Abstract
Setting: GHESKIO (Groupe Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes) clinic, Port-au-Prince, Haiti.
Objective: To evaluate tuberculosis (TB) care continuum outcomes among adolescents.
Design: Among a retrospective cohort of 10–24 year olds diagnosed with active TB, we report completion of the following steps of the TB care continuum stratified by human immunodeficiency virus (HIV) status: diagnosis of microbiologically confirmed TB, initiation of anti-tuberculosis treatment, retention in care at 2 months on anti-tuberculosis treatment, and TB treatment success. Factors associated with attrition at each step were identified using multivariable regression.
Results: A total of 1005 adolescents were diagnosed with active TB; 74 (7%) were HIV-positive at the time of TB diagnosis. HIV-positive patients had poorer outcomes than non-HIV-infected patients: 73% vs. 85% initiated anti-tuberculosis treatment (P < 0.01), 46% vs. 74% were retained in care at 2 months (P < 0.01), and 41% vs. 68% achieved TB treatment success (P < 0.01). Among those who initiated treatment, same-day initiation resulted in less treatment failure. Attrition before treatment initiation was associated with female sex and HIV coinfection. Attrition after treatment initiation was associated with age ⩾16 years and HIV coinfection.
Conclusion: Outcomes across the TB care continuum are suboptimal among adolescents, with only two thirds of patients achieving treatment success. Interventions tailored to adolescents are needed to improve retention in care, particularly for those who are co-infected with HIV.
Keywords: HIV, youth, cascade of care
Abstract
Contexte : Centre de santé, Groupe Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes, Port-au-Prince, Haïti.
Objectif : Evaluer les résultats tout au long de la prise en charge de la tuberculose (TB) parmi les adolescents.
Schéma : Parmi une cohorte rétrospective d'adolescents âgés de 10–24 ans ayant eu un diagnostic de TB active, nous rapportons le déroulement des étapes suivantes des soins de la TB stratifiés par statut virus de l'immunodéficience humaine (VIH) : diagnostic de TB confirmée par microbiologie ; mise en route du traitement de la TB ; rétention en soins à 2 mois sous traitement de TB ; et succès du traitement de TB. Les facteurs associés à l'attrition à chaque étape ont été identifiés grâce à une régression logistique multivariée.
Résultats: Des 1005 adolescents qui ont eu un diagnostic de TB active, 74 (7%) ont été positifs au VIH au moment du diagnostic de TB. Les patients VIH positifs ont eu des résultats plus médiocres comparés aux patients non infectés par le VIH : 73% contre 85% ont mis en route le traitement de TB (P < 0,01), 46% contre 74% sont restés sous traitement à 2 mois (P < 0,01) et 41% contre 68% ont achevé le traitement avec succès (P < 0,01). Parmi ceux qui ont mis en route le traitement, une prise dès le premier jour a abouti à moins d'échec du traitement. L'attrition avant le début du traitement a été associée au sexe féminin et à la coinfection à VIH. L'attrition après mise en route du traitement a été associée à un âge ⩾16 ans et à la coinfection à VIH.
Conclusion : Les résultats au niveau de la continuité des soins de TB sont sousoptimaux parmi les adolescents, dont seulement deux tiers achèvent le traitement avec succès. Des interventions adaptées aux adolescents sont requises pour améliorer la rétention en soins, particulièrement pour ceux qui sont coinfectés par le VIH.
Abstract
Marco de Referencia: El centro du Groupe Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes de Port-au-Prince, en Haití.
Objetivo: Evaluar los resultados del proceso asistencial continuo de la tuberculosis (TB) en los adolescentes.
Método: En una cohorte retrospectiva de jóvenes de 10–24 años de edad con diagnóstico de TB activa, se comunican los resultados logrados en las siguientes etapas del proceso asistencial continuo de la TB, estratificados según la situación frente al virus de la inmunodeficiencia humana (VIH): diagnóstico de TB confirmada microbiológicamente; inicio del tratamiento antituberculoso; retención en la atención a los 2 meses del tratamiento; y el éxito del tratamiento antituberculoso. Se determinaron los factores asociados con el abandono en cada etapa mediante un modelo de regresión multivariante.
