Abstract
Background
The aims of this study were to investigate the incidence and risk factors for the development of bone metastases and prognosis in women with cancer of the uterine cervix using database analysis.
Material/Methods
The National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) database was analyzed for the incidence and survival rates of women diagnosed with uterine cervical cancer in the United States between 2010–2015. Multivariate logistic regression analysis identified risk factors for bone metastases. Kaplan-Meier analysis estimated the overall survival. Proportional hazard regression analysis estimated prognostic factors associated with bone metastases.
Results
There were 19,363 women with uterine cervical cancer, and 469 women were diagnosed with bone metastases on initial diagnosis (2.42%). Increased T-stage, N-stage, non-squamous and non-adenocarcinoma histology, high-grade tumors, and the presence of lung, liver, and brain metastases were all significantly associated with early bone metastases. There were 364 patients with cervical cancer and bone metastases on initial diagnosis who were followed-up for at least one year. Multivariate Cox regression analysis showed that unmarried status and lung, liver, and brain metastases were significantly associated with reduced overall survival. No other significant risk or prognostic associations were found.
Conclusions
SEER data analysis showed that women with uterine cervical cancer had some standard risk factors associated with bone metastases, and with prognosis, but a heterogeneous group of risk factors was also present. The findings of this study may have clinical application in screening for bone metastases in women with cervical cancer.
MeSH Keywords: Neoplasm Metastasis, Risk Factors, SEER Program, Uterine Cervical Neoplasms
Background
Uterine cervical cancer is the fourth most common malignancy in women, with an estimated 528,000 new cases reported worldwide in 2012 [1,2]. On initial diagnosis, 13% of women with uterine cervical cancer are at an advanced stage, which results in reduced patient survival [3,4].
In women with cervical cancer, metastasis to bone is one of the most common sites, which may lead to skeletal-related events, including bone pain and pathological fracture, and to a reduced patient quality of life [5,6]. The incidence of bone metastases in women with uterine cervical cancer has been reported to range from 1.1–29.0% [6–10]. Early diagnosis and proper treatment of bone metastases in cervical cancer can prevent or reduce symptoms such as bone pain [11]. Currently, there are no screening guidelines for the detection of bone metastases in women who present with cervical cancer. The identification of risk factors for the development of bone metastases could ensure that patients with a high risk for developing bone metastases are thoroughly investigated, and, if possible, treated at an early stage or provided with appropriate preventive treatment. A previously published study showed that clinical and pathological factors, including the presence of cervical adenocarcinoma, advanced stage (IIB–IV), were significantly associated with earlier occurrence of bone metastases in cervical cancer, but this study had a small sample size [11]. Therefore, studies with larger study populations are needed.
Although the development of novel systemic therapies has contributed to significant improvements in prognosis for uterine cervical cancer, the presence of bone metastases results in reduced patient survival [6,11]. A series of studies reported the median survival time of 7–12 months among women with cervical cancer who had bone metastases, and more than 60% of patients with cervical cancer died within six months after being diagnosed with bone metastases [6,11–14]. Therefore, there is a need to study the prognostic factors for bone metastases and provide proper treatment to the high-risk patients with cervical cancer [15]. A previously published study that included 68 patients with cancer of the uterine cervix also showed that when compared with elderly patients, young patients with bone metastases who were aged less than 45 years, had a poorer prognosis [16]. It has also been reported that patients with cancer of the uterine cervix who have a higher tumor TNM stage and several sites for bone metastases had a worse survival rate [11]. However, not only are large study sample sizes required, but real-world evidence is still required to determine the prognostic factors for bone metastases in patients with cancer of the uterine cervix.
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) was established in 1973, which includes approximately 30% of the total US population and provides an important data source for epidemiologic analysis [17]. Therefore, the aims of this study were to analyze the SEER database to determine the incidence and the risk factors for bone metastases in women with bone metastases on initial diagnosis of uterine cervical cancer, and to determine the overall survival, prognosis, and risk factors for patients who present with cervical cancer associated with bone metastases.
Material and Methods
Study population
Data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER *Stat 8.3.5 software (https://seer.cancer.gov/data/) was used to access the database. Since the details of metastases were not recorded before 2010, patients with primary cancer of the uterine cervix who were aged ≥18 years at diagnosis, between 2010–2015, were analyzed. The site code ICD-O-3 (International Classification of Diseases for Oncology-3)/WHO 2008 was restricted to ‘cervix uteri.’ The exclusion criteria for patient selection included patients diagnosed with carcinoma in-situ, benign or borderline tumors, patients diagnosed at autopsy or via death certificates, patients who had not been investigated for bone metastases, or who did not undergo follow-up.
Patients who were identified as having histologically-confirmed cervical cancer from 1st January 2010 to 31st December 2015 were included in the analysis of the incidence and risk factors for bone metastases. Women who were diagnosed with cancer of the uterine cervix with bone metastases between 2010–2014 (with at least one year of follow-up) were included to conduct survival analysis and to investigate the prognostic factors for bone metastases.
