Abstract
The Maximum Cumulative Ratio (MCR) quantifies the degree to which a single chemical drives the cumulative risk of an individual exposed to multiple chemicals. Phthalates are a class of chemicals with ubiquitous exposures in the general population that have the potential to cause adverse health effects in humans. This work used the MCR to evaluate coexposures to six phthalates as measured in biomonitoring data from the most recent cycle (2013-2014) of the National Health and Nutrition Examination Survey (NHANES). The values of MCR, Hazard Index (HI), and phthalate-specific Hazard Quotients (HQs) were determined for 2663 NHANES participants aged six years and older by using reverse dosimetry techniques to calculate steady-state doses consistent with concentrations of metabolites of six phthalates in urine and using Tolerable Daily Intake values. There were 21 participants (0.8% of the NHANES sample) with HI>1. Of those, 43% (9/21) would have been missed by chemical-by-chemical assessments (i.e. all HQs were less than one). The mean MCR value in the 21 participants was 2.1. HI and MCR values were negatively correlated (p<0.001) indicating that most participants, especially those with elevated HI values, had their cumulative risks driven by relatively large doses of a single phthalate rather than doses of multiple phthalates. The dominate phthalate varied across participants. Children (aged 6-17years) had a higher HI values (p<0.01) than adults (18+ years). However, the probability of having HI>1 was not driven by age, gender, or ethnicity. The cumulative exposures of concern largely originated from a subset of three of the fifteen possible pairs of the six phthalates. These findings suggest that cumulative exposures were a potential concern for a small portion of the surveyed participants involving a subset of the phthalates explored. The largest risks tended to occur in individuals whose exposures were dominated by a single phthalate.
Keywords: Mixtures, Phthalates, Biomonitoring, NHANES, Cumulative exposures, Maximum cumulative ratio
1. Introduction
Phthalates (esters of phthalic acid) are used as plasticizers in a wide range of consumer goods including vinyl flooring, food packaging, the outer coatings of pills, cosmetics, food containers, pipes and tubing, etc. (NRC, 2008). As plasticizers, phthalates can make nail polish less brittle, allow hair sprays to have more flexibility, and reduce volatility in fragrances. Phthalates are not strongly bound and leaching of the compounds can occur in many of these products (Sathyanarayana, 2008). Human exposure routes include dermal exposures, inhalation, intravenous injection and, most commonly, ingestion (Colacino et al., 2010). In 2008, the National Research Council concluded that phthalates met the conditions to warrant a cumulative risk approach. They stated that the general population is exposed to multiple phthalates which may contribute to a common adverse health outcome (NRC, 2008). Although the NRC report focused on effects related to disrupted male reproductive development known as the “phthalate syndrome”, there was evidence from both animal and human studies that phthalates impact a wide variety of health endpoints (Jurewicz and Hanke, 2011, Lyche et al., 2009, Martino-Andrade and Chahoud, 2009, Meeker and Ferguson, 2011, Pak et al., 2011).
The Hazard Index (HI) is a screening tool for estimating cumulative risks from exposures to multiple chemicals from a common mechanism group. This approach assumes dose addition (EPA, 1986, EPA, 2003, EPA, 2007, Teuschler and Hertzberg, 1995). The HI provides a straightforward method for quantifying risks by relating the intakes of substances to their Reference Values (RfVs) (NRC, 2008). This technique applied to individuals has been previously demonstrated in the literature (Kortenkamp and Faust, 2010, Søeborg et al., 2012). Examples of RfVs for oral exposures include the United States Environmental Protection Agency's (USEPA's) Reference Dose (RfD) and the European Union's Tolerable Daily Intake (TDI). The Hazard Quotient (HQ) is calculated as the ratio of an individual's estimated exposure level to the RfV for that chemical. The chemical-specific HQs are then summed to give an individual's HI.
The Maximum Cumulative Ratio (MCR) is a measure of the contribution of the most dominant chemical to the risks posed by an individual's cumulative exposures to multiple chemicals (Kienzler et al., 2014, Price and Han, 2011, Vallotton and Price, 2016). The MCR along with measures of cumulative exposures can inform risk management decisions and help identify specific combinations of chemicals that result in elevated cumulative risks. The MCR approach has been applied to biomonitoring data on mixtures of dioxin-like chemicals (Han and Price, 2013), exposures to mixtures of chemicals in water (Han and Price, 2011, Price and Han, 2011, Silva and Cerejeira, 2015, Vallotton and Price, 2016), and mixtures in residential indoor air (De Brouwere et al., 2014).
The present study applied the MCR approach to a group of six phthalates from the National Health and Nutrition Examination Survey (NHANES) for the years 2013 and 2014. Reverse dosimetry techniques were used to reconstruct individuals' phthalate exposures from data using metabolite concentrations in their urine along with information about their physiologiesand demographics (Christensen et al., 2014). Using hazard information from each phthalate, MCR values were constructed. The results are presented by age, gender, and ethnicity.
2. Methods and materials
2.1. NHANES data set
Phthalate biomarker data came from the 2013–2014 cycle of NHANES (CDC, 2016a). NHANES is a nationwide survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) and is representative of the general non-institutionalized, civilian population in the United States. This survey has a complex multistage, stratified, random sampling design based on the sampling of counties, households, and household members. NHANES gathers information through interviews, physical examinations, and laboratory tests. Samples of urine are collected from participants six years and older and samples of blood are collected from participants aged one year and older (CDC, 2016b). Urine samples from a subset of participants were analyzed for metabolites of phthalates. Of the 2777 participants sampled for phthalate analysis, 82 participants were missing a necessary metabolite concentration, and an additional 32 were missing either height or weight information. Thus, there were data from a total of 2663 participants from the 2013–2014 NHANES cycle used in this analysis.
