Abstract
Setting: Manicaland Province, Zimbabwe.
Objectives: To compare the utilisation and results of deploying Xpert® MTB/RIF in 13 (one provincial, six district and six rural) hospitals between January and June 2016, when Xpert was recommended only for those with presumptive multidrug-resistant tuberculosis (MDR-TB) and coinfection with human immunodeficiency virus (HIV), and between January and June 2017, when Xpert was recommended for all presumptive TB patients.
Design: This was a cross-sectional study.
Results: Xpert assays averaged 759 monthly in 2016 and 1430 monthly in 2017 (88% increase). Utilisation of Xpert averaged 22% monthly in 2016 and 42% in 2017 (88% increase). In 2017, utilisation of Xpert was significantly higher in provincial (82%) than in district (51%) and rural (26%) hospitals (P < 0.001). The proportion of successful assays that detected TB decreased significantly from 13% in 2016 to 7% in 2017 (a 46% decrease, P < 0.001); this phenomenon was observed in all types of hospital. The proportion of persons detected with rifampicin-resistant TB was similar between hospitals (4% in 2016 and 3% in 2017). The proportion of registered TB cases with bacteriological confirmation increased from 48% in 2016 to 53% in 2017 (P = 0.04).
Conclusion: Xpert use in all presumptive TB patients led to a significant increase in assay numbers and utilisation of Xpert instruments, resulting in more bacteriological confirmation of cases.
Keywords: TB, Xpert® MTB/RIF, SORT IT, rifampicin-resistant TB
Abstract
Contexte : Province de Manicaland, Zimbabwe.
Objectif : Comparer l'utilisation et les résultats du déploiement de l'Xpert® MTB/RIF dans 13 hôpitaux (1 provincial, 6 de district et 6 ruraux) entre janvier et juin 2016, quand l'Xpert a été recommandé seulement pour les patients ayant une présomption de la tuberculose (TB) multirésistante et une coinfection par le virus de l'immunodéficience humaine, et de janvier à juin 2017, quand l'Xpert a été recommandé pour tous les patients présumés TB.
Schéma : Etude transversale.
Résultats : Le nombre moyen de tests Xpert a été de 759 par mois en 2016 et de 1430 par mois en 2017 (augmentation de 88%). L'utilisation de l'instrument a été d'environ 22% par mois en 2016 et de 42% en 2017 (augmentation de 88%). En 2017, l'utilisation de l'instrument a été significativement plus élevée dans l'hôpital provincial (82%) comparé aux hôpitaux de district et ruraux (51% contre 26% ; P < 0,001). La proportion de succès des tests qui ont détecté une TB a significativement diminué de 13% en 2016 à 7% en 2017 (diminution de 46% ; P < 0,001) ; ceci a été observé dans tous les types d'hôpitaux. Les proportions de TB résistantes à la rifampicine ont été similaires entre les hôpitaux (4% en 2016 et 3% en 2017). La proportion des cas de TB enregistrés avec une confirmation bactériologique a augmenté de 48% en 2016 à 53% en 2017 (P = 0,04).
Conclusion : L'utilisation du Xpert pour tous les patients présumés TB, les nombres de tests et l'utilisation de l'instrument Xpert ont significativement augmenté, aboutissant à davantage de confirmation bactériologique des cas.
Abstract
Marco de referencia: La provincia de Manicaland, en Zimbabwe.
Objetivos: Comparar la utilización de los dispositivos Xpert® MTB/RIF y los resultados de su despliegue en 13 hospitales (1 de provincia, 6 distritales y 6 rurales) durante dos períodos: de enero a junio del 2016, cuando se recomendaba la prueba Xpert solo en los casos de presunción de tuberculosis (TB) multirresistente y coinfección por el virus de la inmunodeficiencia humana, y de enero a junio del 2017, cuando se recomendaba la prueba Xpert en todos los pacientes con presunción de TB.
Objetivos: Fue este un estudio transversal.
Resultados: El promedio mensual de pruebas Xpert fue 759 en el 2016 y 1430 en el 2017 (un aumento del 88%). El porcentaje de utilización de los dispositivos Xpert fue en promedio 22% mensual en el 2016 y 42% en el 2017 (un aumento del 88%). En el 2017, la utilización de los dispositivos Xpert fue significativamente mayor en los hospitales de provincia (82%) comparados con los hospitales distritales y los rurales (51% contra 26%; P < 0,001). La proporción de análisis eficaces que detectaban la TB disminuyó de manera notable de 13% en el 2016 a 7% en el 2017 (una disminución del 46%; P < 0,001); esta situación se observó en todos los tipos de hospitales. La proporción de casos detectados de TB con resistencia a rifampicina fue equivalente en todos los hospitales (4% en el 2016 y 3% en el 2017). La proporción de casos de TB con confirmación bacteriológica registrados aumentó del 48% en el 2016 al 53% en el 2017 (P = 0,04).