Resultados: Se diagnosticó TB activa en 1005 adolescentes; 74 de ellos (7%) eran positivos frente al VIH en el momento del diagnóstico de TB. Los pacientes positivos frente al VIH presentaron desenlaces más desfavorables que los pacientes sin infección por el VIH (73% contra 85% inició el tratamiento antituberculoso, P < 0,01; 46% contra 74% continuaba en la atención a los 2 meses, P < 0,01; y 41% contra 68% alcanzó un tratamiento antituberculoso exitoso, P < 0,01). Los jóvenes que iniciaron tratamiento el mismo día de la consulta presentaron menos fracasos terapéuticos. El abandono antes de iniciar el tratamiento se asoció con el sexo femenino y la coinfección por el VIH. El abandono después de haber iniciado el tratamiento se asoció con la edad ⩾16 años y la coinfección por el VIH.
Conclusión: Los resultados a lo largo de la continuidad asistencial de la TB son deficientes en los adolescentes, pues solo dos tercios de ellos alcanzan el éxito terapéutico. Se precisan intervenciones adaptadas a los adolescentes que mejoren la retención en los servicios de atención, sobre todo en los pacientes coinfectados por el VIH.
Tuberculosis (TB) is a leading cause of morbidity and mortality worldwide. In 2016, an estimated 10.4 million people developed active TB and 1.7 million died from the disease.1 TB disproportionally affects persons with human immunodeficiency virus (HIV) infection, with the risk of developing TB approximately 30-fold greater among people living with HIV.1 Haiti is the poorest country in the Western Hemisphere, with the largest number of patients living with TB and HIV in the Caribbean.2,3 In 2016, TB prevalence was 244 per 100 000 population, with an annual incidence rate of 188 cases/100 000 according to estimates by the World Health Organization (WHO).1 The Haitian Ministry of Health has prioritised TB testing and treatment in an effort to improve TB outcomes, particularly among patients co-infected with HIV.4
Substantial research has shown that adolescents with HIV have worse treatment outcomes than any other age group.5–8 An estimated 10 000 adolescents are currently living with HIV in Haiti.9 Adolescents are a ‘missing cohort’ in TB research; limited existing data specific to adolescents suggest that they are at increased risk for new infections and higher rates of treatment failure.10–13 These findings underscore the need for adolescent-specific research on TB prevalence and treatment outcomes, particularly those co-infected with HIV.
The goal of this analysis was to describe the TB care cascade among adolescents with microbiologically confirmed TB and determine the proportion who successfully completed anti-tuberculosis treatment. We also evaluated outcomes, stratified by HIV status, among adolescents who initiated anti-tuberculosis treatment at GHESKIO (Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections) in Port-au-Prince, Haiti, from 2011 to 2014.
METHODS
Study population and setting
We conducted a retrospective cohort study using routinely collected clinic data of all adolescents and young adults aged 10–24 years with microbiologically confirmed TB who received care at GHESKIO between January 2011 and October 2014. The study population included individuals who had microbiologically confirmed TB defined as either a positive acid-fast bacilli (AFB) smear, positive mycobacterial culture, and/or positive Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) and documented HIV testing results. Exclusion criteria included any patients without microbiologically confirmed TB disease. The study was carried out at GHESKIO, one of the first clinics in the world to provide integrated HIV and TB care. Each year, GHESKIO treats approximately 2500 new cases of TB and provides over 20 000 patients with HIV services. GHESKIO's laboratory is the national reference laboratory for mycobacterial culture.