Ethics statement
The SEER database is an open public database, and the release of data from the SEER database does not require informed patient consent because cancer is a reportable disease in every state of the United States. The present study complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and the study was approved by the Research Ethics Board of the Tianjin First Central Hospital (2018N012KY).
Statistical analysis
The following patient demographic and clinical characteristics were included: age (≤40, 41–64 years, and ≥65 years); ethnicity (Caucasian, black, others); marital status (married and unmarried); insurance status (insured and uninsured); primary tumor stage (T-stage: T1, T2, T3 and T4); regional lymph node stage (N-stage: N0 and N1); histology, including squamous, adenocarcinoma, adenosquamous, and other types, including large cell neuroendocrine carcinoma (35 patients), small cell carcinoma, NOS (175 patients), neuroendocrine carcinoma, NOS (86 patients), malignant mesonephroma (23 patients), leiomyosarcoma, NOS (28 patients); tumor grade, G1 or well-differentiated, G2 or moderately-differentiated, and G3, or poorly-differentiated, undifferentiated or anaplastic; and the presence of lung metastases, liver metastases, and brain metastases.
The differences in the incidence of bone metastases between the categorical variables were analyzed by the chi-squared (χ2) test or rank sum test. The risk factors for women with cancer of the uterine cervix with bone metastases at initial diagnosis were determined primarily by univariate logistic regression analysis. Characteristics with P<0.05 in the univariate logistic regression analysis were then further analyzed using a multivariate logistic regression model. The primary outcome of the survival analysis was the overall survival, which was defined from the time of diagnosis of uterine cervical cancer to all causes of death. Kaplan-Meier curves were used to test the overall survival rate, and the log-rank test evaluated the survival differences. Also, multivariate Cox proportional hazard regression analysis was performed for analyzing the prognostic factors for bone metastases. Univariate Cox regression analysis was performed for the surgical treatments of the primary site (yes or no). All statistical analysis was performed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA). MedCalc version 15.2.2 statistical software was used to analyze survival data. A P-value <0.05 was considered as statistically significant.
Results
Demographic and clinical characteristics
A total of 19,363 women with cancer of the uterine cervix met the criteria for inclusion in the study (Figure 1). The mean patient age was 50.86±14.97 years, most patients were Caucasian (74.6%), and 53.0% were unmarried. Among these patients, 364 patients with cervical cancer and bone metastases underwent clinical follow-up for at least one year (mean survival, 9.94±12.38 months), and the mean age was 56.43±13.78 years. The demographic and clinical characteristics of the patients included in the study are shown in Table 1.
Figure 1.
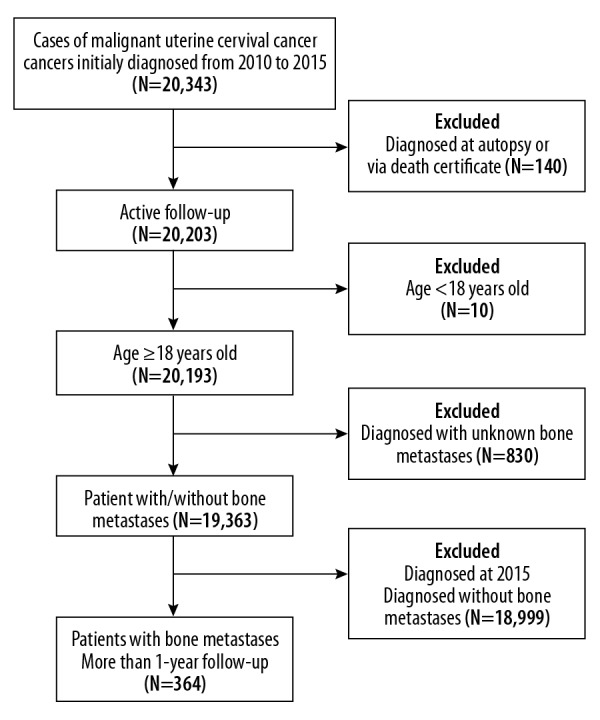
A flow chart showing the study design and patient selection.
Table 1.
