ABSTRACT
Chikungunya fever is an emerging arbovirus infection, representing a serious public health problem. Its etiological agent is the Chikungunya virus (CHIKV). Transmission of this virus is mainly vector by mosquitoes of the genus Aedes, although transmission by blood transfusions and vertical transmission has also been reported. The disease presents high morbidity caused mainly by the arthralgia and arthritis generated. Cardiovascular and neurological manifestations have also been reported. The severity of the infection seems to be directly associated with the action of the virus, but also with the decompensation of preexisting comorbidities. Currently, there are no therapeutic products neither vaccines licensed to the infection CHIKV control, although several vaccine candidates are being evaluated and human polyvalent immunoglobulins anti-CHIKV had been tested. Antibodies can protect against the infection, but in sub-neutralizing concentrations can augment virus infection and exacerbate disease severity. So, the prevention still depends on the use of personal protection measures and vector control, which are only minimally effective.
KEYWORDS: Chikungunya virus, chikungunya virus infection, chikungunya virus pathogenesis, chikungunya fever, arboviruses
Introduction
Chikungunya virus (CHIKV), the etiological agent of chikungunya fever, was isolated for the first time on the African continent in Newala, Tanzania in 1953, from a patient with a symptomatic picture of fever and arthralgia [1]. The origin of the name chikungunya derives from the native language word ‘Makonde’, which means "walking bent" in reference to the curved posture of patients because of the strong joint pains which are a classic symptom of the disease that cause intense polyarthralgia, and is strongly associated with high disease morbidity [2,3].
CHIKV was for a long time considered to belong to the dengue group due to its similarities in symptomatology and transmission form. This is because it uses mosquitoes of the genus Aedes ssp. as vectors of transmission like other arboviruses, and specifically Aedes aegypti and Aedes albopictus. However, after sequencing and phylogenetic analysis studies showed that CHIKV belongs to the family Togaviridae, genus Alphavirus, presenting a monophyletic lineage of African origin [4] which probably evolved from the O'nyong-nyong virus (ONNV) with which it is closely related. This suggests that CHIKV has mutated throughout its evolution giving rise to distinct lineages [5–7].
Phylogenetic analyzes have identified four different CHIKV genotypes: Asian/ Caribbean (AC), West African (WA), Eastern/Central/South African (ECSA) and Indian Ocean Line (IOL), with the latter being responsible for major epidemics on islands of the Indian Ocean and Asia between 2005 and 2011 [8].
Chikungunya fever constitutes a serious public health problem due to the high degree of morbidity, sometimes becoming severe and progressing to chronic form [9]. The disease is characterized by symptoms such as fever, headache, myalgia, severe joint and muscle pain, maculopapular or petechial rashes, and at times as prolonged periods of severe pain in the joint which can be disabling [9]. The acute phase is characterized by high viremia and intense inflammatory response, which coincides with elevated levels of immune mediators and infiltration of immune cells into infected joints and adjacent tissues [10].
Autochthonous transmission of CHIKV in Brazil was detected for the first time in September 2014 in the city of Oiapoque in the State of Amapá, followed by the notification of 2,772 cases over the same year distributed across six Federative Units from three different regions of the country: Amapá and Roraima in the North, the Federal District, Mato Grosso do Sul, and Goiás in the Midwest, and Bahia in the Northeast [11].
In this review we present some recent advances on Chikungunya fever, including aspects of the structure, biology and pathogenesis of the virus, clinical manifestations, epidemiology, diagnosis and treatment of the disease. The literature search was performed using the PubMed (National Institutes of Health, Bethesda, MD, USA) and Scopus (Elsevier, Amsterdam, The Netherlands, www.scopus.com/scopus) electronic databases using the following keywords: Aboviruses, chikungunya fever, Chikungunya virus, biology and pathogenesis, Chikungunya fever epidemiology, diagnosis and treatment of chikungunya fever. Hundreds of articles were found in the researched databases and the most relevant ones were selected, which included more recent studies published in impact journals and were conducted by groups with recognized knowledge in the area.