The six phthalates and associated metabolites included in this analysis were di-n-butyl phthalate (DBP; with metabolite MBP), diisobutyl phthalate (DIBP; with metabolite MIBP), butyl benzyl phthalate (BBP; with metabolite MBZP), di(2-ethylhexyl) phthalate (DEHP; with metabolites MECPP, MEOHP, MEHHP, and MEHP), diisononyl phthalate (DINP; with metabolites MINP and MCOP), and diisodecyl phthalate (DIDP; with metabolite MCNP; Table 1). At the time of this writing, the 2013–2014 NHANES cycle constitutes the most recent publically available NHANES biomonitoring data for these compounds. This combination of phthalates has been explored in previous works (Qian et al., 2015) and is a slight expansion on the five phthalates investigated by Christensen et al. (2014). The set of phthalates selected are due to their wide and varied use in consumer products (Qian et al., 2015).
Table 1.
Data on tolerable daily intakes, uncertainty factors, metabolites, and metabolite detection limits from the 2013–2014 NHANES cycle.
| Phthalate (parent) |
Tolerable daily intakes (μg/kg/d) |
Uncertainty factors |
Metabolite | Limit of detection in NHANES (ng/mL) |
Number of samples below the limit of detection (%) |
|---|---|---|---|---|---|
|
di-n-butyl phthalate (DBP) |
10a | 200a | monobutyl phthalate (MBP)g | 0.4 | 43 (1.6) |
|
diisobutyl phthalate (DIBP) |
1250b | 100b | monoisobutyl phthalate (MIBP)g | 0.8 | 72 (2.7) |
|
butyl benzyl phthalate (BBP) |
500c | 100c | monobenzyl phthalate (MBZP)g | 0.3 | 63 (2.4) |
|
di(2- ethylhexyl) phthalate (DEHP) |
50d | 100d | mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)g | 0.4 | 6 (0.2) |
| mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)g | 0.2 | 13 (0.5) | |||
| mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)g | 0.4 | 9 (0.3) | |||
| mono(2-ethylhexyl) phthalate (MEHP)g | 0.8 | 1003 (37.66) | |||
|
diisononyl phthalate (DINP) |
150e | 100e | monoisononyl phthalate (MINP)h | 0.9 | 1586 (59.56) |
| mono(carboxyoctyl) phthalate (MCOP)g | 0.3 | 3 (0.1) | |||
|
diisodecyl phthalate (DIDP) |
130f | 100f | mono(carboxynonyl) phthalate (MCNP)h | 0.2 | 32 (1.2) |
(EFSA, 2005a);
(Saillenfait et al., 2008);
(EFSA, 2005b);
(EFSA, 2005c);
(EFSA, 2005d);
(CPSC, 2010);
(Qian et al., 2015) only.
2.2. Daily intake dose
Internal Daily Intake (DI) doses of phthalates for NHANES participants were calculated using the methodology presented in Christensen et al. (2014). In brief, the DI was calculated through adjusting metabolite concentrations of phthalates by creatinine concentrations while incorporating other variables such as daily creatinine excretion rates, the molar fraction of a given metabolite that was excreted, and information about the molecular weights of the metabolites and their parent phthalates. Under the assumption of steady state exposures, the DI for each participant i and metabolite k originating from parent phthalate j was calculated using the following equation:
| [1] |
where DIi, j, k (μg/kg/d) in urine is the daily intake dose for metabolite k, 100 is a unit conversion factor, Meti, k (ng/mL) is the metabolite concentration as given in the NHANES data set, Cri (mg/dL) is the creatinine concentration in urine as given in the NHANES data set, CEi (mg/kg/d) is the creatinine excretion per day as calculated by Mage et al. (2008)using information about a participant's age, ethnicity, gender, weight, and height, FUE, i, k(unitless) is the molar fraction of the metabolite excreted, 1000 is a unit conversion factor, MWi, j (mg/mol) is the molecular weight of the parent phthalate, and MWi, j, k (mg/mol) is the molecular weight of the metabolite (Table S1). Among the phthalates that have multiple metabolites (i.e. DEHP and DINP), within an individual i, the value of DIi, j was calculated by taking a weighted mean of the values of DIi, j, k estimated from each metabolite k using FUE, i,k (Christensen et al., 2014, Qian et al., 2015). The weighted mean was determined using the following equation:
| [2] |
where DIi, j is the daily intake dose for phthalate j and nk is the number of metabolites for a given parent phthalate. In this work, nk ∈ {1, 2, 4}.
We used the NHANES convention of setting metabolite concentrations below the Limit of Detection (LOD) to LOD/2. Table 1 gives the LOD for each metabolite and the number (and percentage) of participants with metabolites below the LODs. Table 1 indicates the majority of the metabolites were detectable in > 97% of the surveyed participants. The predictions of DIi, j for each participant, the measurements of metabolite concentrations obtained from NHANES, and the physiological and demographic information used to determine DIi, j values are provided in the Supplementary Data.
2.3. Maximum cumulative ratio
The following equations were used to determine the values of HQ and HI for participant i and phthalate j for N phthalates:
| [3] |
| [4] |
| [5] |
There were six phthalates used in this analysis (i.e. N = 6) and HQM quantifies the maximum HQ among the six phthalates for participant i. The TDIs used in this study and their references are given in Table 1. Five of the six were taken from several sources (EFSA, 2005a, EFSA, 2005b, EFSA, 2005c, EFSA, 2005d, Saillenfait et al., 2008) with DIDP taken from an additional source (CPSC, 2010). Because many Lowest Observed Adverse Effect Level (LOAEL) and No Observed Adverse Effect Level (NOAEL) are based on findings in animal studies, a series of Uncertainty Factors (UFs) are applied to these measures to make them applicable to humans (NRC, 2008). Different toxicological studies have led to different UFs (Table 1).
The MCR is a function of the doses that reach an exposed individual. As a result, values of MCR will vary across individuals in an exposed population ranging from one to N (i.e. MCRi ∈ [1, N]), where N is the number of chemicals considered in the assessment. A value close to one indicates that one chemical was responsible for nearly all of the individual's cumulative risk, and a value of N indicates that the individual receives an equitoxic dose from all chemicals. The value of the MCR can be readily determined using the metrics HI and HQM used for screening assessments. The value of MCR for an individual i in an exposed population is defined as:
| [6] |
The values of HI and MCR can be used to evaluate cumulative exposures in a number of ways. The correlation of MCR ∓ 1 against HI is a useful criterion in evaluating cumulative exposures. A negative correlation (i.e. MCR − 1 declines as HI increases) indicates that the individuals most at risk from cumulative exposures received the majority of their risks from a single chemical and suggests that risk mitigation could focus on the population groups with high exposures to individual phthalates. A positive correlation indicates that cumulative exposures to multiple chemicals drove the highest risks in the population and that tracking cumulative exposures is required to fully characterize risks. The percent reciprocal of MCR (i.e. 100 ∗ HQM, i/HIi) can also be a helpful measure in understanding what percent an individual's HQM contributes to their total hazard.
