Abstract
Cerebral amyloid angiopathy (CAA), in which amyloid accumulates predominantly in the walls of arterioles and capillaries, is seen in most patients with Alzheimer disease (AD) and may contribute to compromise of blood-brain barrier (BBB) function seen in AD. We investigated the effects of CAA on BBB integrity by examining the expression of the endothelial marker CD31, basement membrane protein collagen IV (COL4), tight junction protein claudin-5, and fibrinogen, a marker of BBB leakage, by immunohistochemistry in the occipital cortex of autopsy brains with AD and capillary CAA (CAA type 1; n = 8), AD with noncapillary CAA (CAA type 2; n = 10), and AD without CAA (n = 7) compared with elderly controls (n = 10). Given the difference in pathogenesis of capillary and noncapillary CAA, we hypothesize that features of BBB breakdown are observed only in capillary CAA. We found decreased expression of CD31 in AD subjects with CAA types 1 and 2 compared with AD without CAA and an increase in COL4 in AD without CAA compared with controls. Furthermore, there was increased immunoreactivity for fibrinogen in AD with CAA type 1 compared with controls. These findings suggest that capillary CAA is associated with morphologic and possibly physiologic alterations of the neurovascular unit and increased BBB permeability in AD.
Keywords: Cerebral amyloid angiopathy, Alzheimer disease, Blood-brain barrier, Immunohistochemistry
1. Introduction
Alzheimer disease (AD) is characterized neuropathologically by the accumulation of amyloid-β (Aβ) in the brain parenchyma in the form of senile plaques (SPs) and in the vessels as cerebral amyloid angiopathy (CAA), neurofibrillary tangles (NFTs) composed predominantly of hyperphosphorylated tau, and neuron and synapse loss (Stewart et al., 1992; Thal et al., 2008a; Vinters, 2015; Zlokovic, 2011). In CAA, Aβ deposits in the walls of arteries and arterioles and less commonly capillaries and veins, preferentially in small- to medium-sized blood vessels within the cortex and leptomeninges. In arteries and arterioles, there is replacement of the smooth muscle cells of the media and resultant weakening of the vessel walls (Vinters and Tung, 2015). CAA is seen in approximately 85%e95% of patients with AD and in 20%−40% of the nondemented elderly, with increased prevalence with age in the nondemented population (Charidimou et al., 2012; Vinters, 2001, 2015). It is a significant cause of spontaneous lobar hemorrhages in the elderly and is also associated with ischemic lesions including microinfarcts (Aguilar and Freeman, 2010; Soontornniyomkij et al., 2010). Aβ is formed from the cleavage of amyloid precursor protein (APP) by β-and γ-secretase into 39–43 amino acid peptides (Thal et al., 2008b). Aβ42 and Aβ40 are the predominant forms in parenchymal and vascular amyloid deposits, with a higher Aβ40/Aβ42 ratio in the arteries and arterioles compared with that in SPs and capillaries (Attems et al., 2004; Thal et al., 2008b).
CAA preferentially affects the occipital cortex followed by the frontal, temporal, and parietal cortices (Gilbert and Vinters, 1983; Rosand et al., 2005) with the most severe CAA frequently seen in the parieto-occipital regions (Vinters,1987). Two types of CAA have been described: CAA type 2, in which mainly larger arteriolar vessels of the neocortex and leptomeninges are affected, and CAA type 1, or capillary CAA, in which both capillaries and larger vessels are involved (Carrano et al., 2011; Jeynes and Provias, 2006). The Aβ deposits of capillary CAA may also occasionally extend into the pericapillary neuropil, termed “dyshoric changes” or “dyshoric plaques” (Attems et al., 2010; Richard et al., 2010). Distinct from dyshoric plaques that are seen immediately adjacent to capillary Aβ deposits, pericapillary Aβ refers to parenchymal deposits around capillaries with or without CAA (Attems et al., 2010). Capillary CAA contains both Aβ40 and Aβ42, whereas pericapillary deposits are primarily composed of Aβ42 (Attems et al., 2010). AD patients with CAA type 1 have been shown to demonstrate more widespread SP pathology compared with CAA type 2 (Thal et al., 2010), and CAA type 1, but not CAA type 2, is strongly associated with the apolipoprotein E (APOE) ε4 allele, suggesting a difference in the pathogenesis of CAA types 1 and 2 (Attems and Jellinger, 2004; Attems et al., 2011; Thal et al., 2002).
CAA may both result from and exacerbate AD-related bloodbrain barrier (BBB) dysfunction. Brain microvessels isolated from transgenic mouse models of AD and CAA demonstrate increased BBB leakage, decreased expression of tight junction proteins, and increased matrix metalloproteinases (Hartz et al., 2012). However, few human studies have distinguished capillary CAA and noncapillary CAA in the assessment of BBB integrity in AD. In this study, we attempt to clarify the role of capillary CAA and noncapillary CAA on the alteration of the components of the neurovascular unit in AD brains by examining the immunohistochemical expression of the endothelial marker CD31, basement membrane protein collagen IV (COL4), tight junction protein claudin-5, and fibrinogen, a marker for BBB leakage, in the brains of AD subjects with CAA types 1 and 2. Given the difference in pathogenesis of capillary and noncapillary CAA, we hypothesize that features of BBB breakdown are observed only in capillary CAA. Furthermore, the degree of AD neuropathologic change and cerebrovascular disease is compared between the groups.
2. Materials and methods
2.1. Human subjects and brain tissue
We examined the autopsy brains of 25 subjects with AD in the UCLA Alzheimer Disease Research Center and Easton Center Brain Bank and 10 nondemented elderly subjects. The AD cases were subcategorized into those with CAA type 1 (CAA with capillary involvement), CAA type 2 (CAA without capillary involvement), and without CAA. All underwent complete neuropathologic examination by a neuropathology fellow and neuropathologist (HVV, WHY, or NK) from 2000 to 2015. Standard diagnostic criteria were used to assess the neuropathologic changes of AD (Braak and Braak, 1991; Montine et al., 2012). AD cases demonstrated changes of relatively pure AD except for one case with combined AD and diffuse Lewy body pathology. One patient had familial AD due to a mutation in the APP gene. All AD subjects had a Braak and Braak stage of at least IV-V. The severity of CAA was graded according to the Vonsattel criteria (Vonsattel et al., 1991). All AD patients with CAA had moderate to severe CAA (Vonsattel grade II to III), and none of the elderly controls demonstrated CAA (Table 1). The investigation was conducted in accordance with the guidelines of the institutional review board of the UCLA Medical Center.
Table 1.