Baseline demographic and clinical characteristics for patients diagnosed with uterine cervical cancer.
| Subject characteristics | No. of cervical cancer patients (2010–2015) | No. of cervical cancer patients (2010–2014) | ||
|---|---|---|---|---|
| With bone mets (N=469) (2.42%) | Without bone mets (N=18894) (97.58%) | With bone mets (N=364) (2.27%) | Without bone mets (N=15662) (97.73%) | |
| Age (years) | ||||
| ≤40 | 57 (1.06) | 5299 (98.94) | 49 (1.10) | 4398 (98.90) |
| 41–64 | 285 (2.76) | 10058 (97.24) | 210 (2.46) | 8333 (97.54) |
| ≥65 | 127 (3.47) | 3537 (96.53) | 105 (3.46) | 2931 (96.54) |
| Ethnicity | ||||
| Caucasian | 341 (2.36) | 14096 (97.64) | 260 (2.18) | 11678 (97.82) |
| Black | 81 (3.00) | 2615 (97.00) | 68 (3.00) | 2199 (97.00) |
| Others | 47 (2.31) | 1985 (97.69) | 36 (2.15) | 1636 (97.85) |
| Unknown | 0 (0.00) | 198 (100.00) | 0 (0.00) | 149 (100.00) |
| Marital status | ||||
| Married | 168 (2.13) | 7719 (97.87) | 129 (1.97) | 6406 (98.03) |
| Unmarried | 280 (2.73) | 9985 (97.27) | 220 (2.59) | 8629 (97.41) |
| Unknown | 21 (1.73) | 1190 (98.27) | 15 (1.50) | 987 (98.50) |
| Insurance status | ||||
| Insured | 420 (2.39) | 17150 (97.61) | 325 (2.24) | 14153 (97.76) |
| Uninsured | 38 (3.01) | 1223 (96.99) | 30 (2.71) | 1076 (97.29) |
| Unknown | 11 (2.07) | 521 (97.93) | 9 (2.04) | 433 (97.96) |
| T-stage | ||||
| T1 | 62 (0.59) | 10370 (99.41) | 46 (0.53) | 8621 (99.47) |
| T2 | 83 (1.96) | 4156 (98.04) | 67 (1.92) | 3415 (98.08) |
| T3 | 183 (6.02) | 2859 (93.98) | 143 (5.66) | 2382 (94.34) |
| T4 | 59 (8.07) | 672 (91.93) | 45 (7.54) | 552 (92.46) |
| Unknown | 82 (8.92) | 837 (91.08) | 63 (8.34) | 692 (91.66) |
| N-stage | ||||
| N0 | 119 (0.87) | 13523 (99.13) | 91 (0.80) | 11267 (99.20) |
| N1 | 288 (5.96) | 4544 (94.04) | 220 (5.60) | 3707 (94.40) |
| Unknown | 62 (6.97) | 827 (93.03) | 53 (7.15) | 688 (92.85) |
| Histology | ||||
| Squamous | 269 (2.17) | 12138 (97.83) | 212 (2.06) | 10078 (97.94) |
| Adenocarcinoma* | 90 (1.75) | 5060 (98.25) | 67 (1.57) | 4189 (98.43) |
| Others | 110 (6.09) | 1696 (93.91) | 85 (5.74) | 1395 (94.26) |
| Grade | ||||
| I | 9 (0.42) | 2127 (99.58) | 6 (0.34) | 1751 (99.66) |
| II | 78 (1.30) | 5905 (98.70) | 63 (1.27) | 4895 (98.73) |
| III# | 211 (3.54) | 5744 (96.46) | 165 (3.35) | 4760 (96.65) |
| Unknown | 171 (3.23) | 5118 (96.77) | 130 (2.96) | 4256 (97.04) |
| Lung mets | ||||
| None | 265 (1.43) | 18221 (98.57) | 208 (1.36) | 15112 (98.64) |
| Yes | 185 (22.67) | 631 (77.32) | 139 (21.38) | 511 (78.62) |
| Unknown | 19 (31.15) | 42 (68.85) | 17 (30.36) | 39 (69.64) |
| Liver mets | ||||
| None | 328 (1.73) | 18587 (98.27) | 258 (1.65) | 15400 (98.35) |
| Yes | 128 (30.92) | 286 (69.08) | 94 (27.98) | 242 (72.02) |
| Unknown | 13 (38.24) | 21 (61.76) | 12 (37.50) | 20 (62.50) |
| Brain mets | ||||
| None | 423 (2.20) | 18834 (97.80) | 329 (2.06) | 15609 (97.94) |
| Yes | 30 (38.46) | 48 (61.54) | 19 (31.15) | 42 (68.85) |
| Unknown | 16 (57.14) | 12 (42.86) | 16 (59.26) | 11 (40.74) |
| Surg (prim) | ||||
| None | 435 (5.05) | 8183 (94.95) | 338 (4.77) | 6741 (95.23) |
| Yes | 34 (0.32) | 10677 (99.68) | 26 (0.29) | 8891 (99.71) |
| Unknown | 0 (0.00) | 34 (100.00) | 0 (0.00) | 30 (100.00) |
Including adenosquamous;
including undifferentiated;
BM – bone metastases; mets – metastases; Surg (prim) – surgical treatment of primary site.
