The development of a human cytomegalovirus (HCMV) vaccine to prevent congenital disease and transplantation-related complications is an unmet medical need. While many HCMV vaccine candidates have been developed, partial success in preventing or controlling HCMV infection in women of childbearing age and transplant recipients has been observed with an approach based on envelope glycoprotein B (gB). We introduce a novel vaccine strategy based on the clinically deployable modified vaccinia virus Ankara (MVA) vaccine vector to elicit potent humoral and cellular immune responses by multiple immunodominant HCMV antigens, including gB, phosphoprotein 65, and all five subunits of the pentamer complex. These findings could contribute to development of a multiantigenic vaccine strategy that may afford more protection against HCMV infection and disease than a vaccine approach employing solely gB.
KEYWORDS: ADCC, HLA, cytomegalovirus, glycoprotein B, neutralizing antibodies, pentamer, phosphoprotein 65, polyfunctional T cells, vaccines, vaccinia
ABSTRACT
As human cytomegalovirus (HCMV) is a common cause of disease in newborns and transplant recipients, developing an HCMV vaccine is considered a major public health priority. Yet an HCMV vaccine candidate remains elusive. Although the precise HCMV immune correlates of protection are unclear, both humoral and cellular immune responses have been implicated in protection against HCMV infection and disease. Here we describe a vaccine approach based on the well-characterized modified vaccinia virus Ankara (MVA) vector to stimulate robust HCMV humoral and cellular immune responses by an antigen combination composed of the envelope pentamer complex (PC), glycoprotein B (gB), and phosphoprotein 65 (pp65). We show that in mice, multiantigenic MVA vaccine vectors simultaneously expressing all five PC subunits, gB, and pp65 elicit potent complement-independent and complement-dependent HCMV neutralizing antibodies as well as mouse and human MHC-restricted, polyfunctional T cell responses by the individual antigens. In addition, we demonstrate that the PC/gB antigen combination of these multiantigenic MVA vectors can enhance the stimulation of humoral immune responses that mediate in vitro neutralization of different HCMV strains and antibody-dependent cellular cytotoxicity. These results support the use of MVA to develop a multiantigenic vaccine candidate for controlling HCMV infection and disease in different target populations, such as pregnant women and transplant recipients.
IMPORTANCE The development of a human cytomegalovirus (HCMV) vaccine to prevent congenital disease and transplantation-related complications is an unmet medical need. While many HCMV vaccine candidates have been developed, partial success in preventing or controlling HCMV infection in women of childbearing age and transplant recipients has been observed with an approach based on envelope glycoprotein B (gB). We introduce a novel vaccine strategy based on the clinically deployable modified vaccinia virus Ankara (MVA) vaccine vector to elicit potent humoral and cellular immune responses by multiple immunodominant HCMV antigens, including gB, phosphoprotein 65, and all five subunits of the pentamer complex. These findings could contribute to development of a multiantigenic vaccine strategy that may afford more protection against HCMV infection and disease than a vaccine approach employing solely gB.
INTRODUCTION
Human cytomegalovirus (HCMV) is the leading infectious cause of permanent birth defects in newborns and complications in transplant recipients (1). Yet despite the recognition of HCMV as a major public health concern (2, 3), a vaccine candidate that could prevent HCMV infection and disease remains elusive. While many HCMV vaccine approaches have been developed over the past 5 decades (4), the live-attenuated HCMV vaccine strain Towne and a glycoprotein B subunit vaccine formulated with MF59 adjuvant (gB/MF59) have been tested most extensively for clinical efficacy (5). Towne showed efficacy in reducing disease in solid-organ transplant recipients and in protecting healthy volunteers from low-dose challenge with HCMV strain Toledo (6, 7), but it failed to provide protection against high-dose Toledo challenge (7) and did not prevent primary HCMV infection in mothers whose children attended day care (8). More encouraging were the findings with the gB/MF59 subunit vaccine. In phase II clinical trials, gB/MF59 showed efficacy in reducing HCMV viremia and need for antiviral therapy in solid-organ transplant recipients and provided partial protection (40 to 50%) against primary HCMV infection of postpartum women and adolescents (9–11). While the immune mechanisms of protection mediated by Towne and gB/MF59 remain unclear, the clinical experience obtained with these vaccine candidates provides evidence for vaccine-mediated protection against HCMV infection and disease.
Early HCMV vaccine development focused primarily on gB due to its recognition as a central mediator in HCMV entry and major target of neutralizing antibodies (NAb) based on in vitro studies using fibroblasts (FB) and laboratory HCMV strains, such as Towne and AD169 (12). However, more recent studies with “clinical-like” HCMV strains, such as TB40/E, TR, and VR1814, have shown that the envelope pentamer complex (PC) composed of gH, gL, UL128, UL130, and UL131A is the major target of NAb preventing in vitro HCMV infection of various non-FB cell types, including epithelial cells (EC), endothelial cells, and monocytes/macrophages (13, 15, 19). In contrast to the clinical-like strains, Towne and AD169 are deficient in expressing an intact PC due to mutations in the UL128/130/131A genes, which greatly restricts their host cell tropism and ability to stimulate NAb (16–18). Importantly for vaccine design, NAb targeting predominantly conformational epitopes formed by the UL128/130/131A subunits of the PC are unable to block FB infection, though they are exceptionally potent in preventing HCMV infection of cell types such as EC (19, 20). Based on these discoveries, many preclinical vaccine strategies have been developed to elicit potent NAb by the PC, which is promoted by the assembly of all five PC subunits (21–23). These include strategies based on viral vectors (21, 23, 24), purified protein (22, 23, 25), mRNA vaccines (26), and a conditionally replication-defective AD169-derived vaccine strain (V160) with repaired PC expression that has recently completed phase I clinical evaluation (18, 27).
Major challenges in HCMV vaccine development include the absence of animal models susceptible to HCMV infection, poorly understood protective HCMV immune correlates, and the imperfect protection by naturally acquired HCMV immunity (28–30). Despite the ill-defined protection by natural HCMV immunity, both humoral and cellular immune responses have been implicated in controlling HCMV infection (30, 31). Clinical and surrogate animal studies indicate that the prevention of congenital HCMV infection involves antibodies to different envelope glycoprotein complexes, including gB, gH/gL, and the PC as well as T cells to immunodominant antigens such as phosphoprotein 65 (pp65) (32–39). High-titer NAb, PC-specific NAb, and pp65-specific CD4+ T cells have been associated with reduced risk of intrauterine virus transmission following primary maternal HCMV infection (33, 36, 39–41). In addition, the importance of antibodies in preventing or controlling congenital HCMV infection may be supported by clinical trials with hyperimmunoglobulins (42), although this remains controversial (43). Both humoral and cellular immune responses appear to also be important to control HCMV infection in transplant recipients (44). While T cell responses to pp65 and other immunodominant antigens, such as the immediate early 1 and 2 (IE1 and IE2) proteins, are well known to control HCMV reactivation during transplantation (45), recent findings with the gB/MF59 subunit vaccine in solid-organ transplant recipients indicate an important role of gB-specific antibodies in controlling HCMV viremia (11, 46). These findings suggest that vaccine-mediated prevention of HCMV-associated disease in either newborns or transplant recipients may depend on stimulation of both humoral and cellular immunity.
Modified vaccinia virus Ankara (MVA) is a highly attenuated poxviral vector that is widely used to develop vaccines for infectious disease and cancer due to its excellent safety record, capacity to express multiple antigens, and ability to elicit potent antigen-specific humoral and cellular immunity (47–49). MVA is unable to productively infect most mammalian cells as a consequence of a late block in virus assembly, allowing robust DNA replication and antigen expression without producing infectious progeny (50). We have used MVA previously to express different HCMV antigens, including gB, the PC subunits, or a combination of pp65 and a IE1/2 fusion protein (21, 24, 51, 52). The vector coexpressing pp65 and IE1/2 (MVA-pp65/IE), also known as Triplex, has recently completed clinical phase I evaluation and showed robust expansion of T cells in HCMV-seronegative and -seropositive healthy volunteers (53). In this study, we have used a recently developed novel MVA bacterial artificial chromosome (BAC) clone to construct MVA vectors coexpressing gB, pp65, and all five PC subunits (54). We show that in mice, these MVA vectors elicit robust HCMV humoral and cellular immune responses, including complement-independent and complement-dependent NAb blocking FB and EC infection, humoral responses promoting antibody-dependent cellular cytotoxicity (ADCC), and mouse and human major histocompatibility complex (MHC)-restricted, polyfunctional T cells. In addition, we show that the PC/gB antigen combination of these vectors can enhance the induction of antibodies promoting neutralization of different HCMV strains and ADCC. These findings support the use of MVA to develop a multiantigenic vaccine strategy that could prevent or reduce HCMV infection and associated disease in different target populations, such as newborns and transplant recipients.
RESULTS
Construction of multiantigenic MVA vectors coexpressing the PC subunits, gB, and pp65.
Using the original MVA BAC generated by Cottingham et al. (55), we previously constructed recombinant MVA (rMVA) vectors with expression constructs for all five PC subunits inserted into separate insertion sites (21, 24). These first-generation MVA-PC vectors stimulated robust HCMV NAb responses in mice and rhesus macaques that exceeded those measurable for HCMV-seropositive individuals (21). In order to simplify the vaccine construction and to reduce the number of expression cassettes and insertion sites needed to accommodate the PC subunits, we constructed a novel MVA BAC, termed MVABAC-TK, with the BAC cassette inserted into the thymidine kinase (TK) gene (54). By exploiting this novel BAC in addition to the ribosomal skipping mechanism mediated by 2A peptides of picornavirus (56, 57), we generated MVA vectors expressing the PC subunits via polycistronic expression constructs inserted into only one or two MVA insertion sites (54). These second-generation MVA-PC vectors were as potent as our first-generation MVA-PC vectors in stimulating HCMV NAb responses in mice (54).
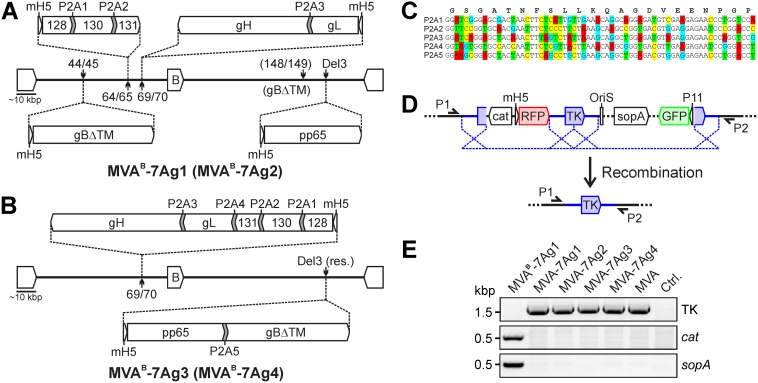
Building on these second-generation MVA-PC vectors, we used MVABAC-TK to generate various multiantigenic MVA vectors that coexpressed all five PC subunits together with gB and pp65. Two concepts of vector construction were employed. The first concept of vector construction used to generate MVAB-7Ag1 and MVAB-7Ag2 (the superscript B indicates that these vectors contained the BAC cassette) was based on the insertion of the seven HCMV antigens into four separate insertion sites. In both MVAB-7Ag1 and MVAB-7Ag2, the UL128/130/131A subunits, gH/gL, and pp65 were inserted as P2A-linked polycistronic and single antigen expression constructs into the intergenic regions (IGR) between MVA 64L and 65L (64/65) and MVA 69R and 70L (69/70) and the MVA deletion 3 (Del3) site (Fig. 1A and Table 1). All of these MVA insertion sites are commonly used insertion sites that have been shown to allow stable antigen expression (24, 52, 58, 59). In contrast, gB was inserted in MVAB-7Ag1 and MVAB-7Ag2 into two different, noncommonly used MVA insertion sites to identify an additional insertion site that would allow stable antigen expression. While gB was inserted in MVAB-7Ag1 into the IGR between MVA 044L and 045L (44/45), it was inserted in MVAB-7Ag2 into the IGR between MVA 148R and 149L (148/149) (Fig. 1A). The commonly used MVA deletion 2 (Del2) site was not used for the vector construction because of previous observations showing frequent antigen instability at this site (52).
FIG 1.
Construction of MVA expressing multiple HCMV antigens. (A and B) Vector construction. Utilizing MVABAC-TK, P2A-linked polycistronic and single antigen expression constructs of the PC subunits (gH, gL, UL128, UL130, and UL131A), gB (without TM), and pp65 were inserted as indicated into different intergenic regions (44/45, 64/45, 69/70, and 148/149), the deletion 3 site (Del3), or a restructured Del3 site (Del3 res.) to generate MVAB-7Ag1, MVAB-7Ag2, MVAB-7Ag3, and MVAB-7Ag4. Different P2A codon sequences (P2A1 to P2A5) were used to link the antigens. mH5, modified H5 promoter; B, BAC vector. (C) P2A codon sequences. The lower 5 lines indicate the different P2A codon sequences with mutated nucleotides (marked in colors) that were used for the vector construction as shown in panels A and B. The upper line shows the amino acid sequence of the P2A peptide. (D and E) BAC removal. A genomic duplication (blue in panel D) was utilized to seamlessly remove the BAC sequences (cat, OriS, sopA, RFP, and GFP) of the different MVA vectors by homologous recombination, resulting in MVA-7Ag1, MVA-7Ag2, MVA-7Ag3, and MVA-7Ag4. TK, thymidine kinase gene; P11, vaccinia virus P11 promoter. Primers P1 and P2 flanking the TK gene and primers specific for cat and sopA were used to confirm the restoration of the TK gene and absence of residual BAC sequences via PCR in DNA isolated from BHK cells infected with rMVA. BHK cells infected with MVAB-7Ag1 containing the BAC vector or parental MVA as well as uninfected cells were analyzed for controls.