Structure and replication of the virus
CHIKV has a single-stranded positive-RNA genome, protected by an icosahedral symmetric capsid, surrounded by a a lipid envelope containing glycoproteins and forming a spherical particle about 70 nm in diameter. The genome presents as a classic messenger RNA with a cap at the end 5’, and poly A-tail at the 3' end, with a size of approximately 11.8 kb. It contains two untranslated regions; a short one at the 5' and a long one at the 3' end with direct repetitions, thought to be associated with virus adaptation to the mosquitoes that serve as vectors for its transmission to vertebrates [12]. The genome has two open reading frames (ORFs); the 5' ORF encodes a precursor protein which after processing gives rise to four non-structural proteins (nsP1, nsP2, nsP3 and nsP4), which together form the viral replication complex. The second ORF, located in the 3' region, encodes the precursor protein which gives rise to five structural proteins after processing: capsid C, envelope glycoproteins (E1, E2, E3) and the 6 K protein [13–15].
In the vertebrate host, CHIKV enters cells by clathrin-mediated endocytosis into a vesicle formed from the plasma membrane of the host cell, followed by fusion dependent on pH variation. However, there is evidence to suggest this virus has other possible pathways of entry specific for each cell type [16]. Viral replication occurs in the cytoplasm associated with vacuoles, formed by the rearrangement of the membranes of cellular organelles. The synthesis of the viral proteins takes place in the endoplasmic reticulum, and the assembly of the virions in the cytoplasm, followed by the acquisition of a lipid bilayer envelope obtained at the time of budding of the viral particles through the plasma membrane of the host cell [17].
The acid environment of the endosome causes conformational changes in the viral envelope that expose the E1 peptide, which in turn promotes the fusion between the endosomal membrane and the viral envelope, releasing the nucleocapsids within the cell, followed by breaking the capsid with the release of the viral genome in the cytoplasm. Replication of viral RNA is preceded by its translation into two precursor polyproteins, one non-structural (ns) P123 and one structural (s) P1234. The non-structural polyprotein is processed into mature non-structural proteins by the activity of viral protease and the host cell itself [18]. Mature nsP1 plays an important role in viral replication since it has multiple enzymatic activities including: ATPase and RNA triphosphatase [19], RNA helicase [17] and protease activity [18]. nsP2 acts to neutralize host cell antiviral responses through multiple mechanisms, including the general transcriptional shutdown of the host cell by inducing Rpb1 subunit degradation of RNA polymerase II [20], inhibition of type 1 interferon production (IFNs) by interfering with the JAK/STAT signaling pathway [21,22] and inducing autophagy [23]. nsP3 is part of the replicase unit and is capable of binding to negatively charged polymers, including RNA [24], as well as promoting interactions with numerous cellular proteins including G3BP [25] and amphiphysins [26]. There is evidence that nsP4 acts as RNA-dependent RNA polymerase and catalyzes the formation of negative-sense, genomic and subgenomic viral RNAs [27].
These non-structural proteins come together to form the viral replication complex that synthesizes full-length genome negative stranded RNA, which serves as a template for replication of 26S subgenomic RNAs and 49S genomic RNAs. Subgenomic RNA leads to expression of the precursor structural polyprotein C-pE2-6 K-E1, which gives rise to the 6 K protein and the capsid protein C which are released upon processing. The E1 and pE2 glycoproteins are generated by further processing, as they associate in the Golgi and are exported to the plasma membrane, where pE2 is cleaved into two units: E2 which functions as adsorption structure of the virus to the cell receptors; and E3, which promotes the folding of E2 and its subsequent association with E1, and has a role in guiding the capsid proteins to mount the virions. The role of the 6 k protein remains ambiguous, but appears to be involved in the assembly and budding stages of the surface virions of infected cells. In addition, this protein is classified as a porphyrin, due to its ability to form cationic ion channels and alter membrane permeability [28]. Assembly of the virions corresponds to incorporating copies of the genomic RNA by the capsid proteins, thereby forming the nucleocapsids which sprout from the plasma membrane of the host cell, and where the envelope glycoproteins are inserted for forming complete virions which are released [9,29,30].
Replication of CHIKV in mosquitoes
In invertebrate hosts, CHIKV has been shown to be able to infect both A. aegypti and A. albopictus by replicating in the epithelial cells of the insect midgut, followed by the release of viral particles from the basal lamina to the hemolymph, through which they are carried to the salivary glands where it continues to replicate and remain for the rest of the insect’s life, then being released in the saliva from where they are transmitted to the vertebrate hosts [31].