Tuberculosis and HIV services at GHESKIO
Tuberculosis diagnosis and treatment services were provided according to the Haitian National TB Programme,4 WHO guidelines,14 and the American Thoracic Society (ATS).15 New patients presenting to GHESKIO were screened for TB using a symptom-based checklist adapted from the WHO,14 which included cough ⩾2 weeks, fever, weight loss, night sweats, hemoptysis and adenopathy. Individuals with cough ⩾2 weeks were referred for same-day clinical evaluation and TB diagnostic testing which included a physical examination and TB history, same-day chest X-ray, and three sputum tests: 1) smear microscopy for AFB on a same-day spot specimen, 2) an Xpert test on a next-day early morning specimen, and 3) a second AFB smear on a next-day spot specimen. Adolescents who presented with TB symptoms started treatment empirically on the same day, before the results of diagnostic testing became available. Treatment initiation based on clinical suspicion followed ATS and Haitian national guidelines, and has been described previously in TB studies from Haiti.15,16 Culture for Mycobacterium tuberculosis was performed for patients with rifampin (R, RMP) resistance on Xpert, and at the physician's discretion for patients with no Xpert results. Xpert testing was introduced as of 2012 and integrated as a first-line anti-tuberculosis test. Spot sputum specimens were stained using the Ziehl-Neelsen method and examined using microscopy for the presence of AFB. The Xpert test was performed directly on clinical samples without prior extraction, according to the manufacturer's instructions. Culture for M. tuberculosis was performed using the BACTEC™ MGIT™ 960 system (BD, Franklin Lakes, NJ, USA).
Patients with first-time active TB were treated with daily RMP, isoniazid (H), ethambutol (E), and pyrazinamide (Z) for 2 months, followed by daily RH for 4 months.17 Patients who were treated for TB due to recurrence or treatment failure with drug-susceptible M. tuberculosis received streptomycin plus RHEZ for 3 months, followed by RHE for 5 months. Patients with drug-resistant TB were treated according to WHO guidelines.17,18
All TB and HIV care for patients aged 10–24 years was provided in the GHESKIO Adolescent Clinic, a dedicated adolescent clinic providing youth-friendly services according to WHO guidelines.19,20 Patients with TB were scheduled for physician visits and medication pick-ups twice per month during the first 2 months of anti-tuberculosis treatment, and monthly visits during the last 4 months of treatment. Follow-up smear microscopy was performed at the end of 2, 5 and 6 months of treatment for new TB cases.
Patients co-infected with HIV all qualified for antiretroviral therapy (ART) due to coinfection with active TB.21 First-line treatment included efavirenz, tenofovir, and lamivudine, and second-line regimens included lopinavir/ritonavir or atazanavir/ritonavir.21 Retention in care and adherence to treatment were encouraged by social support programs, which included HIV-infected peer educators, transportation vouchers and tracing patients who missed clinic appointments by telephone and home visits.
Clinical measurements and tuberculosis care continuum outcomes
At the time of TB diagnosis, demographic measurements included sex, age, residence zone and education level. Clinical measurements included weight, pulmonary or extra-pulmonary TB, HIV status and CD4 cell count at the time of TB diagnosis. Dates for TB diagnosis, treatment initiation and clinic visits as well as HIV testing, ART initiation and clinic visits were also recorded.
Steps in the TB care continuum were as follows: 1) the diagnosis of bacteriologically confirmed TB as defined using at least one positive smear, culture, or Xpert test; 2) TB treatment initiation as defined by recorded pharmacy distribution of an anti-tuberculosis drug regimen; 3) retention in TB care at 2 months as defined by a clinic visit within 1–3 months of date of TB treatment initiation; and 4) TB treatment success, which included treatment completion (defined as completed treatment without evidence of failure, but no negative sputum test available) or cure (defined as having a negative smear at 6-month follow-up tests). Treatment failure was defined as a smear-positive sputum test 5 months from initiating treatment or later; loss to follow-up (LTFU) was defined as treatment interrupted by 2 consecutive months or no clinic visit after 5 months from TB diagnosis; and death as ascertained in the electronic medical records (EMR).
Outcomes were compared by HIV status, age group (younger adolescents aged 10–15, older adolescents aged 16–19, and young adults aged 20–24 years), and delay in TB treatment initiation (initiation ⩾1 day after TB diagnosis). Patients defined as HIV-co-infected had a positive HIV test before or at TB diagnosis.