Subject demographics and neuropathologic assessment
| Case | Age in years |
Gender | Break stage |
CERAD plaque score |
Level of AD pathologic change |
CAA |
|---|---|---|---|---|---|---|
| AD with CAA type 1 |
||||||
| 1 | 79 | M | VI | 3 | High | Severe |
| 2 | 78 | F | VI | 2 | High | Severe |
| 3 | 68 | F | VI | 3 | High | Severe |
| 4 | 101 | M | V | 2 | High | Severe |
| 5 | 73 | M | V | 2 | High | Severe |
| 6 | 75 | M | VI | 3 | High | Severe |
| 7 | 76 | M | VI | 1 | Intermediate | Moderate |
| 8 | 80 | M | VI | 3 | High | Severe |
| AD with CAA type 2 |
||||||
| 1 | 76 | M | VI | 3 | High | Severe |
| 2 | 93 | F | VI | 3 | High | Moderate |
| 3 | 69 | F | VI | 3 | High | Severe |
| 4 | 86 | F | IV-V | 3 | Intermediate-high Moderate | |
| 5 | 82 | F | VI | 3 | High | Severe |
| 6 | 78 | M | VI | 2 | High | Severe |
| 7 | 68 | M | VI | 2 | High | Severe |
| 8 | 64 | F | VI | 1 | Intermediate | Severe |
| 9 | 96 | F | VI | 2 | High | Moderate |
| 10 | 70 | M | VI | 2 | High | Moderate |
| AD without CAA |
||||||
| 1 | 65 | F | VI | 2–3 | High | No CAA |
| 2 | 115 | F | IV-V | 2 | Intermediate-high No CAA | |
| 3 | 81 | F | V | 3 | High | No CAA |
| 4 | 63 | VI, also DLBD | 3 | High | No CAA | |
| 5 | 61 | M | VI | 3 | High | No CAA |
| 6 | 70 | M | VI | 3 | High | No CAA |
| 7 | 93 | M | V-VI | 1–2 | Intermediate-high | No CAA |
| Nondemented elderly |
||||||
| 1 | 71 | M | II | 0 | Low | No CAA |
| 2 | 76 | F | <IV | 0 | Not | No CAA |
| 3 | 66 | M | I | 0 | Low | No CAA |
| 4 | 79 | F | II | 0 | Not | No CAA |
| 5 | 64 | F | <IV | 0 | Not | No CAA |
| 6 | 67 | M | I | 0 | Not | No CAA |
| 7 | 75 | F | III-IV | 0 | Low | No CAA |
| 8 | 65 | M | I-II | 0 | Not | No CAA |
| 9 | 75 | M | I | 0 | Not | No CAA |
| 10 | 89 | M | <IV | 0 | Low | No CAA |
Key: AD, Alzheimer disease; CERAD, Consortium to Establish a Registry for AD; CAA, cerebral amyloid angiopathy; DLBD, diffuse Lewy body disease.
2.2. Neuropathologic examination and image analysis
AD brains were blocked and processed according to the routine UCLA dementia autopsy protocol, including sections from the frontal, temporal, parietal, and occipital cortices, hippocampus, entorhinal cortex and amygdala, basal ganglia, brainstem, and cerebellum. Immunohistochemistry was performed on formalin-fixed paraffin embedded tissue sectioned at 6 mm in thickness with antibodies to β-amyloid 1–42 (1:150, EMD Millipore, rabbit polyclonal, AB5078P), β-amyloid 1–40 (1:400, EMD Millipore, rabbit polyclonal, AB5074P), phospho-tau (1:200, Thermo Fisher, mouse monoclonal, AT8), and alpha-synuclein (EMD Millipore, rabbit polyclonal, AB5038). Sections from the occipital cortex, a region preferentially affected by CAA, were additionally stained with the endothelial marker CD31 (1:20, Dako, mouse monoclonal, Clone JC70A), basement membrane protein COL4 (1:200, Abcam, rabbit polyclonal, ab6586), tight junction protein claudin5 (1:100, Thermo Fisher, mouse monoclonal, 4C3C2), and fibrinogen (1:800, Dako, rabbit polyclonal). Sections were incubated with the primary antibody followed by either horse anti-mouse or horse anti-rabbit secondary antibody conjugated to horseradish peroxidase (MP7402 & MP7401; Vector Laboratories, Burlingame, CA). Antibody reactivity was visualized with N,N’ diaminobenzidine as chromogen (no. SK-4100; Vector Laboratories) and counterstained with hematoxylin. Positive and negative controls for the immunohistochemical studies were performed. Slides were then scanned and digitized using the ScanScope image scanner (Aperio Technologies), and intensity of CD31, COL4, claudin-5, and fibrinogen staining semiquantitatively analyzed using the Positive Pixel Count algorithm in the ImageScope program with the following parameters: hue value of 0.1 and hue width of 0.175 for CD31, COL4, and fibrinogen, and hue value of 0.1 and hue width of 0.5 for claudin-5. The color saturation threshold was 0.19 for CD31, COL4, and fibrinogen, and 0.07 for claudin-5 to minimize detection of background staining. The positivity (percentage of positive pixels) was calculated from the number of positive pixels divided by the total number of pixels (positive and negative) and multiplied by 100. Five random areas each from the gray matter and white matter were evaluated and averaged to assess for staining in each region. Sections were cut at the same thickness and parameters kept constant across groups for each antibody. SPs, NFTs, and Aβ-positive vessels were counted according to the method of Jeynes and Provias (2006) in which 10 contiguous fields, encompassing the full cortical thickness, were examined to assess the density of lesions per field, except at 200× magnification instead of 250 (Table 2).
Table 2.
Density of neuropathologic lesions of Alzheimer disease (AD) per 200× field
| SP Aβ40 | SP Aβ42 |
CAA Aβ40 |
CAA Aβ42 |
Cap CAA Aβ40 |
Cap CAA Aβ42 |
NFT | |
|---|---|---|---|---|---|---|---|
| AD with CAA type 1 | 2.9 | 9.6 | 2.2 | 1.3 | 4.1 | 3.7 | 3.8 |
| AD with CAA type 2 | 5.3 | 16.4 | 1.6 | 0.9 | 0.0 | 0.0 | 7.3 |
| AD without CAA | 3.5 | 12.3 | 0.0 | 0.0 | 0.1 | 0.0 | 4.5 |
| Nondemented elderly | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Key: CAA, cerebral amyloid angiopathy; cap CAA, capillary CAA; NFT, neurofibrillary tangles; SP, senile plaques.
CAA severity has been shown to correlate with atherosclerosis and arteriosclerosis (Ellis et al., 1996; Tian et al., 2004; Yarchoan et al., 2012). Assessment of the degree of atherosclerosis was based on gross and microscopic estimates of stenosis of the major branches of the circle of Willis, including basilar and vertebral arteries, on a 4-point scale (0 = none; 1 = mild, <20%; 2 = moderate, 20%−50%; 3 = severe; >50%). We assessed arteriolosclerosis in the subcortical white matter vessels to avoid overlap with CAA-affected vessels. The degree of arteriolosclerosis was evaluated by calculating the sclerotic index (SI) of arteriolar vessels with exterior diameters ranging from 50 to 200 mm in the white matter, as described by Yamamoto et al. (2009) except examined on COL4 immunostained sections in 10 arteriolar vessels per case.