The CHIKV throughout its evolution underwent genetic variations resulted in increased virulence and transmissibility by the vectors, being the main a point mutation in the gene that encodes the E1 glycoprotein. It has been proven that Ae. albopictus is more efficient in the transmission of the mutant strain (E1-226 V), which belongs to genotype IOL, isolated in 2006 in Reunion Islands, belonging to the French Republic in the Indian Ocean, which is derived from wild genotype ECSA, common ancestor (E1-226A and E1-98A). This strain has a point mutation characterized by a substitution of alanine for valine at position 226 of the viral E1 gene, which is related with decrease of cholesterol dependence for infection of the arthropod vector [8]. This new characteristic of the virus increased its infectivity to Ae. albopictus, since it replicates more efficiently in the midgut of the insect with dissemination to the salivary glands from where it is transmitted to the vertebrate hosts. Although this mutation does not change the transmission of the virus by Ae. aegypti, it increases the number of vectors with potential to spread the disease in geographic regions that are also infested by Ae. albopictus, as it happens in Brazil [32,33]. Studies have shown that this mutation is associated with recent outbreaks of Chikungunya fever worldwide [13,34,35].
On the other hand, A. aegypti showed to be more efficient in the transmission of the Asian/Caribbean genotype mutant strain (E1-98T), isolated in New Caledonia in Asia, which has an alanine substitution for threonine in position 98 in the E1 glycoprotein gene of the viral envelope, also derived from its wild common ancestor (E1-226A and E1-98A). This study was carried out for both vectors under equal and controlled conditions of infection and showed a significant reduction of the extrinsic incubation period of CHIKV in both vectors that were able to transmit the virus much earlier from two days after the infection onset [36].
Epidemiology
The evidence points to Africa being the origin of CHIKV, with its first identification in Tanzania in 1952, from where the virus spread to the Asian continent, where the first outbreak of chikungunya fever was reported in the Philippines in 1954. In addition, sporadic cases of the disease were reported in Africa and Asia in subsequent years during the last century [10,37]. The virus then re-emerged in 2004 initially in Kenya, and later spread to the African continent and to Indian Ocean countries, resulting in millions of cases of the infection; there was severe clinical presentation of the disease when it was first reported, along with death in Suriname [37].The virus was subsequently introduced even in temperate regions, causing an outbreak in Italy, in addition to reported cases of the disease in the Americas, including in the United States in travelers, but mainly in areas of Asia [38].
Numerous CHIKV outbreaks have been reported on almost every continent since the mid-2000s, and they have been identified and confirmed in more than 40 countries. It is estimated that about 2 million people contracted infection by this pathogen in the last 10 years [39]. Currently, chikungunya fever occurs on the Asian continent in: Cambodia, East Timor, India, Indonesia, Laos, Malaysia, Maldives, Myanmar, Pakistan, Philippines, Réunion, Seychelles, Singapore, Taiwan, Thailand and Vietnam; in the African continent: Benin, Burundi, Cameroon, Central African Republic, Comoros, Democratic Republic of Congo, Equatorial Guinea, Guinea, Kenya, Madagascar, Malawi, Mauritius, Mayotte, Nigeria, Senegal, South Africa, Sudan, Tanzania, Uganda and Zimbabwe [40].
In Europe, an outbreak initially occurred in 2007 in Northeast Italy, followed by other regions with smaller proportions of cases such as: France, Belgium, Switzerland, Germany and England. In the Americas, imported cases by travelers were first reported, mainly in the Caribbean islands, and later established with endemic patterns in some countries of region due to favorable climatic conditions and establishment of the vector mosquito [4,41]. The resurgence of CHIKV in the Americas was announced in December 2013, with diagnosis of the first cases on the island of Saint Martin, from where the virus spread to several Caribbean countries such as Martinique, Guadeloupe, Dominican Republic and the British Virgin Islands, with notification of about 1.1 million cases of chikungunya fever within a year, including severe cases with deaths. The true impact of the disease is unclear due to inadequate surveillance and under diagnosis. The evolution of the virus, globalization which favors travel and climate change can increase the virus spread, further aggravating the problem [3,42].