Statistical analysis
Data were abstracted from the EMR and TB registers and entered into a central electronic database, de-identified and converted to a Microsoft Excel data set (MicroSoft, Redmond, WA, USA). Among adolescents diagnosed with TB, we measured the number and proportion who achieved each step of the TB care cascade. Among adolescents who started anti-tuberculosis treatment, we also measured the number and proportion in each TB treatment outcome category, and stratified by age group, HIV status and timing of TB treatment initiation. Differences between treatment success and all other treatment outcomes were assessed using the χ2 test. We assessed the binary outcome, attrition from TB care, in two multivariable logistic regressions: 1) between TB diagnosis and TB treatment initiation, given TB diagnosis; and 2) between TB treatment initiation and TB treatment success, given treatment initiation. Attrition was defined as LTFU, treatment failure or death. We calculated unadjusted and adjusted odds ratios (ORs) for attrition from TB care to evaluate risk factors for attrition. Age, sex, residence, education, timing of treatment initiation, and HIV status at TB diagnosis were included in adjusted models based on their previous association with TB treatment outcomes.11,22 Variables were treated categorically. Weight was excluded due to a high proportion of missing data and our hypothesis that weight may not be missing at random. Statistical analyses were conducted using Stata, v 14 (Stata Corp College Station, TX, USA).
Ethics
This analysis was approved by the Institutional Review Board at Weill Cornell Medicine, New York, NY, USA, and the Ethics Board at GHESKIO, Port-au-Prince, Haiti.
RESULTS
Patient characteristics
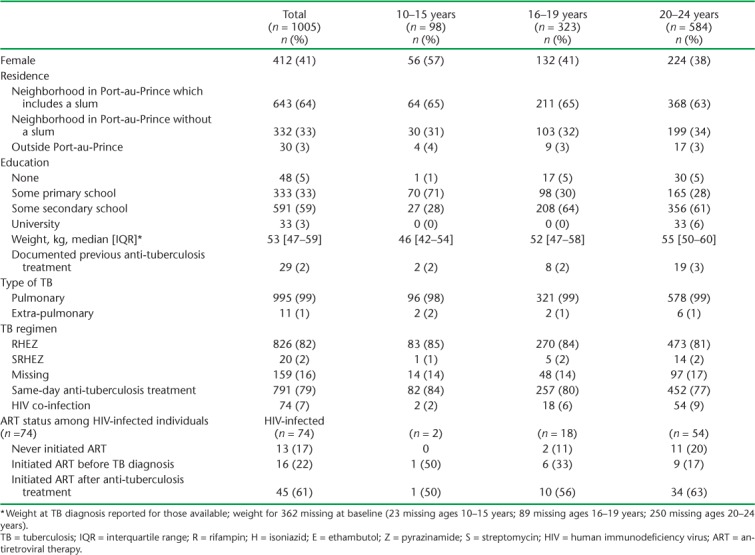
A total of 1005 individuals were diagnosed with bacteriologically confirmed TB and tested for HIV. Seventy-four patients (7%) were co-infected with HIV at the time of TB diagnosis. The proportion of patients co-infected with HIV increased with age: 2/98 (2%) among young adolescents aged 10–15 years, 18/323 (6%) among older adolescents aged 16–19 years, and 54/584 (9%) among young adults aged 20–24 years (Table 1).
TABLE 1.
Baseline characteristics of 1005 adolescents and young adults with microbiologically confirmed TB by age group
TB care continuum outcomes
The Figure illustrates the TB care continuum stratified by HIV status. Among 1005 patients diagnosed with active TB, 846 (84%) started anti-tuberculosis treatment, with a mean time to initiation of 2 days. Of those who initiated treatment, 722 (72% of the total cohort and 85% of those who initiated anti-tuberculosis treatment) were retained at 2 months after starting anti-tuberculosis treatment, and 662 (66% of the total cohort, 78% of those who started anti-tuberculosis treatment, and 92% of those retained at 2 months) had successful TB outcomes.
FIGURE.