2.3. Statistical analysis
The intensity of CD31, COL4, claudin-5, and fibrinogen immunoreactivity as well as sclerotic index (SI) were compared between the subgroups of AD patients and nondemented elderly subjects using the nonparametric Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Differences in degree of atherosclerosis between the groups were assessed using the X2 test. Associations between density of SPs, NFTs, and CAA-involved vessels were assessed using multiple linear regression analysis corrected for age and gender. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographics
The demographic characteristics of subjects with AD and elderly controls are shown in Table 1. There were 8 subjects with AD and CAA type 1 (average age = 78.8 ± 9.8 [SD] years, range = 68 to 101), 10 subjects with CAA type 2 (average age = 78.2 ± 10.9 years, range ¼ 64–96), 7 AD patients without CAA (average age = 78.3 ± 19.8, range = 61–115), and 10 nondemented elderly controls (average age difference in age 72.7 ± 7.8, range = 64–89). There was no significant between the groups.
3.2. Immunohistochemical analysis
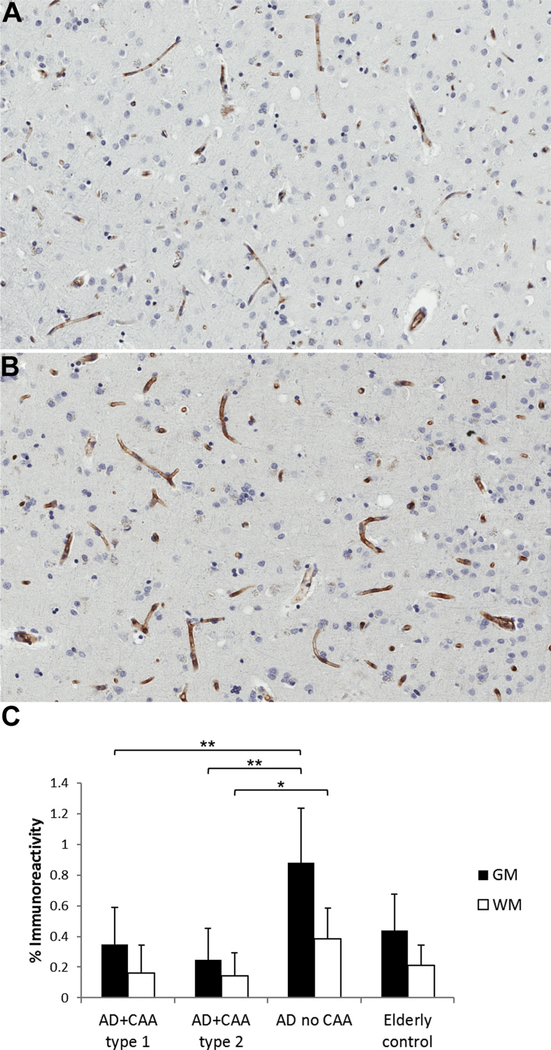
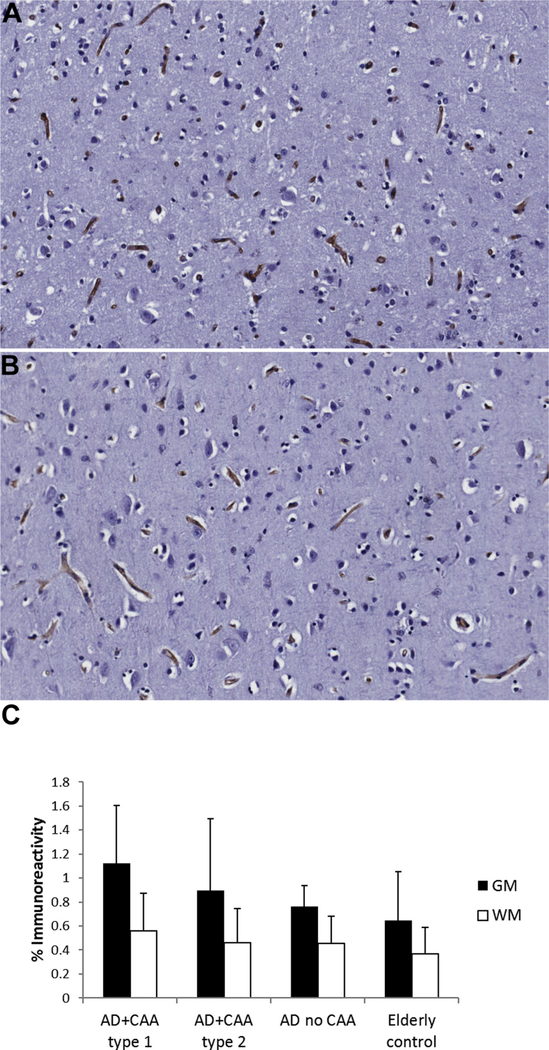
CD31 immunoreactivity was decreased in the brains of both AD subjects with CAA type 1 and AD subjects with CAA type 2 compared with AD subjects without CAA in the gray matter (p < 0.01) (Fig. 1). This decrease was also seen in the white matter in AD patients with CAA type 2 compared with AD subjects without CAA (p < 0.05). The specificity of the commercially available claudin-5 antibody has been demonstrated in prior studies, including in human brain tissue (Pienaar et al., 2015). Claudin-5 showed diffuse immunostaining restricted to the endothelium as reported previously (Viggars et al., 2011). There was no difference in the endothelial expression of the tight junction protein claudin-5 between the groups (Fig. 2).
Fig. 1.
Immunostaining for CD31 in the gray and white matter of AD subjects with CAA type 1, AD subjects with CAA type 2, AD subjects without CAA, and nondemented elderly subjects demonstrates (A) decreased staining for CD31 in the gray matter of AD subjects with CAA type 2 and (not shown) AD subjects with CAA type 1 compared with (B) AD subjects without CAA. This decrease is also seen in the white matter for AD patients with CAA type 2 compared with AD patients without CAA. (C) CD31 staining as mean ± SD. * p < 0.05, ** p < 0.01. Abbreviations: AD, Alzheimer disease; CAA, cerebral amyloid angiopathy; GM, gray matter; WM, white matter.
Fig. 2.
Immunohistochemistry for claudin-5 in the gray matter of (A) AD subjects with cerebral amyloid angiopathy (CAA) type 1, (not shown) AD subjects with CAA type 2, (not shown) AD subjects without CAA, and (B) nondemented elderly subjects demonstrates no significant difference in endothelial claudin-5 immunoreactivity between the groups. The white matter also shows no difference in claudin-5 staining. (C) Claudin-5 staining as mean ± SD. Abbreviations: AD, Alzheimer disease; CAA, cerebral amyloid angiopathy; GM, gray matter; WM, white matter.