In Brazil, the first case of chikungunya fever was notified in 2010 as being imported by a traveler from the island of Sumatra, Indonesia. However, no confirmatory laboratory tests such as virus isolation or viral genome detection by PCR were performed. The diagnosis was only established based on the typical symptomatology of infection with marked polyarthritis [43].
The first autochthonous cases of the disease were reported in the city of Oiapoque, in the state of Amapá, on September 13, and seven days later, in the city of Feira de Santana, in the State of Bahia, both in the year 2014. However, even although of the introduction of the virus has occurred almost simultaneously in the country, the sources of the virus form different. The virus introduced in Amapá came from the Caribbean and was identified as being from the Asian genotype, while the virus introduced in Bahia came from Angola, Africa and was identified as being from the East-South-Central Africa genotype [34,35,44]. In the same year, cases of the disease were reported in Brasília, Mato Grosso do Sul, Roraima and Goiás [11,45]. Until 2015, the disease has spread throughout the country, mainly in the Northeast region, where the tropical climate is very favorable to the development of the vectors [46].
In 2016 alone, 271,824 suspected cases of the disease were reported distributed across 2,829 municipalities in the country in different regions, corresponding to an incidence rate of 133.0 cases/100 thousand inhabitants, a record of 196 deaths, with 91.0% of cases in Northeast region. The State of Rio Grande do Norte reported 21,638 suspected cases of the disease with 37 deaths, accounting for the second highest death toll in the country. Death cases were reported at all ages (0 to 98 years), but most of them were in the elderly, with a median age of 62 years. Fortunately, there was a significant reduction in the incidence rate of the disease in 2017, declining to 1.8 cases/100 inhabitants [47,48].
Thus, as happens with other arbovirus infections, there is considerable risk of transmission CHIKV by blood transfusions, due to short periods of asymptomatic viremia in populations with extremely high incidence of infection. This type of transmission is difficult to prove from the obstacle in discarding vector transmission in blood recipients with arbovirose [49]. However, estimates of the transmission risk of the virus were carried out during the epidemic on Réunion Island, indicating an average risk of 132 cases per 100,000 blood donations [50].
CHIKV infection is mainly transmitted by hematophagous arthropods, being the mosquitoes of genus Aedes the main vectors, having the species, A. aegypti and A. albopictus as the most important transmitters [35]. The vertical transmission was reported for the first time in 2005, during the epidemic that reached Reunion Island (French overseas district in the Indian Ocean), where 84 cases were reported of infected women during pregnancy, with some newborns presenting severe complications after birth [51].
Another study, also carried out in Réunion, found that in pregnant women with CHIKV infection, confirmed by serology and RT-PCR and who had viremia during the pre- or intrapartum period, presented vertical transmission observed exclusively in in near-term deliveries (35–40 wk). Caesarean section had no protective effect on transmission, suggesting that transmission during passage through the birth canal does not seem to be important [52]. There are also reports of cases in three different countries from Central and South American, during the peak of the epidemic that hit Latin America [53] and in Brazil where the results of imaging exams were suggestive of encephalitis and presence of CHIKV was detected by RT-PCR in cerebrospinal fluid, blood, urine and saliva [54].
The anthropophilic character of the vectors of CHIKV and their wide distribution, coupled with the increase of intercontinental travels that allow the transit of viremic people, facilitates the spread of the virus, with permanent risk of epidemics outbreak worldwide. This makes this pathogen a global threat not only to public health, but also of countries economy due to incapacitation of the disease for work.
Clinical manifestations
Chikungunya fever can present a very varied clinical picture in which about 3 to 25% of infected individuals have no clinically evident symptoms of disease [10]. When symptomatic, the disease appears after an average incubation period of three days and usually with abrupt onset characterized by a triad of: high fever above 39 °C, rash and arthralgia, often accompanied by headache, photophobia, joint swelling and conjunctivitis, and being more rarely associated with severe neurological, cardiovascular and hemorrhagic manifestations [3,30,55]. The peak clinical manifestations of the disease coincide with a peak in viremia, and most infected individuals become incapacitated to work for weeks to months because of decreased dexterity, loss of mobility and reaction [56]. At least 40% of the patients still present anti-IgM against CHIKV 18 months after the onset of the disease, thus showing the high chronic potential of the infection [2].