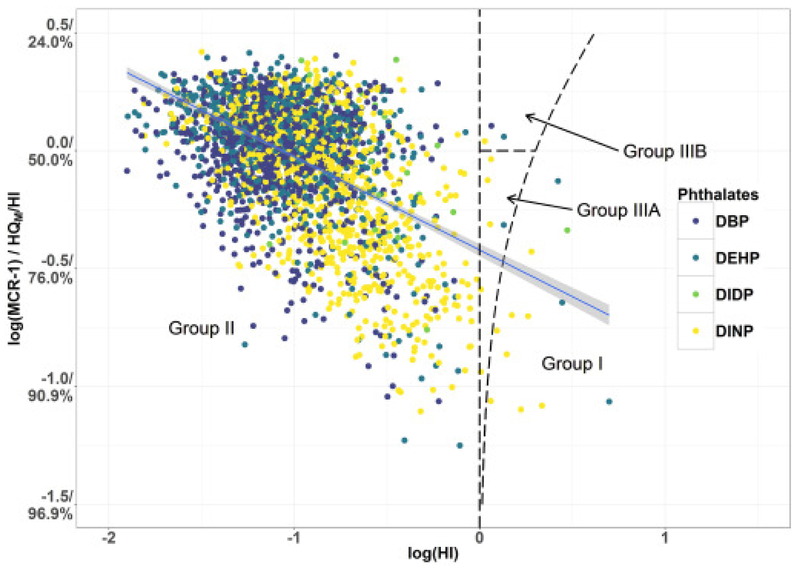
The determination of the values of HI and MCR enables categorization of the surveyed participants into four groups as seen in Table 2 (Price et al., 2012a, Price et al., 2012b). Group I are those participants having one or more phthalate exposures that exceeds the respective benchmarks (HQM > 1). Group II are participants with minimal risks under an additive model of toxicity (HI ≤ 1). The participants that did not fall into either Groups I or II, are participants with cumulative phthalate exposures that are of a potential concern. However, none of the phthalates would have been identified under a single-chemical assessment. These mixtures are identified as Group III. Group III can be divided into two subgroups. Group IIIA includes those mixtures with an MCR < 2. In these mixtures, the majority of the risk to a participant was driven by one phthalate. The remaining mixtures fall into Group IIIB where no single phthalate results in the majority of risk. In this study, MCR is determined for each participant. The relative number of participants who fall in the groups along with the ranges of HI for each group can provide additional insight on the patterns of exposure. Unlike earlier publications on MCR and HI, this work plots the values of MCR − 1 versus HI for the surveyed participants on a log-log plot. However, transformation of the data is not necessary to calculate the MCR. The MCR and the HI were regressed against each other to visualize the dominance of a single given chemical as a function of hazard. The log-transform was taken to leave the variables unbounded in a regression setting. Like the MCR-HI plots, log-log plots of MCR − 1 and HI result in the four groups falling into contiguous regions. In particular, the value of log(MCR − 1) = 0 is the dividing line between Group IIIA and Group IIIB, allowing for a more intuitive interpretation.
Table 2.
Group names, definition and descriptions from HI and MCR values for each participant.
| Group | Total hazard |
Individual chemical hazard |
MCR | Description |
|---|---|---|---|---|
| I | HI > 1 | HQM > 1 | – | The mixture presents a potential risk already based on individual components |
| II | HI ≤ 1 | HQM < 1 | – | The assessment does not identify a concern |
| IIIA | HI > 1 | HQM < 1 | MCR < 2 | The majority of the risk offered by the mixture is driven by one component |
| IIIB | HI > 1 | HQM < 1 | MCR ≥ 2 | The potential risk is driven by multiple components |
Reproduced from Table 2 of Vallotton and Price (2016).
The pattern of exposures received by participants in Group III potentially provides insights into specific combinations of chemicals that result in cumulative exposures of concern (Price et al., 2012a, Price et al., 2012b, Vallotton and Price, 2016).
NHANES reports the demographics of the surveyed participants. This allows for identifying sub-populations that are at elevated risk. In this study we investigated age-related differences by dividing the population into school age (6 to 17 years of age) and adults (18 years of age and above). NHANES does not include phthalate biomonitoring data on children under the age of six years. The population was also divided by gender and ethnicity (i.e., Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other – including multi-racial).
The impact of multiple alternative approaches for evaluating hazard were investigated. First, MCNP, the metabolite of DIDP, and MINP, one of the two metabolites of DINP, were omitted from the assessment as was done in Christensen et al. (2014); second, non-detects were set to zero; third, DI was calculated using urine flow rates instead of a creatinine adjusted concentration (Aylward et al., 2016). The equation used to predict the daily intake of a phthalate using flow rates is:
| [7] |
where, UFRi (ml/min) is the urinary flow rate for participant i as reported by NHANES. Additionally, we repeated the analysis using a toxicity equivalency approach (Varshavsky et al., 2016). This was done to assess the impacts of the varying UFs used in setting the standards that are used to calculate the TDIs. This approach determine a potency-weighted sum of androgen disruptors using relative potency factors calculated from benchmark doses for androgen disruption (NRC, 2008) for the phthalates. This approach does not use uncertainty factors. A detailed description of the methodologies used in the alternative assessments is provided in the Supplementary Data.
All analyses and visualizations were conducted in R (version 3.2.2) using the packages ggplot2 (version 2.2.0) and survey (version 3.31).
3. Results
3.1. Visualizing hazards and the maximum cumulative ratio
The MCR calculated among the 2663 participants ranged from 1.1 to 3.6. Because six phthalates were considered, MCR can range between 1 and 6. The finding that MCR values were all at or below 3.6 indicated that none of the exposed participants received the same level of risk from the six phthalates. That is, for each subject, a subgroup of phthalates had a dominant influence on the participant's value of HI. HI ranged from 0.01 to 5.0 but exceeded one for only 21 (0.8%) of surveyed participants. The values of HQ, HI, and MCR for each individual are provided in the Supplementary Data.