Incidence of bone metastases
In total, 469 women with cancer of the uterine cervix were diagnosed with bone metastases on initial diagnosis (2.42%). Patients ≥65 years of age (3.47%) presented with a significantly increased incidence of bone metastases when compared with patients in the lower age groups (χ2=63.55, P<0.001). Moreover, patients with unmarried status (χ2=6.62, P=0.01), higher T stage (χ2=469.49, P<0.001), N stage (χ2=428.72, P<0.001), non-squamous or non-adenocarcinoma (χ2=116.15, P<0.001), poor differentiated grade (χ2=107.18, P<0.001) and with lung (χ2=1548.09, P<0.001), liver (χ2=1497.94, P<0.001) and brain metastases (χ2=446.54, P<0.001) showed higher BM incidence than the others. However, the incidence of bone metastases showed no significant differences between different ethnic groups (χ2=4.10, P=0.13) and between insurance status (χ2=1.93, P=0.17) (Table 1).
Risk factors for developing bone metastases
Univariate logistic analysis showed that the following factors were all significantly associated with the development of bone metastases: older patient age (OR=3.34; 95% CI, 2.44–4.58; P<0.001), unmarried status (OR=1.29; 95% CI, 1.06–1.56; P=0.01), higher T-stage (OR=14.69; 95% CI, 10.20–21.15; P<0.001), higher N-stage (OR=7.20; 95% CI, 5.80–8.94; P<0.001), non-squamous and non-adenocarcinoma histological type (OR=2.93; 95% CI, 2.33–3.67; P<0.001), poorly-differentiated grade (G3) (OR=8.68; 95% CI, 4.45–16.95; P<0.001), the presence of lung metastases (OR=20.16; 95% CI, 16.44–24.72; P<0.001), liver metastases (OR=25.36; 95% CI, 20.04–32.09; P<0.001), and brain metastases (OR=27.83; 95% CI, 17.46–44.36; P=0.01).
Multivariate logistic regression analysis showed that a higher T-stage, a higher N-stage, non-squamous and non-adenocarcinoma histological type, higher grade, and the presence of lung, liver, and brain metastases were all significantly associated with the early development of bone metastases (Table 2, Figure 2).
Table 2.
Univariate and multivariate logistic regression analysis for the associated risk factors for developing bone metastases in patients diagnosed with uterine cervical cancer between 2010–2015.
| Subject characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (years) | ||||
| ≤40 | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| 41–64 | 2.63 (1.98–3.51) | <0.001 | 1.44 (0.95–2.18) | 0.08 |
| ≥65 | 3.34 (2.44–4.58) | <0.001 | 1.36 (0.84–2.21) | 0.22 |
| Marital status | ||||
| Married | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| Unmarried | 1.29 (1.06–1.56) | 0.01 | 0.99 (0.74–1.33) | 0.94 |
| Unknown | NA | NA | NA | NA |
| T-stage | ||||
| T1 | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| T2 | 3.34 (2.40–4.65) | <0.001 | 1.97 (1.25–3.10) | 0.003 |
| T3 | 10.71 (8.00–14.32) | <0.001 | 3.86 (2.52–5.91) | <0.001 |
| T4 | 14.69 (10.20–21.15) | <0.001 | 4.05 (2.36–6.96) | 0.001 |
| Unknown | NA | NA | NA | NA |
| N-stage | ||||
| N0 | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| N1 | 7.20 (5.80–8.94) | <0.001 | 2.89 (2.09–4.00) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Histology | ||||
| Squamous | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| Adenocarcinoma* | 0.80 (0.63–1.02) | 0.07 | 1.17 (0.81–1.69) | 0.40 |
| Others | 2.93 (2.33–3.67) | <0.001 | 1.87 (1.24–2.82) | 0.003 |
| Grade | ||||
| I | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| II | 3.12 (1.56–6.24) | 0.001 | 1.66 (0.73–3.77) | 0.23 |
| III# | 8.68 (4.45–16.95) | <0.001 | 2.72 (1.23–6.04) | 0.01 |
| Unknown | NA | NA | NA | NA |
| Lung metastases | ||||
| None | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| Yes | 20.16 (16.44–24.72) | <0.001 | 5.00 (3.54–7.06) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Liver metastases | ||||
| None | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| Yes | 25.36 (20.04–32.09) | <0.001 | 6.25 (4.10–9.54) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Brain metastases | ||||
| None | 1 (Reference) | 1.00 | 1 (Reference) | 1.00 |
| Yes | 27.83 (17.44–44.36) | <0.001 | 7.21 (3.10–16.74) | <0.001 |
| Unknown | NA | NA | NA | NA |
Including adenosquamous;
including undifferentiated;
NA – not available. All factors with unknown data were removed from the univariate and multivariate logistic regression model.
Figure 2.