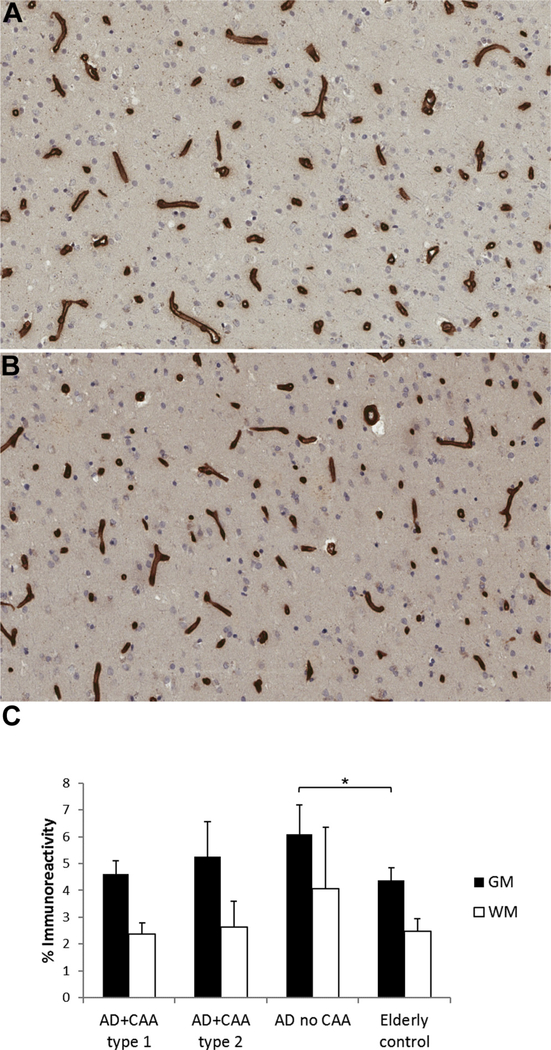
Although COL4 was increased in the gray matter in AD subjects without CAA compared with elderly controls (p < 0.05) (Fig. 3), this increase was not seen in AD whshown a decrease in tight junction proteins, including claudini either CAA type 1 or 2. No difference in vascular COL4 immunoreactivity was seen in the white matter. Kalaria and Hedera (1995) have shown vessel profiles with negative staining for CD31 and CD34, another vascular endothelial marker, but retained staining for COL4 (Kalaria and Hedera, 1995) and increased hydroxyproline, the main component of COL4, in microvessels in AD brains (Kalaria and Pax, 1995). We also found a decrease in CD31 and increase in COL4 in AD brains, but this varied with the presence or absence of CAA.
Fig. 3.
Immunohistochemistry for collagen IV (COL4) in the gray and white matter of AD subjects with CAA type 1, AD subjects with CAA type 2, AD subjects without CAA, and nondemented elderly subjects shows increased COL4 staining in the gray matter in (A) AD subjects without CAA compared with (B) elderly controls. (C) COL4 staining as mean ± SD, * p < 0.05. Abbreviations: AD, Alzheimer disease; CAA, cerebral amyloid angiopathy; WM, white matter; GM, gray matter.
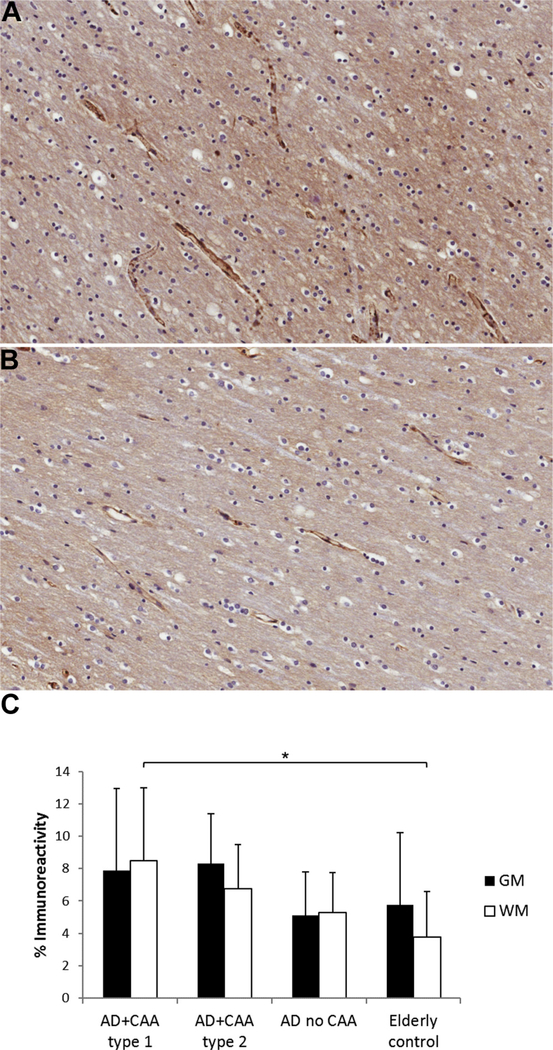
Fibrinogen immunoreactivity was significantly increased in the white matter of AD patients with CAA type 1 compared with elderly controls (p < 0.05). However, there was no significant difference in the brains of AD subjects with CAA type 2 and AD subjects without CAA compared with elderly controls (Fig. 4) suggesting that the presence of capillary CAA is associated with BBB dysfunction in AD brains. There was no significant difference in fibrinogen immunoreactivity in the gray matter among the groups.
Fig. 4.
Immunostaining for fibrinogen in the gray and white matter of AD subjects with CAA type 1, AD subjects with CAA type 2, AD subjects without CAA, and nondemented elderly subjects demonstrates increased vascular and parenchymal fibrinogen immunoreactivity in the white matter of (A) AD patients with CAA type 1 compared with (B) elderly controls. (C) Fibrinogen staining as mean ± SD, *p < 0.05. Abbreviations: AD, Alzheimer disease; CAA, cerebral amyloid angiopathy; WM, white matter, GM, gray matter.
3.3. Assessment of cerebrovascular disease
Atherosclerosis has been demonstrated to correlate with AD pathology in a study of 1000 dementia cases, including more than 400 with a primary diagnosis of AD, with over 77% of AD patients demonstrating atherosclerosis of the circle of Willis compared with 47% in control brains (Yarchoan et al., 2012). Similarly, we found that 83% (50% mild, 13% moderate, and 21% severe) of AD patients had some degree of atherosclerosis of the circle of Willis, significantly higher compared with 50% (30% mild, 20% moderate, 0% severe) of elderly controls. There was no difference in the prevalence of atherosclerosis between the subgroups of AD patients. Furthermore, there was no significant difference in the degree of arteriolosclerosis of white matter arterioles as measured by the SI between the different groups (AD with CAA type 1: 0.25 ± 0.04 [SD]; AD with CAA type 2: 0.22 ± 0.05; AD without CAA: 0.21 ± 0.04; elderly controls: 0.24 ± 0.06).