Fig. 1 presents a plot of log(MCR − 1) versus the log(HI) for the 2663 participants. In this plot, lower values on the vertical axis for a participant (i.e. the MCR − 1 axis), indicate that their risk was dominated by exposures to one phthalate. To aid in this understanding the vertical axis also provides the percent contribution of HI from the HQM. There were 12 participants in Group I, 2642 participants in Group II, six participants in Group IIIA, and three participants in Group IIIB (Table 3). Group I designates participants whose HQM > 1 and therefore would be successfully identified in a chemical-by-chemical assessment. The mean (range) of HI values among surveyed participants in Group I was 2.2 (1.1–5.0). There were fewer participants in Group III than Group I. Group III identifies participants with a potentially hazardous internal dose that could only be properly identified through a cumulative assessment. The mean (range) of HI values among surveyed participants in Group III was 1.1 (1.0–1.4). As shown in Table S2 in the Supplementary Data, the log transformations of MCR − 1 and HI had a larger Pearson's correlation coefficient than other transformation combinations indicating that the use of the log transformations of MCR − 1 and HI improves the determination of correlations over the use of MCR and HI used in some earlier publications. The use of log transformed value of HI also increased the normality of the parameter as quantified by the Wilks-Shapiro W statistic.
Fig. 1.
Plot of log(HI) versus log(MCR−1) (with HQM/HI) of six phthalates for 2663 participants from the 2013–2014 NHANES cycle identified by the phthalate that produced the HQM. Regions corresponding to Groups I, II, IIIA, and IIIB and the linear regression between log(HI) with log(MCR−1) with 95% confidence interval (slope = − 0.395 and intercept = − 0.421) are provided.
Table 3.
Frequency of the phthalates producing the HQM for all participants in the 2013–2014 cycle of NHANES by Group.
| I | II | IIIA | IIIB | Total | |
|---|---|---|---|---|---|
| BBP | 0 | 0 | 0 | 0 | 0 |
| DBP | 0 | 958 | 0 | 1 | 959 |
| DEHP | 3 | 712 | 1 | 1 | 717 |
| DIBP | 0 | 0 | 0 | 0 | 0 |
| DIDP | 1 | 25 | 0 | 0 | 26 |
| DINP | 8 | 947 | 5 | 1 | 961 |
| Total | 12 | 2642 | 6 | 3 | 2663 |
3.2. Phthalates that drive cumulative exposures
In Fig. 1, the data are identified by which phthalate produced the HQM. Only four of the six phthalates produced HQM in at least one participant. The collective internal doses of most participants were either driven by DBP, DINP, DIDP, or DEHP (Table 3). BBP or DIBP did not produce HQM for any participant. Although DBP and DINP produced the HQM for the majority of participants, the frequency at which certain phthalates produced the HQMchanged as a function of HI and Group. Among those participants with lower HI values (i.e. Group II), internal dosages were primarily driven by DBP. Among participants at larger HI values (i.e. Groups I and III) dosages were primarily driven by DEHP and DINP. Among Groups I and III, the odds of hazardous mixtures being driven by one chemical was approximately 4:3. Group III (i.e. those participants that would have been missed by a chemical-by-chemical assessment) and Group I (i.e. participants that would be correctly identified as at-risk by a chemical-by-chemical assessment) were dominated by DINP.
For six phthalates, there are 15 possible unique pairs. However, this analysis suggested that some pairs of phthalates were more important in terms of defining cumulative risk than others. The phthalates which produced the top HQs in each of the 21 participants with HI > 1 were determined. Table 4 presents the frequency of these pairs of phthalates. As Table 4indicates, only four of the six phthalates appeared in the top two HQs for the surveyed participants among the 21 participants with HI > 1. A total of 19 of the 21 pairs involved only three phthalates: DEHP, DINP, and DIDP.
Table 4.
Frequency of pairs of phthalates that produced the top two HQs in the 21 participants with HI > 1.
| DEHP | DINP | DIDP | DBP | |
|---|---|---|---|---|
| DEHP | 12 | 2 | 1 | |
| DINP | – | 5 | 1 | |
| DIDP | – | – | 0 | |
| DBP | – | – | – |
3.3. The decline of MCR with HI
There was a distinct downward trend between HI and MCR when both parameters were plotted on a log scale. Fig. 1 includes a linear regression model with 95% confidence intervals. Although this work does not prove the linearity of this relationship as fit with linear regression parameters (Table S3), this trend elucidates the relationship between the two parameters and informs us about the importance of a single chemical in determining cumulative risk. As HI values increase, the values of MCR tend to decrease. That is, as hazards to individual phthalates increase, the participant is more likely to have a single hazard quotient dominate their HI. Among participants, the data were subset to the individual phthalates that produced the HQM and a linear regression model was refit. After the refitting, HQM from DEHP, DBP, and DINP produced a statistically significant negative correlation between HI and MCR, while DIDP did not produce a statistically significant negative correlation (Fig. S2). This lack of significance was likely due to the limited number of surveyed participants in which DIDP produced the HQM (Table S3).