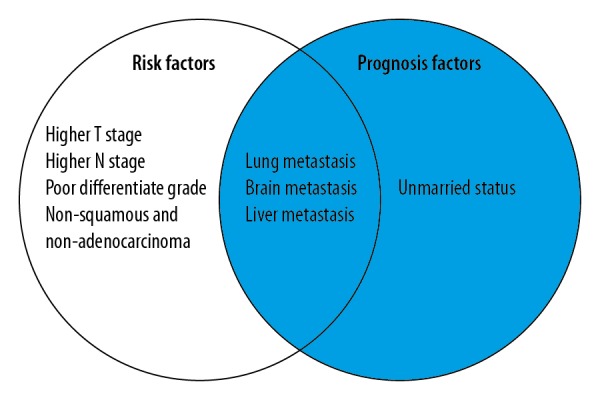
The homogeneous and heterogeneous risk factors and prognosis factors associated with bone metastases in patients with cancer of the uterine cervix. All the factors included in the left circle represent the risk factors for developing bone metastases. The factors included in the right circle represent the risk factors that were positively associated with overall risk of mortality for patients with uterine cervical cancer with bone metastases.
Survival analysis and prognostic factors for bone metastases
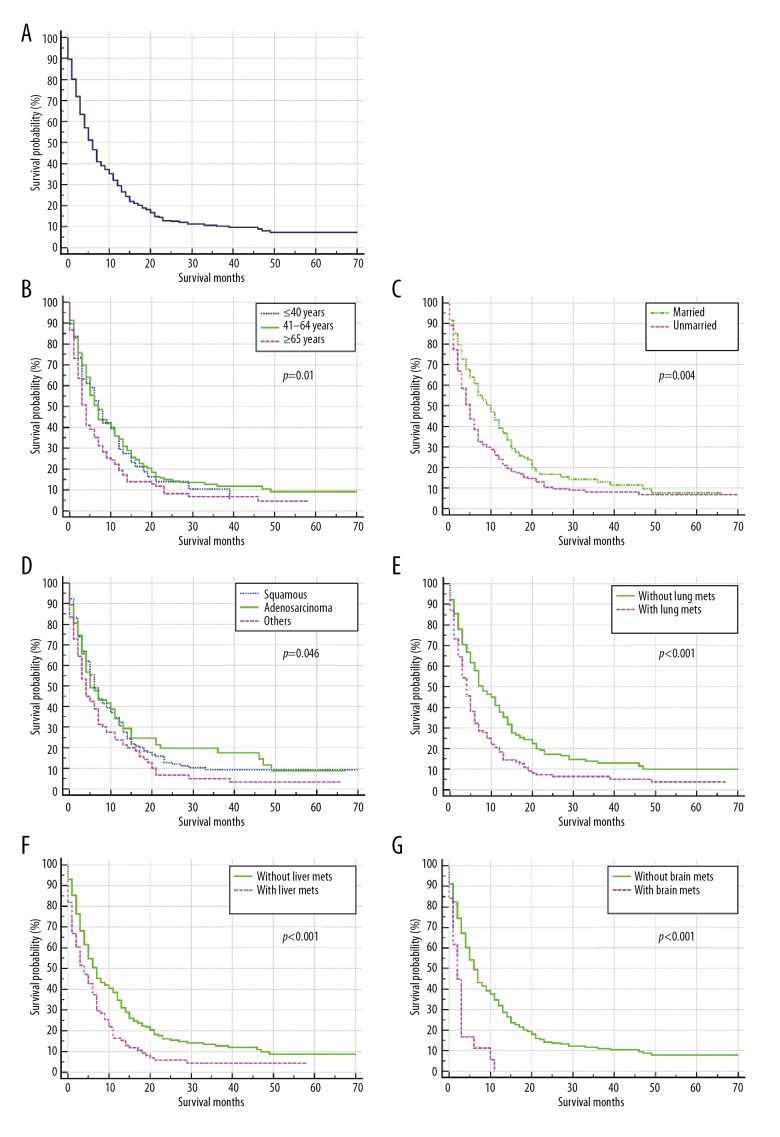
When women with cancer of the uterine cervix were diagnosed with bone metastases, their survival was significantly reduced. The overall one-year and three-year survival rate of the cohort was 85% and 69%, respectively, which decreased to 32% and 11%, respectively following a diagnosis of bone metastases. Of the 364 patients with cervical cancer who had bone metastases on initial diagnosis (diagnosed between 2010–2014), the median overall survival time was 6.00 months (95% CI, 5.05–6.95 months) (Figure 3A). Kaplan-Meier analysis showed the overall survival was significantly reduced in women of older age (P=0.01) (Figure 3B), unmarried status (P=0.004) (Figure 3C), non-squamous and non-adenocarcinoma histological type (P=0.046) (Figure 3D) and with lung metastases (P<0.001) (Figure 3E), liver metastases (P<0.001) (Figure 3F) and brain metastases (P<0.001) (Figure 3G). Patients with surgical treatment of the primary site did not have a significantly increased overall survival compared with those without surgery (P>0.05).
Figure 3.