TABLE 1.
Primer pairs for recombination
| Primer pair (5′–3′)a | Insertion siteb | Genome positionb |
|---|---|---|
| GAATATGACTAAACCGATGACCATTTAAAAACCCCTCTCTAGCTTTCACTAAAAATTGAAAATAAATACAAAGGTTC | 44L/45L | 37331 |
| ATAATGTTTTTATATTATACATGTTCTAAAAGAATAATCGATACAGTTTACTAGTATAAAAAGGCGCGCC | ||
| AATTGTACTTTGTAATATAATGATATATATTTTCACTTTATCTCATTTGATTTTTATAAAAATTGAAAATAAATACAAAGGTTC | 64L/65L | 56742 |
| ATTCCGAAATCTGTACATCATGCAGTGGTTAAACAAAAACATTTTTATTCCTAGTATAAAAAGGCGCGCC | ||
| ATATGAATATGATTTCAGATACTATATTTGTTCCTGTAGATAATAACTAAAAATTTTTATCTAGTATAAAAAGGCGCGCC | 69R/70L | 63812 |
| GGAAAATTTTTCATCTCTAAAAAAAGATGTGGTCATTAGAGTTTGATTTTTATAAAAATTGAAAATAAATACAAAGGTTC | ||
| ATTGATAATATAAATATGAGCATTAGTATTTCTGTGGATTAATAGATTTTTATAAAAATTGAAAATAAATACAAAGGTTC | 148R/149L | 137504 |
| TTATGAGGTATTTAGAGATTAGAGATGATTAATGATCCCCATACTAGAAATTTTTATCTAGTATAAAAAGGCGCGCC | ||
| TTGGGGAAATATGAACCTGACATGATTAAGATTGCTCTTTCGGTGGCTGGTAAAAAATTGAAAATAAATACAAAGGTTC | Del3 | 149342 |
| ACAAAATTATGTATTTTGTTCTATCAACTACCTATAAAACTTTCCAAATACTAGTATAAAAAGGCGCGCC | ||
| GGAAAGAATCTACTCATCTAAACGATTTAGTAAACTTGACTAAATCTTAATTTTTATAAAAATTGAAAATAAATACAAAGGTTC | Del3 res. (163R/167R) | 148381- 151019 |
| TAAATTTTAAGTTTTACGTGGTAAGTTTTAATATTTAACTAATACATTAGCTAGTATAAAAAGGCGCGCC |
Underlined sequences were used to mediate recombination.
Insertion sites and genome positions based on GenBank accession no. U94848.
The second concept of vector construction used to generate the vectors MVAB-7Ag3 and MVAB-7Ag4 (Fig. 1B) was based on the insertion of the seven HCMV antigens in only two MVA insertion sites. In both MVAB-7Ag3 and MVAB-7Ag4, all five PC subunits were inserted together as P2A-linked polycistronic expression constructs into IGR 69/70. Additionally, pp65 and gB were inserted in both MVAB-7Ag3 and MVAB-7Ag4 as P2A-linked polycistronic expression constructs; however, while the pp65/gB expression construct was inserted in MVAB-7Ag3 into the traditional Del3 site, it was inserted in MVAB-7Ag4 into a restructured Del3 site (Del3 res.), which was generated by deleting the MVA genome sequence between the two essential MVA genes 163R and 167R (Fig. 1B and Table 1). Such a restructured Del3 site has been proposed to promote antigen stability within MVA (60).
All HCMV gene sequences used for vector construction were codon optimized for vaccinia virus to enhance their stability within MVA (58). In addition, all 2A signal sequences were based on the 2A peptide of porcine teschovirus 1 (P2A), as this 2A signal sequence has been shown to mediate highly efficient polyprotein cleavage in different mouse and human cell lines as well as different animal models (56). Different coding sequences were used for the P2A signal sequences between different antigens to prevent homologous recombination (Fig. 1C) (54). In all constructs, gH was inserted with its transmembrane (TM) domain to express a membrane-tethered PC, since we have shown previously that this form of the PC when expressed by MVA is more immunogenic than a soluble form of the PC without gH TM, at least when considering the numbers of immunizations required to elicit robust NAb (21, 61). In contrast, gB was inserted in all vectors without its TM (gBΔTM), as there is evidence that a soluble form of gB provides enhanced immunogenicity compared to full-length gB (51). Another consideration for including gB without its TM was to eliminate its fusogenic function, as this may potentially be associated with cellular cytotoxicity (51). The used gBΔTM gene encodes 698 amino acids (aa) of the gB N terminus, a portion of the gB protein that contains the antigenic domains AD1, AD2, AD4, and AD5 but does not contain AD3 (62). While all of these gB antigenic domains are known as targets of humoral immunity, only antibodies targeting gB AD2 have been correlated with protection (46, 63).
All seven-antigen MVA vectors (MVAB-7Ag1, MVAB-7Ag2, MVAB-7Ag3, and MVAB-7Ag4) (Fig. 1A and B) were further modified by removing the BAC sequences via homologous recombination of a genomic duplication (Fig. 1D and E) to generate MVA-7Ag1, MVA-7Ag2, MVA-7Ag3, and MVA-7Ag4 without any unwanted bacterial vector sequences (no superscript B, indicating the removal of the BAC sequences) (64–66). For controls, MVA vectors were generated that expressed all five PC subunits together with gB (MVAB-PC/gB), the PC subunits together with pp65 (MVAB-PC/pp65), or only the PC subunits (MVAB-PC), gB (MVAB-gB), or pp65 (MVAB-pp65) using identical MVA insertion sites and expression constructs as used for the construction of MVAB-7Ag1 (Fig. 1A).
Characterization of HCMV antigen expression by multiantigenic MVA vectors.
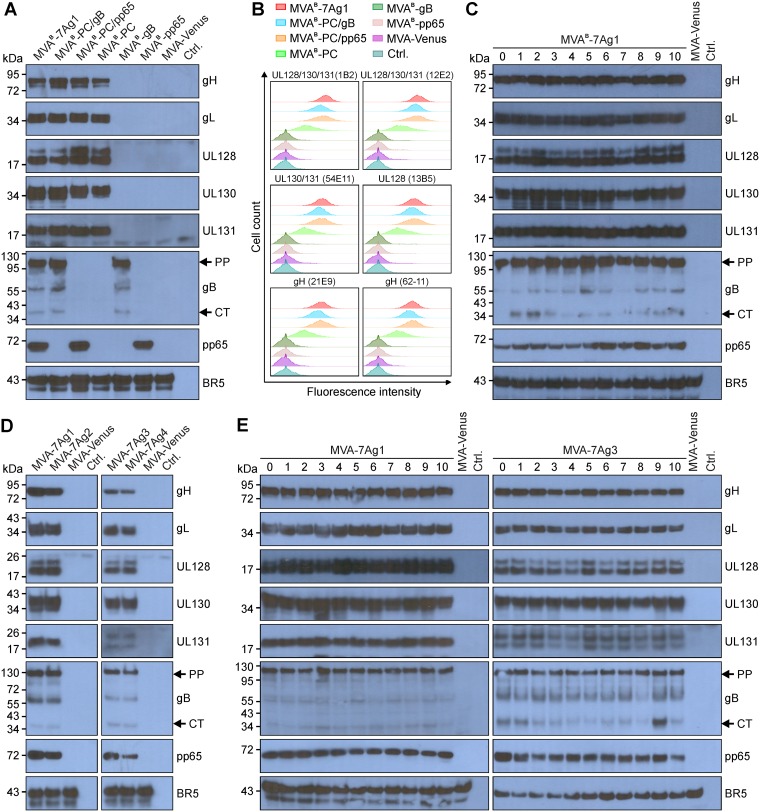
To characterize the expression of the PC subunits, gB, and pp65 when simultaneously expressed by MVA, we compared HCMV antigen expression by MVAB-7Ag1 and the control vectors in infected chicken embryo fibroblasts (CEF) via immunoblotting. As expected, while MVAB-7Ag1 expressed all seven HCMV antigens, the control vectors expressed only antigen subsets (PC/gB, PC/pp65, or PC) or single antigens (gB or pp65) (Fig. 2A). The detectable protein sizes of the individual HCMV antigens appeared consistent with published molecular weight values (gH, ∼85 kDa; gL, ∼35 kDa; UL128, ∼15 kDa; UL130, ∼38 kDa; UL131A, ∼18 kDa [67]; gBΔTM, ∼110-kDa precursor protein and ∼35-kDa C-terminal cleavage product [51]; pp65, ∼65 kDa [68]), factoring in that some of the expressed antigens contained C-terminal P2A peptide remnants (∼2 kDa). Similar to previous observations with a monoclonal antibody (MAb) specific for the C-terminally located gB AD1 (21, 51), gBΔTM was primarily detected as a full-length ∼110-kDa precursor form and in minor amounts also as an ∼35-kDa C-terminal furin cleavage product that contained only AD1. The detected gB protein species of intermediate size most likely represents degradation products or immature forms of the gB precursor proteins (Fig. 2A).
FIG 2.
HCMV antigen expression by multiantigenic MVA vectors. (A and B) Immunoblot (A) and flow cytometry (B) analyses were used to compare the HCMV antigen expression by MVAB-7Ag1 and the control vectors (MVAB-PC/gB, MVAB-PC/pp65, MVAB-PC, MVAB-gB, and MVAB-pp65). Monoclonal (gH, UL130, gB, and pp65) and polyclonal (gL, UL128, and UL131A) antibody preparations were used to detect the HCMV antigens in whole-cell lysates of MVA-infected CEF via immunblotting (A). NAb specific for epitopes formed by UL128/130/131A (1B2 and 12E2), UL130/131A (54E11), UL128 (13B5), or gH (21E9 and 62-11) were used to detect cell surface expression of the PC subunits on live, nonpermeabilized BHK cells infected with rMVA (B). (C to E) Immunoblot analysis as described for panel A was used to evaluate the HCMV antigen expression by MVAB-7Ag1 following 10 (lanes 0 to 10) virus passages in CEF (C), by MVA-7Ag1, MVA-7Ag2, MVA-7Ag3, and MVA-7Ag4 following BAC vector removal (D), and by MVA-7Ag1 and MVA-7Ag3 following 10 virus passages in CEF (E). Vaccinia virus BR5 was detected in panels A and C to E for loading control. Arrows in panels A and C to E indicate the precursor protein (PP) and C-terminal cleavage product (CT) of gB. CEF infected with MVA expressing the fluorescence marker Venus (MVA-Venus) or uninfected cells (Ctrl.) were analyzed as additional controls for panels A and C to E. The x axis in B represents the log10 of fluorescence intensity, and the y axis represents cell count.
The levels of expression of the individual HCMV antigens by MVAB-7Ag1 and all control vectors were comparable to one another, indicating that coexpression of the PC subunits, gB, and pp65 by MVA does not significantly impact the expression of the individual HCMV antigens (Fig. 2A). In addition, baby hamster kidney (BHK) cells infected with MVAB-7Ag1 and the control vectors were efficiently stained with a panel of previously isolated NAb specific for the UL128/130/131A subunits and gH (Fig. 2B) (20), suggesting that the PC subunits when coexpressed with gB and pp65 by MVA retain the ability to efficiently assemble into protein complexes that form neutralizing epitopes. Moreover, all HCMV antigens of MVAB-7Ag1 were expressed to comparable levels following 10 virus passages in CEF (Fig. 2C), indicating that all seven HCMV antigens are stably maintained in MVAB-7Ag1.
Following BAC removal, we confirmed that all multiantigenic vectors (MVA-7Ag1, MVA-7Ag2, MVA-7Ag3, and MVA-7Ag4) expressed the seven HCMV antigens (Fig. 2D). However, HCMV antigen expression of MVA-7Ag3 and MVA-7Ag4, especially UL131A, appeared lower than antigen expression of MVA-7Ag1 and MVA-7Ag2, although this has not been investigated in detail. Moreover, we confirmed that all seven HCMV antigens of MVA-7Ag1 and MVA-7Ag3 were expressed at comparable levels following 10 virus passages in CEF (Fig. 2E), suggesting that the seven HCMV antigens are stably maintained in these vectors following BAC vector removal. The two protein bands detected for UL128 for all multiantigenic MVA vectors and for UL131A for MVA-7Ag3 and MVA-7Ag4 have been observed previously with our second-generation MVA-PC vectors and appear to be specifically associated with the P2A-linked polycistronic expression constructs (54). These results in sum indicate that all five PC subunits, gB, and pp65 of HCMV can be efficiently and stably coexpressed by MVA.
Enhanced HCMV NAb induction in C57BL/6 mice by MVA-vectored PC/gB antigen combination.
