Abstract
Objective
To investigate the psychosocial etiology of physical frailty by examining the influence of chronic stress and perceived control.
Method
Using population-based samples of older adults from the Health and Retirement Study, this study employed structural equation modeling in cross-sectional (N = 5,250) and longitudinal (N = 2,013) samples to estimate the effects of chronic stress and socioeconomic status (SES) on baseline frailty and change in frailty status over 4 years and the extent to which perceived control mediates or moderates effects of chronic stress.
Results
Perceived control fully mediated effects of chronic stress and partially mediated effects of SES on both baseline frailty and change in frailty. Multigroup analyses revealed that the mediating role of perceived control was consistent across age, gender, and racial/ethnic subgroups. There was no evidence to support a moderating role of perceived control in the chronic stress and frailty relationship.
Discussion
Findings provide novel evidence for a mediating role of perceived control in pathways linking SES and chronic stress to frailty, further underscoring the importance of psychosocial constructs to the development and progression of frailty in older adults.
Keywords: Mediation analysis, Phenotypic frailty, Physical frailty, Psychosocial
Geriatric frailty is conceptualized as an age-related state of extreme vulnerability to environmental or physical stressors, thought to result from dysregulation in multiple physiologic systems that impair the body’s ability to maintain homeostasis (Fried et al., 2001, 2009). Although there are several conceptually and empirically distinct definitions and operational models of frailty (Cesari, Gambassi, van Kan, & Vellas, 2014; Cigolle, Ofstedal, Tian, & Blaum, 2009; Fried et al., 2001; Rockwood et al., 1999), studies have consistently implicated physical frailty as an important risk factor for adverse geriatric outcomes including mortality, falls, disability, and hospitalization (Ensrud et al., 2007; Xue, Walston, Fried, & Beamer, 2011), as well as increased use of health services (Makary et al., 2010).
In spite of frailty’s substantial health and economic consequences, its etiology has yet to be fully elucidated. Research into the pathophysiology of physical frailty (Fried et al., 2001) has implicated chronic systemic inflammation and abnormal or exaggerated neuroendocrine alterations (Leng & Fried, 2009; Yao, Li, & Leng, 2011): Frail older adults have a phenotypic profile that consists of elevated levels of inflammatory markers including interleukin-6 and C-reactive protein (Leng & Fried, 2009; Walston et al., 2002), as well as dysregulated cortisol secretion (Varadhan et al., 2008). Notably, neuroendocrine and immune dysregulation have also been linked to chronic stress exposure (McEwen & Stellar, 1993). This observation suggests that chronic stress may contribute to the development of frailty (Cesari, Vellas, & Gambassi, 2013), potentially by initiating allostatic mechanisms leading to the multisystemic dysregulation characteristic of physical frailty (Gruenewald, Seeman, Karlamangla, & Sarkisian, 2009). Furthermore, researchers have identified chronic stress as a plausible mechanism explaining frailty’s well-established associations with sociodemographic factors and markers of social stratification including socioeconomic status (Szanton, Seplaki, Thorpe, Allen, & Fried, 2010) and race/ethnicity (Hirsch et al., 2006). In spite of these conceptual linkages, the relationship of chronic stress to physical frailty remains one of the least developed areas in empirical frailty research.
Investigation of the potential etiologic contribution of psychological factors to frailty has been similarly limited. Although some evidence suggests that the psychological constructs of positive affect (Ostir, Ottenbacher, & Markides, 2004; Park-Lee, Fredman, Hochberg, & Faulkner, 2009) and psychological well-being (Gale, Cooper, Deary, & Sayer, 2014; Woo, Goggins, Sham, & Ho, 2005) are associated with frailty, conclusions from these studies are limited by inconsistent operational models of frailty and the potential for bias and confounding relating to cross-sectional study designs. One exception is a longitudinal study by Gale and colleagues (2014), who found that psychological well-being was associated with lower risk of incident frailty in an English cohort. It is unclear, however, whether similar findings would be observed in the U.S. population and whether frailty is related to other well-validated psychological constructs including perceived control, which has been considered a component of successful aging (Rowe & Kahn, 1987). Supporting a potential relationship with frailty, low control has been associated with several components of physical frailty including poorer grip strength (Infurna & Gerstorf, 2014) and physical exhaustion in older adults (Dulin, Hanson, & King, 2013).
In addition to a potentially direct association with physical frailty, perceived control could also moderate effects of chronic stress on frailty. Although chronic stress is generally associated with adverse outcomes, there is evidence of selective vulnerability, whereby certain individuals are more or less prone to its pathogenic effects (Cohen, Janicki-Deverts, & Miller, 2007), potentially as a function of psychological factors (Cohen & Wills, 1985; McEwen & Stellar, 1993). In prior studies, perceived control buffered adverse effects of stress exposures and low socioeconomic status (SES) on health outcomes, including in older adults (Pudrovska, Schieman, Pearlin, & Nguyen, 2005; Turiano, Chapman, Agrigoroaei, Infurna, & Lachman, 2014). Although, there is also some evidence for mediation (Pudrovska et al., 2005), there is greater support for a moderating role of control in relation to health. Focusing on the constructs of chronic stress and perceived control, the present study aimed to develop empirically based insights into the potential psychosocial etiology of physical frailty—a syndrome of physiological dysregulation that results in shrinking, weakness, slowing, exhaustion, and low physical activity (Fried et al., 2001).
Using population-based samples of community-dwelling older adults from the Health and Retirement study in conjunction with structural equation modeling (SEM), we sought to develop and test models of the influence of chronic stress and perceived control, as well as socioeconomic status and other sociodemographic variables, on baseline frailty and change in frailty status over a 4-year period. Based on conceptual models and prior studies (Gale et al., 2014; Glei, Goldman, Chuang, & Weinstein, 2007), we hypothesized that chronic stress would be associated with greater frailty severity at baseline as well as increase in frailty severity over time. Conversely, we hypothesized that perceived control would be associated with lower frailty severity at baseline and smaller increases in frailty severity over time. We further hypothesized that perceived control would moderate (rather than mediate) this relationship, such that effects of chronic stress on frailty would be attenuated at high levels of control. We also evaluated potential differences in these models across age, gender, and race/ethnicity. There are important psychosocial and health differences between young and older segments within the older adult population (Baltes & Smith, 2003), and perceived control is known to decrease with age (Lachman & Firth, 2004). Furthermore, there are differences in both prevalence of frailty and perceptions of control by gender and race (Infurna & Mayer, 2015; Shaw & Krause, 2001). These findings suggest that associations of SES, chronic stress, and control with frailty may vary along demographic lines, although related evidence is insufficient to state specific hypotheses regarding the nature of these differences.