Kaplan-Meier analysis of overall survival (OS) among patients with cancer of the uterine cervix who were diagnosed with bone metastases, stratified with other risk factors, and the presence of metastases to other sites. (A) Overall survival (OS) for the total population studied. (B) Overall survival (OS) stratified by age. (C) Overall survival (OS) stratified by marital status. (D) Overall survival (OS) stratified by histological grade of the cervical carcinoma. (E) Overall survival (OS) stratified by the presence of lung metastases. (F) Overall survival (OS) stratified by the presence of liver metastases. (G) Overall survival (OS) stratified by the presence of brain metastases. UCC – uterine cervical cancer; Lung mets – lung metastases; Livermets – liver metastases; Brain mets – brain metastases.
Multivariate Cox regression analysis showed that unmarried status (HR=1.34; 95% CI, 1.04–1.72; P=0.02) was associated with poor overall survival, with a median survival time of only five months, while the median survival time of married patients was ten months. Also, the presence of lung metastases (HR=1.48; 95% CI, 1.15–1.89; P=0.002), liver metastases (HR=1.42; 95% CI, 1.07–1.87; P=0.02), and brain metastases (HR=2.64; 95% CI, 1.57–4.43; P<0.001) were all associated with poor prognosis (Table 3, Figure 2).
Table 3.
Multivariate Cox regression analysis for the associated risk factors for developing bone metastases in patients diagnosed with uterine cervical cancer between 2010–2014.
| Subject characteristics | Survival, Median (IQR), months | HR (95% CI) | P-value |
|---|---|---|---|
| Age (years) | |||
| ≤40 | 7.00 (4.50–9.50) | 1 (Reference) | 1.00 |
| 41–64 | 7.00 (5.84–8.16) | 0.87 (0.61–1.24) | 0.44 |
| ≥65 | 4.00 (3.16–4.85) | 1.18 (0.88–1.58) | 0.44 |
| Marital status | |||
| Married | 10.00 (7.10–12.90) | 1 (Reference) | 1.00 |
| Unmarried | 5.00 (4.11–5.89) | 1.34 (1.04–1.72) | 0.02 |
| Unknown | NA | NA | NA |
| Histology | |||
| Squamous | 6.00 (4.83–7.17) | 1 (Reference) | 1.00 |
| Adenocarcinoma* | 5.00 (2.33–7.67) | 0.90 (0.65–1.24) | 0.51 |
| Others | 4.00 (2.90–5.10) | 1.18 (0.88–1.58) | 0.27 |
| Lung metastases | |||
| None | 8.00 (5.87–10.12) | 1 (Reference) | 1.00 |
| Yes | 4.00 (3.05–4.95) | 1.48 (1.15–1.89) | 0.002 |
| Unknown | NA | NA | |
| Liver metastases | |||
| None | 7.00 (5.65–8.34) | 1 (Reference) | 1.00 |
| Yes | 4.00 (2.26–5.74) | 1.42 (1.07–1.87) | 0.02 |
| Unknown | NA | NA | NA |
| Brain metastases | |||
| None | 6.00 (5.05–6.96) | 1 (Reference) | 1.00 |
| Yes | 2.00 (0.64–3.37) | 2.64 (1.57–4.43) | <0.001 |
| Unknown | NA | NA | NA |
Including adenosquamous.
IQR – interquartile range; HR – hazard ratio; CI – confidence interval; NA – not available. All factors with unknown data were removed from Cox and Kaplan-Meier model.
Discussion
To our knowledge, the present study involved the analysis of the largest population of women with cancer of the uterine cervix with bone metastases conducted at this time. The National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) database was analyzed for the incidence and survival rates of women diagnosed with uterine cervical cancer in the United States between 2010–2015, and included 19,363 women with cervical cancer who had an incidence of bone metastases at diagnosis of 2.42% (N=469).
Patients with cancer of the uterine cervix have been reported to have a relatively lower incidence of bone metastases when compared with cancer of the breast, lung, and prostate [17–19]. The present study showed that 2.42% of the study cohort of women with cervical cancer had bone metastases when initially diagnosed, which was a similar finding to that of the study conducted by Thanapprapasr et al. (1.1%) [6], but was lower than that found in other studies [8,20,21]. The variety of detection methods used may partly cause the difference in the reported incidence of bone metastases in women with cervical cancer [6,7,22]. However, using the clinical data from the SEER database, it was not possible to determine which imaging or other detection methods were used to identify bone metastases. There have been few previously reported studies on the risk factors for bone metastases in women with cancer of the uterine cervix, but a previous study showed that older age and a higher International Federation of Gynecology and Obstetrics (FIGO) stage was significantly associated with the development of bone metastases [11]. Also, in the present study, analysis of the large patient sample size showed that a higher tumor grade and stage, including a higher N-stage, non-squamous and non-adenocarcinoma histological type, and the presence of lung, liver, and brain metastases were also significantly associated with the development of bone metastases. These factors might be of clinical value in identifying patients with cervical cancer who are at high risk for the occurrence of bone metastases. For women who are diagnosed with cervical cancer, skeletal radiographic scanning for high-risk patients with cervical cancer might be used in screening for the presence of bone metastases.