The rash may be of a maculopapular or petechial type, which in the most severe cases extends to more than 90% of the skin’s surface and disappears three to four days later [57]. Polyarthralgia is the most obvious and distinctive symptom associated with CHIKV infection when compared to other arbovirus infection [30]. Most patients complain of strong joint pains and present swelling with severe stiffness in the morning, consistent with inflammatory arthritis [57]. Ocular manifestations can occur less frequently such as conjunctivitis, uveitis, episcleritis and retinitis [58]. Usually the frame regresses spontaneously within 7–10 days, with rash disappearance followed by palmoplantar desquamation [59]. Atypical cases of the disease are reported less frequently, reaching particularly elderly and immunocompromised patients, in whom the disease may present ocular changes [60], cardiovascular involvement [61], syndrome of shock syndrome with severe generalized purpuric lesions and bullous dermatosis [62] and severe neurological manifestations, including Guillain-Barré syndrome [63].
Strong symmetrical joint pains involving more than one joint, especially during the acute phase, are reported in the fingers, wrists, elbows, ankles and knees being the most commonly affected, often causing severe disabling pain [64]. Synovitis with periarticular edema along with large joint effusions are reported by about 15% of infected individuals [65]. Joint pains associated with chikungunya fever begin to improve after the first week of infection in most patients, but in some cases, it persists for a longer time, even lasting up to 3 years, with alternating periods of improvement and exacerbation with edema formation [66,67]. Some patients develop bone erosions because of arthritis. The cause of which is yet to be determined but appears to be associated with osteoclastogenesis by a mechanism involving differentiation of CD14 + monocytes into osteoclasts which promote bone resorption, although appears to be a less common event. However, that ability to cause erosive bone lesions is a characteristic that distinguishes CHIKV from other arthritogenic alphaviruses [68].
It was shown that, pregnant women with CHIKV infection confirmed by laboratory tests, that presented viremia in the pre- or intrapartum period, transmitted the virus to their children. The newborns were asymptomatic at birth, as onset of symptoms after a period of 3-7 days with fever, prostration, and thrombocytopenia. Severe disease consisting mainly of encephalopathy some with compatible pathological findings as cerebral edema and cerebral hemorrhage [52]. Both vertical and perinatal transmission were associated with severe disease, after birth, presented extensive bullous cutaneous eruption, intravascular coagulation, hypoactivity, progressing to generalized seizures. There have also was reported cases of meningoencephalitis, intracerebral hemorrhage, requiring prolonged neonatal hospitalization, with need of intubation and assisted ventilation and even cases of death [51,53,54].
The risk of vertical transmission appears to be greater when the viremia peak in the maternal infection occurs in the period closest to delivery [52]. However, the virus can be established in the fetus in any trimester of pregnancy, possibly through the transplacental route, and may also occur during passage through the birth canal. However, caesarean section has not been indicated as a protective factor for the newborn. Neurological complications are quite variable in congenital and neonatal infections, including encephalopathy with long-term neurocognitive deficits, and may also result in severe sequelae, such as cerebral palsy [69]. In addition to neuroinvasive disease, some neonates develop hemorrhagic syndrome, necrotizing enterocolitis, hemodynamic disorders, ventricular dysfunction, pericarditis, and/or coronary artery dilatation [70,71]. There is no evidence yet that the virus is transmitted through breast milk [56,72].
In adults, complications are more associated with persistent arthralgia [73,74]. However, more severe complications with the involvement of multiple organs may cause cardiac manifestations, hepatitis, and neurological pathologies such as encephalitis that can lead to death, especially in patient’s newborns, the elderly, and in those with some underlying disease, and immunosuppressed patients. Neuroinvasion by CHIKV results in convulsions, altered mental status, flaccid paralysis, and can lead to death [10,70,75].
CHIKV infection is also associated with autoimmune manifestations such as Guillain Barre syndrome. The infection is characterized by high viremia that can reach concentrations of 108 plaque forming virus (PFU)/mL in peripheral blood. The highest viral loads are found in the elderly and newborns, suggesting that greater severity of the disease is associated with greater virus replication due to immaturity of the immune response in the neonate and immunological ineffectiveness in the elderly, in addition to viral escape, or both [41,73,74].