Method
Sample
The present study utilized cross-sectional and longitudinal data from the Health and Retirement Study (HRS) collected in 2006, 2010, and 2012. (The present study did not utilize the 2008 wave given that it did not incorporate a measure of chronic stress.) The HRS is a representative panel of U.S. households that surveys adults aged 50 and older and their spouses on issues concerning employment and labor force, retirement, and health, on a biennial basis. HRS participants considered eligible for the present study included respondents aged 65 and older at baseline who consented to physical tests administered during the in-home survey visit and completed a self-administered psychosocial questionnaire. Based on previously established criteria (Cigolle et al., 2009; Fried et al., 2001), we excluded those with a history of stroke (10%), severe cognitive impairment (1%), and multiple depressive symptoms (12%) given that despite symptomatic resemblance, these conditions are likely to be sequelae of conditions distinct from frailty. Multiple depressive symptoms was defined as ≥4 on the modified Center for Epidemiologic Studies Depression Scale (CES-D), and severe cognitive impairment was defined as ≤8 on an instrument modeled after the Mini-Mental State Examination. The final cross-sectional sample included 5,250 respondents and the longitudinal sample, 2,013 respondents.
Measures
Sociodemographic variables
Sociodemographic covariates included age (continuous in years), gender (male or female), and race/ethnicity (Black, White, and Other). Due to sample size limitations, the Black and Other categories were combined into a single racial/ethnic minority category for SEM analyses.
Frailty
Frailty was operationalized using the validated clinical phenotype model developed by Fried and colleagues (2001), which has been widely utilized in frailty studies including those investigating psychological factors (Gale et al., 2014; Park-Lee et al., 2009). The phenotypic model classifies individuals as frail, prefrail, and nonfrail/robust based on the following five components: unintentional weight loss, weakness, slowness, exhaustion, and low energy expenditure. The presence of three or more components indicates the presence of frailty. One or two components indicate a prefrail state, and zero component indicates a nonfrail state. Unintentional weight loss was assessed using a direct measurement of weight loss over time. More specifically, it defined unintentional weight loss as a 10% or greater weight loss in the previous 2 years. If prior weight data were not available, a body mass index of less than 18.5kg/m2 was used as the defining criteria (Bandeen-Roche et al., 2006). Weakness was assessed using participants’ average grip strength score measured with hand dynamometers. Weakness was defined as grip strength scores in the lowest 20% of the sample distribution, adjusting for gender and body mass index (Fried et al., 2001). Slowness was assessed using a measure of walking speed over an 8-foot distance, and defined as a walking speed score in the lowest 20% of the sample distribution, adjusting for gender and standing height (Fried et al., 2001). Participants who refused or did not complete the grip or walking tests due to safety concerns (noted by the participant or the interviewer) or physical limitations were also classified as having weakness or slowness (Bandeen-Roche et al., 2015). Exhaustion was defined by a response of “Yes” to at least one of two items from the modified CES-D that asked participants to identify whether they “felt activities were efforts” and “could not get going”. Low energy expenditure was assessed using a weighted activity scale based on respondents’ self-reported frequency of participation in mild, moderate, and vigorous physical activity (0 = hardly ever/never; 1 = 1–3 times a month; 2 = once a week; 3 = more than once a week), developed in prior studies of HRS participants (Cigolle et al., 2009; Lohman, 2014). Low energy expenditure was defined as an activity scale score falling in the lowest 20% of the sample distribution, stratified by gender.
Perceived control
Perceived control was measured as a latent variable using a previously validated measure (Lachman and Weaver, 1998) that asked respondents to rate their level of agreement with a series of control-related statements on a six-point Likert scale ranging from strongly disagree to strongly agree. The measure consists of two subscales, which were utilized as indicators of the latent construct. Personal mastery reflects a general sense of efficacy in attaining goals and was measured using five items (e.g., “I can do just about anything I really set my mind to”). Perceived constraints, which reflect a belief that outcomes are beyond one’s control, were similarly measured with five items (e.g., “I often feel helpless in dealing with the problems of life”). Scale scores were derived by calculating a mean across the respective scale items, with perceived constraints items reverse scored. Final scores for each indicator were set as missing if there were more than three items with missing values (Smith et al., 2013). Internal consistency was high for both mastery (α = .89, .90) and low perceived constraints (α = .84, .83) scales in the cross-sectional and longitudinal samples, respectively.
Chronic stress
The HRS asked respondents to indicate whether they were enduring a series of eight “current and ongoing” stressful situations lasting 12 months or longer (1 = no, didn’t happen), and if so, to rate how upsetting each situation was on a 3-point scale (2 = yes, but not upsetting; 3 = yes, somewhat upsetting; 4 = yes, very upsetting). Thus, this measure reflects both the presence and subjective severity of stressors. The situations included ongoing difficulties relating to the following: personal health (in self), physical or emotional health in spouse/child, alcohol or drug use in family member, work, finances, housing, close relationships, and caregiving. A composite score was estimated by calculating an unweighted sum of respondents’ scores (Smith et al., 2013).
Socioeconomic status
SES was measured as a latent variable comprised of two indicators: total education years completed and total nonhousing financial wealth. The measure of nonhousing financial wealth represented the net value of all investments and savings, after subtracting debt value (Moldoff et al., 2013). To reduce skewness in the nonhousing financial wealth variable, extreme outliers were winsorized using the 5th and 95th percentiles before being standardized (Ghosh & Vogt, 2012).