Also, the identification of the prognostic factors associated with the presence of bone metastases in women with cervical cancer might help physicians to provide targeted treatment strategies for patients at different levels of risk and improve patient survival and quality of life. To the best of our knowledge, this study is the first to show that lung, liver, and brain metastases were negatively associated with overall survival in patients with cervical cancer who have bone metastases. The results were similar to the findings from studies conducted in other cancers with bone metastases, such as breast cancer [23,24].
Previous studies have shown that the number of metastatic sites was an independent prognostic factor and that more sites for tumor metastases resulted in a worse patient prognosis [24,25]. The present study also showed unmarried status was one of the independent prognosis factors for bone metastases and was negatively associated with overall survival in patients with cancer of the uterine cervix. The following potential explanations are possible. Firstly, married patients may have more opportunity to get prompt diagnosis and treatment due to increased financial support compared with unmarried patients [26,27]. Married patients may have better support from their family after tumor diagnosis, as is depression and stress have been reported to be significantly associated with tumor metastases [28,29]. Married patients may have lower opportunity to be infected by human papillomavirus (HPV), which is the main cause of cervical cancer [16,30].
It has been previously reported that patients with cancer of the uterine cervix who present at under 45 years had shorter overall survival compared with older patients [16]. However, contrary to this previous report, we found no significant correlation between age and overall survival when age was stratified into three subgroups. Also, previously published SEER-based analysis has shown that surgery can promote the overall survival of breast [31], and colorectal cancer [25]. However, no similar association was found in the present study. Further investigations with larger patient population numbers, should be performed to investigate these results further.
This study had several limitations. Firstly, although the presence or absence of bone metastases at the time of the initial diagnosis of cervical cancer was analyzed in the present study, the patients who developed bone metastases later in their disease course could not be analyzed and were not included in the study. Therefore, the real incidence of bone metastases on initial diagnosis of women with cancer of the uterine cervix could have been underestimated. Furthermore, due to the unspecified detection methods used for bone metastases in the cases included in the SEER database, we were not able to compare the different methods. Also, symptoms of bone metastases, including bone pain and pathological fracture have been accepted as the important prognostic factors in other malignancies, but in patients with cervical cancer with bone metastases, these symptoms were not described in detail in the public SEER database and the association between bone symptoms and survival could not be analyzed.
Conclusions
Cancer of the uterine cervix has a relatively lower incidence of metastasis to bone when compared with several other types of cancer. As this study has shown, there are some standard or recognized risk factors associated with bone metastases, and with prognosis, in women with cervical cancer, but a heterogeneous group of risk factors is also present. Risk factors, including an increased stage (T-stage and N-stage), non-squamous and non-adenocarcinoma histological type, poor tumor grade, and the presence of lung, liver and brain metastases, were found to be significantly associated with the finding of bone metastases at the initial diagnosis of cervical cancer. Prognostic factors, including unmarried status, lung metastases, liver metastases and brain metastases, were found to be significantly associated with poor prognosis in cancer of uterine cervix with initial bone metastases. The results of this real-world study may have clinical application in screening for bone metastases when patients present with cervical cancer, with the aim of developing targeted therapy to prevent the development of metastasis to bone in women who present with cancer of the uterine cervix.
Footnotes
Conflicts of interest
None.