Conclusión: Al utilizar la prueba Xpert en todos los pacientes con presunción clínica de TB se aumentó considerablemente el número de pruebas y la utilización de los dispositivos Xpert, lo cual ha dado lugar a una mayor proporción de casos con confirmación bacteriológica.
Zimbabwe has one of the world's highest tuberculosis (TB) burdens, with an estimated TB incidence of 208 cases per 100 000 population and an associated TB-HIV (human immunodeficiency virus) coinfection rate of 67% in 2016.1 For many years, the diagnosis of pulmonary TB has relied on sputum smear microscopy, although the test has poor sensitivity and does not detect drug-resistant TB.2 The introduction of the Xpert® MTB/RIF assay (Cepheid Inc, Sunnyvale, CA, USA) is a game changer in this regard: Xpert is a fully automated, commercially available nucleic-acid amplification test for sputum and other specimens requiring minimal laboratory expertise, with results available within 2 h.3 Sensitivity and specificity for the diagnosis of Mycobacterium tuberculosis are high, and the assay also detects rifampicin (RMP) resistant TB (RR-TB).
Early studies showed that the diagnosis of TB using Xpert was feasible and accurate,4 prompting the World Health Organization (WHO) in 2010 to strongly recommend its widespread use; priority was initially given to those with presumptive multidrug-resistant TB (MDR-TB, defined as TB resistant to isoniazid and RMP) and those co-infected with HIV.5 Based on additional worldwide field experience, the WHO made a futher recommendation in 2013 that Xpert be considered as the initial diagnostic test for all people requiring investigations for TB.6
Zimbabwe has been scaling up implementation of Xpert since 2011; by the end of 2016 there were 120 sites in the country using this technology. At the time of the initial deployment of the instruments, national guidelines were in line with those first recommended by WHO in 2010. In late 2016, the national guidelines were changed to reflect the more recent WHO guidance that Xpert be used as the initial diagnostic test for all patients with presumptive TB.
In the context of a busy African district hospital, one four-module Xpert instrument is capable of performing 12 assays per day (based on an 8 h working day),7 and if there are no technical problems, 264 assays could potentially be performed per month. The Xpert assay, with higher diagnostic sensitivity, should allow a higher proportion of TB patients to be bacteriologically confirmed and this might encourage more patients with presumptive TB to come forward for diagnostic investigation, potentially improving TB control in the community. However, there are several challenges with Xpert implementation, of which the transport of specimens from peripheral sites to laboratories housing the Xpert instruments is one.8 Since the first deployment of Xpert instruments in Zimbabwe, there has been no formal evaluation of their utilisation and results at the provincial level, nor has there been any investigation as to whether the number of assays performed had increased following the adoption of the new national guidelines to test all patients with presumptive TB.
Manicaland Province, Eastern Zimbabwe, has seven districts, all of which have deployed Xpert technology since 2014. This provides us with an opportunity to evaluate the utilisation of the Xpert instruments when Xpert use was restricted to individuals with presumptive MDR-TB or with TB-HIV coinfection, and compare this with its current and broader use for all patients with presumptive TB. This will provide useful information for the National TB Programme (NTP) in scaling up and further decentralising Xpert technology in the country.
The aim of the present study was to evaluate the utilisation and results of deploying Xpert instruments in Manicaland Province, Zimbabwe, in relation to the change of national guidelines in late 2016. The two time periods for the study were from January to June 2016, when Xpert was recommended only for those with presumptive MDR-TB and HIV coinfection, and from January to June 2017, when Xpert was recommended for all patients with presumptive TB. Specific objectives were to determine, in each of the two periods: 1) the number of assays and instrument utilisation rates per month, 2) the results of Xpert assays, and 3) the numbers and proportions of registered TB cases who were bacteriologically confirmed.
METHODS
Study design
This was a cross-sectional study of Xpert assays using secondary data.