Analytic Strategy
Preliminary data screening revealed a small number of multivariate outliers in the cross-sectional and longitudinal samples (1.3% and 2.0%, respectively). Sensitivity analyses excluding these outliers revealed no substantive changes in estimates, providing justification for retaining the identified cases and avoiding loss of power. All variables were missing values on less than 2.5% of total cases in both analytic samples. As there was no evidence of systematic nonresponse, missingness was treated as random with missing values estimated using pairwise-present covariance matrices (Muthén & Muthén, 2011).
The SEM analysis proceeded in two stages, beginning with a confirmatory factor analysis to evaluate the fit of the measurement model for the latent constructs of control and SES, followed by an assessment of the hypothesized structural models. Next, we tested a structural mediation model in which baseline frailty (using the cross-sectional sample) and change in frailty (longitudinal sample) were predicted by SES and chronic stress, with the latent construct of perceived control acting as a mediator of both associations (Figures 1 and 2). The significance of mediated effects was evaluated using bias-corrected bootstrap confidence intervals and standard errors (Shrout & Bolger, 2002). Next, we tested a model in which perceived control moderated relationships of chronic stress to baseline frailty and change in frailty. To do so, we tested the significance of a product term for the interaction of the latent perceived control variable and the manifest chronic stress variable, adjusting for SES and other model covariates. Lastly, we examined the invariance of the mediation model across age (65–79 years vs 80 years and older), gender, and racial/ethnic subgroups (White vs. combined racial/ethnic minority), comparing model parameters in unconstrained and constrained models using chi-square difference tests (Kline, 2011). Due to sample size limitations, invariance across racial/ethnic subgroups was tested in the cross-sectional sample alone. In a supplemental analysis, we examined the consistency of results using an alternative chronic stress measure derived from scales measuring constructs of everyday discrimination, major lifetime discrimination, stressful life events, and lifetime trauma. An additional analysis examined manifest measures of each dimension of control (i.e., mastery and low perceived constraints) in separate models.
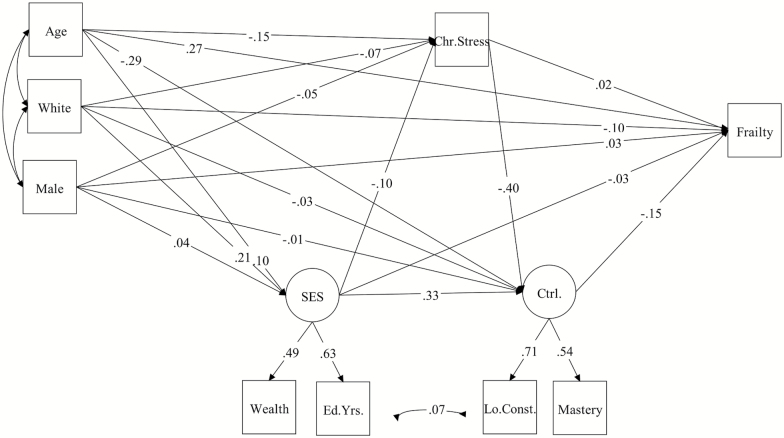
Figure 1.
Standardized path coefficients (β) for cross-sectional mediation model (N = 2,013).
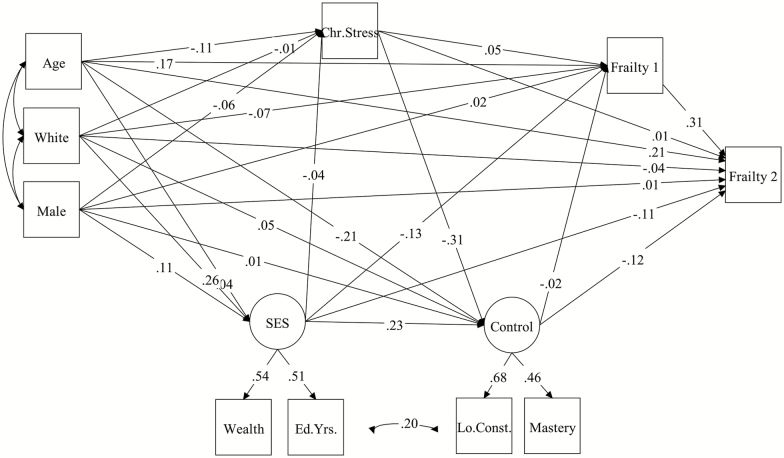
Figure 2.
Standardized path coefficients (β) for longitudinal mediation model (N = 5,250).
Due to the ordinal nature of the dependent variable, path coefficients for the structural models were estimated using weighted least squares means and variance adjusted estimation techniques (Muthén, du Toit, & Spisic, 1997). The measurement and structural models were assessed using goodness-of-fit criteria that compared covariance matrices between the hypothesized model and observed data. Standard fit indexes including the Chi-square goodness-of-fit test, the root mean square error of approximation (RMSEA), and the comparative fit index (CFI) were employed. Evidence of acceptable fit is given by RMSEA values less than or equal to .05 (MacCallum, Browne, & Sugawara, 1996) and CFI values of .90 or greater (Hu & Bentler, 1999). Model respecifications were made if recommended by modification indexes and aligned with theoretical models. A sample weight, based on the product of respondents’ psychosocial questionnaire and physical measure weights, was applied to all structural models. These weights were a product of HRS respondent weights and nonresponse adjustment factors and have been described in detail elsewhere (Smith et al., 2013). All SEM analyses adjusted for sample strata and clustering of data resulting from the inclusion of respondents from the same household. We did not adjust for sampling error computation unit (SECU). Analyses were performed using Mplus, version 7.3 (Muthén & Muthén, 2011).
Results
Table 1 presents demographic characteristics as well as scores on SES, perceived control, and chronic stress for the cross-sectional and longitudinal samples. In line with other studies of physical frailty (Bandeen-Roche et al., 2015; Fried et al., 2001), the weighted prevalence of frailty was 6.8% in the cross-sectional sample. Baseline frailty prevalence was lower in the longitudinal sample (3.7%), which may be a reflection of selection bias as only those with follow-up data were included. Frailty prevalence at follow-up was 8.1%. In bivariate analyses (not shown), greater frailty severity was directly associated with greater age and minority race/ethnicity and inversely associated with education years and nonhousing financial wealth (all p < .001). No significant differences were found in frailty severity as a function of gender.