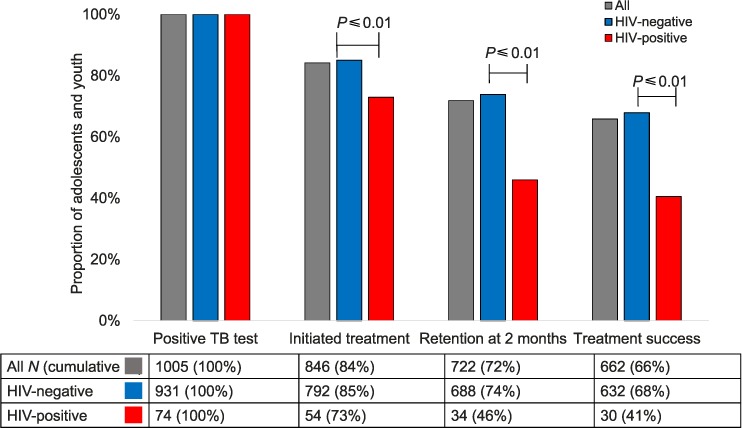
TB treatment continuum among adolescents and young adults aged 10–24 years with microbiologically confirmed TB in Haiti. TB = tuberculosis; HIV = human immunodeficiency virus.
Outcomes were poorer in HIV-positive patients than in HIV-negative patients at every step in the TB care continuum: 73% (54/74) vs. 85% (792/931) started anti-tuberculosis treatment (P < 0.01); 46% (34/74) vs. 74% (688/931) were retained at 2 months (P < 0.01); and 41% (30/74) vs. 68% (632/931) had successful treatment outcomes (P < 0.01) (Figure). Among the 54 HIV co-infected patients who did start anti-tuberculosis treatment, 5 (9%) were lost to follow-up within 2 months of initiating, 1 (1.8%) died, and 1 (1.8%) transferred care to another clinic.
TB treatment outcomes by HIV status, age group, and timing of treatment
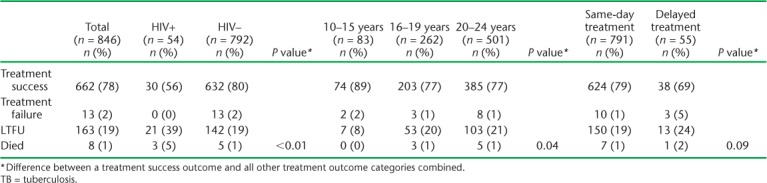
Table 2 gives TB treatment outcomes for a subset of the total study population—the 846 patients who initiated anti-tuberculosis treatment. A total of 78% (662/846) had successful TB outcomes, 2% (13/846) failed treatment, 19% (163/846) were lost to follow-up and 1% (8/846) died.
TABLE 2.
TB treatment outcomes for 846 patients with microbiologically confirmed TB who started treatment
Among those who started treatment, 6% (54/846) were HIV-positive and 94% (792/846) were HIV-negative. Among the HIV-TB co-infected patients who started anti-tuberculosis treatment (n = 54), 27 had initiated TB treatment before ART, 10 initiated treatment within 3 months after starting ART, 10 initiated treatment >3 months after starting ART, and 7 were lost to care before initiating ART.
A higher proportion of younger adolescents had successful TB treatment outcomes than older adolescents and young adults: respectively 89% (74/83), 77% (203/262), and 77% (385/501) were cured or completed treatment by age group (P = 0.04). The worst treatment outcomes occurred among HIV co-infected patients aged 16–24 years. Of these 72 patients, 53 (74%) initiated anti-tuberculosis treatment, and 39 (54%) had successful TB outcomes.
Among individuals who started anti-tuberculosis treatment, 93% (791/846) initiated medication on the same day they presented with symptoms based on clinical suspicion and before the results of testing that ultimately confirmed TB diagnosis became available. Among those who did not start the same day as presentation, the median time to initiation was 12 days (interquartile range 7–19). A higher proportion of patients who received same-day treatment than those who initiated treatment after the day of presentation had successful TB treatment outcomes (624/791, 79% vs. 38/55, 69%; P = 0.09) (Table 2).