To characterize the immunogenicity of the PC subunits, gB, and pp65 when coexpressed by MVA, HCMV humoral and cellular immune responses induced by MVAB-7Ag1 and the control vectors were compared following immunization of C57BL/6 wild-type mice. As an additional control to better gauge the magnitude of the T cell responses, we included the clinically evaluated MVA-pp65/IE vector (53).
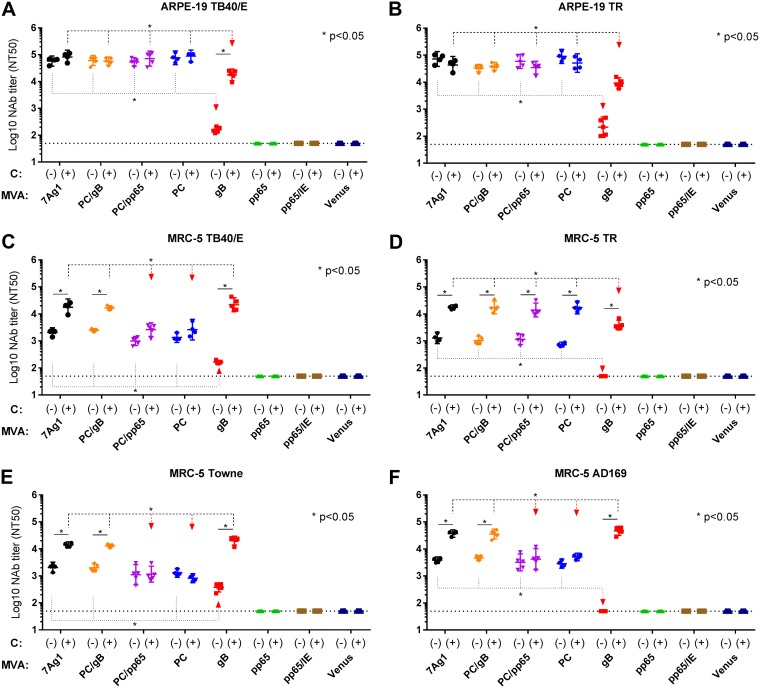
Because gB-specific antibodies are known to mediate neutralization in part through complement (69, 70), NAb induced by the different MVA vectors in C57BL/6 mice were measured in heat-inactivated immune sera in the absence or presence of an exogenous source of complement. EC-specific NAb titers were evaluated on ARPE-19 cells against the clinical-like HCMV strains TB40/E and TR (71). As shown in Fig. 3A and B, MVAB-7Ag1 and all control vectors expressing the PC subunits (MVAB-PC/gB, MVAB-PC/pp65, and MVAB-PC) stimulated potent and comparable complement-independent EC-specific NAb responses against both TB40/E and TR that dramatically exceeded those elicited by the MVAB-gB control vector. Complement-independent EC-specific NAb induced by MVAB-gB were of only very low potency. While complement did not enhance the potency of EC-specific NAb elicited by MVAB-7Ag1 and all control vectors expressing the PC subunits, it substantially enhanced the potency of EC-specific NAb induced by MVAB-gB. Yet EC-specific NAb induced by MVAB-gB that interfered with TB40/E and TR infection in the presence of complement did not reach the potency of EC-specific NAb elicited by MVAB-7Ag1 and all control vectors expressing the PC subunits. These results suggest that MVA coexpressing the PC subunits, gB, and pp65 can elicit potent EC-specific NAb responses in C57BL/6 mice that appear to be dominated by the potency of complement-independent NAb that target the PC.
FIG 3.
HCMV NAb induction by MVAB-7Ag1 and control vectors in C57BL/6 mice. C57BL/6 mice (n = 4 or 5) were immunized three times in 4-week intervals by the intraperitoneal (i.p.) route with MVAB-7Ag1 or the control vectors (MVAB-PC/gB, MVAB-PC/pp65, MVAB-PC, MVAB-gB, or MVAB-pp65) and HCMV immune responses were evaluated 1 week postimmunization. MVA-pp65/IE and MVA-Venus were used as additional controls. NAb titers (NT50; geometric mean titer) were measured in mouse immune sera on ARPE-19 or MRC-5 cells against HCMV strain TB40/E (A and C), TR (B and D), Towne (E), or AD169 (F) in the absence (−) or presence (+) of 5% guinea pig complement (abbreviated C). Dotted lines indicate the lowest serum dilution analyzed. Bars represent geometric means with 95% confidence intervals. Statistical significance of differences between NAb titers were calculated using two-way ANOVA, followed by Sidak's multiple-comparison test.
FB-specific NAb responses induced by the different MVA vectors in C57BL/6 mice were measured on MRC-5 cells against HCMV TB40/E and TR and additionally against the laboratory strains Towne and AD169. As shown in Fig. 3C to F, MVAB-7Ag1 and all control vectors expressing the PC subunits (MVAB-PC/gB, MVAB-PC/pp65, and MVAB-PC) elicited comparable complement-independent FB-specific NAb responses against all HCMV strains that exceeded those induced by the MVAB-gB control vector. Similar to the observations with EC, complement-independent FB-specific NAb induced by MVAB-gB were only very low or undetectable. In the presence of complement, NAb elicited by MVAB-7Ag1 and the control vectors expressing gB (MVAB-PC/gB and MVAB-gB) showed significantly enhanced potency against FB infection by all HCMV strains. In contrast, while complement enhanced the potency of NAb elicited by the control vectors expressing the PC subunits without gB (MVAB-PC/pp65 and MVAB-PC) to interfere with FB infection by HCMV strain TR, it did not enhance their potency to prevent FB infection by HCMV TB40/E, AD169, or Towne. NAb responses induced by MVAB-7Ag1 and the control vectors expressing gB (MVAB-PC/gB and MVAB-gB) that inhibited FB infection by the HCMV strains in the presence of complement were generally comparable to each other, and they exceeded those induced by the control vectors expressing the PC subunits without gB (MVAB-PC/pp65 and MVAB-PC). The only exception were the complement-dependent FB-specific NAb responses measured with HCMV TR, where NAb induced by MVAB-7Ag1 and all control vectors expressing the PC subunits (MVAB-PC/gB, MVAB-PC/pp65, and MVAB-PC) were similar to each other but higher than those induced by MVAB-gB. These results overall demonstrated that MVAB-7Ag1 and the MVAB-PC/gB control vector elicited comparable NAb responses that inhibited FB infection by the different HCMV strains more potently and consistently than NAb induced by the control vectors expressing the PC subunits without gB (MVAB-PC/pp65 and MVAB-PC) or only gB (MVAB-gB). These results for C57BL/6 mice suggest that MVA coexpressing the PC subunits, gB, and pp65 can stimulate potent FB-specific NAb responses against different HCMV strains, which appear to be enhanced by the antigen combination of the PC subunits and gB.
Improved ADCC induction in C57BL/6 mice by MVA-vectored PC/gB antigen combination.
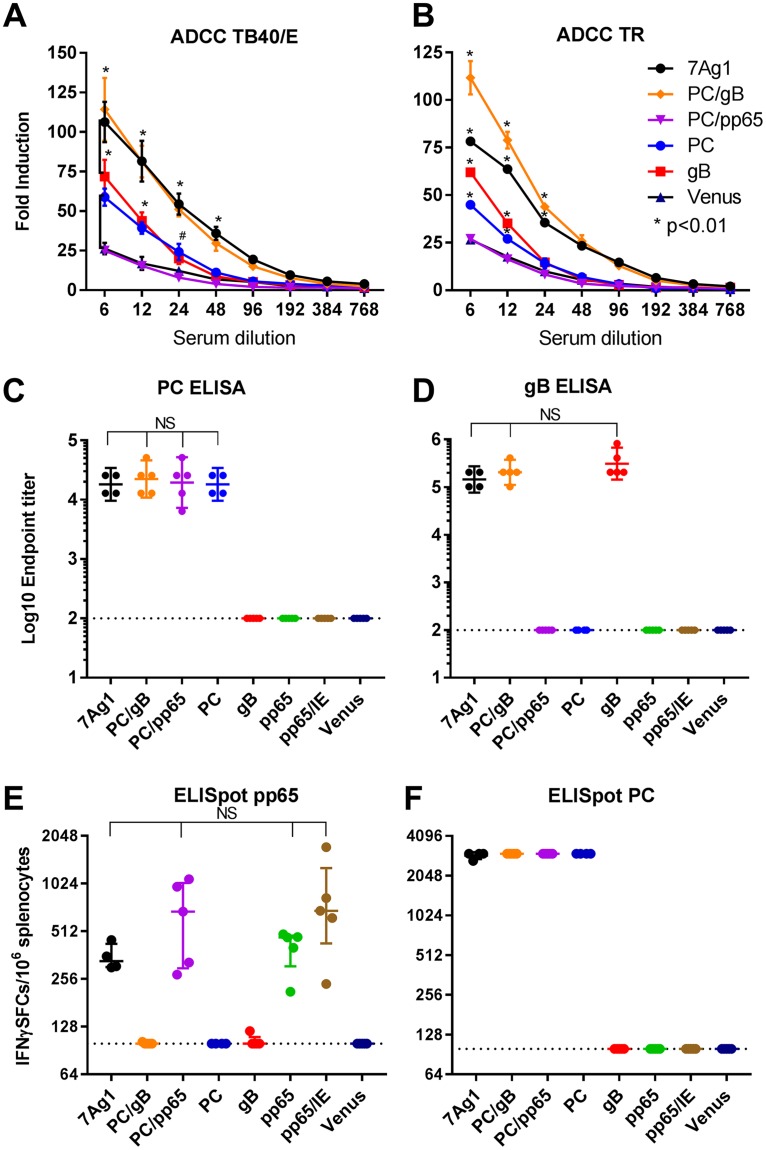
Since ADCC in addition to antibody-mediated neutralization could be an important humoral immune function to provide protection against HCMV (70), we evaluated the immune sera of the immunized C57BL/6 mice for potency in promoting ADCC using a commercially available, surrogate reporter assay. Using this assay, the potency of the immune sera antibodies in mediating ADCC was measured using ARPE-19 cells infected with TB40/E or TR. As shown in Fig. 4A and B, MVAB-7Ag1 and the control vector MVAB-PC/gB stimulated antibody responses that showed higher potency in promoting ADCC against TB40/E and TR than antibodies induced by the control vectors expressing the PC subunits without gB (MVAB-PC/pp65 and MVAB-PC) or only gB (MVAB-gB). While antibodies induced by MVAB-7Ag1 and MVAB-PC/gB showed comparable potencies in promoting ADCC against TB40/E, antibodies stimulated by MVAB-7Ag1 appeared slightly less potent in mediating TR-specific ADCC than antibodies induced by MVAB-PC/gB. This suggests that the incorporation of pp65 in MVAB-7Ag1 interferes to a minor extent, and dependent upon HCMV strain, with the immunogenicity of the PC subunits and gB to induce antibodies that promote ADCC. In addition, antibodies induced by the control vector MVAB-PC/pp65 did not show measurable potency in promoting ADCC, which may support the notion that pp65 can interfere in some instances with the immunogenicity of the PC to elicit ADCC-promoting antibodies. Importantly, MVAB-7Ag1 and the control vectors stimulated comparable levels of PC-specific and gB-specific binding antibodies (Fig. 4C and D), indicating that coexpression of the HCMV antigens by MVAB-7Ag1 does not substantially influence the immunogenicity of the PC and gB in eliciting humoral immunity. Only slightly lower gB-specific binding antibodies were induced by MVAB-7Ag1 than by the control vectors MVAB-PC/gB and MVAB-gB, yet this did not reach statistical significance. These observations with C57BL/6 mice show that MVA coexpressing the PC subunits, gB, and pp65 can induce HCMV humoral immune responses that promote ADCC, and the PC/gB antigen combination has an additive effect on ADCC stimulation.
FIG 4.
ADCC and T cell stimulation by MVAB-7Ag1 and control vectors in C57BL/6 mice. The induction of ADCC (A and B), antigen-specific binding antibodies (C and D), and mouse MHC-restricted T cells (E and F) was evaluated in mice immunized with MVAB-7Ag1 or the control vectors (MVAB-PC/gB, MVAB-PC/pp65, MVAB-PC, MVAB-gB, or MVAB-pp65) as described in the legend to Fig. 3. (A and B) Serial dilutions of the mouse immune sera were tested for activity to promote ADCC following antibody binding to ARPE-19 cells infected with TB40/E (A) or TR (B) using an ADCC surrogate reporter assay. Bars represent SDs of duplicates. (C and D) PC-specific (C) and gB-specific (D) binding IgG endpoint titers were evaluated via ELISA using purified gB and PC protein. Dotted lines indicate the minimum serum dilution analyzed. Bars represent geometric means with 95% confidence intervals. (E and F) Ex vivo T cell responses were determined by IFN-γ ELISpot assay utilizing previously described mouse H2-b-restricted immunoreactive peptides of pp65 (E) and the PC subunits (F). Horizontal lines represent median values with interquartile ranges. Statistical significance of differences comparing each group in panels C to F was calculated using one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test (NS, P > 0.05). SFCs, spot-forming units. Note that in panel F the measured IFN-γ responses, with only a single exception, were higher than the threshold, and therefore, differences could not be evaluated.