Table 1.
Cross-Sectional (N = 5,250) and Longitudinal (N = 2,013) Sample Characteristics
| Cross-sectional sample | Longitudinal samplea | |||
|---|---|---|---|---|
| Unweighted % | Weighted % | Unweighted % | Weighted % | |
| Gender | ||||
| Male | 42.7 | 44.8 | 45 | 45.3 |
| Female | 57.3 | 55.2 | 55 | 54.7 |
| Race | ||||
| White | 84.7 | 89 | 88.4 | 92.1 |
| Black | 12.1 | 7.7 | 9.3 | 5.7 |
| Other | 3.2 | 3.3 | 2.3 | 2.2 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 72.8 (6.8) | 72.1 (6.9) | 72.8 (5.9) | 73.1 (6.1) |
| SES | ||||
| Wealth (in 10,000) | 12.1 (20.4) | 12.6 (20.9) | 15.1 (23.5) | 15.8 (24.3) |
| Education (years) | 12.7 (3.0) | 12.9 (2.9) | 12.9 (2.8) | 13.1 (2.8) |
| Perceived control | ||||
| Mastery (1–6) | 4.8 (1.1) | 4.8 (1.1) | 4.9 (1.0) | 4.9 (1.0) |
| Low constraints (1–6) | 4.9 (1.1) | 4.9 (1.1) | 5.1 (0.9) | 5.0 (0.9) |
| Chronic stress (8–32) | 11.2 (3.2) | 11.3 (3.2) | 10.8 (2.8) | 10.8 (2.9) |
Notes: SES = socioeconomic status; SD = standard deviation.
Wealth refers to nonhousing financial wealth.
aRepresents baseline characteristics (2006).
Evaluation of Measurement and Mediation Structural Models
Cross-sectional sample
A CFA employing robust maximum likelihood estimation showed adequate fit for the overall measurement model in the cross-sectional sample, χ2 (1) = 13.69, p < .001; CFI = .99; RMSEA = .039. The structural model in the cross-sectional sample (Model 1) was also a good fit to the data, χ2 (11) = 117.35, p < .001; CFI = .952; RMSEA = .043. Based on modification indices, a covariance of residuals was added between low constraints and education years, and resulted in a significant improvement in model fit, Δχ2 (1) = 9.77, p <.001.
As hypothesized, chronic stress was related to baseline frailty (β = .08), with the effect being almost entirely indirect through perceived control (β = .06). Perceived control also had a direct effect on lower baseline frailty (β = −.15). As with control, the results showed a significant indirect effect of SES on baseline frailty through control (β = −.05), and a small but significant indirect effect through both chronic stress and control (β = −.006).
Longitudinal sample
The measurement model showed excellent fit in the longitudinal sample, χ2 (1) = .17, p = .68; CFI = 1.0; RMSEA = .00. The overall structural model predicting change in frailty (Model 2) was also a good fit to the data, χ2 (12) = 34.28, p < .001; CFI = .96; RMSEA = .043. (Due to modification indices suggested in the cross-sectional model, the original model included a covariance between the residuals for low constraints and education years.) As with the prior model, the direct effect of chronic stress on change in frailty was not significant (β = −.01); however, there was a significant indirect effect of chronic stress on change in frailty operating through control (β = .05). Control was also found to have a direct effect on change in frailty (β = −.15) but not on baseline frailty (β = −.02). Contrary to the cross-sectional model, SES had a direct effect on change in frailty (β = −.10), as well as an indirect effect through perceived control (β = −.04), consistent with partial mediation. However, an indirect effect through chronic stress and perceived control (β = −.004) was not significant. Path coefficients in structural models are shown in Table 2 and Figures 1 and 2.
Table 2.
Standardized Probit Regression Coefficients (β) for Mediation Model in Full-Sample and Multigroup Analyses
| Full-sample analyses | Selected multigroup analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 5 | Model 7 | Model 9 | |||||||
| CS. | Long. | Gender | Race/Ethnicity | Age | |||||||
| Effect | N = 5,250 | N = 2,013 | Male | Female | p Valuea | White | Minority | p Valuea | Young | Old | p Valuea |
| n = 2,243 | n = 3,007 | n = 4,446 | n = 804 | n = 4,318 | n = 932 | ||||||
| Frail2←Frail1 | — | .31** | — | — | — | — | — | — | — | — | — |
| Frail2←C.Stress | — | .05 | — | — | — | — | — | — | — | — | — |
| Frail2←Ctrl. | — | −.15* | — | — | — | — | — | — | — | — | — |
| Frail2←SES | — | −.10† | — | — | — | — | — | — | — | — | — |
| Frail2←Age | — | .20** | — | — | — | — | — | — | — | — | — |
| Frail2←Male | — | .01 | — | — | — | — | — | — | — | — | — |
| Frail2←White | — | −.04 | — | — | — | — | — | — | — | — | — |
| Frail1←C.Stress | .02 | .05 | .03 | .00 | NS | .01 | .17 | NS | −.01 | −.01 | NS |
| Frail1←Ctrl. | −.16** | −.02 | −.10 | −.20** | NS | −.17** | .06 | NS | −.16* | −.31** | NS |
| Frail1←SES | −.03 | −.12 | −.14† | .05 | .03 | −.03 | −.09 | NS | −.06 | −.02 | NS |
| Frail1←Age | .27** | .18** | .27** | .28** | NS | .28** | .27* | NS | — | — | — |
| Frail1←Male | .03 | .02 | — | — | — | .04 | .01 | NS | .01 | .12 | NS |
| Frail1←White | −.10** | −.09* | −.07 | −.14** | NS | — | — | — | −.11** | −.07 | NS |
| Ctrl.←C.Stress | −.40** | −.33** | −.44** | −.36** | NS | −.40** | −.39** | NS | −.41** | −.25** | NS |
| Ctrl.←SES | .33** | .24** | .33** | .27** | NS | .32** | .37 | NS | .34** | .28† | NS |
| Ctrl.←Age | −.29** | −.20** | −.21** | −.36** | NS | −.30** | −.16 | NS | — | — | — |
| Ctrl.←Male | −.01 | .03 | — | — | — | −.00 | −.15 | NS | −.01 | .14† | .05 |
| Ctrl.←White | −.03 | .05 | −.01 | −.04 | NS | — | — | — | −.03 | −.10 | NS |
| C.Stress←SES | −.10* | −.07 | −.19** | −.01 | .02 | −.07 | −.37* | .03 | −.13* | .09 | .02 |
| C.Stress←Age | −.15** | −.10* | −.14** | −.14** | NS | −.15** | −.22** | NS | — | — | — |
| C.Stress←Male | −.05† | −.04 | — | — | — | −.07* | 11 | NS | −.04 | −.12† | NS |
| C.Stress←White | −.07* | −.01 | −.10** | −.03 | NS | — | — | — | −.05† | −.16** | .03 |
Notes: Results of Models 3 and 4 (cross-sectional and longitudinal moderation models) are not presented. Models 6 and 10 (longitudinal subsamples) are not presented, as results were highly similar to Models 5 and 9.