Setting
General setting
Zimbabwe is a land-locked country in the southern part of Africa. It has a population of 13 million and a per capita gross domestic product (GDP) of US$924, compared to US$1588 for sub-Saharan Africa as a whole.9,10 The country is divided into 10 administrative provinces, covering 63 health districts.
Zimbabwe National Tuberculosis Control Programme
National TB control efforts are coordinated by the Zimbabwe NTP. The programme is represented at the national level by a central unit and at the provincial and district levels by provincial and district TB coordinators. The diagnosis and treatment of TB are offered free of charge at public health facilities, and the country is fully compliant with international TB control policies and WHO TB guidelines.11–13
Study sites
Manicaland Province is made up of seven districts. Within these districts, there is a provincial hospital (which also serves as a district hospital), six urban district hospitals and six rural mission hospitals, each of which has had a functioning 4-module Xpert instrument in place since before 2016. The management of patients with presumptive TB is standardised as follows: individuals with presumptive TB submit two sputum specimens to one of the health facilities in the district, and these are transported by a transport courier system to the health facility laboratories for diagnosis, including those with an Xpert instrument. At the 13 hospital laboratories with Xpert instruments, patients initially underwent both smear microscopy and an Xpert assay. However, in late 2016, the use of smear microscopy for initial diagnosis was discontinued and only Xpert has been used since then, with one sputum specimen being tested per patient. To monitor the process, all patient and sputum specimen data are recorded in Xpert registers.
Study samples
The study included assays from 13 Xpert instruments in Manicaland Province, evaluated from January to June 2016, when Xpert was recommended only for those with presumptive MDR-TB and HIV coinfection, and from January to June 2017, when Xpert was recommended for all patients with presumptive TB.
Data variables, data sources and data collection
Data variables included the site of the Xpert instrument (provincial, district or rural hospital), year, month, number of assays per month, instrument utilisation per month, successful assays, assays detecting M. tuberculosis (MTB), drug-susceptible MTB, RR-TB or indeterminate status and unsuccessful assays, registered TB cases and bacteriologically confirmed cases. Monthly utilisation of each instrument was determined by the number of assays performed per month compared with the expected target of 264 assays per month. Sources of data were the Xpert registers in each of the hospital laboratories. Data were collected into a structured proforma between August 2017 and March 2018.
Analysis and statistics
Data were double-entered from the forms into EpiData (v 3.1 for entry and v 2.2.2.182 for analysis; EpiData Association, Odense, Denmark). Numbers and frequencies of assays and utilisation rates per month were described over the two time periods by type of hospital for Manicaland Province; Xpert results were described for the two 6-month periods by province and type of hospital. All comparisons were made using the χ2 test; the level of significance was set at 5% (P < 0.05).
Ethics approval
Permission for the study protocol was obtained from the Provincial Medical Director, Manicaland, Mutare, and the NTP of Zimbabwe, Harare. Ethics approval was obtained from the Medical Research Council of Zimbabwe and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As routinely collected aggregate data were used, informed patient consent was not required and no potential harm or confidentiality issues were anticipated.
RESULTS
The number of Xpert assays performed monthly in the province during the two time periods are shown in Figure 1. In 2016, monthly assays ranged from 631 to 846, with a monthly average of 759. In 2017, monthly assays ranged from 1126 to 1771, with a monthly average of 1430 (over the 6-month period, 88% more assays were performed than in the previous year).
FIGURE 1.
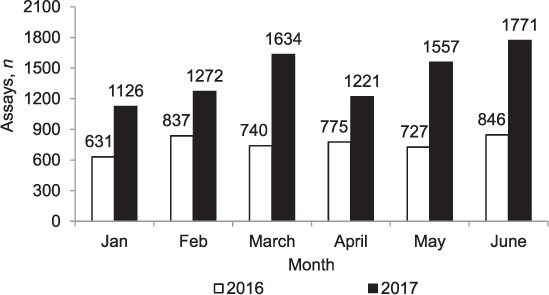
Number of Xpert® MTB/RIF assays performed each month in Manicaland Province, Zimbabwe, January–June 2016 and January–June 2017. The instrument was non-functional in two hospitals (Birchenough and Chipinge) between March and May 2016 and in two hospitals (Makoni and Bonda) between April and May 2017.
Monthly utilisation rates of Xpert in the province during the two time periods are shown in Figure 2. In 2016, utilisation rates varied from 18.4% to 24.7%, with a monthly average of 22.1%. In 2017, utilisation rates varied from 32.8% to 51.6%, with a monthly average of 41.7% (over the 6-month period, utilisation rates were 88% higher than in the previous year).