Factors associated with attrition across the TB care continuum
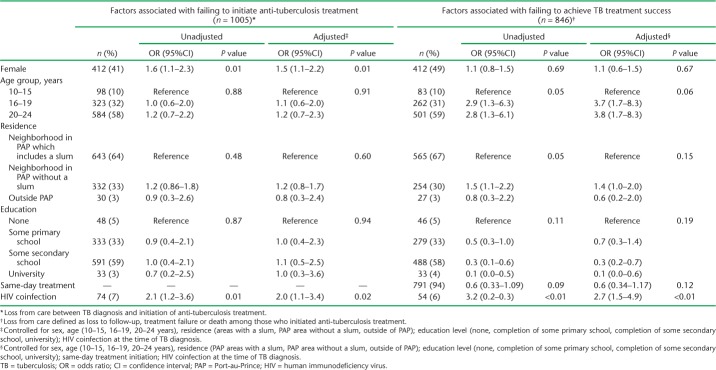
In multivariable analysis, females were less likely than males to initiate anti-tuberculosis treatment (adjusted OR [aOR] 1.5, 95% confidence interval [CI] 1.1–2.2); however, there were no sex differences in attrition after treatment initiation. Older adolescents and young adults were more likely than younger adolescents (age 10–15 years) to be lost from care after treatment initiation (respectively aOR 3.7, 95%CI 1.7–8.3 and aOR 3.8, 95%CI 1.7–8.3). HIV-infected patients had higher attrition than non-HIV-infected patients between both diagnosis and initiation and between initiation and treatment success (aOR 2.0, 95%CI 1.1–3.4 and aOR 2.7, 95%CI 1.5–4.9, respectively) (Table 3).
TABLE 3.
Factors associated with failing to initiate anti-tuberculosis treatment and failing to achieve TB treatment success
DISCUSSION
This analysis identifies critical gaps in the TB care continuum among adolescents and young adults that lead to suboptimal treatment outcomes. The analysis also underscores the increased vulnerability of HIV-TB co-infected adolescents. Only 66% of all adolescents diagnosed with bacteriologically confirmed TB and 41% of adolescents co-infected with HIV achieved TB completion or cure. TB treatment outcomes were significantly worse among older adolescents (aged 16–24 years) and among patients co-infected with HIV.
Our results indicate that adolescents who start treatment have outcomes similar to those of adults on anti-tuberculosis treatment in Haiti.22–24 This is encouraging, considering that adolescents have worse treatment outcomes for similarly stigmatised diseases such as HIV.25,26 However, our study also includes data on the number of adolescents lost between TB testing and initiating anti-tuberculosis treatment, part of the care cascade that is rarely captured,24,27 and shows that the greatest loss (16%) of patients occurred between diagnosis and treatment initiation. Interventions to increase treatment initiation among adolescents could improve outcomes.
Our results are similar to studies that report poor outcomes among adolescents with TB in other countries. In a study of 878 adolescents and adults in Botswana, adolescents aged 10–19 years were three times more likely to be lost from TB care than adults. Furthermore, HIV-infected patients in this study were twice as likely to be lost to care.11 It should be noted that GHESKIO provides extensive services to retain patients, including peer support groups, transport vouchers and patient tracking. These services could be one of the reasons why adolescent treatment outcomes are similar to those of adults despite the exacerbated challenges faced by adolescents. These services may not be routinely available in resource-limited settings.
Reasons for poor outcomes are likely multifactorial. Adolescence is an inherently high-risk period in a young person's life, when decision-making abilities and sense of personal risk are not fully developed.28–30 Adolescents lack financial autonomy and problem solving and long-term planning skills.31,32 Other reasons include external factors, including poverty and an unstable home life.33–35 Adolescents who are co-infected with HIV face even greater challenges of adhering to complex concurrent treatment regimens, drug side effects, and the compounded stigma of HIV and TB co-infection which may impact their coping skills and result in poor retention and adherence.
Interventions are needed to engage adolescents early in the TB treatment continuum. Same-day TB treatment initiation is one feasible clinic-based intervention, which, in this study, was associated with a 10% increase in successful outcomes. Same-day TB treatment initiation can be based on clinical guidelines while waiting for diagnostic testing results, or be based on Xpert testing, which processes same-day results. While same-day Xpert testing is not widely available, clinical diagnoses using chest X-ray may be feasible to evaluate symptoms and enable same-day treatment initiation. Programs should provide ongoing training for providers to minimise overdiagnosis of TB. Research on same-day ART initiation among HIV-infected adults has been shown to increase retention and viral suppression and could translate to better treatment outcomes in adolescent patients with TB.36
The study had several limitations. First, reasons for LTFU are not included, as they were not routinely documented in the EMR. We have data on documented death, but the number of undocumented deaths is unknown and is likely to be underreported. Similarly, outcomes of undocumented patient transfers are unknown, as Haiti does not have a national EMR to track patients who seek care elsewhere. This could cause an overestimation of the proportion of patients lost from care in our study. Finally, patients with a negative first sputum sample who did not return for testing of a second sputum sample, but were in fact TB-infected, would not have been included in our study. This may have resulted in selection bias and falsely low estimates of patients lost before initiating treatment.