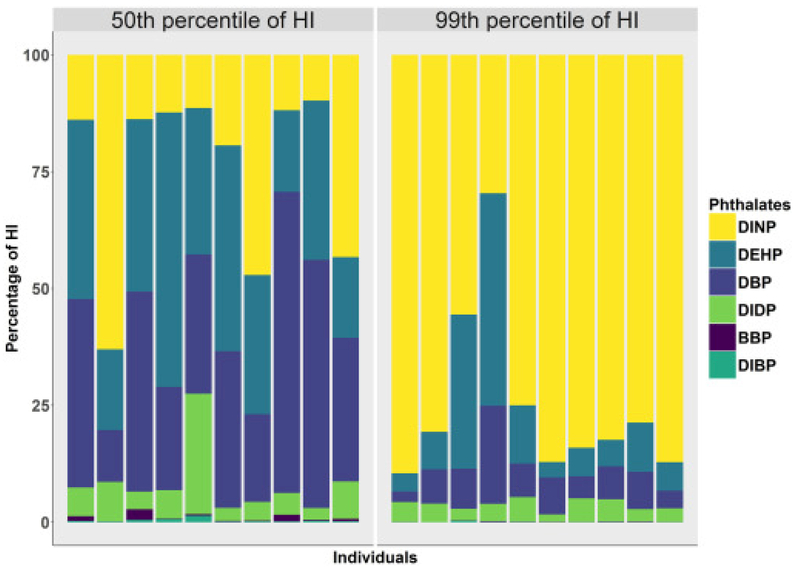
This trend of increasing dominance by a single phthalate could be seen within select participants (Fig. 2). Among ten participants with HI values from the 49.8–50.2th percentiles of HI for the population, a few of the phthalates had HQ values that made up the majority of the participants' HIs. This was evidenced by the mean MCR value (2.1) among those 10 participants. Among ten participants from the 98.8–99.2th percentiles of HI, most had a phthalate that provides > 50% of their HI values. The median MCR values for those around the 50th and 99th percentile groups were 2.2 and 1.2, respectively. Thus, the median MCR values for the individuals around the 50th percentile was 0.8 times larger than those around the 99th percentile, meaning that participants at the higher ends of hazard are driven more by individual chemicals within a given mixture compared to participants within the midranges of hazard.
Fig. 2.
Percent contribution to HI as quantified by the HQ from each of the six phthalates for ten participants ranging from the 49.8–50.2th percentiles of HI and ten participants ranging from the 98.8–99.2th percentiles of HI.
3.4. The effect of demographics on MCR and HI
After adjusting for the NHANES study design using survey weights, there was a statistically significant difference in the HI means between children (aged six to 17 years) and adults (aged 18 + years); the means were 0.19 and 0.14, respectively (p = 0.002; Table S4). Differences in metabolite concentrations between children and adults are in line with previous works (Zota et al., 2014). Future work should investigate the differences in metabolite concentrations between children and adults. The frequency (percent) of children and adults with an HI > 1 were eight (1.1%) and 13 (0.7%), respectively (Table 5). After adjusting for NHANES weights, there was no difference in the proportion of children versus adults with HI > 1 (Table S4). However, the small fraction of those participants with HI > 1 limits our ability to identify differences. Among children with HI ≤ 1, MCR ranged from 1.1–3.5 compared with 1.1–3.6 among adults. Among children with HI > 1, MCR ranged from 1.1–2.3 compared with 1.1–2.1 among adults. Within both age groups the negative correlation of MCR − 1 and HI was statistically significant (Table S3, Fig. S3).
Table 5.
Frequency, percentage, and HI of total participants and participants by Group and age group.
| Group | Frequency (%) | Hazard index | ||||
|---|---|---|---|---|---|---|
| 6–17 | 18 + | Total | Mean | Min | Max | |
| I | 3 (0.4) | 9 (0.5) | 12 (0.5) | 2.2 | 1.1 | 5.0 |
| II | 741 (98.9) | 1901 (99.3) | 2642 (99.2) | 0.1 | 0.0 | 1.0 |
| III | 5 (0.7) | 4 (0.2) | 9 (0.3) | 1.1 | 1.0 | 1.4 |
| Total | 749 (100) | 1914 (100) | 2663 (100) | 0.2 | 0.0 | 5.0 |
| I or III | 8 (1.1) | 13 (0.7) | 21 (0.8) | 1.7 | 1.0 | 5.0 |
Although disparities of exposure to phthalates based on gender, especially when presented by ethnicity, have been reported (Branch et al., 2015), there was little evidence of gender differences observed in the cumulative risk indices investigated in this study (Table S4). There were ten males and eleven females with HI > 1. This difference was not statistically significant (Table S4). As with age, there is an inverse relationship between HI and MCR when the data were subset to each gender (Fig. S4). The mean HI (range) among women was 0.2 (0.01–5.0) while the mean HI (range) among men was 0.2 (0.01–2.8). Among both females and males with HI ≤ 1, MCR ranged from 1.1–3.6. Among females with HI > 1, MCR ranged from 1.1–2.3 compared with 1.1–2.1 among males. After adjusting for the study design, there was no statistically significant difference in the HI means between men and women (Table S4).
Lastly, MCR and HI plots can be presented by an NHANES ethnicity variable exploring Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other (including multi-racial) ethnicities (Fig. S5). There were no statistically significant differences in mean HI or in the proportions of participants with HI > 1 among ethnic groups (Table S4). Like the previous participant characteristics, there was a downward trend of MCR as a function of HI for all ethnic groups (Table S4).
The alternative approaches produced similar results as the analyses presented here (Table S5). The lack of impact from dropping the two metabolites was expected, due to the HQ values for DIDP being small compared to those from DEHP, DBP, and DINP. Dropping MINP from the calculation of DINP did result in slightly higher predicted doses for that phthalate. The lack of impact from setting non-detects to zero was expected, because detection limits for the phthalates were low in comparison to the compounds' toxicity standards. Using the urinary flow rate method resulted in an increase in the fraction of participants with HI > 1 from 0.8% to 1.5%. Like in the original analyses, the majority of participants fell into Group I with a lesser frequency falling into Group IIIA and IIIB. The phenomenon of the decreasing in MCR and total weighted dose is independent of uncertainty factors. The total potency-weighted dose plotted against MCR − 1 shows the same declining trends as HI (Fig. S1). This suggests that there is increasing dominance of a single phthalate for individuals with elevated exposures expressed either as HI or as a sum of androgen disruptors (see Supplementary Data). A negative correlation between MCR and total toxicity equivalences of dioxin-like compounds measured in biomonitoring data has been observed in previous works (Han and Price, 2013).