Pathogenesis
The main form of CHIKV transmission is through introduction of the virus into the skin during the bite of an infected Aedes aegypti or Aedes albopictus mosquitoes, although some cases of maternal-fetal transmission have been reported in the recent epidemic [52]. The initial site of viral replication appears to be cutaneous fibroblasts and macrophages while viral particles are captured by dendritic cells (DCs), including Langerhan cells from the skin and reach to the closest lymph nodes where they are transferred to monocytes and macrophages which enter the bloodstream, leading the virus to target several organs [30,76,77] Figure 1.
Figure 1.
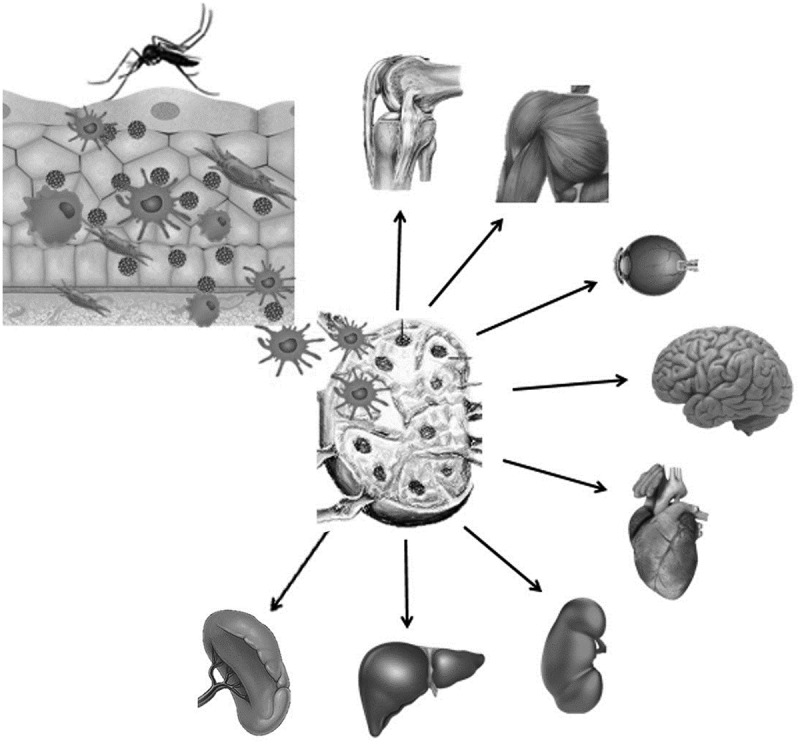
Schematic representation of the pathogenesis of chikungunya virus infection in the human host.
Notes: The virus is introduced into the skin by the bite of the infected mosquito, where it initiates replication in macrophages and cutaneous fibroblasts. Viral particles are captured by dendritic cells from the skin that carry them to the nearest lymph nodes where they will infect macrophages and monocytes. Infected monocytes enter the bloodstream carrying the viruses to multiple organs such as: liver, kidneys, heart, brain, eyes, skeletal muscles, and joints, where they replicate and induce inflammatory response.
In both mouse and human models, it has been observed that fibroblasts are permissive to the virus with replication, with fibroblasts being the main type of cell infected in target tissues such as muscles, joints and skin. The detection of viral antigens by immunohistochemistry, in tissue of both newborns and adult mice, revealed fibroblast as the main target cell of the CHIKV, which explains its tropism by muscles, joints, connective tissue and skin, classic symptomatic organs of the disease in human. The molecular basis of this tropism is currently unknown but appears to be the result of a combination of interactions between virus and host cell and lower intrinsic ability of that cell type to control CHIKV infection. Two critical factors for viral replication were identified: the neonatal status, and deficient signaling to type I IFN, both associated with high viral load, greater tissue distribution, and disease greater severity. Similar fact occurs in humans, regarding neonatal status, but the relevance of the deficiency in the production type I INFN for severe infection in humans still needs to be demonstrated. However, the fact that severe infections in humans are observed exclusively in individuals with underlying conditions renders this hypothesis enough attractive [78,79].