3.4. Associations with AD pathologic change
In the assessment of AD neuropathologic changes in subjects with AD and CAA type 1, NFTs were significantly associated with Aβ40 immunoreactive noncapillary CAA (R2 = 0.727, p < 0.05) with increase in density of Aβ40-positive vessels with increase in density of NFTs, but there were no associations with SPs or capillary CAA. There was also a positive association between noncapillary CAA and capillary CAA on Aβ42 immunohistochemistry (R2 = 0.903, p < 0.05).
4. Discussion
Cerebrovascular dysfunction has increasingly been recognized to play a significant role in the pathogenesis of AD and other dementias (Nelson et al., 2016). The normal BBB is characterized by endothelial cells with abundant mitochondria, few pinocytic vesicles, and interendothelial tight junctions (Claudio, 1995). AD patients demonstrate morphologic abnormalities of the neurovascular unit such as endothelial atrophy, degeneration of smooth muscle cells, and disruption and thickening of the basement membrane (Farkas and Luiten, 2001; Kalaria and Hedera, 1995). Zipser et al. (2007) have demonstrated thinning and focal loss of endothelial cells highlighted by factor VIII in brains with severe AD changes, suggesting that endothelial damage plays a role in leakage of plasma proteins. Ultrastructural studies have shown decreased mitochondrial area and density in the cerebral capillary endothelium, increased pinocytotic vesicles, greater numbers of interendothelial junctions per unit of vessel length, increased numbers of pericyte profiles per vessel profile, and increased cleft index, suggesting a leakiness of the BBB in AD brains (Baloyannis and Baloyannis, 2012; Claudio, 1995; Stewart et al., 1992).
In CAA, Aβ is deposited in the basement membranes of capillary walls and between smooth muscle cells in the tunica media (Carare et al., 2013). Deposition of amyloid in the vessel walls may both result from and contribute to BBB dysfunction via direct interaction of toxic Aβ with the neurovascular unit, impaired transvascular clearance across the BBB, and perivascular drainage of Aβ (Carare et al., 2013; Ramanathan et al., 2015; Zipfel et al., 2009). Although some ultrastructural studies of predominantly arteriolar CAA have demonstrated a thinned but relatively preserved endothelium, examination of capillary CAA has found shrinkage and degeneration of endothelial cells and vessel occlusion, leading to blood flow disturbances and ischemic injury (Attems and Jellinger, 2004; Thal et al., 2008a, 2009; Vinters et al., 1994). Kalaria and Hedera (1995) have shown loss of staining of the endothelial markers CD31 and CD34 in capillaries but retained basement membrane COL4 immunoreactivity in AD brains. However, other studies have demonstrated no difference in CD31 staining between moderate-to-severe AD, subclinical AD, and control patients in frontal and temporal cortices (Lepelletier et al., 2017). Zhang et al. (1998) found no difference in endothelial markers in brains with severe arterial and arteriolar CAA, with or without AD, compared with controls. These variances may be due to the degree of AD pathologic change, regional differences, and severity and type of CAA examined. We found decreased CD31 immunostaining in both AD patients with CAA type 1 and AD patients with CAA type 2 compared with AD patients without CAA, suggesting that endothelial degeneration is associated with both types of CAA even though the arterial endothelial basement membrane appears to be relatively spared from direct deposition of Aβ (Keable et al., 2016). Few studies have differentiated the 2 types of CAA in evaluating the association between CAA and BBB dysfunction (Carrano et al., 2011). Carrano et al. (2012, 2011) have shown a decrease in tight junction proteins, including claudin-5, occludin, and zonula occludens-1, in amyloid-laden capillaries that showed increased fibrinogen leakage and were furthermore surrounded by NADPH oxidase-2 immunopositive activated microglia; this suggested that neuroinflammation and reactive oxygen species contribute to the endothelial toxicity of Aβ and disruption of the BBB in capillary CAA. We did not detect differences in claudin-5 expression between elderly controls and AD patients with or without CAA, but we did not discriminate between vessels with and without amyloid deposition. Interestingly, exposure of Aβ42 to brain microvessel endothelial cells in rats have shown to result in redistribution of claudin-5 from the plasma membrane to the cytoplasm without change in protein levels, suggesting that alterations in the localization of tight junction proteins may also contribute to BBB dysfunction (Marco and Skaper, 2006).
Collagen content in cerebral microvessels increases with age, leading to vessel wall stiffness and luminal narrowing, likely predisposing to ischemic injury (Uspenskaia et al., 2004). Thickening of the capillary basement membrane with increase in collagen content, which could disrupt transport into and out of the brain, has also been reported in the brains of subjects with AD and Parkinson disease (Farkas et al., 2000; Kalaria and Pax, 1995; Tian et al., 2006). Furthermore, these vascular changes appear to occur early in the course of disease as COL4, perlecan, and fibronectin, components of the perivascular extracellular matrix, were increased in both subclinical AD subjects and AD patients compared with controls, without a further increase in AD patients compared with those with subclinical AD (Lepelletier et al., 2017). However, other studies have shown no difference in the collagen content in the cerebral vasculature. Keable et al. (2016) found no difference in COL4 and fibronectin staining between young, elderly, and CAA brains, many with AD. A different group demonstrated that the decrease in vascular smooth muscle actin immunoreactivity in AD patients was exacerbated by arteriolar CAA but found no difference in collagen staining in leptomeningeal arteries and arterioles among AD patients with CAA, AD patients without CAA, and elderly controls (Merlini et al., 2016). We found increased COL4 immunoreactivity in cortical vessels in AD brains without CAA, but not in AD brains with CAA, compared with elderly controls. Interestingly, Tian et al. (2006) have shown a negative correlation between degree of CAA on Aβ42 (but not Aβ40 or total Aβ) staining and COL4 immunoreactivity in the frontal cortex when only examining leptomeningeal arteries. Thus, the apparent discrepant results may be partially explained by the difference in degree and type of CAA and the type of vessels examined. These studies suggest that unlike some other microangiopathies in which deposition of collagen in the vessel wall, or fibrosis, accompanies the degeneration of smooth muscle cells, the replacement of the vessel wall with Aβ instead of increased collagen may underlie the increased risk of vessel rupture and hemorrhage in CAA (Zhang et al., 1998).