CS = cross-sectional; C.Stress = chronic stress; Ctrl = perceived control; Frail1 = frailty at baseline; Frail2 = frailty at time 2; Long = longitudinal; NS = not significant; SES = socioeconomic status.
aProbability value for critical ratio (z score) of difference between parameters across groups.
† p < .05. *p < .01. **p < .001.
Supplemental analyses using an alternative measure of stress incorporating lifetime stressors and trauma provided similar findings with respect to the mediating role of perceived control in associations of chronic stress and SES with baseline frailty and change in frailty. Analyses employing separate measures of mastery and low constraints were generally consistent with prior findings, showing significant indirect effects of chronic stress on baseline frailty and change in frailty through both measures. However, the indirect effects of SES on baseline and change in frailty were generally weaker and not significant (see Supplementary Material). Although these differences may be substantive, they could also be due to increased measurement error in the manifest measures relative to the latent measure.
Evaluation of moderation models
Results for the model evaluating moderation in the cross-sectional sample (Model 3) showed that the interaction between chronic stress and perceived control in predicting baseline frailty (N = 5,129) was not significant (B = −.006, p = .95). Similar results were observed in the longitudinal sample (Model 4; N = 1,986) examining the interactive effect of chronic stress and control on change in frailty (B = −.004, p = .94).
Multigroup analyses
Multigroup analyses were conducted (Table 2) to examine potential differences in the structural mediation models across age, gender, and race/ethnicity. Multigroup analyses were not performed for the moderation model as it was not supported in the overall sample. Given highly similar results for the cross-sectional and longitudinal samples, only the former are presented. The results showed that there were no significant differences in indirect effects of chronic stress and SES through perceived control by age, gender, and race/ethnicity. However, there was a direct effect of SES on frailty for men but not for women. In addition, there were stronger direct effects of SES on chronic stress in the younger group (65–80), men, and racial/ethnic minorities.
Discussion
The objective of the present study was to address key knowledge and methodological gaps that exist within the extant literature concerning the psychosocial etiology of physical frailty and to develop empirically based insights using population-based samples of older adults from the HRS. We found that chronic psychosocial stress was associated with greater severity of frailty at baseline and over a 4-year period in a large and nationally representative cohort of older adults. As noted, there are few prior empirical studies of the effects of acute or chronic stressors on physical frailty. Peek, Howrey, Ternent, Ray, and Ottenbacher (2012) previously reported that health and financial stressors, but not a measure of stressors reflecting negative life events experienced in the prior year, were associated with frailty over time in older Mexican American adults.
In the present study, we found that associations of chronic stress with baseline frailty and change in frailty over time were entirely indirect through perceived control. In other words, greater levels of chronic stress were associated with lower levels of control, which in turn were associated with greater severity of frailty. Notably, this finding was also obtained when examining a composite measure of various types of stress (discrimination, stressful life events, and traumatic experiences) over the life course. Although there is no existing evidence with which to substantiate this specific pathway to frailty, other studies have reported a mediating role of perceived control in associations between financial-based stressors and adverse health outcomes in older adults (Barbareschi, Sanderman, Kempen, & Ranchor, 2008; Pudrovska et al., 2005). Furthermore, this finding appears consistent with conceptual models of stress reactivity including the stress process model (Pearlin, Lieberman, Menaghan, & Mullan, 1981). These models posit that stress alone does not explain adverse health outcomes. Rather, the effects of stress on health are mediated by its adverse effects on psychological processes, which in turn could result in greater stress reactivity and cumulative physiological burden (e.g., allostatic load) possibly contributing, over the long term, to the pathogenesis of frailty.
The association of perceived control with frailty observed here is consistent with a sizeable literature linking control with better psychological and physical health outcomes in old age (Dulin et al., 2013; Infurna & Gerstorf, 2014) as well as mounting evidence linking positive psychological constructs with frailty (Gale et al., 2014; Ostir et al., 2004; Park-Lee et al., 2009) or its outcomes (Dent & Hoogendijk, 2014).
Consistent with prior findings linking SES and frailty (Szanton et al., 2010), we found direct and indirect effects of SES on baseline frailty and change in frailty. SES exerted an indirect effect on frailty through perceived control, such that greater SES was associated with greater control, which in turn was associated with lower severity of frailty. There was also an indirect effect of SES on baseline frailty, but not change in frailty, through multiple mediators of chronic stress and perceived control. These findings parallel prior studies that have observed a mediating role of control in the SES and health relationship (Barbareschi et al., 2008; Bosma et al., 2005).