Several types of cutaneous manifestations occur including erythematous, maculopapular or morbilliform rash that regress within 3–4 days without leaving marks [80]. Severe bullous skin lesions that resemble staphylococcal scalded skin syndrome are also observed located in the trunk and limbs, although mainly in children. Such manifestations could be a consequence of the inflammatory response in the skin with the mobilization of resident cells such as keratinocytes, melanocytes and dermal fibroblasts [81–83].
Ocular manifestations have also been reported, with the presence of viral RNA detected by reverse transcription polymerase chain reaction quantitative real time (qRT-PCR) on the corneal-scleral borders of asymptomatic viremic individuals. In addition, infectious virus was isolated from all positive samples by molecular method and viral antigens were detected in specimens of the cornea and scleral, iris, ciliary body and oculomotor muscles [84]. In another study, CHIKV RNA was detected by qRT-PCR from an aqueous humor sample obtained from a patient with granulomatous uveitis, that two months ago presented symptoms compatible with chikungunya fever [85].
The Intradermal inoculation of CHIKV in mice showed that as it occurs in humans, the fibroblast is the preferred target cell of the virus and that it was able to infect the ocular tissues of the same affected areas in the human eye [79]. In human infection, CHIKV infects the fibroblasts present in the scleral connective tissue, in the stroma of the smooth muscles of the ciliary bodies, in the stroma of the iris and among the fibers of the ocular muscle, where it actively replicates with the production of infectious infections. particles [84]. This suggests that the damage to ocular tissues is possibly due to the direct action of the virus.
Neurological manifestations associated with chikungunya fever, including encephalitis, encephalopathies, peripheral neuropathies and Guillain-Barré syndrome, have also been reported. However, the mechanisms by which it develops remains unknown [86]. Studies in animal models show that strains of the Asian / Caribbean and ECSA genotypes of the CHIKV were able to infect astrocytes and oligodendrocytes of rats, increasing the expression of apoptotic genes [63]. In addition, it is believed that the immune response also contributes to neuronal lesions [63,87], once the astrocytes can produce IFN and pro-apoptotic factors, being able to cause damage to the hematoencephalic barrier [88].
The severity of the infection seems to be directly associated with the action of the virus, but also with the decompensation of preexisting comorbidities [89]. Joint involvement in the acute and chronic phase is the main clinical manifestation [90]. It is believed that multiple factors, including viral persistence in the joints, trigger mechanisms involving chronic inflammation, with elevated levels of immune mediators and infiltration of immune cells in adjacent joints and tissues [10,91].
The pathophysiology of bullous skin lesions is still unknown. However, the viral load found in the fluid collected from blisters is at least 20 times higher than that found in serum. It is not yet known if these lesions have only one or several pathogenic pathways. These lesions appear to be secondary to virus-induced pathological changes in the epidermis that result in focal necrosis, ballooning, vacuolization or nuclear rupture of the cells, followed by an immune response with leukocyte infiltration. It is speculated that additional factors such as drugs or their metabolites can modulate this response by breaking the balance between cytotoxic phenomena and their regulators, favoring cytotoxicity against cutaneous cells infected with viruses. At least two mechanisms are proposed to explain the origin of the bullous lesions of the skin associated with chikungunya fever; virus-induced keratinocyte necrosis, followed by a cytotoxic immune response; or a possible modulation of drug-mediated cytotoxicity, such as paracetamol [92].
Although antibodies have a crucial role in protecting against infectious diseases, in some arbovirus infections, especially those caused by flaviviruses, it has been shown that antibodies with low affinity or in low titers can augment virus infection and exacerbate disease severity through of a phenomenon known as antibody-mediated enhancement [93]. A recent study has demonstrated the enhancement of CHIKV attachment and infection in primary human monocytes and B cells in the presence of sub-neutralizing levels of anti-CHICKV antibodies. Experimental infection in adult mice also demonstrated enhanced CHICK infection, besides more severe disease and increased lethality, mediated by low titles of CHIKV antibodies . These questions brings caution to the phenomenon and advocates the need for careful vaccine design and extensive pre-clinical trials [94].
However, the enhanced CHIKV infection did not augment viral replication in primary human monocytes and B cells, suggesting that this quick shutdown of viral replication could act as a putative mechanism employed by CHIKV for chronic persistence [94].