Accompanying the structural alterations of the neurovascular unit, AD brains demonstrate BBB dysfunction early in the disease process (Viggars et al., 2011; Zlokovic, 2011). BBB permeability increases with age and is increased even further in vascular dementia and AD (Farrall and Wardlaw, 2009). BBB breakdown has been shown to precede SP development in mouse models of AD (Ujiie et al., 2003) and possibly even precede cognitive decline, suggesting that BBB breakdown may be an early event in the pathogenesis and progression of dementia (Kalaria, 1999; Skoog et al.,1998). Levels of serum proteins normally excluded from the brain, such as fibrinogen, have been shown to be increased in the brain parenchyma and blood vessels of AD patients and mouse models of AD, with levels appearing to correlate with Aβ pathology and Braak stage (CortesCanteli et al., 2015; Fiala et al., 2002; Hultman et al., 2013; Viggars et al., 2011). In a study that showed a correlation between increases in prothrombin leakage with Braak stage, no difference in prothrombin levels was seen between AD with and without CAA, suggesting prothrombin leakage may be independent of or precede the development of CAA (Zipser et al., 2007). On the other hand, Wisniewski et al. (1997) demonstrated increased permeability to albumin only in vessels laden with amyloid or surrounded by amyloid plaques. Another group reported a strong association between increased fibrinogen deposition and severity of CAA as well as with the homozygous APOE ε4 genotype (Hultman et al., 2013).
We found a significant increase in fibrinogen immunoreactivity in the white matter of AD patients with CAA type 1 but not in AD patients with CAA type 2 or AD subjects without CAA, implicating capillary CAA in the BBB leakage seen in AD. Interestingly, no difference was seen in the gray matter, where vessels are preferentially involved by CAA. As there were no differences in the degree of arteriolosclerosis between the groups, the differences cannot be solely attributed to hypertensive arteriopathy, which also increases vascular permeability (Kalaria, 1999; Wardlaw et al., 2003).
In addition to gray matter, AD patients show significant pathologic change in the white matter, previously attributed largely to cerebrovascular disease but which may also be due to amyloid pathology (Roher et al., 2003). AD brains show increased interstitial fluid (ISF) in the white matter; furthermore, decreased perivascular drainage of ISF and solutes is associated with advancing age, APOE ε4 allele, AD, and CAA (Roher et al., 2003; Weller et al., 2015). This is postulated to result from decreased motive force from stiffening of the vessel walls with age and deposition of proteins such as Aβ within the basement membranes of the perivascular drainage pathways (Weller et al., 2015). White matter abnormalities seen on MRI, such as white matter hyperintensities and dilated white matter perivascular spaces, have been used as a marker of small vessel disease and may reflect impaired ISF drainage (Charidimou et al., 2015; Weller et al., 2015). These white matter changes are commonly seen in dementia and have been thought to result from arteriolosclerosis and ischemia, with associated BBB dysfunction and leakage of plasma proteins; more recently, impaired drainage of ISF, especially in association with CAA, has been implicated (Weller et al., 2015, 2008). It has been further demonstrated that the frequency and severity of dilatation of white matter perivascular spaces correlate with severity of CAA and cortical Aβ load, suggesting that CAA may impede white matter ISF drainage in AD (Charidimou et al., 2014; Roher et al., 2003; van Veluw et al., 2015). The vascular basement membrane plays an important role in the formation and maintenance of the BBB (Morris et al., 2014), and ISF formation is dependent on active solute transport across the BBB (Bakker et al., 2016). Thus, CAA may be the link between impaired perivascular Aβ drainage and BBB dysfunction.
CAA type 1 and CAA type 2 are distinct not only morphologically but genetically and possibly in pathogenesis, with CAA type 1 strongly associated with the APOE ε4 allele (Attems and Jellinger, 2004; Richard et al., 2010; Thal et al., 2010), whereas CAA type 2 may be associated with the APOE ε2 allele, especially in nondemented individuals (Love et al., 2014). AD patients with CAA type 1 also demonstrate more widely distributed SPs (Attems et al., 2011). Most AD patients show some degree of CAA, with approximately a quarter having severe CAA, but only half demonstrate capillary CAA (Ellis et al., 1996; Thal et al., 2008a). Slightly over 10% of elderly nondemented subjects demonstrate capillary CAA (Thal et al., 2008a). We found a positive association between Aβ42 immunoreactive capillary CAA and noncapillary CAA in the occipital cortex, in agreement with Jeynes and Provias (2006) who also showed positive correlations between capillary CAA and noncapillary CAA in the occipital and temporal cortices. In addition, our cohort demonstrated a positive association between NFTs and noncapillary CAA on Aβ40 immunohistochemistry. Previous studies have shown both Braak stages and Aβ42 positive plaques to have a high correlation with the severity of Aβ42 immunoreactive capillary CAA, with only a low correlation with noncapillary CAA (Attems and Jellinger, 2004; Attems et al., 2004). Tian et al. (2003) demonstrated a negative association between SPs and CAA when examining CAA in 4 different cortical regions combined. However, Jeynes and Provias (2006) demonstrated a positive correlation between CAA Aβ42 and Aβ8–17,40 peptide forms of SPs in the superior temporal cortex and between 3 different amyloid peptide forms of CAA and SP Aβ40 in the occipital cortex, although SP Aβ42 was negatively correlated with capillary CAA. The somewhat discrepant associations between CAA and AD pathology may be due to differences in severity and type of CAA, the examination of different Aβ peptide forms, and regional variation (Attems et al., 2004; Jeynes and Provias, 2006; Tian et al., 2003).
The APOE ε4 allele is the strongest genetic risk factor in sporadic AD and interacts with known vascular risk factors for AD such as hypertension and diabetes mellitus (Liu et al., 2013; van der Flier and Scheltens, 2005). Unfortunately, only 4 of our AD patients had APOE genotypes available; 3 subjects had the ε3/ε4 genotype (2 in the AD without CAA group and 1 in the AD with CAA type 2 group) and 1 AD subject with CAA type 1 had a homozygous ε4/ε4 genotype. However, the strong association of AD and capillary CAA with the APOE ε4 allele has already been demonstrated (Thal et al., 2010; Yu et al., 2015), and our sample size would have been too small to detect a difference, which is 1 limitation of this study (Boche et al., 2008). Another limitation is the patchy nature of CAA and SPs. For consistency, we evaluated only cortical CAA, but the focus on either cortical or leptomeningeal vessels in different studies in the assessment of CAA may also underlie the variability in results.
Vascular changes are a significant component of morbidity in AD and other dementias. Impaired clearance of Aβ via degradation, transport across the BBB, and clearance along perivascular drainage pathways through the vascular basement membranes may lead to accumulation of Aβ in the form of SPs and CAA in AD (Nelson et al., 2016; Zlokovic, 2011). Stiffening of vessels from arteriolosclerosis and changes in the basement membrane components from aging and cerebrovascular disease may underlie impaired perivascular clearing (Keable et al., 2016; Weller et al., 2009). The role of CAA in the pathogenesis of AD is unclear, but Aβ deposition and BBB dysfunction may lead to further decreased clearance of Aβ, exacerbating Aβ accumulation, increased propensity for hemorrhage, and leakage of blood-derived proteins into the brain parenchyma resulting in neuronal dysfunction (Zlokovic, 2011). Understanding the pathogenesis of CAA, an important substrate of dementia, and its relationship to BBB breakdown, what may be an early event in AD, will help shed light on mechanisms of neurodegeneration and ultimately facilitate development of potential preventative and therapeutic strategies.