Potent stimulation of pp65- and PC-specific T cells by multiantigenic MVA in C57BL/6 mice.
Taking advantage of previously described mouse H2-b-restricted immunodominant peptides (72), we evaluated by gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assay whether the C57BL/6 mice immunized with MVAB-7Ag1 and the control vectors developed antigen-specific T cell responses. As shown in Fig. 4E and F, MVAB-7Ag1 and the control vectors expressing pp65 (MVAB-PC/pp65 and MVAB-pp65) showed comparable potencies in eliciting H2-b-restricted pp65-specific T cell responses. In addition, H2-b-restricted pp65-specific T cell responses elicited by MVAB-7Ag1 were similar to those induced by the clinically evaluated MVA-pp65/IE vector (53). Only slightly lower pp65-specific T cell responses were elicited by MVAB-7Ag1 and MVAB-pp65 than by MVAB-PC/pp65 and MVA-pp65/IE, although this was not significant considering the limited number of animals. Interestingly, although the PC subunits are not known as dominant T cell targets (73), mice immunized with MVAB-7Ag1 and all PC-expressing control vectors showed exceptionally high and comparable levels of H2-b-restricted T cells specific for the PC subunits. We also evaluated gB-specific T cell stimulation by the MVA vectors using H2-b-restricted immunodominant peptides of gB, yet results were ambiguous due to high-level background responses (data not shown). In sum, these results suggest that the individual HCMV antigens of MVA coexpressing the PC subunits, gB, and pp65 can be efficiently processed in C57BL/6 mice to stimulate antigen-specific T cell responses.
Stimulation of potent NAb and T cell responses by multiantigenic MVA in BALB/c mice.
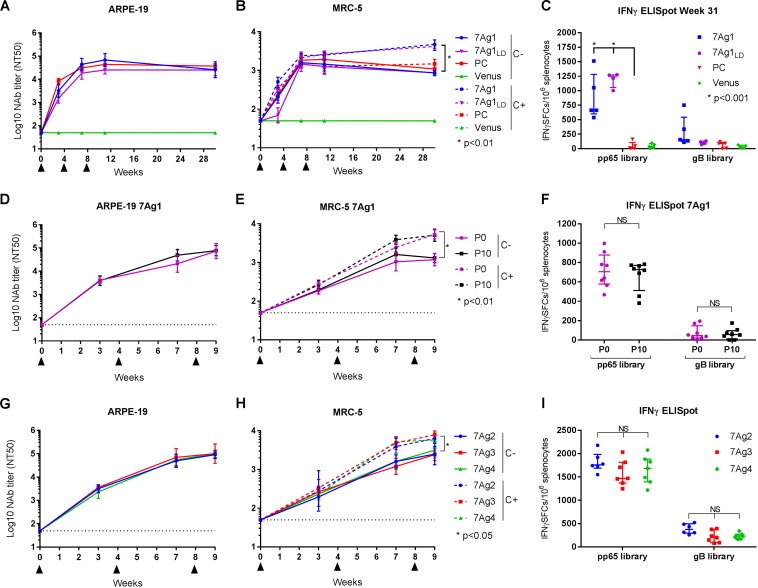
Following BAC removal, we evaluated the potency of the multiantigenic MVA vectors to stimulate NAb and T cell responses in BALB/c mice, which we commonly use to evaluate antigen-specific immunity elicited by rMVA. To investigate the durability of NAb induced by MVA coexpressing the PC subunits, gB, and pp65, HCMV NAb responses induced in BALB/c mice by immunization with MVA-7Ag1 in either standard or low dose were evaluated over a period of 30 weeks. As controls, BALB/c mice were immunized (in the standard dose) with MVAB-PC or MVA-Venus. NAb titers were measured in the absence or presence of complement against TB40/E on MRC-5 and ARPE-19 cells. As shown in Fig. 5A and B, MVA-7Ag1, in standard and low doses, and the MVAB-PC control vector stimulated robust FB- and EC-specific NAb responses that remained relatively stable during the entire 30-week period. Potent NAb responses were detectable after only one or two immunizations with either MVA-7Ag1 or MVAB-PC, and these responses remained at comparable levels after an additional booster immunization with the vectors. While NAb induced by MVA-7Ag1 (standard and low doses) and MVAB-PC showed similar potencies in preventing EC and FB infection in the absence of complement, NAb induced by MVA-7Ag1 inhibited FB infection in the presence of complement significantly more potently than NAb induced by MVAB-PC. These observations for BALB/c mice are consistent with those for C57BL/6 mice and suggest that the antigen combination of the PC subunits and gB in MVA-7Ag1 enhances HCMV NAb induction.
FIG 5.
HCMV immune stimulation by multiantigenic MVA vectors in BALB/c mice. BALB/c mice were immunized three times i.p. in 4-week intervals (triangles) with the multiantigenic MVA vectors, and HCMV NAb and T cell responses were evaluated. HCMV NAb titers were measured at the indicated time points against HCMV TB40/E on ARPE-19 cells in the absence of complement, and on MRC-5 cells in the absence or presence of 5% guinea pig complement. T cell responses were determined ex vivo by IFN-γ ELISpot assay utilizing pp65 and gB peptide libraries. Panels A to C show NAb and T cell responses of BALB/c mice (n = 4 or 5) immunized with MVA-7Ag1 in either standard dose (5 × 107 PFU) or low dose (LD; 1 × 107 PFU) or control vectors MVA-PC and MVA-Venus in standard dose. NAb titers shown in panels A and B were measured over a period of 30 weeks. Ex vivo T cell responses shown in panel C were measured at week 31 after an additional booster immunization at week 30. Panels D to F show NAb and T cell responses of BALB/c mice (n = 8) immunized with MVA-7Ag1 that was derived before (P0) and after (P10) 10 virus passages on CEF. Panels G to I show NAb and T cell responses of BALB/c mice (n = 6 or 7) immunized with MVA-7Ag2, MVA-7Ag3, or MVA-7Ag4. Bars in panels A, B, D, E, G, and H represent geometric means with 95% confidence intervals. Bars in panels C, F, and I represent median values with interquartile ranges. Statistical significance of differences comparing each group in panels A, B, D, E, G, and H was calculated using ANOVA followed by Sidak's multiple-comparison test. Differences between vaccine groups in panels C, F, and I were calculated by multiple t test (NS, P > 0.05).
After an additional booster immunization of the BALB/c mice at week 30 with MVA-7Ag1 and the control vectors (MVAB-PC and MVA-Venus), ex vivo T cell responses were evaluated at the peak effector phase by IFN-γ ELISpot assay using pp65 and gB peptide libraries. As shown in Fig. 5C, BALB/c mice immunized with MVA-7Ag1 in either standard or low dose developed robust levels of pp65-specifc T cell responses. Surprisingly, slightly lower pp65-specifc T cell responses were detected in mice immunized with the standard dose of MVA-7Ag1 than in mice immunized with a low dose of MVA-7Ag1, although this did not reach statistical significance given the limited number of animals. In contrast, gB-specific T cells were detected at low levels only in mice immunized with the standard dose of MVA-7Ag1, whereas they were undetectable in mice immunized with a low dose of MVA-7Ag1. This may suggest that the pp65- and gB-specific T cell responses induced by MVA-7Ag1 can differ in dependence on immunization dose. As expected, MVAB-PC and MVA-Venus did not induce measurable pp65- or gB-specific T cells.
In a follow-up experiment, we verified that MVA-7Ag1 derived before and after 10 virus passages on CEF stimulated comparable NAb and T cell responses in BALB/c mice (Fig. 5D to F), suggesting that the HCMV antigens in MVA-7Ag1 retain their immunogenicity following extensive vector propagation. In addition, EC- and FB-specific NAb and pp65- and gB-specific T cell responses similar to those induced in BALB/c mice by MVA-7Ag1 were stimulated in BALB/c mice by MVA-7Ag2, MVA-7Ag3, and MVA-7Ag4 (Fig. 5G to I), suggesting that the different seven-antigen MVA vectors have similar potencies in eliciting HCMV immunity. Only slightly lower pp65 and gB-specific T cell responses were measured for the MVA-7Ag3 and MVA-7Ag4 vaccine groups than for the MVA-7Ag2 vaccine group, yet this did not reach significance. These results for BALB/c mice support that MVA simultaneously expressing all five PC subunits, gB, and pp65 can stimulate robust HCMV humoral and cellular immunity.
Potent HCMV-specific T cell stimulation by multiantigenic MVA in HLA transgenic mice.
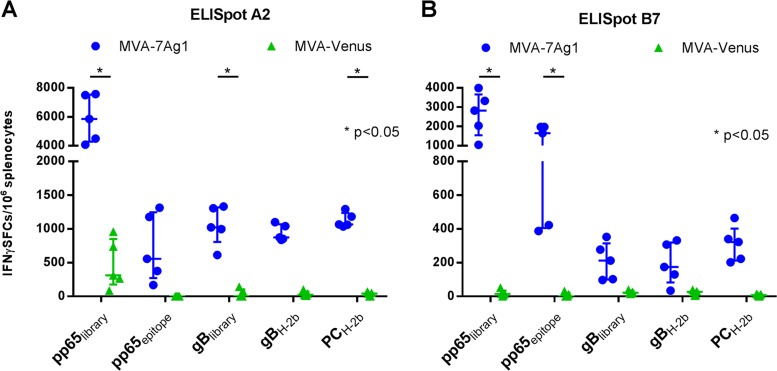
To address whether pp65 when coexpressed together with gB and the PC subunits by MVA can efficiently stimulate T cells to epitopes presented by human MHC class I molecules, T cell induction by MVA-7Ag1 was evaluated in A2 and B7 human leukocyte antigen (HLA) transgenic mice. These transgenic mice are deficient in expressing mouse MHC class I molecules and contain transgenes of the commonly expressed HLA-A*0201 (A2) or HLA-B*0702 (B7) class I molecules (74, 75). When studying cellular immune responses to HCMV, these transgenic mice are an invaluable tool to evaluate HLA class I-restricted T cell responses because of their capacity to efficiently process and present immunodominant epitopes of pp65 and other HCMV proteins in the context of HLA alleles (59, 76–78). A2 and B7 transgenic mice were immunized with MVA-Venus as a control. T cell responses were evaluated by IFN-γ ELISpot assay using pp65 and gB peptide libraries, A2- and B7-restricted immunodominant peptide epitopes of pp65, and H2-b-restricted peptides of gB and the PC subunits. As shown in Fig. 6, T cell responses were detected in MVA-7Ag1-immunized A2 and B7 mice with all peptide stimuli, although particularly high levels of T cell responses were detected with the pp65 peptide library. The observed H-2b-restricted responses may represent CD4+ T cells stimulated by mouse MHC class II presentation, or they may represent CD8+ T cells stimulated via residual mouse MHC class I molecule expression (74).
FIG 6.
Human MHC-restricted T cell responses elicited by MVA-7Ag1. Transgenic C57BL/6 mice (n = 5) expressing HLA-A*0201 (A2) (A) or HLA-B*0702 (B7) (B) class I molecules were immunized two times in a 4-week interval with MVA-7Ag1 or control vector MVA-Venus. One week following the booster immunization, antigen-specific T cell responses were determined by IFN-γ ELISpot assay using pp65- and gB-specific peptide libraries, HLA-A*0201- or HLA-B*0702-restricted immunodominant peptide epitopes of pp65, or pools of mouse H-2b-restricted peptides of gB or the PC. Bars represent median values with interquartile ranges. Significance of the difference between the groups was calculated using two-way ANOVA followed by Sidak's multiple-comparison test.
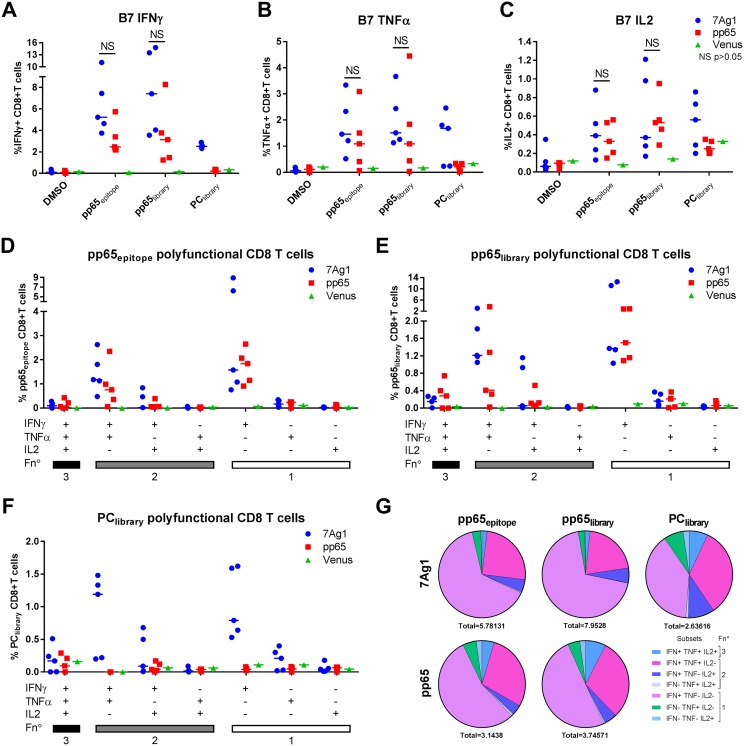
Since polyfunctionality of T cells has been implicated in preventing HCMV infection (79), we evaluated whether the antigen-specific T cells induced by MVA-7Ag1 in A2 and B7 mice were polyfunctional. A2 and B7 mice were immunized with MVA-7Ag1 or the control vectors MVAB-pp65 and MVA-Venus, and T cell responses were evaluated by IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin 2 (IL-2) intracellular flow cytometry staining using HLA-A*0201- and HLA-B*0702-restricted pp65 peptide epitopes and pp65 and PC peptide libraries. As shown in Fig. 7A to C, MVA-7Ag1 and MVAB-pp65 stimulated IFN-γ-, TNF-α-, and IL-2-producing pp65-specific T cell responses in B7 mice, with the largest amounts of pp65-specific T cells producing IFN-γ and the smallest amounts of pp65-specific T cells producing IL-2. Generally, pp65-specific T cells were detected at higher levels in MVA-7Ag1-immunized B7 mice than in MVAB-pp65-immunized B7 mice (Fig. 7A to F), although this was not significant. In contrast, the proportion of polyfunctional pp65-specific T cells producing two or three cytokines appeared slightly reduced in MVA-7Ag1-immunized B7 mice compared to MVAB-pp65-immunized B7 mice (Fig. 7G). Interestingly, MVA-7Ag1-immunized B7 mice also developed IFN-γ-, TNF-α-, and IL-2-producing PC-specific T cells (Fig. 7A to F), and approximately half of the PC-specific T cells stimulated by MVA-7Ag1 in B7 mice were polyfunctional (Fig. 7G).
