et al. A prospective, multicenter, randomized, double-blind study comparing ertapenem and ceftriaxone followed by appropriate oral therapy for complicated urinary tract infections in adults. Urology. 2002;60:16–22. doi: 10.1016/s0090-4295(02)01664-3. [DOI] [PubMed] [Google Scholar]
- 32.Indian Council of Medical Research. Department of Health Research. New Delhi, India: 2017. Treatment Guidelines for Antimicrobial Use in Common Syndromes. [Google Scholar]
- 33.Grabe M, Bartoletti R, Johansen TE, Cai T, Cek M, Koves B, et al. Guidelines on Urological Infections. European Association of Urology. Limited Update. 2015 Mar [Google Scholar]
- 34.Chapman AL, Seaton RA, Cooper MA, Hedderwick S, Goodall V, Reed C, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: A consensus statement. J Antimicrob Chemother. 2012;67:1053–62. doi: 10.1093/jac/dks003. [DOI] [PubMed] [Google Scholar]