Acknowledgements
This work was supported by the UCLA transdisciplinary seed grant.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Aguilar MI, Freeman WD, 2010. Spontaneous intracerebral hemorrhage. Semin. Neurol 30, 555–564. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA, 2004. Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology - a pilot study. Acta Neuropathol. 107, 83–90. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger K, Thal DR, Van Nostrand W, 2011. Review: sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol 37, 75–93. [DOI] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA, 2004. Amyloid b peptide 1–42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 107, 283–291. [DOI] [PubMed] [Google Scholar]
- Attems J, Yamaguchi H, Saido TC, Thal DR, 2010. Capillary CAA and perivascular Aβ-deposition: two distinct features of Alzheimer‘s disease pathology. J. Neurol. Sci 299, 155–162. [DOI] [PubMed] [Google Scholar]
- Bakker ENTP, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AWJ, Weller RO, Carare RO, 2016. Lymphatic clearance of the brain: perivascular, paravascular, and significance for neurodegenerative diseases. Cell. Mol. Neurobiol 36, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis SJ, Baloyannis IS, 2012. The vascular factor in Alzheimer‘s disease: a study in Golgi technique and electron microscopy. J. Neurol. Sci 322, 117–121. [DOI] [PubMed] [Google Scholar]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JAR, 2008. Consequence of Aβ immunization on the vasculature of human Alzheimer‘s disease brain. Brain 131, 3299–3310. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO, 2013. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol 39, 593–611. [DOI] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJM, van der Vies SM, Rozemuller AJM, van Horssen J, de Vries HE, 2011. Amyloid beta induces oxidative stress-mediated bloodbrain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal 15, 1167–1178. [DOI] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJM, van Der Vies SM, Van Horssen J, De Vries HE, Rozemuller AJM, 2012. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener. Dis 10, 329–331. [DOI] [PubMed] [Google Scholar]
- Charidimou A, Gang Q, Werring DJ, 2012. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry 83, 124–137. [DOI] [PubMed] [Google Scholar]
- Charidimou A, Hong YT, Jäger HR, Fox Z, Aigbirhio FI, Fryer TD, Menon DK, Warburton EA, Werring DJ, Baron JC, 2015. White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke 46, 1707–1709. [DOI] [PubMed] [Google Scholar]
- Charidimou A, Jäger RH, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Werring DJ, 2014. White matter perivascular spaces are related to cortical superficial siderosis in cerebral amyloid angiopathy. Stroke 45, 2930–2935. [DOI] [PubMed] [Google Scholar]
- Claudio L, 1995. Ultrastructural features of the blood-brain barrier in biopsy tissue from Alzheimer‘s disease patients. Acta Neuropathol. 91, 6–14. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Mattei L, Richards AT, Norris EH, Strickland S, 2015. Fibrin deposited in the Alzheimer‘s disease brain promotes neuronal degeneration. Neurobiol. Aging 36, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A, 1996. Cerebral amyloid angiopathy in the brains of patients with Alzheimer‘s disease: the CERAD experience, part XV. Neurology 46, 1592–1596. [DOI] [PubMed] [Google Scholar]
- Farkas E, De Jong GI, de Vos RAI, Jansen Steur ENH, Luiten PGM, 2000. Pathological features of cerebral cortical capillaries are doubled in Alzheimer‘s disease and Parkinson‘s disease. Acta Neuropathol. 100, 395–402. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PGM, 2001. Cerebral microvascular pathology in aging and Alzheimer‘s disease. Prog. Neurobiol 64, 575–611. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM, 2009. Blood-brain barrier: ageing and microvascular disease - systematic review and meta-analysis. Neurobiol. Aging 30, 337–352. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV, 2002. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer‘s disease brain and damage the blood-brain barrier. Eur. J. Clin. Invest 32, 360–371. [DOI] [PubMed] [Google Scholar]
- Gilbert JJ, Vinters HV, 1983. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke 14, 915–923. [DOI] [PubMed] [Google Scholar]
- Hartz AMS, Bauer B, Soldner ELB, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klunemann HH, Schuierer G, Schlachetzki F, 2012. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 43, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman K., Strickland S., Norris EH., 2013. The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer‘s disease patients. J. Cereb. Blood Flow Metab 33, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B, Provias J, 2006. The possible role of capillary cerebral amyloid angiopathy in Alzheimer lesion development: a regional comparison. Acta Neuropathol. 112, 417–427. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, 1999. The blood-brain barrier and cerebrovascular pathology in Alzheimer‘s disease. Ann. N. Y. Acad. Sci 893, 113–125. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Hedera P, 1995. Differential degeneration of the cerebral microvasculature in Alzheimer‘s disease. Neuroreport 6, 477–480. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Pax AB, 1995. Increased collagen content of cerebral microvessels in Alzheimer‘s disease. Brain Res. 705, 349–352. [DOI] [PubMed] [Google Scholar]
- Keable A, Fenna K, Yuen HM, Johnston DA, Smyth NR, Smith C, Salman RAS, Samarasekera N, Nicoll JAR, Attems J, Kalaria RN, Weller RO, Carare RO, 2016. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim. Biophys. Acta 1862, 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepelletier F-X, Mann DMA, Robinson AC, Pinteaux E, Boutin H, 2017. Early changes in extracellular matrix in Alzheimer‘s disease. Neuropathol. Appl. Neurobiol 43, 167–182. [DOI] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K, Yamada M, McCarron M, Minett T, Matthews F, Greenberg S, Mann D, Kehoe PG, 2014. Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am. J. Neurodegener. Dis 3, 19–32. [PMC free article] [PubMed] [Google Scholar]
- Marco S, Skaper SD, 2006. Amyloid beta-peptide1–42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci. Lett 401, 219–224. [DOI] [PubMed] [Google Scholar]
- Merlini M, Wanner D, Nitsch RM, 2016. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer‘s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 131, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, 2012. National Institute on Aging-Alzheimer‘s Association guidelines for the neuropathologic assessment of Alzheimer‘s disease: a practical approach. Acta Neuropathol. 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AWJ, Carare RO, Schreiber S, Hawkes CA, 2014. The cerebrovascular basement membrane: role in the clearance of β-amyloid and cerebral amyloid angiopathy. Front. Aging Neurosci 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV, 2016. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer‘s disease. Biochim. Biophys. Acta 1862, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar IS, Lee CH, Elson JL, McGuinness L, Gentleman SM, Kalaria RN, Dexter DT, 2015. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson‘s disease. Neurobiol. Dis 74, 392–405. [DOI] [PubMed] [Google Scholar]
- Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV, 2015. Impaired vascularmediated clearance of brain amyloid beta in Alzheimer‘s disease: the role, regulation and restoration of LRP1. Front. Aging Neurosci 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E, Carrano A, Hoozemans JJ, van Horssen J, van Haastert ES, Eurelings LS, de Vries HE, Thal DR, Eikelenboom P, van Gool WA, Rozemuller AJM, 2010. Characteristics of dyshoric capillary cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol 69, 1158–1167. [DOI] [PubMed] [Google Scholar]
- Roher AE, Kuo Y-M, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA, 2003. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer‘s disease. Mol. Med 9, 112–122. [PMC free article] [PubMed] [Google Scholar]
- Rosand J, Muzikansky A, Kumar A, Wisco JJ, Smith EE, Betensky RA, Greenberg SM, 2005. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann. Neurol 58, 459–462. [DOI] [PubMed] [Google Scholar]
- Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K, 1998. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer‘s disease and vascular dementia. Neurology 50, 966–971. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, Vinters HV, 2010. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 20, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Hayakawa K, Akers MA, Vinters HV, 1992. A morphometric study of the blood-brain barrier in Alzheimer‘s disease. Lab. Invest 67, 734–742. [PubMed] [Google Scholar]
- Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N, 2009. Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol. Aging 30, 1936–1948. [DOI] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H, 2002. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol 61, 282–293. [DOI] [PubMed] [Google Scholar]
- Thal DR, Griffin WST, Braak H, 2008a. Parenchymal and vascular Aβ-deposition and its effects on the degeneration of neurons and cognition in Alzheimer‘s disease. J. Cell. Mol. Med 12, 1848–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Griffin WST, de Vos RA, Ghebremedhin E, 2008b. Cerebral amyloid angiopathy and its relationship to Alzheimer‘s disease. Acta Neuropathol. 115, 599–609. [DOI] [PubMed] [Google Scholar]
- Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kölsch H, Del Tredici K, Attems J, Ghebremedhin E, 2010. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer‘s disease. Acta Neuropathol. 120, 169–183. [DOI] [PubMed] [Google Scholar]
- Tian J, Shi J, Bailey K, Mann DMA, 2003. Negative association between amyloid plaques and cerebral amyloid angiopathy in Alzheimer‘s disease. Neurosci. Lett 352, 137–140. [DOI] [PubMed] [Google Scholar]
- Tian J, Shi J, Bailey K, Mann DMA, 2004. Relationships between arteriosclerosis, cerebral amyloid angiopathy and myelin loss from cerebral cortical white matter in Alzheimer‘s disease. Neuropathol. Appl. Neurobiol 30, 46–56. [DOI] [PubMed] [Google Scholar]
- Tian J, Shi J, Smallman R, Iwatsubo T, Mann DMA, 2006. Relationships in Alzheimer‘s disease between the extent of Aβ deposition in cerebral blood vessel walls, as cerebral amyloid angiopathy, and the amount of cerebrovascular smooth muscle cells and collagen. Neuropathol. Appl. Neurobiol 32, 332–340. [DOI] [PubMed] [Google Scholar]
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA, 2003. Bloodebrain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10, 463–470. [DOI] [PubMed] [Google Scholar]
- Uspenskaia O, Liebetrau M, Herms J, Danek A, Hamann GF, 2004. Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci. 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Scheltens P, 2005. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry 76 (Suppl 5), v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, Macklin EA, Rozemuller AJ, Gurol ME, Greenberg SM, Viswanathan A, Martinez-Ramirez S, 2015. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J. Cereb. Blood Flow Metab 36, 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM, Garwood C, Drew D, Shaw PJ, Ince PG, 2011. Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: a study in the MRC-CFAS population neuropathology cohort. Neurosci. Lett 505, 25–30. [DOI] [PubMed] [Google Scholar]
- Vinters HV, 1987. Cerebral amyloid angiopathy. A critical review. Stroke 18, 311–324. [DOI] [PubMed] [Google Scholar]
- Vinters HV, 2001. Cerebral amyloid angiopathy: a microvascular link between parenchymal and vascular dementia? Ann. Neurol 49, 691–693. [DOI] [PubMed] [Google Scholar]
- Vinters HV, 2015. Emerging concepts in Alzheimer‘s disease. Annu. Rev. Pathol 10, 291–319. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Secor DL, Read SL, Frazee JG, Tomiyasu U, Stanley TM, Ferreiro JA, Akers MA, 1994. Microvasculature in brain biopsy specimens from patients with Alzheimer‘s disease: an immunohistochemical and ultrastructural study. Ultrastruct. Pathol 18, 333–348. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Tung S, 2015. Cerebral amyloid angiopathy: clinicopathologic features and pathogenesis In: Dorovini-Zis K (Ed.), The Blood-Brain Barrier in Health and Disease, Vol. 2 Pathophysiology and Pathology. CRC Press, Boca Raton, pp. 299–327. [Google Scholar]
- Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP, 1991. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann. Neurol 30, 637–649. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sandercock PAG, Dennis MS, Starr J, 2003. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34, 806–811. [DOI] [PubMed] [Google Scholar]
- Weller RO, Boche D, Nicoll JAR, 2009. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer‘s disease and their potential impact on therapy. Acta Neuropathol. 118, 87–102. [DOI] [PubMed] [Google Scholar]
- Weller RO, Hawkes CA, Kalaria RN, Werring DJ, Carare RO, 2015. White matter changes in dementia: role of impaired drainage of interstitial fluid. Brain Pathol. 25, 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO, 2008. Perivascular drainage of amyloid-b peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer‘s disease. Brain Pathol. 18, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Vorbrodt AW, Wegiel J, 1997. Amyloid angiopathy and bloodbrain barrier changes in Alzheimer‘s disease. Ann. N. Y. Acad. Sci 826, 161–172. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ihara M, Tham C, Low RWC, Slade JY, Moss T, Oakley AE, Polvikoski T, Kalaria RN, 2009. Neuropathological correlates of temporal pole white matter hyperintensities in CADASIL. Stroke 40, 2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VMY, Trojanowski JQ, Arnold SE, 2012. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 135, 3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, Wilson RS, Arvanitakis Z, Farfel JM, De Jager PL, Bennett DA, Schneider JA, 2015. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol. Aging 36, 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, Lempessi H, Olsson Y, 1998. Amyloid angiopathy of the human brain: immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol. 96, 558–563. [DOI] [PubMed] [Google Scholar]
- Zipfel GJ, Han H, Ford AL, Lee JM, 2009. Cerebral amyloid angiopathy progressive disruption of the neurovascular unit. Stroke 40, 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG, 2007. Microvascular injury and blood-brain barrier leakage in Alzheimer‘s disease. Neurobiol. Aging 28, 977–986. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2011. Neurovascular pathways to neurodegeneration in Alzheimer‘s disease and other disorders. Nat. Rev. Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]