4. Discussion
This work investigated recent data on exposures to six phthalates. The data indicated that 0.8% of the surveyed participants (12 in Group I and 9 in Group III) had HI values of concern. While this percentage was small, it may correspond to a large number of individuals in the US population. This analysis also suggested that exposures of concern in some individuals would be missed if only using a chemical-by-chemical exposure risk assessment were used (Group III). However, the analysis also showed that the participants in Group III tended to have smaller HI values than participants in Group I (mean HI value of 1.1 versus 2.2, respectively). HI values in Group III did not exceed 1.4. Thus a phthalate-by-phthalate assessment approach that ignored additive effects of the combined exposures still identified all participants with HI values > 1.4. Among the 15 possible unique pairs of the six phthalates, only three pairs were strongly associated with values of HI > 1 (Table 4): DEHP:DINP, DINP:DIDP, and DIDP:DEHP. This suggests that toxicological studies of phthalate interactions should focus on the phthalates in these pairs. Finally, although the time period and set of metabolites differed, the absence of strong ethnicity and gender differences in these data disagree with earlier findings and may warrant additional study (Branch et al., 2015, Zota et al., 2014).
The total fraction of the survey population with HI > 1 was 0.8%. The probability of having HI > 1 was similar for adults and children aged six years and older, males and females, and across different ethnicities. The hazards to participants in Groups I and III were largely driven by DINP. We found a significant negative correlation between the log(MCR − 1) and the log(HI). This negative correlation implies that health impacts in the most at-risk portion of the population were largely driven by exposures to a single phthalate and not cumulative exposures to multiple phthalates.
This was the first application of the MCR approach to assess cumulative phthalate exposures. This was the second analysis to apply the MCR approach to biomonitoring data (see Han and Price, 2013) and the first publication that applied MCR to biomonitoring data of short half-life compounds. This was the first work to explore the four groups defined by MCR and HI (i.e., Group I, Group II, Group IIIA, and Group IIIB) for estimated exposures using biomonitoring data. This was also the first work to use the graphing technique of visualizing both HI and the MCR on a log-log scale. By fitting both variables on log scales, the relationship between the two parameters were more clearly displayed and quantified (e.g., the correlation between log(HI) and log(MCR − 1)) than the approaches used in earlier publications (Price and Han, 2011, Han and Price, 2011).
Much of this work was based on approaches published by Qian et al. (2015) and Christensen et al. (2014). Christensen et al. (2014) combined cycles from 2005–2006 and 2007–2008 and explored five phthalates from eight metabolites. Qian et al. (2015) used only the 2007–2008 cycle and incorporated DIDP and a second metabolite of DINP (i.e. MINP) for a total of six phthalates from ten metabolites. Christensen et al. (2014) investigated hazard while Qian et al. (2015) investigated dose. This work looked at the six phthalates chosen by Qian et al. (2015) while looking at the measures of hazard found in Christensen et al. (2014). Our findings differ from these earlier works in key ways. Christensen et al. (2014) found that overall hazard was mostly driven by DEHP with the mean HI in the population being 0.3 with hazards mostly coming from exposures to DEHP and to a lesser extent DINP. In this study we found the mean HI to be 0.15 with DINP now being the dominant contributor among participants with HI > 1. The drop in overall hazard, as witnessed from drops in internal daily intake doses from 2005–2008 to 2013–2014, is in line with overall trends of declining levels of metabolites of some phthalates seen in other works (Gyllenhammar et al., 2017, Johns et al., 2016, Zota et al., 2014). We did agree with Qian et al.’s (2015) finding that coexposures among the six phthalates changed as a function of daily intake. Those participants at the upper ends of exposure (as quantified by the daily intake dose) were primarily driven by one phthalate for the 2007–2008 NHANES cycle, while coexposure patterns remained fairly consistent when presented by age, ethnicity, and gender. This negative correlation has been seen in a number of other studies of risk ranging from cumulative exposures to mixtures of chemicals in water and indoor air (Price and Han, 2011, Han and Price, 2011, De Brouwere et al., 2014, Silva and Cerejeira, 2015, Vallotton and Price, 2016). Our work confirmed this pattern by demonstrating a robust negative correlation between MCR and HI.
This cycle of the NHANES data did not sample or analyze phthalate metabolites in children under the age of six. This lack of data is of particular concern due to hand-to-mouth and object-to-mouth behavior, especially with children's toys that may contain phthalates (Sathyanarayana, 2008). If infants and small children had sources of exposure that differ from the older children in this study, then these findings may not be relevant to that age group. NHANES is also a national survey. Thus, small local populations with unique exposure sources may not be reflected. Exposure patterns for phthalates can differ between geopolitical regions (Giovanoulis et al., 2016, Wang et al., 2015); thus, these findings may not be appropriate for populations outside of the U.S.
The NHANES data are cross-sectional snapshots of metabolite concentrations. The use of cross sectional data to characterize long-term doses is known to introduce a bias in the measurement of inter-individual variation in long-term doses due to the data reflecting both inter- and intra-individual variation in metabolite concentrations (Braun et al., 2012, Pleil and Sobus, 2013). Intra-individual variation is larger for rapidly cleared compounds than persistent compounds. The phthalates and their metabolites are typically fully excreted in the urine or feces within one to two days (Koch and Calafat, 2009, LaKind et al., 2014). DEHP metabolite concentrations decline with fasting duration (Aylward et al., 2011). We have not attempted to correct the predictions of interindividual variation in the DI for this effect; however, we acknowledge that measures of the percentages of the upper and lower tails of the distributions are likely to be overestimated. While this was of little concern for the lower tail, it was an important issue for the evaluation of the doses predicted for the upper tails of the population. As a result, the true fractions of the populations with HI > 1 are likely to be lower than the values presented here.
The HQs and the HI for each individual were dependent on the value of TDI for each phthalate derived through UFs. DBP has the largest UFs among the six phthalates (Table 1). DBP was the driver for only one participant (i.e., DBP produced the HQM) among the participants with HI values greater than one. Thus the larger UF value for DBP did not impact the findings of HI values > 1.
This work assumed that there was a one-to-one correspondence between the metabolites and the phthalates from which they originate and that each metabolite can be used to determine the dose of the parent compound based on a measured fraction of a chemical excreted as a specific metabolite (FUE). Each of the ten metabolites in this analysis has only one parent phthalate. For the metabolites originating from DIBP, DBP, and BBP, the value of FUE was 70% or greater. However, for the metabolites originating from DINP and DIDP, the values were less 10%. The smaller values for these two chemicals increases the uncertainty in their DI values. We also assume that the value of FUE within the participants do not vary as a function of age, race, or ethnicity (Anderson et al., 2011, Koch and Bolt, 2004, Koch et al., 2007). Finally, the values of HQ, HI, and MCR for the surveyed participants were dependent of the choice of the toxicity values. The toxicity values used here were generally based on common endpoints (Christensen et al., 2014, Varshavsky et al., 2016).