Source of support: The present study was sponsored by Natural Science Foundation of China (81602363, 81702161, 81801781, 81872184), Natural Science Foundation of Tianjin Science and Technology Committee China (17JCQNJC11000), Natural Science Foundation of Tianjin Medical University (2016KYZQ10), Russian Foundation of Basic Research (15-29-01338)
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics, 2011. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Google Scholar]
- 3.Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43. doi: 10.3802/jgo.2016.27.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauri A, Messiah SE, Bouzoubaa LA, et al. Cervical cancer sociodemographic and diagnostic disparities in Florida: A population-based study (1981–2013) by stage at presentation. Ethn Health. 2018 doi: 10.1080/13557858.2018.1471669. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31(4):515–28. doi: 10.1016/s0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 6.Thanapprapasr D, Nartthanarung A, Likittanasombut P, et al. Bone metastasis in cervical cancer patients over a 10-year period. Int J Gynecol Cancer. 2010;20(3):373–78. doi: 10.1111/IGC.0b013e3181d4a0a1. [DOI] [PubMed] [Google Scholar]
- 7.Disibio G, French SW. Metastatic patterns of cancers: Results from a large autopsy study. Arch Pathol Lab Med. 2008;132(6):931–39. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 8.Babar S, Rockall A, Goode A, et al. Magnetic resonance imaging appearances of recurrent cervical carcinoma. Int J Gynecol Cancer. 2007;17(3):637–45. doi: 10.1111/j.1525-1438.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Karim FW, Kida M, Wentz WB, et al. Bone metastasis from gynecologic carcinomas: a clinicopathologic study. Gynecol Oncol. 1990;39(2):108–14. doi: 10.1016/0090-8258(90)90414-g. [DOI] [PubMed] [Google Scholar]
- 10.Fulcher AS, O’Sullivan SG, Segreti EM, et al. Recurrent cervical carcinoma: Typical and atypical manifestations. Radiographics. 1999;19:S103–16. doi: 10.1148/radiographics.19.suppl_1.g99oc19s103. [DOI] [PubMed] [Google Scholar]
- 11.Yoon A, Choi CH, Kim HJ, et al. Contributing factors for bone metastasis in uterine cervical cancer. Int J Gynecol Cancer. 2013;23(7):1311–17. doi: 10.1097/IGC.0b013e31829da127. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama T, Mabuchi S, Shimura K, et al. Prognostic factors for survival in cervical cancer patients with bone metastasis. Eur J Gynaecol Oncol. 2015;36(3):290–93. [PubMed] [Google Scholar]
- 13.Ratanatharathorn V, Powers WE, Steverson N, et al. Bone metastasis from cervical cancer. Cancer. 1994;73(9):2372–79. doi: 10.1002/1097-0142(19940501)73:9<2372::aid-cncr2820730921>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama T, Tsukamoto N, Imachi M, et al. Bone metastasis from cervix cancer. Gynecol Oncol. 1989;32(1):72–75. doi: 10.1016/0090-8258(89)90853-6. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri H, Takahashi M, Wakai K, et al. Prognostic factors and a scoring system for patients with skeletal metastasis. J Bone Joint Surg Br. 2005;87(5):698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 16.Nartthanarung A, Thanapprapasr K, Udomsubpayakul U, et al. Age and survival of cervical cancer patients with bone metastasis. Asian Pac J Cancer Prev. 2014;15(19):8401–4. doi: 10.7314/apjcp.2014.15.19.8401. [DOI] [PubMed] [Google Scholar]
- 17.Xiong Z, Deng G, Huang X, et al. Bone metastasis pattern in initial metastatic breast cancer: A population-based study. Cancer Manag Res. 2018;10:287–95. doi: 10.2147/CMAR.S155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira MB, Mello FC, Paschoal ME. The relationship between lung cancer histology and the clinicopathological characteristics of bone metastases. Lung Cancer. 2016;96:19–24. doi: 10.1016/j.lungcan.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Zhang C, Guo Q, et al. The homogeneous and heterogeneous risk factors for the morbidity and prognosis of bone metastasis in patients with prostate cancer. Cancer Manag Res. 2018;10:1639–46. doi: 10.2147/CMAR.S168579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Disibio G, French SW. Metastatic patterns of cancers: Results from a large autopsy study. Arch Pathol Lab Med. 2008;132(6):931–39. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Karim FW, Kida M, Wentz WB, et al. Bone metastasis from gynecologic carcinomas: A clinicopathologic study. Gynecol Oncol. 1990;39(2):108–14. doi: 10.1016/0090-8258(90)90414-g. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal U, Dahiya P, Chauhan A, et al. Scalp metastasis in carcinoma of the uterine cervix--a rare entity. Gynecol Oncol. 2002;87(3):310–12. doi: 10.1006/gyno.2002.6829. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Zhang C, Zhang J, et al. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: A SEER based study. Oncotarget. 2017;8(16):26368–79. doi: 10.18632/oncotarget.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537–48. doi: 10.1007/s10549-016-4066-7. [DOI] [PubMed] [Google Scholar]
- 25.Luo D, Liu Q, Yu W, et al. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: A population-based study. Int J Colorectal Dis. :2018. doi: 10.1007/s00384-018-3091-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zhu MX, Qi SH. Marital status and survival in patients with renal cell carcinoma. Medicine (Baltimore) 2018;97(16):e0385. doi: 10.1097/MD.0000000000010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchioni M, Martel T, Bandini M, et al. Marital status and gender affect stage, tumor grade, treatment type and cancer specific mortality in T1–2 N0 M0 renal cell carcinoma. World J Urol. 2017;35(12):1899–905. doi: 10.1007/s00345-017-2082-9. [DOI] [PubMed] [Google Scholar]
- 28.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone SC, Rossetti RA, Lima AM, et al. HPV associated tumor cells control tumor microenvironment and leukocytosis in experimental models. Immun Inflamm Dis. 2014;2(2):63–75. doi: 10.1002/iid3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537–48. doi: 10.1007/s10549-016-4066-7. [DOI] [PubMed] [Google Scholar]