FIG 7.
HLA-B*0702-restricted polyfunctional CD8+ T cells elicited by MVA-7Ag1. HLA-B*0702 (B7) transgenic mice were immunized two times in 4-week intervals with MVA-7Ag1, MVAB-pp65 (n = 5), or MVA-Venus (n = 1). One week postimmunization, antigen-specific T cell responses were evaluated by multicytokine ICS following stimulation with pp65- and PC-specific peptide libraries or an HLA-B*0702-restricted immunodominant pp65 peptide epitope. (A to C) Shown are the percentages of CD8+ T cells secreting IFN-γ (A), TNF-α (B), or IL-2 (C) following stimulation of splenocytes from B7 immunized mice with different stimuli. (D to F) Frequency of antigen-specific CD8+ T cells producing all combinations of IFN-γ, TNF-α, and IL-2 cytokines following in vitro stimulation with the HLA-B*0702-restricted pp65 peptide epitope (D), pp65-specific peptide library (E), or PC-specific peptide library (F) in MVA-7Ag1, MVAB-pp65, or MVA-Venus immunized B7 mice. Horizontal bars represent the median values. (G) Pie charts show the relative contribution of each polyfunctional subset among the total polyfunctional response to the pp65 epitope, pp65 library, or PC library in MVA-7Ag1- or MVAB-pp65-immunized B7 mice. Each pie chart represents the mean response across the immunized mice to the three different antigen stimulations. The average total percentage of CD8+ T cells responding to the peptide stimulation is shown under each pie chart. Polyfunctional subsets and functions (Fn°) are indicated in the color key. Significance of the difference between the groups was calculated using multiple t test.
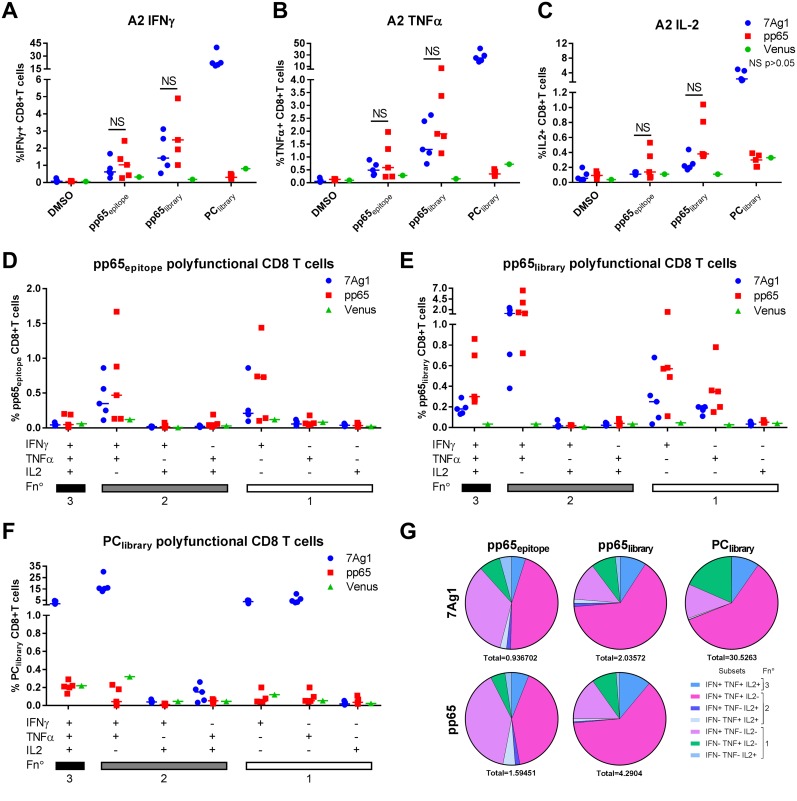
IFN-γ-, TNF-α-, IL-2-producing pp65-specific T cell responses induced by MVA-7Ag1 in A2 mice were lower than the pp65-specific T cell responses induced by MVA-7Ag1 in B7 mice and generally lower than those induced by MVAB-pp65 in A2 mice (Fig. 8A to F), although the difference was not significant with the number of animals evaluated. Yet unlike the pp65-specific T cells induced by MVA-7Ag1 and MVAB-pp65 in B7 mice, the pp65-specific T cells induced by these vectors in A2 mice were mainly polyfunctional (Fig. 8D to G). Most strikingly, however, were the impressive levels of IFN-γ-, TNF-α-, and IL-2-producing PC-specific T cells induced by MVA-7Ag1 in A2 mice, reaching up to 45% of the total CD8+ T cell population (Fig. 8A to F). In addition, more than half of these high-level PC-specific T cells induced MVA-7Ag1 in A2 mice were polyfunctional (Fig. 8G). These results for transgenic mice suggest that pp65 and the PC subunits expressed by MVA-7Ag1 can be efficiently processed and presented by different HLA molecules to stimulate polyfunctional T cell responses.
FIG 8.
HLA-A*0201-restricted polyfunctional CD8+ T cells elicited by MVA-7Ag1. HLA-A*0201 (A2) transgenic mice were immunized two times in 4-week intervals with MVA-7Ag1, MVAB-pp65 (n = 5), or MVA-Venus (n = 1). One week postimmunization, antigen-specific T cell responses were evaluated by multicytokine ICS following stimulation with pp65- and PC-specific peptide libraries or an HLA-A*0201-restricted immunodominant pp65 peptide epitope. (A to C) Shown are the percentages of CD8+ T cells secreting IFN-γ (A), TNF-α (B), or IL-2 (C) following stimulation of splenocytes from A2 immunized mice with different stimuli. (D to F) Frequency of antigen-specific CD8+ T cells producing all combinations of IFN-γ, TNF-α, and IL-2 cytokines following in vitro stimulation with the HLA-A*0201-restricted peptide epitope (D), pp65-specific peptide library (E), or PC-specific peptide library (F) in MVA-7Ag1-, MVAB-pp65-, or MVA-Venus-immunized B7 mice. Horizontal bars represent median values. (G) Pie charts show the relative contribution of each polyfunctional subset among the total polyfunctional response to the pp65 epitope, pp65 library, or PC library in MVA-7Ag1- or MVAB-pp65-immunized A2 mice. Each pie chart represents the mean response across the immunized mice to the three different antigen stimulations. The average total percentage of CD8+ T cells responding to the peptide stimulation is shown under each pie chart. Polyfunctional subsets and functions (Fn°) are indicated in the color key. Significance of the difference between the groups was calculated using multiple t test.
DISCUSSION
Although the precise HCMV immune correlates of protection remain unclear, vaccine-mediated prevention of HCMV disease in newborns and transplant recipients may depend on the stimulation of both antibody and T cell responses (31, 44). While subunit vaccine design has primarily focused on the immunodominant antigens gB, pp65, and IE1/2 (5, 10, 80), more recent attention has been given to the PC due to its recognition as a major target of NAb preventing in vitro HCMV infection of many cell types that are thought to be important for HCMV natural history (21–24). However, a multiantigenic vaccine candidate that combines all five PC subunits with gB, pp65, and/or the IE1/2 proteins has been introduced only recently by an approach based on mRNA vaccines (26). In addition, the recently developed conditionally replication-defective AD169-derived vaccine strain V160 with restored PC expression provides the possibility to stimulate HCMV immunity by all relevant antigens that are potentially involved in the protection against HCMV infection (27). In this study, we have introduced a novel vaccine approach based on a recently developed BAC of the well-characterized and clinically deployable MVA vaccine vector to stimulate robust HCMV humoral and cellular immune responses by an antigen combination composed of the PC, gB, and pp65 in different strains of mice (54).
One major advantage in utilizing MVA as a vaccine platform is its versatile expression system and large capacity to accommodate foreign DNA sequences, allowing simultaneous expression of multiple antigens by a single MVA vector (21, 49). By taking advantage of these traits of MVA in addition to highly efficient and markerless manipulation by BAC technology (21, 54, 55), two main concepts of MVA vector construction were exploited to generate different rMVA vectors simultaneously expressing the seven HCMV antigens via P2A-linked polycistronic and single antigen expression constructs inserted into four (MVAB-7Ag1 and MVAB-7Ag2) or only two (MVAB-7Ag3 and MVAB-7Ag4) MVA insertion sites. In addition, using a procedure based on homologous recombination of genomic duplication (66), the BAC vector sequences of these recombinant multiantigenic MVA vectors could be removed without leaving behind any bacterial vector sequences, which may simplify the investigational new drug process and approval of these vectors for human use. All of the multiantigenic vectors showed expression of the seven HCMV antigens, and we confirmed that the PC subunits polycistronically expressed by MVAB-7Ag1 efficiently assembled into complexes that formed a variety of neutralizing epitopes. We did not find any evidence that coexpression of the PC subunits, gB, and pp65 by MVA influences the expression of the individual HCMV antigens. In addition, MVA-7Ag1 and MVA-7Ag3 with removed BAC vector showed stable HCMV antigen expression during extensive virus passaging in CEF, suggesting that both concepts of MVA vector construction allow stable coexpression of the PC subunits, gB, and pp65 in a cell type that is acceptable by the Food and Drug Administration for MVA vaccine production.
While all of the multiantigenic MVA vectors showed potent and comparable abilities to elicit NAb and T cell responses in BALB/c mice, MVAB-7Ag1 and its vector-free derivative MVA-7Ag1 were exploited most extensively to characterize the immunogenicity of the PC subunits, gB, and pp65 when coexpressed by MVA. Using MVAB-7Ag1/MVA-7Ag1, we demonstrated in different mouse strains (C57BL/6, BALB/c, and A2 and B7 HLA transgenic mice) that MVA coexpressing the seven HCMV antigens can stimulate robust complement-independent and complement-dependent EC-specific and FB-specific NAb responses, humoral immune responses promoting ADCC, and mouse and human polyfunctional MHC-restricted T cell responses by the individual HCMV antigens. In addition, compared to control vectors expressing only the PC subunits or gB, MVAB-7Ag1 showed substantially enhanced potency to elicit antibodies that promote in vitro neutralization of different HCMV strains and ADCC. These observations suggest that a PC/gB antigen combination when expressed by MVA can augment the induction of cross-NAb responses, a finding that may be of general importance for HCMV vaccine development considering that the NAb responses observed in the recent gB/MF59 phase II trial in postpartum HCMV-seronegative women were only very weak and particularly ineffective against heterologous HCMV strains (63). Moreover, recent observations indicate an important role of non-NAb responses in the protection mediated by gB/MF59 in postpartum women, which may suggest a beneficial vaccine effect through enhanced ADCC stimulation by a PC/gB antigen combination. Particularly intriguing were the high-level PC-specific T cell responses induced by MVA-7Ag1 in C57BL/6 and HLA transgenic mice, since the PC subunits have not been described as dominant T cell targets in HCMV-seropositive individuals (73). Polyfunctional CD8+ T cells able to secrete multiple effector cytokines have been associated with immunological control of different pathogens. In addition, polyfunctional CD8+ T cells, especially those directed toward pp65 and IE1, have been associated with control of HCMV viremia (81, 82). Consequently, it will be important to investigate the implications of the unusual PC-specific T cell responses in more detail.