CONCLUSION
Our study is one of few to report treatment outcomes among adolescents with bacteriologically confirmed TB disease, and emphasises the vulnerability of TB-infected adolescents, particularly those co-infected with HIV. Same-day TB treatment initiation is helpful, but not sufficient. Enhanced clinical support services, and external social and community support for adolescents are needed to optimise treatment outcomes and prevent further transmission of both HIV and TB.37,38
Acknowledgments
Funding was gratefully received from the following: Fogarty International Center Grant# D43TW010062, Grant# K24AI098627, MAC AIDS Foundation.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization Global tuberculosis control, 2017. Geneva, Switzerland: WHO; 2017. WHO/HTM/TB/2017.23. [Google Scholar]
- 2.Joint United National Programme on HIV/AIDS (UNAIDS) ‘15 by 15’ a global target achieved. Geneva, Switzerland: UNAIDS; 2015. http://www.unaids.org/en/resources/documents/2015/15_by_15_a_global_target_achieved Accessed July 2018. [Google Scholar]
- 3.Andrews B E. Prevalence and correlates of HIV testing among Caribbean youth. Int J STD AIDS. 2011;22:722–7226. doi: 10.1258/ijsa.2011.011088. [DOI] [PubMed] [Google Scholar]
- 4.Haitian Ministry of Health and Population National Programme for Tuberculosis Control, 2009–2015. Port-au-Prince, Haiti: MoHP; 2009. [Google Scholar]
- 5.Lee S, Hazra R. Achieving 90-90-90 in paediatric HIV: adolescence as the touchstone for transition success. J Int AIDS Soc. 2015;18(Suppl 6):S20257. doi: 10.7448/IAS.18.7.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idele P, Gillespie A, Porth T et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 7.Kim S H, Gerver S M, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28:1945–1956. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamb M.R, Fayorsey R, Nuwagaba-Biribonwoha et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28:559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations Children's Fund Children and AIDS. Statistical Update. New York, NY, USA: UNICEF; 2015. [Google Scholar]
- 10.McKenna S B, Theunissen M, Nachman S, Frick M, Furin J. The missing cohort: adolescents in tuberculosis research. Int J Tuberc Lung Dis; 45th Union World Conference on Lung Health; Barcelona, Spain. 28 October–1 November 2014; 2014. p. S206. [Abstract no OAP-207-30] [Google Scholar]
- 11.Enane L A, Lowenthal E D, Arscott-Mills T et al. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis. 2016;20:1320–1325. doi: 10.5588/ijtld.16.0060. [DOI] [PubMed] [Google Scholar]
- 12.Isaakidis P, Paryani R, Khan S et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLOS ONE. 2013;8:e68869. doi: 10.1371/journal.pone.0068869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon J A, McKenna L, Shah T, Kampann B. Recent developments and future opportunities in the treatment of tuberculosis in children. Clin Infect Dis. 2015;61(Suppl 3):S188–S99. doi: 10.1093/cid/civ582. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Systematic screening for active tuberculosis: principles and recommendations. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.04. [PubMed] [Google Scholar]
- 15.Blumberg H M, Burman W J, Chaisson R E et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald D W, Desvarieux M, Severe P et al. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet. 2000;356:1470–1474. doi: 10.1016/S0140-6736(00)02870-1. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.6. [PubMed] [Google Scholar]
- 18.World Health Organization Treatment of tuberculosis guidelines. 4th ed. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 19.Reif L K, Bertrand R, Benedict C et al. Impact of a youth-friendly HIV clinic: 10 years of adolescent outcomes in Port-au-Prince, Haiti. J Int AIDS Soc. 2016;19:20859. doi: 10.7448/IAS.19.1.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Making health services adolescent friendly: developing national quality standards for adolescent friendly health services. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 21.World Health Organization Rapid advice: antriretroviral therapy for HIV infection in adults and adolescents. Geneve, Switzerland: WHO; 2009. [Google Scholar]