The mechanisms through which perceived control and SES influence frailty were not directly investigated in the present study and represent an important area for future inquiry. For example, perceived control could impact the development or progression of frailty by influencing autonomic, neuroendocrine, and inflammatory responses to stress (Sanz & Villamarín, 2001). There is some evidence that control might influence coping strategies (Caplan & Schooler, 2007) and in turn levels of psychological distress, which has been linked to systemic inflammation (Duivis, Vogelzangs, Kupper, de Jonge, & Penninx, 2013) and other aspects of physiologic dysregulation. It is also possible that low perceived control and low SES may affect frailty by promoting behavioral risk factors such as sedentariness (Cesari et al., 2015) and poor nutrition (Bartali et al., 2006), which have been linked with frailty and its components. Lachman and Firth (2004), for instance, found that perceived control is positively associated with exercise and engagement in leisure activities, which are predictive of positive health and psychological outcomes. More recently, Infurna and Gerstorf (2014) reported that physical activity mediated associations of control with grip strength as well as measures of cardiometabolic risk factors in the HRS. Importantly, behavioral and physiologic mechanisms are not mutually exclusive pathways, but instead may intersect to influence frailty status.
The finding that perceived control mediated effects of chronic stress and SES on baseline frailty and change in frailty is particularly important in light of the observation, consistent with prior research (Lachman & Firth, 2004), that perceived control diminished with age. We found that the mediating role of perceived control in the chronic stress and frailty relationship was generally equivalent across age, gender, and racial/ethnic subgroups. Nevertheless, advanced age, female gender, and minority race/ethnicity are themselves established frailty risk factors (Fried et al., 2001; Hirsch et al., 2006), and careful attention should be given to these groups when performing and designing frailty-based research and interventions. Although the mediation model was generally equivalent across groups, we did find that the unmediated effect of SES on baseline frailty and chronic stress was greater for men than for women. Furthermore, the effect of SES on chronic stress was greater for the younger and racial/ethnic minority subgroups. These findings extend existing evidence documenting disparities in the SES and stress relationship (Warnecke et al., 2008; Wilkinson & Marmot, 2003).
Contrary to our hypothesis, we found no evidence that perceived control moderates the relationship of chronic stress to frailty. This finding generally parallels that of another study using HRS data (Ward, 2012), in which perceived control did not moderate effects of SES on self-reported health. It remains possible, however, that control or other psychological resources may exhibit stress-buffering effects on frailty in other older adult populations, including those in institutionalized settings (Dent and Hoogendijk, 2014).
The results of this study must be interpreted in light of study limitations. First, despite incorporating a longitudinal sample, data were limited to two time points. Thus, future studies employing repeated-measures designs over an extended period are necessary to further delineate the role of psychological resources in frailty trajectories. Related to the longitudinal sample, it is unclear whether survival bias or other selection factors influenced results. Second, the community-dwelling sample limits the generalizability of results to more impaired older adults including those residing in long-term care facilities where frailty prevalence is expected to be greater than in the community (Freiheit et al., 2011). In light of prior findings suggesting the importance of perceived control to the health of institutionalized older adults (Langer and Rodin, 1976), future studies are necessary to examine whether the findings observed here extend to these vulnerable populations. Third, SEM is useful for investigating complex mechanisms involving latent variables and multiple indirect effects, yet it also imposes strict assumptions regarding its parametric structure. Future work might examine similar questions using alternative approaches to mediation analysis (e.g., Valeri & Vanderweele, 2013). Lastly, due to software limitations, our analyses did not adjust for the SECU. These limitations are balanced by several study strengths including a large nationally representative cohort, an integrative model incorporating SES, stress, and control, and the analysis of both mediation and moderation.
In summary, we found indirect effects of SES and chronic stress on baseline frailty and change in frailty through perceived control in population-based samples of U.S. older adults. These findings advance the empirical evidence for an association of psychosocial factors with physical frailty in older adults and further underscore the health significance of psychological resources including perceived control in older adulthood. Future work should explore the potential value of interventions that may increase older adults’ sense of control and possibly forestall the development, or slow the progression, of frailty. In addition, our findings suggest that health interventions targeting stress exposure or management may wish to evaluate the impact of stress on older adults’ perceptions of control. Further examination of the role of psychosocial factors including stress and psychological resources is an important avenue in research ultimately aimed at reducing the burden of frailty in older adults.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
C. L. Seplaki was supported by Mentored Research Scientist Development Award number K01AG031332 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Supplementary Material
References
- Baltes P. B., & Smith J (2003). New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology, 49, 123–135.doi:10.1159/000067946 [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K., Seplaki C. L., Huang J., Buta B., Kalyani R. R., Varadhan R., … Kasper J. D. (2015). Frailty in older adults: A nationally representative profile in the United States. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 70, 1427–1434. doi:10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K. Xue Q. Ferrucci L. Walston J. Guralnik J. Chaves P., … Fried L (2006). Phenotype of frailty: Characterization in the women’s health and aging studies. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 262–266. doi:10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- Barbareschi G., Sanderman R., Kempen G. I., Ranchor A. V. (2008). The mediating role of perceived control on the relationship between socioeconomic status and functional changes in older patients with coronary heart disease. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, P353–P361. [DOI] [PubMed] [Google Scholar]
- Bartali B., Frongillo E. A., Bandinelli S., Lauretani F., Semba R. D., Fried L. P., Ferrucci L. (2006). Low nutrient intake is an essential component of frailty in older persons. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H. Van Jaarsveld C. H. Tuinstra J. Sanderman R. Ranchor A. V. Van Eijk J. T., & Kempen G. I (2005). Low control beliefs, classical coronary risk factors, and socio-economic differences in heart disease in older persons. Social Science & Medicine, 60, 737–745. doi:S0277953604002850 [DOI] [PubMed] [Google Scholar]