In contrast to the recently introduced multiantigenic mRNA vaccines (26), we observed only minimal interference of in vivo immunogenicity of the PC subunits, gB, and pp65 when coexpressed by MVA. More specifically, we did not observe a dramatic reduction in pp65-specific T cell stimulation through interference with the PC subunits, as observed recently for the mRNA vaccine approach (26). We rather observed a differential intensity of recognition of the PC using two different HLA transgenic mouse strains. In A2 mice, the slightly decreased CD8 response to pp65 in MVA-7Ag1-immunized mice compared to the response to pp65 in MVAB-pp65-immunized mice may have been the consequence of the extraordinary response to the PC accounting for up to 45% of the total CD8+ T cell compartment. In contrast, in B7 mice the response to the PC was not as strong as in A2 mice, which may explain why the pp65-specific T cell responses induced by MVA-7Ag1 in B7 mice were similar to or even of higher magnitude than those induced by MVAB-pp65. A possible explanation for the disparities in the level of recognition of the same antigens between the two transgenic strains may be the intrinsic differences of the genetic background of the two strains of transgenic mice (74, 75). The most notable indication for antigen interference was the apparent reduced ability of MVAB-7Ag1 to stimulate ADCC-promoting antibody responses compared to the control vector MVAB-PC/gB, whereas this appeared to depend on the targeted HCMV strain. This suggests that pp65 may have a marginal impact on the immunogenicity of the PC and/or gB to stimulate HCMV humoral immunity upon MVA-vectored coexpression. Compared to the pp65-specific T cell responses induced by all multiantigenic MVA vectors, we generally observed only low-level gB-specific T cell stimulation by these vectors, which may be associated with epitope competition, but it may also be consequence of the chosen antigenic form of the gB protein. One way to overcome potential antigen interference of the multiantigenic MVA vectors could be prime-boost immunization strategies with single-antigen MVA vectors, although such strategies may complicate the translatability of the multiantigenic vaccine approach. In addition, vector-specific immunity may potentially limit the ability of the MVA vaccine approach to stimulate or boost HCMV immune responses by repeated immunization or antigen codelivery using separate vectors (51, 83).
A major challenge in HCMV vaccine development remains the quandary of imperfect protection by naturally acquired HCMV immunity (28). Congenital HCMV infection can occur following primary infection in HCMV-seronegative women, but it can also be a consequence of nonprimary infection in HCMV-seropositive women, resulting from either reinfection or virus reactivation (84). Because of the imperfect protection by natural HCMV immunity, it is argued that a vaccine candidate capable of eliciting HCMV immune responses similar to those induced during natural infection may not significantly alter the outcome of congenital infection and disease, particular in populations with high HCMV seroprevalence (28). Based on a single controversial study, HCMV immunity acquired through natural infection is suggested to reduce the risk of congenital infection in future pregnancies by 69% (28, 29). In addition, while intrauterine HCMV transmission following primary maternal infection is estimated to occur with a frequency at 30 to 40%, intrauterine transmission following nonprimary maternal infection is estimated to occur with a frequency of only 1 to 2% (85). Besides reducing the risk of intrauterine transmission, preconceptional natural HCMV immunity is also considered to reduce the severity of congenital disease (86), although this remains controversial (28). Hence, despite being imperfect, the protection afforded by preexisting HCMV immunity against congenital infection and disease appears to be substantial. Based on this premise, it can be hypothesized that the incidence of congenital infection and severity of congenital disease may be reduced by a vaccine candidate able to stimulate robust HCMV humoral and cellular immune responses in women without preexisting naturally acquired HCMV immunity.
While antibody responses are generally considered the primary immune component involved in the prevention of infection/reinfection, T cell responses are well known as the primary immune response mediating suppression of viral replication and reactivation. This general concept of protection by HCMV immunity may suggest that vaccine-mediated prevention of congenital infection in HCMV-seronegative women depends primarily on the induction of antibody responses to envelope glycoprotein complexes such as the PC and gB (33, 36, 41). Preexisting antibody responses may provide protection against congenital HCMV infection through immune mechanisms that include prevention of maternal HCMV acquisition at mucosal membranes, virus dissemination to the placenta, or intrauterine virus transmission at the fetal-maternal interface (20, 30, 38, 41). While antibody-mediated protection against HCMV infection may primarily involve NAb responses that interfere with virus entry or promote complement-dependent viroloysis, it may also involve non-NAb that promote ADCC and complement-dependent cytotoxicity as recently suggested (63). However, considering the imperfect protection provided by naturally acquired HCMV immunity, antibody responses will most likely not provide complete protection against HCMV infection of either the mother or the developing fetus. Vaccine-mediated stimulation of T cells may therefore be particularly important as a secondary immune response to protect against congenital infection in HCMV-seronegative women by promoting antibody production and maturation and enhancing virus containment and clearance once HCMV acquisition has occurred at mucosal membranes.
Since most cases of congenital infection, in particular in populations with high HCMV seroprevalence, occur following nonprimary maternal infection (86), HCMV-seropositive women in addition to HCMV-seronegative women may potentially benefit from vaccination prior to pregnancy. Vaccination of HCMV-seropositive women may boost or augment immune responses induced during natural infection, thereby potentially improving protection against congenital infection as a consequence of nonprimary maternal infection. Because congenital HCMV infection following nonprimary maternal infection can result from either reinfection or virus reactivation (28, 84), vaccine-mediated protection against congenital infection in HCMV-seropositive women may equally depend on the stimulation of humoral and cellular immune responses. While the induction of antibodies may be primarily required to protect against congenital infection as a result of maternal reinfection, the induction of T cells may be primarily required to prevent congenital infection as a result of maternal virus reactivation. Hence, the protection against congenital infection in HCMV-seropositive women may be at least in part mediated though immune mechanisms that resemble those mediating protection against HCMV infection in transplant recipients. In this context it is important to note that HCMV antigens expressed from plasmids or viral vectors such as MVA may provide the possibility to stimulate immune responses that are quantitatively or qualitatively different from those elicited by HCMV during natural infection. This hypothesis may be supported by our finding that MVA-7Ag1 can stimulate exceptionally high and polyfunctional PC-specific T-cell responses in HLA transgenic mice that are not known to be present in HCMV-seropositive individuals (73).
In sum, we have generated different multiantigenic MVA vaccine vectors simultaneously expressing the PC subunits, gB, and pp65 that stimulate robust HCMV humoral and cellular immune responses in mice. These observations support the use of MVA to develop a multiantigenic vaccine approach to protect against congenital HCMV infection and HCMV-associated disease in transplant recipients. Because MVA has an excellent safety record and proven ability to stimulate antigen-specific humoral and cell-mediated responses in animals and humans, these multiantigenic MVA vectors may form the basis of a vaccine candidate to prevent or control HCMV infection in different target populations.
MATERIALS AND METHODS
Cells and viruses.
BHK, ARPE-19, and MRC-5 cells were purchased from the American Type Culture Collection (ATCC). Chicken embryo fibroblasts (CEF) were obtained from Charles River. All rMVA vectors were derived from MVABAC-TK using BAC technology (54), with the exception of MVA-Venus and MVA-pp65/IE, which were constructed previously by homologous recombination using traditional transfection/infection method (52). All MVA virus stocks were prepared following virus propagation on CEF (21, 51). HCMV TB40/E was derived from TB40/Ewt-GFP BAC DNA (87), a kind gift from Thomas Shenk (Princeton University). HCMV TR expressing green fluorescent protein (GFP) was obtained from Jay Nelson (Oregon Health & Science University). HCMV Towne (RC2940) was a kind gift from Edward Mocarski (88). HCMV AD169 was obtained from John Zaia (City of Hope). TB40/E and TR virus stocks were prepared following propagation on ARPE-19 cells, whereas Towne and AD169 virus stocks were prepared following propagation in MRC-5 cells (21).
Recombinant MVA vectors.
MVABAC-TK-derived rMVA expressing HCMV antigens were generated via en passant mutagenesis in GS1783 Escherichia coli cells using specific transfer constructs and site-specific primers for homologous recombination (24, 54, 89, 90). Table 1 shows the primers that were used to mediate the gene insertion by en passant mutagenesis as well as the genome positions of the used MVA insertion sites. HCMV gene sequences encoding the PC subunits or pp65 were based on HCMV strain TB40/E (TB40/E-BAC; GenBank accession no. EF999921). The gene sequence expressing a truncated, form of gB in which the TM had been deleted (gBΔTM) encoded the N-terminal 698 amino acids (aa) of gB of HCMV strain Merlin (GenBank accession no. AY446894.2). All HCMV gene sequences were codon optimized for vaccinia virus expression using the Codon Optimization Tool from Integrated DNA Technologies (IDT), and runs of more than three of the same nucleotide type in a row were subsequently silently mutated to enhance the stability of the antigen sequences within MVA (58). Codon-optimized and P2A-linked HCMV gene sequences were synthesized by Genescript. Detailed sequence maps of all transfer plasmids and BAC constructs were generated by Vector NTI (Invitrogen). All recombinant BAC vectors were verified by PCR and restriction fragment length analysis, and all inserted HCMV gene sequences were verified by sequencing. Virus reconstitution from the recombinant BAC vectors was performed with BHK cells as described using fowl pox virus as a helper virus (24, 55).
BAC vector removal.
BAC removal of MVABAC-TK-derived rMVA by homologous recombination of a genomic duplication was performed similarly to a previously described procedure (64–66). Using en passant mutagenesis (89, 90), an ∼1.4-kbp genomic duplication of bp 69313 to 70703 of MVA (GenBank accession no. AY603355.1) that was homologous to ∼0.7-kbp sequences on either site of the BAC vector was inserted in direct orientation between the chloramphenicol resistance marker (cat) and the mini-F replicon (OriS, repE, sopA/B/C, cos, loxP) (Fig. 1) (54). A red fluorescent protein (RFP) marker was inserted along with the genomic duplication into the BAC vector so that each vector sequence (cat and mini-F replicon) separated by the genomic duplication contained a florescence marker (either RFP or GFP) (Fig. 1), whereby the GFP marker originated from the construction of MVABAC-TK (54). Upon virus reconstitution, virus with removed BAC sequences was identified by the absence of RFP and GFP expression. Plaque purification was performed to eliminate residual virus containing remaining vector sequences and to obtain pure rMVA vectors without BAC sequences. Confluent CEF in a 96-well plate format were infected with 20 PFU of MVA per plate. At 3 to 5 days postinfection, virus from wells showing viral foci without signs of RFP and GFP expression was prepared by standard freeze-thaw technique and expanded on CEF into larger virus amounts. Expanded virus was tested by PCR using primers (5′-TGG ATG ACA ACT CAA ACA TCT GC-3′ and 5′-TTTCCTCCTCGTTTGGATCTCAC-3′) that flank the TK insertion to confirm the complete removal of the BAC vector sequences (Fig. 1). Primers specific for bacterial resistance marker cat (5′-TGC CAC TCA TCG CAG TAC TG-3′ and 5′-AGG CAT TTC AGT CAG TTG CTC-3′) or the sopA element of the mini-F replicon (5′-TTA ACT CAG TTT CAA TAC GGT GCA G-3′ and 5′-TGGGGTTTCTTCTCAGGCTATC-3′) were used to confirm the absence of residual vector sequences (Fig. 1). PCR fragments amplified from the TK site were sequenced to verify the restoration of the MVA TK gene locus (64, 66).
Immunoblotting.
HCMV antigens in whole-cell lysates of rMVA-infected CEF cells were detected by Immunoblot using standard methods (21). HCMV gL and UL131A were detected using peptide-specific rabbit polyclonal antisera (21), UL128 was detected using rabbit polyclonal antiserum raised against glutathione S-transferase (GST)-tagged UL128 (PTA-8474; ATCC), and gH, gB, and pp65 were detected using MAb AP86 (91), MAb p7-17 (92), and MAb 28-103 (68), respectively, kindly provided by William Britt (University of Alabama at Birmingham). UL130 was detected using MAb 3C5 (17), a kind gift from Thomas Shenk (Princeton University). Vaccinia virus BR5 was detected using MAb 19C2 (93).
Flow cytometry.
Cell surface flow cytometry analysis of rMVA-infected BHK cells by NAb was performed as described previously (20). Briefly, BHK cells (70 to 90% confluent) were infected with rMVA at a multiplicity of infection (MOI) of 5. At 4 h postinfection, rMVA-infected BHK cells (live, nonpermeabilized) were stained with 25 μg/ml of NAb and secondary antibody Alexa Fluor 647 goat anti-mouse IgG (Life Technologies) at a dilution of 1/2,000. Fifteen thousand events were collected using a BD FACSCelesta flow cytometer (BD) and analyzed with FlowJo software (Tree Star).
Mouse immunization.
The Institutional Animal Care and Use Committee (IACUC) of the Beckman Research Institute of City of Hope approved protocol 98004 assigned for this study. All study procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (94) and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals (95). BALB/c and C57BL/6 mice were purchased from the Jackson Laboratory. HLA-A*0201 HHD II H-2Db/β2 microglobulin double-knockout (74) (A2) and HLA-B*0702 H-2KbDb double-knockout (75) (B7) transgenic mice on a C57BL/6 background were obtained from F. Lemonnier (Institut Pasteur, France) and bred at the City of Hope Animal Research Center. Mice were vaccinated two or three times in 4-week intervals with the rMVA vectors by the intraperitoneal (i.p.) route with either 5 × 107 PFU or 1 × 107 PFU (low dose). Blood samples for humoral immune analysis were collected by retro-orbital bleeding. Splenocytes for cellular immune analysis were isolated by standard procedure, whereby red blood cells (RBSs) were removed using RBC lysis buffer (BioLegend).
HCMV neutralization.