- 22.McGreevy J, Jean Juste M A, Severe P et al. Outcomes of HIV-infected patients treated for recurrent tuberculosis with the standard retreatment regimen. Int J Tuberc Lung Dis. 2012;16:841–845. doi: 10.5588/ijtld.11.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Global tuberculosis report, 2016. Haiti Tuberculosis Profile. Geneva, Switzerland: WHO; 2016. WHO/HTM/TB/2016.13. [Google Scholar]
- 24.Desvarieux M, Hyppolite P R, Johnson W D, Pape J W. A novel approach to directly observed therapy for tuberculosis in an HIV-endemic area. Am J Public Health. 2001;91:138–141. doi: 10.2105/ajph.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations Children's Fund Children and AIDS: statistical update. UNAIDS 2017 estimates. UNICEF; 2017. https://data.unicef.org/wp-content/uploads/2017/11/HIVAIDS-Statistical-Update-2017.pdf Accessed August 2018. [Google Scholar]
- 26.United Nations Children's Fund Current status + progress: turning the tide against AIDS will require more concentrated focus on adolescents and young people. Geneva Switzerland: WHO; 2017. https://data.unicef.org/topic/hivaids/adolescents-young-people/# Accessed July 2018. [Google Scholar]
- 27.Mauch V, Weil D, Munim A et al. Structure and management of tuberculosis control programs in fragile states—Afghanistan, DR Congo, Haiti, Somalia. Health Policy. 2010;96:118–127. doi: 10.1016/j.healthpol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Reisner S L, Mimiaga M J, Skeer M, Perkovich B, Johnson C V, Safren S A. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17:14–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Machado J K, Sant-Anna M J, Coates V, Almeida F J, Berezin E N, Omar H A. Brazilian adolescents infected by HIV: epidemiologic characteristics and adherence to treatment. ScientificWorldJournal. 2009;9:1273–1285. doi: 10.1100/tsw.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy D A, Moscicki A B, Vermund S H, Muenz L R. Psychological distress among HIV(+) adolescents in the REACH study: effects of life stress, social support, and coping. The Adolescent Medicine HIV/AIDS Research Network. J Adolesc Health. 2000;27:391–398. doi: 10.1016/s1054-139x(00)00158-0. [DOI] [PubMed] [Google Scholar]
- 31.Tanney M R, Naar-King S, MacDonnel K. Depression and stigma in high-risk youth living with HIV: a multi-site study. J Pediatr Health Care. 2012;26:300–305. doi: 10.1016/j.pedhc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutumba M, Musiime V, Lepkwoski J M et al. Examining the relationship between psychological distress and adherence to anti-retroviral therapy among Ugandan adolescents living with HIV. AIDS Care. 2016;28:807–815. doi: 10.1080/09540121.2015.1131966. [DOI] [PubMed] [Google Scholar]
- 33.Kalichman S C, Kalichman M O, Cherry C. Forget about forgetting: structural barriers and severe non-adherence to antiretroviral therapy. AIDS Care. 2017;29:418–422. doi: 10.1080/09540121.2016.1220478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haynes R B, Ackloo E, Sahota N, McDonald H P, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD000011.pub3. CD000011. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwlaat R, Wilczynski N, Navarro T et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11) doi: 10.1002/14651858.CD000011.pub4. CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig S P, Dorvil N, Devieux J G et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLOS Med. 2017;14:e1002357. doi: 10.1371/journal.pmed.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.United Nations Children's Fund HIV and adolescents: guidance for HIV testing, and counseling, and care for adoelscents living with HIV. Geneva, Switzerald: WHO; 2015. [Google Scholar]
- 38.US Agency for International Development, President's Emergency Plan For AIDS Relief Best practices for adolescent-and-youth-friendly HIV services. A compendium of selected projects in PEPFAR-supported countries. Washington DC, USA: USAID, PEPFAR, Measure Evaluation; 2017. [Google Scholar]