FIGURE 2.
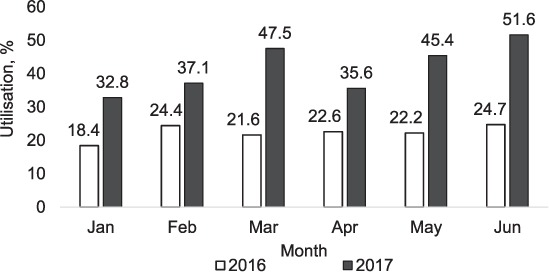
Utilisation rates* of Xpert® MTB/RIF instruments per month in Manicaland Province, Zimbabwe, January–June 2016 and January–June 2017. The instrument was non-functional in two hospitals (Birchenough and Chipinge) between March and May 2016 and in two hospitals (Makoni and Bonda) between April and May 2017. *Utilisation rates were calculated by dividing the total number of assays by the total number of expected assays. The number of expected assays per instrument per month was 264, based on a four-module machine with a throughput of 12 assays/day and an average of 22 working days/month. An 8 h working day model was assumed.
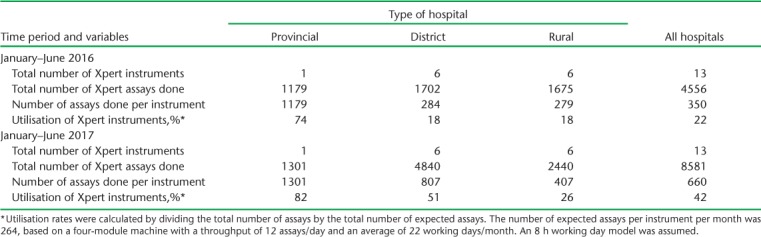
The number of assays performed and utilisation rates at the provincial, district and rural hospitals during the two time periods are shown in Table 1. Instrument numbers remained the same in the two periods. For each type of hospital, the total number of assays performed, the number of assays per instrument and utilisation rates increased in 2017 compared with 2016. In each of the two time periods, more assays and better utilisation rates were observed from provincial to district to rural hospitals, while in 2017, the utilisation rate was 82% in the provincial hospital, 51% in the district hospitals and 26% in the rural hospitals (P < 0.001).
TABLE 1.
Xpert® MTB/RIF assays performed and instrument utilisation rates at provincial, district and rural hospitals in Manicaland Province, Zimbabwe, January–June 2016 and January–June 2017
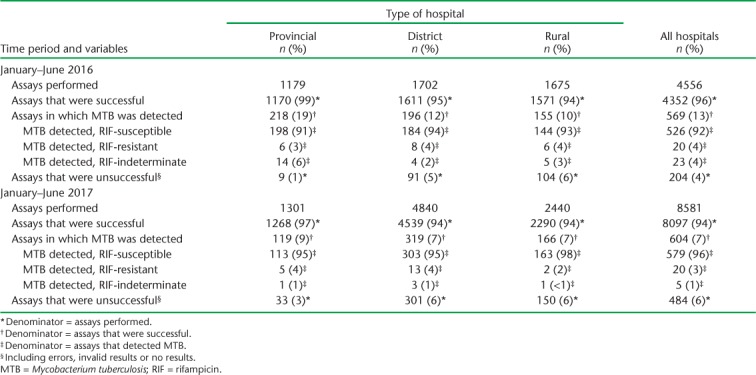
Xpert results at provincial, district and rural hospitals during the two time periods are shown in Table 2. The proportion of successful assays was higher in the provincial than in the district and rural hospitals, and this was mirrored by a higher proportion of unsuccessful assays in the latter. In 2017, there were significantly more unsuccessful assays in the provincial hospital than in 2016 (P < 0.001), while there was little difference in district and rural hospitals in this regard. The proportion of successful assays that detected MTB decreased significantly overall by 46% in 2017 compared with 2016 (P < 0.001); significant decreases were observed in each of the three different types of hospital (P < 0.01). The proportions of patients detected with RR-TB were similar in each time period (4% in 2016 and 3% in 2017).
TABLE 2.