NAb titers at which 50% HCMV infection was inhibited (NT50) were determined by microneutralization assay performed similarly to previous descriptions (20, 21). Briefly, heat-inactivated immune sera with or without the addition of 5% guinea pig complement were serially 2-fold diluted in 75-μl volumes using complete growth medium for ARPE-19 or MRC-5 cells depending on the cell type used in the assay. Dilutions ranged from 1:25 to 1:204,800. Diluted serum was mixed with 75 μl of complete growth medium containing approximately 2,400 PFU of HCMV. After 2 h of incubation, virus-serum mixtures were added in duplicate (50 μl) to ARPE-19 or MRC-5 cells seeded the day before at 1.5 × 104/well in a clear-bottom polystyrene 96-well plate (Corning) that contained 50 μl per well of complete growth medium. Cells were grown for 48 h and fixed in methanol-acetone. Infected cells were stained by immunostaining using mouse anti-HCMV IE1 Ab (p63-27 [96]; kindly provided by William Britt) and the Vectastain ABC kit (Vector Laboratories). The substrate was 3,3′-diaminobenzidine (DAB; Vector Laboratories). Plates were analyzed by an automated system using an DMi8 inverted microscope equipped with a linear motorized stage (Leica). IE1-positive nuclei per field of view using a 5× objective were counted using ImagePro Premier (Media Cybernetics). For each dilution the average number of positive nuclei in duplicate was calculated. The percent neutralization titer for each dilution was calculated as follows: NT = [1 − (number of positive nuclei with immune sera/number of positive nuclei with preimmune sera)] × 100. The titers that gave 50% neutralization were calculated by determining the linear slope of the graph plotting NT versus plasma dilution by using the next higher and lower NT values that were closest to 50% neutralization.
Surrogate ADCC reporter assay.
ADCC was measured by mouse FcγRIV (mFcγRIV) reporter bioassay (Promega). This reporter assay allows measurement of ADCC without the need to isolate NK cells from peripheral blood mononuclear cells (PBMCs) and without variability in mFcγRIV amounts (97). mFcγRIV is the main receptor involved in ADCC in mice and is most closely related to human FcγRIIIa, the primary Fc receptor involved in ADCC in humans (98). ARPE-19 cells were infected with HCMV at an MOI of 5 for 24 h, and infected cells were transferred to white, flat-bottomed 96-well plates. After 24 h, pooled heat-inactivated serum from immunized mice was 2-fold diluted in assay buffer (RPMI supplemented with 4% low-IgG serum) and added to the target cells. Engineered mFcγRIV cells expressing a luciferase reporter driven by a nuclear factor of activated T cells response element were added to each well and incubated for 6 h, following which Bio-Glo reagent was added. Plates were developed using a BioTeck Cytation3 luminometer. Fold induction was calculated relative to luciferase activity in the absence of serum.
ELISA.
PC and gB binding antibodies in immune sera of rMVA-immunized mice were quantified by enzyme-linked immunosorbent assay (ELISA) using purified PC or gB protein that was derived by expression from plasmids (PC) or rMVA (gB) in mammalian cells (51, 61). ELISA microtiter wells (Costar) were coated with 1 μg/ml of purified protein and blocked with 1% bovine serum albumin (BSA)–phosphate-buffered saline (PBS), and 2-fold serum dilutions were added in duplicates. Microtiter plates were developed using anti-mouse IgG horseradish peroxidase conjugate (Sigma-Aldrich), followed by addition of 3,3′,5,5′-tetramethylbenzidine (Thermo-Fisher). Absorbance at 450 nm was measured using a FilterMax F3 microplate reader (Molecular Devices). Endpoint titers were calculated as the highest serum dilution to produce an absorbance higher than the geometric mean plus three times the standard deviation of the absorbance obtained using serum from 5 naive mice that was diluted 100 times.
Peptides.
Peptide libraries of pp65 and gB were composed of 15-mers with an 11-aa overlap. The pp65 peptide library was obtained from NIH (AIDS Reagent Program, Division of AIDS, NIAID). The gB peptide library was purchased from JPT Peptide Technologies GmbH (Berlin, Germany). Peptide libraries of gH, gL, UL130, and UL131A were composed of 15-mers with a 9-aa overlap and purchased from Genscript. The UL128 peptide library was composed of 15-mers with an 11-aa overlap and produced at the City of Hope Core Facility (99). Equal amounts of peptides of the individual PC subunits were used to create the PC peptide library. The human MHC class I-restricted immunodominant peptides HLA-A*0201 pp65(495–503) and HLA-B*0702 pp65(265–275) were described previously (100). To detect mouse H2-b-restricted pp65-specifc T cells, a mixture of previously identified 11 immunoreactive peptides was used (72). To detect mouse H-2b-restricted PC-specific T cells, a mixture of gH (PHGWKESHTTSGLHR and TQGVINIMYMHDSDD), gL (LIRYRPVTPEAANSV), UL128 (NKLTSCNYNPLYLEA), UL130 (KLTYSKPHDAATFYC), and UL131A (LNYHYDASHGLDNFD) immunodominant peptides was used (72). All H-2b-restricted peptides were synthesized at City of Hope Core Facility.
ELISpot assay.
T cell detection by ELISpot assay was performed according to the manufacturer's instructions (Millipore). Briefly, Millipore Multiscreen–IP filter plates were coated with capture antibody (mouse IFN-γ ELISpot development module; R&D Systems) and blocked with 1% BSA–5% sucrose in PBS. Splenocytes (∼5 × 104 to 2 × 105) in RPMI medium were added to each well and incubated overnight at 37°C in the presence of 2 μg/ml of peptides and 1 μg/ml of CD28 and CD49d costimulatory Ab (Biolegend). After 24 h, plates were incubated overnight at 4°C with detection antibody and developed using ELISpot blue color module (R&D Systems). Spots were counted using an AID EliSpot reader. The number of spots obtained in wells in which 2 μl of dimethyl sulfoxide (DMSO) was added was subtracted from each well. An arbitrary number of 3,000 spots was assigned to wells in which the number of spots was too high to be counted.
Intracellular cytokine staining.
Multicolor intracellular cytokine staining (ICS) was performed according to standard protocol. Briefly, 2.5 × 106 splenocytes were stimulated with peptides (1 μg/ml), 0.1% DMSO, or phorbol myristate acetate (PMA)-ionomycin (BD Biosciences) for 1.5 h at 37°C. Anti-mouse CD28 and CD49d antibodies (1 μg/ml; BioLegend) were added as costimulation. Brefeldin A (3 μg/ml; eBioscience) was added, and the cells were incubated for additional 16 h at 37°C. Cells were fixed using Cytofix buffer (BD Biosciences) and surface staining was performed using fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD3, BV650 anti-mouse CD8a, and peridinin chlorophyll protein (PerCP)-Cy5.5 anti-mouse CD4 (BD Biosciences). Following cell permeabilization using Cytoperm buffer (BD Biosciences), ICS was performed using allophycocyanin (APC)-conjugated anti-mouse IFN-γ, phycoerythrin (PE)-conjugated anti-mouse TNF-α, and PE-CF594 anti-mouse IL-2 (BD Biosciences). Events were acquired using a BD FACSCelesta flow cytometer (2 × 105cells/tube). Analysis was performed using FlowJo. Percentages of polyfunctional subsets were calculated by applying FlowJo Boolean combination gating.
Statistics.
GraphPad Prism software version 5.0 (GraphPad) was used for statistical analysis. NAb titers and IFN-γ-secreting T cells in each group were compared using one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test, while differences between NAb titers were calculated using two-way ANOVA followed by Sidak's multiple-comparison test.
ACKNOWLEDGMENTS
We thank Tomasz Adamus and Dayson Moreira for their help with using the luminometer. We are grateful to Weimin Tsai and Ferdinand Kos for their assistance with the flow cytometer. We also thank Chandran Ramakrishna for helping with the use of the ELISpot reader.
This research was funded by U.S. Public Health Service grant R01 AI103960 to Don J. Diamond, who was partially supported by CA077544 and CA181045. This research was partially supported by Helocyte Inc. The City of Hope Cancer Center is supported by the National Cancer Institute of the National Institutes of Health under award number CA33572.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Don J. Diamond, Felix Wussow, and Flavia Chiuppesi receive royalty payments from Helocyte Inc. Don J. Diamond chairs the Scientific Advisory Board of Helocyte Inc., and he has an equity interest in Helocyte Inc.
REFERENCES
- 1.Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 325:417–470. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. 2000. Vaccines for the 21st century: a tool for decisionmaking. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin S. 2015. The history of vaccination against cytomegalovirus. Med Microbiol Immunol 204:247–254. doi: 10.1007/s00430-015-0388-z. [DOI] [PubMed] [Google Scholar]
- 5.Schleiss MR. 2016. Cytomegalovirus vaccines under clinical development. J Virus Erad 2:198–207. [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin SA, Higgins R, Kurtz JB, Morris PJ, Campbell DA Jr, Shope TC, Spector SA, Dankner WM. 1994. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 58:1176–1178. [PubMed] [Google Scholar]
- 7.Plotkin SA, Starr SE, Friedman HM, Gonczol E, Weibel RE. 1989. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis 159:860–865. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- 8.Adler SP, Starr SE, Plotkin SA, Hempfling SH, Buis J, Manning ML, Best AM. 1995. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis 171:26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM, Turley CB, Stanberry LR, Patel SM, McNeal MM, Pichon S, Amegashie C, Bellamy AR. 2016. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 34:313–319. doi: 10.1016/j.vaccine.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britt WJ, Vugler L, Butfiloski EJ, Stephens EB. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol 64:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 86:7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Wussow F, Chiuppesi F, Contreras H, Diamond DJ. 2017. Neutralization of human cytomegalovirus entry into fibroblasts and epithelial cells. Vaccines (Basel) 5(4):E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu TM, Wang D, Freed DC, Tang A, Li F, He X, Cole S, Dubey S, Finnefrock AC, ter Meulen J, Shiver JW, Casimiro DR. 2012. Restoration of viral epithelial tropism improves immunogenicity in rabbits and rhesus macaques for a whole virion vaccine of human cytomegalovirus. Vaccine 30:7469–7474. doi: 10.1016/j.vaccine.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiuppesi F, Wussow F, Johnson E, Bian C, Zhuo M, Rajakumar A, Barry PA, Britt WJ, Chakraborty R, Diamond DJ. 2015. Vaccine-derived neutralizing antibodies to the human cytomegalovirus gH/gL pentamer potently block primary cytotrophoblast infection. J Virol 89:11884–11898. doi: 10.1128/JVI.01701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, Newell M, Tran E, Ortiz J, La Rosa C, Herrmann A, Longmate J, Chakraborty R, Barry PA, Diamond DJ. 2014. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 10:e1004524. doi: 10.1371/journal.ppat.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, Preite S, Fuschillo D, Percivalle E, Sallusto F, Gerna G, Corti D, Lanzavecchia A. 2014. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A 111:17965–17970. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen Y, Monroe J, Linton C, Archer J, Beard CW, Barnett SW, Palladino G, Mason PW, Carfi A, Lilja AE. 2014. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine 32:3796–3804. doi: 10.1016/j.vaccine.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Wussow F, Yue Y, Martinez J, Deere JD, Longmate J, Herrmann A, Barry PA, Diamond DJ. 2013. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol 87:1322–1332. doi: 10.1128/JVI.01669-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann I, Wen Y, Ciferri C, Schulze A, Fuhner V, Leong M, Gerber A, Gerrein R, Nandi A, Lilja AE, Carfi A, Laux H. 2015. Expression of the human cytomegalovirus pentamer complex for vaccine use in a CHO system. Biotechnol Bioeng 112:2505–2515. doi: 10.1002/bit.25670. [DOI] [PubMed] [Google Scholar]
- 26.John S, Yuzhakov O, Woods A, Deterling J, Hassett K, Shaw CA, Ciaramella G. 2018. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 36:1689–1699. doi: 10.1016/j.vaccine.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Freed DC, He X, Li F, Tang A, Cox KS, Dubey SA, Cole S, Medi MB, Liu Y, Xu J, Zhang ZQ, Finnefrock AC, Song L, Espeseth AS, Shiver JW, Casimiro DR, Fu TM. 2016. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med 8:362ra145. doi: 10.1126/scitranslmed.aaf9387. [DOI] [PubMed] [Google Scholar]
- 28.Britt WJ. 2017. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 91:e02392-16. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler KB, Stagno S, Pass RF. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 30.Permar SR, Schleiss MR, Plotkin SA. 17 January 2018. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol doi: 10.1128/JVI.00030-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itell HL, Nelson CS, Martinez DR, Permar SR. 2017. Maternal immune correlates of protection against placental transmission of cytomegalovirus. Placenta 60(Suppl 1):S73–S79. doi: 10.1016/j.placenta.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach MR, Yan D, Vij R, Hongo JA, Nakamura G, Vernes JM, Meng YG, Lein S, Chan P, Ross J, Carano R, Deng R, Lewin-Koh N, Xu M, Feierbach B. 2014. A neutralizing anti-gH/gL monoclonal antibody is protective in the guinea pig model of congenital CMV infection. PLoS Pathog 10:e1004060. doi: 10.1371/journal.ppat.1004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, Sallusto F, Lanzavecchia A, Corti D, Gerna G. 2013. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 8:e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bialas KM, Tanaka T, Tran D, Varner V, Cisneros De La Rosa E, Chiuppesi F, Wussow F, Kattenhorn L, Macri S, Kunz EL, Estroff JA, Kirchherr J, Yue Y, Fan Q, Lauck M, O'Connor DH, Hall AH, Xavier A, Diamond DJ, Barry PA, Kaur A, Permar SR. 2015. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci U S A 112:13645–13650. doi: 10.1073/pnas.1511526112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee A, Harrison CJ, Britt WJ, Bewtra C. 2001. Modification of maternal and congenital cytomegalovirus infection by anti-glycoprotein b antibody transfer in guinea pigs. J Infect Dis 183:1547–1553. doi: 10.1086/320714. [DOI] [PubMed] [Google Scholar]