- Caplan L. J., & Schooler C (2007). Socioeconomic status and financial coping strategies: The mediating role of perceived control. Social Psychology Quarterly, 70, 43–58. [Google Scholar]
- Cesari M., Vellas B., Gambassi G. (2013). The stress of aging. Experimental Gerontology, 48, 451–456. doi:10.1016/j.exger.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Cesari M., Gambassi G., van Kan G. A., Vellas B. (2014). The frailty phenotype and the frailty index: Different instruments for different purposes. Age and Ageing, 43, 10–12. doi:10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- Cesari M. Vellas B. Hsu F. C. Newman A. B. Doss H. King A. C.…Pahor M; LIFE Study Group (2015). A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 70, 216–222. doi:10.1093/gerona/glu099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigolle C. T., Ofstedal M. B., Tian Z., Blaum C. S. (2009). Comparing models of frailty: The Health and Retirement Study. Journal of the American Geriatrics Society, 57, 830–839. doi:10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- Cohen S. Janicki-Deverts D., & Miller G. E (2007). Psychological stress and disease. The Journal of the American Medical Association, 298, 1685–1687. doi:10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Cohen S., & Wills T (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–357. doi:10.1037//0033-2909.98.2.310 [PubMed] [Google Scholar]
- Dent E., Hoogendijk E. O. (2014). Psychosocial factors modify the association of frailty with adverse outcomes: a prospective study of hospitalised older people. BMC Geriatrics, 14, 108. doi:10.1186/1471-2318-14-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis H. E., Vogelzangs N., Kupper N., de Jonge P., Penninx B. W. (2013). Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology, 38, 1573–1585. doi:10.1016/j.psyneuen.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Dulin P. L., Hanson B. L., King D. K. (2013). Perceived control as a longitudinal moderator of late-life stressors on depressive symptoms. Aging & Mental Health, 17, 718–723. doi:10.1080/13607863.2013.784956 [DOI] [PubMed] [Google Scholar]
- Ensrud K. E. Ewing S. K. Taylor B. C. Fink H. A. Stone K. L. Cauley J. A.,… for the Study of Osteoporotic Fractures Research Group. (2007). Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62, 744–751. doi:10.1093/gerona/62.7.744 [DOI] [PubMed] [Google Scholar]
- Freiheit E. A., Hogan D. B., Strain L. A., Schmaltz H. N., Patten S. B., Eliasziw M., Maxwell C. J. (2011). Operationalizing frailty among older residents of assisted living facilities. BMC Geriatrics, 11, 23. doi:10.1186/1471-2318-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried L. P. Tangen C. M. Walston J. Newman A. B. Hirsch C. Gottdiener J.,… Cardiovascular Health Study Collabor. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56, M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Fried L. P. Xue Q. L. Cappola A. R. Ferrucci L. Chaves P. Varadhan R.…Bandeen-Roche K (2009). Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64, 1049–1057. doi:10.1093/gerona/glp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R. Cooper C. Deary I. J., & Sayer A. A (2014). Psychological well-being and incident frailty in men and women: the English Longitudinal Study of Ageing. Psychological Medicine, 44, 697–706. doi:10.1017/S0033291713001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., & Vogt A (2012). Outliers: An evaluation of methodologies. Paper presented at the Joint Statistical Meetings (pp. 3455–3460). Retrieved from https://www.amstat.org/sections/srms/proceedings/y2012/files/304068_72402.pdf [Google Scholar]
- Glei D. A., Goldman N., Chuang Y. L., Weinstein M. (2007). Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosomatic Medicine, 69, 769–776. doi:10.1097/PSY.0b013e318157cba6 [DOI] [PubMed] [Google Scholar]
- Gruenewald T. L., Seeman T. E., Karlamangla A. S., Sarkisian C. A. (2009). Allostatic load and frailty in older adults. Journal of the American Geriatrics Society, 57, 1525–1531. doi:10.1111/j.1532-5415.2009.02389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C. Anderson M. L. Newman A. Kop W. Jackson S. Gottdiener J., … Fried L. P (2006). The association of race with frailty: The cardiovascular health study. Annals of Epidemiology, 16, 545–553. doi:10.1016/j.annepidem.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Hu L., & Bentler P. M (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. doi:10.1080/10705519909540118 [Google Scholar]
- Infurna F. J., Gerstorf D. (2014). Perceived control relates to better functional health and lower cardio-metabolic risk: The mediating role of physical activity. Health Psychology, 33, 85–94. doi:10.1037/a0030208 [DOI] [PubMed] [Google Scholar]
- Infurna F. J., Mayer A. (2015). The effects of constraints and mastery on mental and physical health: Conceptual and methodological considerations. Psychology and Aging, 30, 432–448. doi:10.1037/a0039050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. B. (2011). Principles and practice of structural equation modeling (3rd ed.). New York, NY: Guilford Press. [Google Scholar]
- Lachman M. E., & Firth K. M (2004). The adaptive value of feeling in control during midlife. In Brim O. G. Ryff C. D., & Kessler R. C. (Eds.), How healthy are we? A national study of well-being at midlife (pp. 320–349). Chicago, IL: University of Chicago Press. [Google Scholar]
- Lachman M. E., & Weaver S. L (1998). The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology, 74, 763–773. doi: 10.1037/0022-3514.74.3.763 [DOI] [PubMed] [Google Scholar]
- Langer E. J., & Rodin J (1976). The effects of choice and enhanced personal responsibility for the aged: A field experiment in an institutional setting. Journal of Personality and Social Psychology, 34, 191–198. doi:10.1037/0022-3514.34.2.191 [DOI] [PubMed] [Google Scholar]
- Leng S. X., & Fried L. P (2009). Inflammatory markers and frailty: Basic understanding and clinical applications. In Fulop T. Franceschi C. Hirokawa K., & Pawelec G. (Eds.), Handbook on immunosenescence (pp. 1293–1303). Netherlands: Springer. doi:10.1007/978-1-4020-9063-9_62 [Google Scholar]
- Lohman M. (2014). Frailty and depression: A latent trait analysis (Doctoral dissertation, Virginia Commonwealth University). Retrieved November 19, 2014 from VCU Theses and Dissertations. Paper 3324. Retrieved from http://scholarscompass.vcu.edu/etd/3324
- MacCallum R. C. Browne M. W., & Sugawara H. M (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1, 130–149. doi:10.1037/1082-989X.1.2.130 [Google Scholar]
- Makary M. A., Segev D. L., Pronovost P. J., Syin D., Bandeen-Roche K., Patel P.,…, Fried L. P. (2010). Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons, 210, 901–908. doi:10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. doi:10.1001/archinte.153.18.2093 [PubMed] [Google Scholar]
- Moldoff M. Meijer E. Chien S. Hayden O. Hurd M. Main R., … St. Clair P (2013). RAND HRS income and wealth imputation, Version M. Santa Monica, CA: RAND Corporation, Center for the Study of Aging; Retrieved from http://hrsonline.isr.umich.edu/modules/meta/rand/randincwlth/randiwm.pdf [Google Scholar]
- Muthén L. K., & Muthén B. O (2011). Mplus user’s guide. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Muthén B. du Toit S. H. C., & Spisic D (1997). Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. Unpublished technical report.