Xpert® MTB/RIF assays and results at provincial, district and rural hospitals in Manicaland Province, Zimbabwe, January–June 2016 and January–June 2017
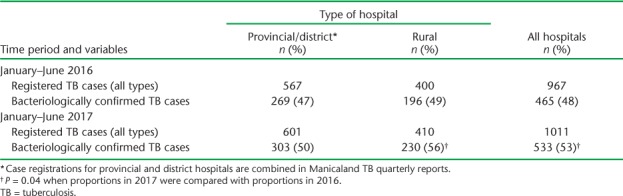
Compared with 2016, there was a slight increase in TB cases in 2017 in all types of hospital, and a significant increase in numbers and proportions with bacteriologically confirmed TB in rural hospitals and in all hospitals combined (Table 3).
TABLE 3.
Registered TB cases (all types) and bacteriologically confirmed TB cases at provincial, district and rural hospitals in Manicaland Province, Zimbabwe, January–June 2016 and January–June 2017
DISCUSSION
This is the first study from Zimbabwe to assess the use of Xpert before and after the WHO recommendation that the assay be used as the first investigation in all patients with presumptive TB. There were some interesting findings.
First, and not unexpectedly, the number of assays and the utilisation rates of the instruments all increased in 2017 compared with 2016, when Xpert was only recommended for patients with presumptive MDR-TB or those with coinfection. Despite this improvement, utilisation rates remained low, and only once, in the month of June 2017, did they exceed 50% overall for the province. We do not know the reasons for this. In 2016 and 2017, instrument malfunctions occurred in two hospitals for 1–3 months, and there may have been other problems related to unstable electricity or interrupted supplies of cartridges.14 The lowest utilisation rates in each period occurred during the month of January, when there is heavy rainfall, and this may have affected patient access to health facilities, disrupted the transport of sputum specimens to hospital laboratories and exacerbated power supply failures. Poor instrument utilisation is a problem that is not limited to Zimbabwe; they have been reported in other countries, and particularly as use of the new technology has started to be scaled up.15,16
Second, in both 2016 and 2017 there were more Xpert assays and better utilisation rates in provincial than in district hospitals, which in turn performed more assays than in rural hospitals; these differences were particularly significant in 2017. In the provincial hospital, these differences might have been due to greater numbers of patients and therefore greater demand for testing, better transport and infrastructure and more skilled human resources. In the rural hospitals, utilisation was generally poor, similar to experiences with Xpert elsewhere at the primary care level, probably related to the various programmatic challenges of health care delivery at this level of the health care system.17
Third, the proportion of unsuccessful assays (4% overall in 2016 and 6% in 2017) was no worse than in other countries.15,16,18 However, the observed increase in unsuccessful assays at the provincial hospital in Zimbabwe in 2017, and the higher proportion at the district and rural hospitals, need to be carefully monitored. Unsuccessful assays may have several reasons: one study in Swaziland found power supply issues to be the predominant challenge.15
Finally, MTB positivity rates decreased by almost half in 2017 compared with 2016. This pattern has been found in other settings when screening is expanded from high-risk patients only to the inclusion of all presumptive TB patients.19 MTB positivity rates in the province in 2017 were also quite low, at 7%, compared with reports from other countries,15–19 and this should also be monitored regularly. It is reassuring to note that the proportion of RR-TB remained low, and was similar in the two study periods, and that the overall proportion of registered TB cases with bacteriological confirmation increased.
The strengths of the study were that data were obtained from within the routine health services of Manicaland Province; the two 6-month comparisons were in the same calendar months, i.e., January to June, accounting as far as possible for seasonal changes; and the conduct and reporting of the study was in line with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.20 Limitations were mainly related to the fact that we did not obtain other potentially useful data to further inform the programme, such as numbers of presumptive TB patients in Manicaland Province during the two 6-month periods; more detailed reasons for poor utilisation of instruments, including their functionality, error codes and modular performance; and possible reasons for the differences between hospitals.
This study highlights three important programmatic implications. First, it is important for central, provincial and district TB coordinators to regularly monitor utilisation rates of Xpert instruments so as to evaluate their proper deployment and effective use. In hospitals where the volume of assays is at or near to full capacity, there is probably a need for an additional instrument; in hospitals where the volume of assays falls well below capacity, the reasons need to be identified and corrected. Second, it is unacceptable to have Xpert instruments non-functional for 2–3 months. Systems, including quality assurance, should be in place for hospitals to report problems as early as possible, and a sufficient budget is required to support instrument maintenance, repairs, replacements and cartridge procurement, all of which are necessary for full and continuous functionality of Xpert technology. Finally, the programme needs to monitor MTB positivity rates and check that these are appropriate and not too high or too low for what is known about the TB epidemic in the country.