- 36.Boppana SB, Britt WJ. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis 171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 37.Schleiss MR, Berka U, Watson E, Aistleithner M, Kiefmann B, Mangeat B, Swanson EC, Gillis PA, Hernandez-Alvarado N, Fernandez-Alarcon C, Zabeli JC, Pinschewer DD, Lilja AE, Schwendinger M, Guirakhoo F, Monath TP, Orlinger KK. 2017. Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol 24:e00300-16. doi: 10.1128/CVI.00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson CS, Cruz DV, Tran D, Bialas KM, Stamper L, Wu H, Gilbert M, Blair R, Alvarez X, Itell H, Chen M, Deshpande A, Chiuppesi F, Wussow F, Diamond DJ, Vandergrift N, Walter MR, Barry PA, Cohen-Wolkowiez M, Koelle K, Kaur A, Permar SR. 2017. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2(13):94002. doi: 10.1172/jci.insight.94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lilleri D, Fornara C, Furione M, Zavattoni M, Revello MG, Gerna G. 2007. Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J Infect Dis 195:1062–1070. doi: 10.1086/512245. [DOI] [PubMed] [Google Scholar]
- 40.Fornara C, Cassaniti I, Zavattoni M, Furione M, Adzasehoun KMG, De Silvestri A, Comolli G, Baldanti F. 2017. Human cytomegalovirus-specific memory CD4+ T-cell response and its correlation with virus transmission to the fetus in pregnant women with primary infection. Clin Infect Dis 65:1659–1665. doi: 10.1093/cid/cix622. [DOI] [PubMed] [Google Scholar]
- 41.Lilleri D, Kabanova A, Lanzavecchia A, Gerna G. 2012. Antibodies against neutralization epitopes of human cytomegalovirus gH/gL/pUL128-130-131 complex and virus spreading may correlate with virus control in vivo. J Clin Immunol 32:1324–1331. doi: 10.1007/s10875-012-9739-3. [DOI] [PubMed] [Google Scholar]
- 42.Nigro G, Adler SP, La Torre R, Best AM. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 43.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 44.Anderholm KM, Bierle CJ, Schleiss MR. 2016. Cytomegalovirus vaccines: current status and future prospects. Drugs 76:1625–1645. doi: 10.1007/s40265-016-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Rosa C, Diamond DJ. 2012. The immune response to human CMV. Future Virol 7:279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baraniak I, Kropff B, McLean GR, Pichon S, Piras-Douce F, Milne RSB, Smith C, Mach M, Griffiths PD, Reeves MB. 2018. Epitope-specific humoral responses to human cytomegalovirus glycoprotein-B vaccine with MF59: anti-AD2 levels correlate with protection from viremia. J Infect Dis 217:1907–1917. doi: 10.1093/infdis/jiy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert SC. 2013. Clinical development of modified vaccinia virus Ankara vaccines. Vaccine 31:4241–4246. doi: 10.1016/j.vaccine.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Draper SJ, Cottingham MG, Gilbert SC. 2013. Utilizing poxviral vectored vaccines for antibody induction-progress and prospects. Vaccine 31:4223–4230. doi: 10.1016/j.vaccine.2013.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cottingham MG, Carroll MW. 2013. Recombinant MVA vaccines: dispelling the myths. Vaccine 31:4247–4251. doi: 10.1016/j.vaccine.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Sutter G, Moss B. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A 89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, La Rosa C, Maas R, Ly H, Brewer J, Mekhoubad S, Daftarian P, Longmate J, Britt WJ, Diamond DJ. 2004. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol 78:3965–3976. doi: 10.1128/JVI.78.8.3965-3976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Martinez J, Zhou W, La Rosa C, Srivastava T, Dasgupta A, Rawal R, Li Z, Britt WJ, Diamond D. 2010. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 28:1547–1557. doi: 10.1016/j.vaccine.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Rosa C, Longmate J, Martinez J, Zhou Q, Kaltcheva TI, Tsai W, Drake J, Carroll M, Wussow F, Chiuppesi F, Hardwick N, Dadwal S, Aldoss I, Nakamura R, Zaia JA, Diamond DJ. 2017. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 129:114–125. doi: 10.1182/blood-2016-07-729756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wussow F, Chiuppesi F, Meng Z, Martinez J, Nguyen J, Barry PA, Diamond DJ. 2018. Exploiting 2A peptides to elicit potent neutralizing antibodies by a multi-subunit herpesvirus glycoprotein complex. J Virol Methods 251:30–37. doi: 10.1016/j.jviromet.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cottingham MG, Andersen RF, Spencer AJ, Saurya S, Furze J, Hill AV, Gilbert SC. 2008. Recombination-mediated genetic engineering of a bacterial artificial chromosome clone of modified vaccinia virus Ankara (MVA). PLoS One 3:e1638. doi: 10.1371/journal.pone.0001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. 2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt LS, Earl PL, Xiao W, Americo JL, Cotter CA, Vogt J, Moss B. 2009. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J Virol 83:7176–7184. doi: 10.1128/JVI.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manuel ER, Wang Z, Li Z, La Rosa C, Zhou W, Diamond DJ. 2010. Intergenic region 3 of modified vaccinia ankara is a functional site for insert gene expression and allows for potent antigen-specific immune responses. Virology 403:155–162. doi: 10.1016/j.virol.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyatt LS, Earl PL, Moss B. 13 February 2018. Generation of recombinant vaccinia viruses. Curr Protoc Protein Sci. https://currentprotocols.onlinelibrary.wiley.com/doi/full/10.1002/cpps.33. [DOI] [PubMed]
- 61.Chiuppesi F, Wussow F, Scharf L, Contreras H, Gao H, Meng Z, Nguyen J, Barry PA, Bjorkman PJ, Diamond DJ. 2017. Comparison of homologous and heterologous prime-boost vaccine approaches using modified vaccinia Ankara and soluble protein to induce neutralizing antibodies by the human cytomegalovirus pentamer complex in mice. PLoS One 12:e0183377. doi: 10.1371/journal.pone.0183377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burke HG, Heldwein EE. 2015. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog 11:e1005227. doi: 10.1371/journal.ppat.1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson CS, Huffman T, Jenks JA, Cisneros de la Rosa E, Xie G, Vandergrift N, Pass RF, Pollara J, Permar SR. 30 April 2018. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1800177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wussow F, Fickenscher H, Tischer BK. 2009. Red-mediated transposition and final release of the mini-F vector of a cloned infectious herpesvirus genome. PLoS One 4:e8178. doi: 10.1371/journal.pone.0008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cottingham MG, Gilbert SC. 2010. Rapid generation of markerless recombinant MVA vaccines by en passant recombineering of a self-excising bacterial artificial chromosome. J Virol Methods 168:233–236. doi: 10.1016/j.jviromet.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Tischer BK, Kaufer BB, Sommer M, Wussow F, Arvin AM, Osterrieder N. 2007. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol 81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Britt WJ, Auger D. 1985. Identification of a 65 000 dalton virion envelope protein of human cytomegalovirus. Virus Res 4:31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- 69.Loomis RJ, Lilja AE, Monroe J, Balabanis KA, Brito LA, Palladino G, Franti M, Mandl CW, Barnett SW, Mason PW. 2013. Vectored co-delivery of human cytomegalovirus gH and gL proteins elicits potent complement-independent neutralizing antibodies. Vaccine 31:919–926. doi: 10.1016/j.vaccine.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Britt WJ, Vugler L, Stephens EB. 1988. Induction of complement-dependent and -independent neutralizing antibodies by recombinant-derived human cytomegalovirus gp55-116 (gB). J Virol 62:3309–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy E, Shenk T. 2008. Human cytomegalovirus genome. Curr Top Microbiol Immunol 325:1–19. [DOI] [PubMed] [Google Scholar]
- 72.Shedlock DJ, Talbott KT, Wu SJ, Wilson CM, Muthumani K, Boyer JD, Sardesai NY, Awasthi S, Weiner DB. 2012. Vaccination with synthetic constructs expressing cytomegalovirus immunogens is highly T cell immunogenic in mice. Hum Vaccin Immunother 8:1668–1681. doi: 10.4161/hv.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. 1997. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohrlich PS, Cardinaud S, Firat H, Lamari M, Briand P, Escriou N, Lemonnier FA. 2003. HLA-B*0702 transgenic, H-2KbDb double-knockout mice: phenotypical and functional characterization in response to influenza virus. Int Immunol 15:765–772. doi: 10.1093/intimm/dxg073. [DOI] [PubMed] [Google Scholar]
- 76.Krishnan A, Wang Z, Srivastava T, Rawal R, Manchanda P, Diamond DJ, La Rosa C. 2008. A novel approach to evaluate the immunogenicity of viral antigens of clinical importance in HLA transgenic murine models. Immunol Lett 120:108–116. doi: 10.1016/j.imlet.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong J, Rist M, Cooper L, Smith C, Khanna R. 2008. Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus. PLoS One 3:e3256. doi: 10.1371/journal.pone.0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lacey SF, La Rosa C, Kaltcheva T, Srivastava T, Seidel A, Zhou W, Rawal R, Hagen K, Krishnan A, Longmate J, Andersson HA, St John L, Bhatia R, Pullarkat V, Forman SJ, Cooper LJ, Molldrem J, Diamond DJ. 2011. Characterization of immunologic properties of a second HLA-A2 epitope from a granule protease in CML patients and HLA-A2 transgenic mice. Blood 118:2159–2169. doi: 10.1182/blood-2011-04-349951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibson L, Barysauskas CM, McManus M, Dooley S, Lilleri D, Fisher D, Srivastava T, Diamond DJ, Luzuriaga K. 2015. Reduced frequencies of polyfunctional CMV-specific T cell responses in infants with congenital CMV infection. J Clin Immunol 35:289–301. doi: 10.1007/s10875-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schleiss MR, Permar SR, Plotkin SA. 2017. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin Vaccine Immunol 24:e00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giménez E, Munoz-Cobo B, Solano C, Amat P, de la Camara R, Nieto J, Lopez J, Remigia MJ, Garcia-Noblejas A, Navarro D. 2015. Functional patterns of cytomegalovirus (CMV) pp65 and immediate early-1-specific CD8(+) T cells that are associated with protection from and control of CMV DNAemia after allogeneic stem cell transplantation. Transpl Infect Dis 17:361–370. doi: 10.1111/tid.12391. [DOI] [PubMed] [Google Scholar]
- 82.Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, Osborne RJ, Sparks SD, Palmer SM, Weinhold KJ. 2016. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med 193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altenburg AF, van Trierum SE, de Bruin E, de Meulder D, van de Sandt CE, van der Klis FRM, Fouchier RAM, Koopmans MPG, Rimmelzwaan GF, de Vries RD. 2018. Effects of pre-existing orthopoxvirus-specific immunity on the performance of modified vaccinia virus Ankara-based influenza vaccines. Sci Rep 8:6474. doi: 10.1038/s41598-018-24820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 85.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 86.Wang C, Zhang X, Bialek S, Cannon MJ. 2011. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 52:e11–e13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 87.O'Connor CM, Murphy EA. 2012. A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny. J Virol 86:9854–9865. doi: 10.1128/JVI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cherrington JM, Mocarski ES. 1989. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol 63:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 90.Tischer BK, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 91.Simpson JA, Chow JC, Baker J, Avdalovic N, Yuan S, Au D, Co MS, Vasquez M, Britt WJ, Coelingh KL. 1993. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J Virol 67:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Britt WJ. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 93.Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, Griffiths G. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol 68:130–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 95.Office of Laboratory Animal Welfare. 2015. Public Health Service policy on the humane care and use of laboratory animals. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- 96.Andreoni M, Faircloth M, Vugler L, Britt WJ. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods 23:157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 97.Parekh BS, Berger E, Sibley S, Cahya S, Xiao L, LaCerte MA, Vaillancourt P, Wooden S, Gately D. 2012. Development and validation of an antibody-dependent cell-mediated cytotoxicity-reporter gene assay. MAbs 4:310–318. doi: 10.4161/mabs.19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nimmerjahn F, Ravetch JV. 2006. Fcgamma receptors: old friends and new family members. Immunity 24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 99.Chiuppesi F, Kaltcheva T, Meng Z, Barry PA, Diamond DJ, Wussow F. 2017. Identification of a continuous neutralizing epitope within UL128 of human cytomegalovirus. J Virol 91:e01857-16. doi: 10.1128/JVI.01857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diamond DJ, York J, Sun JY, Wright CL, Forman SJ. 1997. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 90:1751–1767. [PubMed] [Google Scholar]