Because the data suggested that the cumulative exposures to the typical individual were of relatively low concern (i.e. HI values in the surveyed participants averaged 0.15), future work should include investigations into the sources of DEHP, DINP, and DIDP that drive high levels of exposure in small portions of the general population. This work could include investigating functional use and production of these three phthalates. The investigation should also seek to determine if there are overlaps between the populations with high exposures of these three compounds.
Data from previous NHANES cycles can be incorporated to investigate population-wide temporal trends in hazards of phthalates across the years. Mitigation and substitution strategies of phthalates can change over time (Bui et al., 2016, Giovanoulis et al., 2016). Thus, ongoing monitoring and analyses are appropriate going forward. Finally, the absence of biomonitoring data in children under the age of six suggests the need for new strategies for characterizing aggregate phthalate exposures in infants and small children.
5. Conclusions
This was the first work to visualize the MCR and HI measures on a log-log scale and the second work to apply the MCR metric to biomonitoring data. An HI > 1 from the six phthalates only occurred in a small fraction of the participants (0.8%). While a majority of these participants could be identified by a chemical-by-chemical assessment, approximately 43% of subjects with HI > 1 would not be considered to be at-risk if the six phthalates were investigated separately. We conclude that performing a cumulative assessment had a modest but measureable impact in the evaluation of hazards among six phthalates in individuals aged six years and older.
Supplementary Material
Acknowledgments
Funding
This research was supported in part by an appointment of Jeanette Reyes to the Postdoctoral Research Program at the National Center for Environmental Assessment, Office of Research and Development, USEPA administered by the Oak Ridge Institute for Science and Education through Interagency Agreement No. DW-89-92298301 between the U.S. Department of Energy and the USEPA.
Footnotes
Declaration of interest
The authors declare they have no actual or potential competing financial interests.
Disclaimer
This manuscript has been reviewed by the U.S. Environmental Protection Agency and approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Appendix A. Supplementary data
References
- Anderson WAC, Castle L, Hird S, Jeffery J, Scotter MJ, 2011. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem. Toxicol. 49 (2011), pp. 2022–2029. 10.1016/j.fct.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Aylward LL, Lorber M, Hays SM, 2011. Urinary DEHP metabolites and fasting time in NHANES. J. Expo. Sci. Environ. Epidemiol 21, 615–624. doi: 10.1038/jes.2011.28. [DOI] [PubMed] [Google Scholar]
- Aylwrd LL, Hays SM, Zidek A. 2016. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry J. Expo. Sci. Environ. Epidemiol (2016), pp. 1–9; doi: 10.1038/jes.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch F, Woodruff TJ, Mitro SD, Zota AR. 2015. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ. Heal. 14:57; doi: 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. 2012. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environ. Health Perspect. 120:739–745; doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TT, Giovanoulis G, Cousins AP, Magnér J, Cousins IT, de Wit CA. 2016. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 541:451–467; doi: 10.1016/j.scitotenv.2015.09.036. [DOI] [PubMed] [Google Scholar]
- CDC. 2016a. 2013-2014 Laboratory Data Overview. Natl. Heal. Nutr. Exmination Surv Available: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2013 [accessed 31 May 2017]. [Google Scholar]
- CDC. 2016b. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [accessed 3 January 2017]. [Google Scholar]
- Christensen KLY, Makris SL, Lorber M. 2014. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul. Toxicol. Pharmacol 69:380–389; doi: 10.1016/j.yrtph.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, et al. 2010. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod. Toxicol. 30:313–321; doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Colacino JA, Harris TR, Schecter A. 2010. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect. 118:998–1003; doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPSC (United States Consumer Product Safety Commission). 2010. Overview of Phthalates Toxicity. Bethesda, Maryland. [Google Scholar]
- De Brouwere K, Cornelis C, Arvanitis A, Brown T, Crump D, Harrison P, Jantunen M, Price P, Torfs R. 2014. Application of the maximum cumulative ratio (MCR) as a screening tool for the evaluation of mixtures in residential indoor air. Sci. Total Environ, 479–480 (2014), pp. 267–276; doi: 10.1016/j.scitotenv.2014.01.083 [DOI] [PubMed] [Google Scholar]
- EFSA. 2005a. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Butylbenzylphthalate (BBP) for use in food contact materials. Eur. Food Saf. Auth. J 1–14; doi: 10.2903/j.efsa.2004.84. [DOI] [Google Scholar]
- EFSA. 2005b. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Di-isononylphthalate (DINP) for use in food contact materials. Eur. Food Saf. Auth. J 1–18; doi: 10.2903/j.efsa.2004.84. [DOI] [Google Scholar]
- EFSA. 2005c. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the Commission related to Bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials. Eur. Food Saf. Auth. J 1–20; doi: 10.2903/j.efsa.2006.314. [DOI] [Google Scholar]
- EPA. 2007. Concepts, Methods, and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report). U.S. Environmental Protection Agency, Washington, DC; doi:EPA/600/R-06/013F. [Google Scholar]
- EPA. 2003. Framework for Cumulative Risk Assessment. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office, Washington, DC; doi:EPA/630/P-02/001F. [Google Scholar]
- EPA. 1986. Guidance for health risk from exposure to chemical mixtures. U.S. Environmental Protection Agency. Fed Reg 51:34014. [Google Scholar]
- Giovanoulis G, Alves A, Papadopoulou E, Cousins AP, Schütze A, Koch HM, et al. 2016. Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ. Res. 151:80–90; doi: 10.1016/j.envres.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Glynn A, Jönsson BAG, Lindh CH, Darnerud PO, Svensson K, et al. 2017. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ. Res. 153:48–54; doi: 10.1016/j.envres.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Han X, Price PS. 2013. Applying the maximum cumulative ratio methodology to biomonitoring data on dioxin-like compounds in the general public and two occupationally exposed populations. J. Expo. Sci. Environ. Epidemiol. 23:343–9; doi: 10.1038/jes.2012.74. [DOI] [PubMed] [Google Scholar]