- Ostir G. V., Ottenbacher K. J., Markides K. S. (2004). Onset of frailty in older adults and the protective role of positive affect. Psychology and Aging, 19, 402–408. doi:10.1037/0882-7974.19.3.402 [DOI] [PubMed] [Google Scholar]
- Park-Lee E., Fredman L., Hochberg M., Faulkner K. (2009). Positive affect and incidence of frailty in elderly women caregivers and noncaregivers: Results of Caregiver-Study of Osteoporotic Fractures. Journal of the American Geriatrics Society, 57, 627–633. doi:10.1111/j.1532-5415.2009.02183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin L. I., Lieberman M. A., Menaghan E. G., Mullan J. T. (1981). The stress process. Journal of Health and Social behavior, 22, 337–356. [PubMed] [Google Scholar]
- Peek M. K., Howrey B. T., Ternent R. S., Ray L. A., Ottenbacher K. J. (2012). Social support, stressors, and frailty among older Mexican American adults. The Journals of Gerontology, Series B, Psychological Sciences and Social Sciences, 67, 755–764. doi:10.1093/geronb/gbs081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudrovska T., Schieman S., Pearlin L. I., Nguyen K. (2005). The sense of mastery as a mediator and moderator in the association between economic hardship and health in late life. Journal of Aging and Health, 17, 634–660. doi:10.1177/0898264305279874 [DOI] [PubMed] [Google Scholar]
- Rockwood K. Stadnyk K. MacKnight C. McDowell I. Hébert R., & Hogan D. B (1999). A brief clinical instrument to classify frailty in elderly people. The Lancet, 353, 205–206. doi:10.1016/S0140-6736(98)04402-X [DOI] [PubMed] [Google Scholar]
- Rowe J. W., & Kahn R. L (1987). Human aging: Usual and successful. Science, 237, 143–149. doi:10.1126/science.3299702 [DOI] [PubMed] [Google Scholar]
- Sanz A., & Villamarín F (2001). The role of perceived control in physiological reactivity: Self efficacy and incentive value as regulators of cardiovascular adjustment. Biological Psychology, 56, 219–246. doi:10.1016/S0301-0511(01)00095-3 [DOI] [PubMed] [Google Scholar]
- Shaw B. A., & Krause N (2001). Exploring race variations in aging and personal control. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56, S119–S124. doi:10.1093/geronb/56.2.S119 [DOI] [PubMed] [Google Scholar]
- Shrout P. E., Bolger N. (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 7, 422–445. [PubMed] [Google Scholar]
- Smith J. Fisher G. Ryan L. Clarke P. House J., & Weir D (2013). Psychosocial and lifestyle questionnaire 2006–2010 (No. Documentation Report Core Section LB). Ann Arbor, MI: Produced and distributed by the University of Michigan; with funding from the National Institute on Aging (grant number NIA U01AG009740). [Google Scholar]
- Szanton S. L., Seplaki C. L., Thorpe R. J., Jr, Allen J. K., Fried L. P. (2010). Socioeconomic status is associated with frailty: The Women’s Health and Aging Studies. Journal of Epidemiology and Community Health, 64, 63–67. doi:10.1136/jech.2008.078428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano N. A. Chapman B. P. Agrigoroaei S. Infurna F. J., & Lachman M (2014). Perceived control reduces mortality risk at low, not high, education levels. Health Psychology, 33, 883–890. doi:10.1037/hea0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L., Vanderweele T. J. (2013). Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychological Methods, 18, 137–150. doi:10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R., Walston J., Cappola A. R., Carlson M. C., Wand G. S., Fried L. P. (2008). Higher levels and blunted diurnal variation of cortisol in frail older women. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 63, 190–195. [DOI] [PubMed] [Google Scholar]
- Walston J. McBurnie M. Newman A. Tracy R. P. Kop W. J. Hirsch C. H. … Fried L. P; Cardiovascular Health Study (2002). Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the cardiovascular health study. Archives of Internal Medicine, 162, 2333–2341. doi:10.1001/archinte.162.20.2333 [DOI] [PubMed] [Google Scholar]
- Ward M. M. (2012). Sense of control and sociodemographic differences in self-reported health in older adults. Quality of Life Research, 21, 1509–1518. doi:10.1007/s11136-011-0068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke R. B. Oh A. Breen N. Gehlert S. Paskett E. Tucker K. L., … Hiatt R. A (2008). Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health, 98, 1608–1615. doi:10.2105/AJPH.2006.102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., & Marmot M. G (2003). Social determinants of health: The solid facts (2nd ed.). Geneva, Switzerland: World Health Organization; ISBN: 978-9289013710 [Google Scholar]
- Woo J., Goggins W., Sham A., Ho S. C. (2005). Social determinants of frailty. Gerontology, 51, 402–408. doi:10.1159/000088705 [DOI] [PubMed] [Google Scholar]
- Xue Q. L., Walston J. D., Fried L. P., Beamer B. A. (2011). Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the women’s health and aging study. Archives of Internal Medicine, 171, 1119–1121. doi:10.1001/archinternmed.2011.252 [DOI] [PubMed] [Google Scholar]
- Yao X., Li H., Leng S. X. (2011). Inflammation and immune system alterations in frailty. Clinics in Geriatric Medicine, 27, 79–87. doi:10.1016/j.cger.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.