In conclusion, with the introduction of new guidelines recommending Xpert use as the first diagnostic tool in patients with presumptive TB, the volume of assays and utilisation of Xpert instruments has increased significantly, in parallel with a decrease in MTB positivity rates and an increase in registered cases with bacteriologically confirmed TB. There is a clear gradation from provincial to rural hospitals, with the latter needing more attention to improve the use of this new technology. Policy implications are discussed.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Médecins Sans Frontières (MSF). The specific SORT IT programme that resulted in this publication was implemented by the Centre for Operational Research, The Union, Paris, France. Mentorship and the coordination/facilitation of this particular SORT IT workshop was provided through the Centre for Operational Research, The Union, Paris, France; the Department of Tuberculosis and HIV, The Union, Paris, France; the University of Washington, School of Public Health, Department of Global Health, Seattle, WA, USA; and AMPATH, Eldoret, Kenya.
This course was funded by the UK Department for International Development (DFID), London, UK; La Fondation Veuve Emile Metz-Tesch, Luxembourg City, Luxembourg; the United States Agency for International Development, Washington DC, USA, through Challenge TB; The Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund) and the National AIDS Council Zimbabwe, Harare, Zimbabwe. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization Global tuberculosis report, 2017. Geneva, Switzerland: WHO; 2017. WHO/HTM/TB/2017.23. [Google Scholar]
- 2.Reid M J A, Shah N S. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 3.Boehme C C, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme C C, Nicol M P, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Policy statement: automated realtime time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.4. [PubMed] [Google Scholar]
- 6.World Health Organization Xpert MTB/RIF assay for diagnosis of pulmonary and extra-pulmonary TB in adults and children. 2013. Policy update. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.16. [Google Scholar]
- 7.World Health Organization Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. Geneva, Switzerland: WHO; 2014. [PubMed] [Google Scholar]
- 8.Ministry of Health and Child Care External National Tuberculosis Control Programme Review for Zimbabwe. Harare, Zimbabwe: Ministry of Health and Child Care; 2016. [Google Scholar]
- 9.Zimbabwe National Statistics Agency Zimbabwe population census, 2012. Harare, Zimbabwe: ZIMSTAT; 2013. [Google Scholar]
- 10.World Bank Gross domestic product (GDP) per capita (current $US): data. Washington DC, USA: World Bank; 2016. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD?view=chart Accessed July 2018. [Google Scholar]
- 11.Ministry of Health and Child Care National TB guidelines. Zimbabwe National TB Control Programme. 4th ed. Harare, Zimbabwe: Ministry of Health and Child Care; 2010. [Google Scholar]
- 12.World Health Organization Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 13.World Health Organization Treatment of tuberculosis. Guidelines for treatment of drug-susceptible tuberculosis and patient care. 2017 update. Geneva, Switzerland: WHO; 2017. WHO/HTM/TB/2017.05. [Google Scholar]
- 14.Trébucq A, Enarson D A, Chiang C-Y et al. Xpert MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuberc Lung Dis. 2011;15:1567–1571. doi: 10.5588/ijtld.11.0392. [DOI] [PubMed] [Google Scholar]
- 15.Sikhondze W, Dlamini T, Khumalo D et al. Countrywide roll-out of Xpert MTB/RIF in Swaziland: the first three years of implementation. Public Health Action. 2015;5:140–146. doi: 10.5588/pha.15.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonganibeia A, Harries A D, Merilles O E A, Tarataake T, Tiira T, Kienene T. Impact of laboratory practice changes on the diagnosis of tuberculosis with the introduction of Xpert MTB/RIF in Kiribati. Hawaii J Med Public Health. 2018;77:30–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Clouse K, Page-Shipp L, Dansey H et al. Implementation of Xpert MTB/RIF for routine point-of-care diagnosis of tuberculosis at the primary care level. S Afr Med J. 2012;102:805–807. doi: 10.7196/samj.5851. [DOI] [PubMed] [Google Scholar]
- 18.Gounder A, Gounder S, Reid S A. Evaluation of the implementation of the Xpert MTB/RIF assay in Fiji. Public Health Action. 2014;4:179–183. doi: 10.5588/pha.14.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardizzoni E, Fajardo E, Saranchuk P et al. Implementing the Xpert MTB/RIF diagnostic test for tuberculosis and rifampicin resistance: outcomes and lessons learned in 18 countries. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0144656. e0144656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]