- Han X, Price PS. 2011. Determining the maximum cumulative ratios for mixtures observed in ground water wells used as drinking water supplies in the United States. Int. J. Environ. Res. Public Health 8:4729–4745; doi: 10.3390/ijerph8124729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD. 2016. Exposure Assessment Issues in Epidemiology Studies of Phthalates. Environ. Int. 150:137–143; doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. 2011. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 24:115–141; doi: 10.2478/s13382-011-0022-2. [DOI] [PubMed] [Google Scholar]
- Kienzler A, Berggren E, Bessems J, Bopp S, Van Der Linden S, Worth A. 2014. Assessment of Mixtures - Review of Regulatory Requirements and Guidance.; doi: 10.2788/84264. [DOI] [Google Scholar]
- Koch HM, Becker K, Wittassek M, Seiwert M, Angerer J, Kolossa-Gehring M. 2007. Di-n-butylphthalate and butylbenzylphthalate F urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children J. Expo. Sci. Environ. Epidemiol, 17 (2007), pp. 378–387; doi: 10.1038/sj.jes.7500526 [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. 2009. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. 364:2063–78; doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M. 2010. No TitleCombined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int. J. Androl. 33:463–474; doi: 10.1111/j.1365-2605.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Sobus JR, Goodman M, Barr DB, Fürst P, Albertini RJ, et al. 2014. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ. Int. 73:195–207; doi: 10.1016/j.envint.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, et al. 2009. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health. B. Crit. Rev 12:225–249; doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Mage DT, Allen RH, Kodali A. 2008. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Expo. Sci. Environ. Epidemiol. 18:360–368; doi: 10.1038/sj.jes.7500614. [DOI] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Chahoud I. 2009. Reproductive toxicity of phthalate esters. Mol. Nutr. Food Res. 54:148–157; doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. 2011. Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in u.s. adults and adolescents from the national health and nutrition examination survey (NHANES) 2007–2008. Environ. Health Perspect. 119:1396–1402; doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2008. Phthalates and Cumulative Risk Assessment: The Task Ahead. Committee on the Health Risks of Phthalates, National Research Council, Washington, DC. [Google Scholar]
- Pak VM, McCauley LA, Pinto-Martin J. 2011. Phthalate Exposures and Human Health Concerns: A Review and Implications for Practice. AAOHN J 59:228–235; doi: 10.1177/216507991105900505. [DOI] [PubMed] [Google Scholar]
- Pleil JD, Sobus J. 2013. Estimating Lifetime Risk from Spot Biomarker Data and Intraclass Correlation Coefficients (ICC). J. Toxicol. Environ. Heal. Part A Curr. Issues 76:747–766; doi: 10.1080/15287394.2013.821394. [DOI] [PubMed] [Google Scholar]
- Price P, Dhein E, Hamer M, Han X, Heneweer M, Junghans M, et al. 2012a. A decision tree for assessing effects from exposures to multiple substances. Environ. Sci. Eur. 24:26; doi: 10.1186/2190-4715-24-26. [DOI] [Google Scholar]
- Price P, Han X, Junghans M, Kunz P, Watts C, Leverett D. 2012b. An application of a decision tree for assessing effects from exposures to multiple substances to the assessment of human and ecological effects from combined exposures to chemicals observed in surface waters and waste water effluents. Environ. Sci. Eur. 24:34; doi: 10.1186/2190-4715-24-34. [DOI] [Google Scholar]
- Price PS, Han X. 2011. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int. J. Environ. Res. Public Health 8:2212–2225; doi: 10.3390/ijerph8062212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Chen M, Kransler KM, Zaleski RT. 2015. Assessment of chemical coexposure patterns based upon phthalate biomonitoring data within the 2007/2008 National Health and Nutrition Examination Survey. J. Expo. Sci. Environ. Epidemiol. 25:249–55; doi: 10.1038/jes.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillenfait AM, Sabaté JP, Gallissot F. 2008. Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod. Toxicol. 26:107–115; doi: 10.1016/j.reprotox.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S 2008. Phthalates and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 38:34–49; doi:doi.org/ 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Silva E, Cerejeira MJ. 2015. Concentration addition-based approach for aquatic risk assessment of realistic pesticide mixtures in Portuguese river basins. Environ. Sci. Pollut. Res. 22:6756–6765; doi: 10.1007/s11356-014-3857-9. [DOI] [PubMed] [Google Scholar]
- Søeborg T, Frederiksen H, Andersson AM. 2012. Cumulative risk assessment of phthalate exposure of Danish children and adolescents using the hazard index approach. Int. J. Androl. 35:245–252; doi: 10.1111/j.1365-2605.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- Teuschler LK, Hertzberg RC. 1995. Current and future risk assessment guidelines, policy, and methods development for chemical mixtures. Toxicology 105:137–144; doi: 10.1016/0300-483X(95)03207-V. [DOI] [PubMed] [Google Scholar]
- Vallotton N, Price PS. 2016. Use of the Maximum Cumulative Ratio As an Approach for Prioritizing Aquatic Coexposure to Plant Protection Products: A Case Study of a Large Surface Water Monitoring Database. Environ. Sci. Technol. 50:5286–5293; doi: 10.1021/acs.est.5b06267. [DOI] [PubMed] [Google Scholar]
- Varshavsky JR, Zota AR, Woodruff TJ. 2016. A novel method for calculating potency-weighted cumulative phthalates exposure with implications for identifying racial/ethnic disparities among U.S. reproductive-aged women in NHANES 2001–2012. Environ. Sci. Technol, 50 (2016), pp. 10616–10624; doi: acs.est.6b00522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang H, Zhou W, Chen Y, Zhou Y, Jiang Q. 2015. Urinary excretion of phthalate metabolites in school children of China: Implication for cumulative risk assessment of phthalate exposure. Environ. Sci. Technol. 49:1120–1129; doi: 10.1021/es504455a. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect. 122:235–241; doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.