Immune response of host
It is well established that type I interferons (IFNs) play a crucial role in an efficient and protective immune response against CHIKV infection, which begins with recognition of the virus by pathogen-associated molecular patterns receptors (PAMPS) which recognize conserved molecular motifs in the virus that are exposed to standard recognition receptors (PRRS) of its host. The two main types of PRRS that recognize viruses are the TLRs found in the plasma membrane and endosome, and RLRs (inducible retinoic acid receptors) found in the cytoplasm. This recognition of the virus as a foreign agent triggers a mechanism in the cell that results in the production of type 1 interferons (IFN-α and IFN-β) and other immune mediators, which then triggers an immune response aiming at elimination of the aggressor agent [79,95,96].
There is a potential protective effect of both IFN-α and IFN-β which are mainly produced by leukocytes and fibroblasts, respectively, and which are present in increased levels in patients who can control the infection by eliminating the virus. On the other hand, patients with defective production of IFN are more susceptible to the virus and are at greater risk of developing severe infection [13,97]. This protective role of type I IFNs is given by the fact that they activate the transcription of genes that are activated by interferons (ISGs), which are very important for the antiviral response and contain IFN-sensitive promoting elements. However, as in other viruses, it is possible that CHIKV has developed mechanisms to modulate the immune response. One of the main candidates is the nsP2 non-structural protein that acts on inhibiting host type I IFN receptor synthesis, being able to turn off the signal, thereby decreasing its expression and activation of other antiviral mediators and weakens the immune response in face of the pathogen [9,33,95].
The manifestation of fever common to all patients with chikungunya virus is due to the production of proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α), which have pyretic action [98]. Serum levels of these cytokines are elevated in the acute phase of infection, but return to baseline levels after the viremia phase [99,100]. In addition to the interferon-β (IFN-β) produced by infected fibroblasts, these cytokines induce high expression of prostaglandin, which in turn further increases inflammation and manifestation of arthralgia due to inflammation and tissue destruction in the joints [30,98].
The inflammatory response coincides with elevated levels of immune mediators and infiltration of immune cells into infected joints and surrounding tissues [10]. A meta-analysis study reveals the existence of high levels of proinflammatory cytokines in serum and plasma of patients from different regions of the world with arthritis caused by CHIKV, including IFN-α, IFN-γ and IL-6, as well as IL-4 and IL-10 anti-inflammatory cytokines and chemokines such as IP-10 and monocyte chemoattractant protein 1 (MCP-1) [101].
Differences between the cytokine profile of the acute and chronic disease have been observed with elevated levels of IL-1Ra, IL-6, IL-7, IL-8, IL-12 and IL-15 in the acute phase, and only elevated levels of IL-6, GM-CSF and IL-17 in chronic arthritis [96,102]. The action of IL-17 can generate chronic inflammation of the extracellular matrix and promote bone destruction through stimulating production of IL-6, TNF, IL-1, matrix metalloproteinases and receptor activator of nuclear factor kappa-Β ligand (RANKL) [103]. IL-6 was found to be essential for the persistence of CHIK-induced arthritis through the regulation of RANKL, resulting in osteoclastogenesis [98,102,104]. Some patients with CHIK-induced arthritis have subchondral bone erosion, joint effusion and thickening of the joints that may persist for years [104,105]. It has been observed that human CHIKV-infected synovial fibroblasts secrete immune mediators, including IL-6 which induces ostoclastogenesis, since they recruit and induce the differentiation of CD14 + monocytes into osteoclasts. CHIKV-infected osteoclasts secrete high levels of IL-6 promoting positive feedback, which results in progression of the arthralgia/arthritis and mediates the pains in multiple joints due to its migratory properties [68].
In addition, studies in mice and humans show that persistent arthritis caused by chikungunya results in increased numbers of activated and effector circulating T cells [57], thus suggesting that T cells play an important role in the pathogenesis of induced arthritis by this pathogen [106]. In addition, a greater number of natural killer cells in peripheral blood have been reported in patients with persistent arthritis caused by chikungunya when compared to healthy individuals [10]. Thus, the evidence indicates that the most severe cases of chikungunya fever depend on multiple factors including viral persistence mechanisms and associated factors such as reactivation of the virus in the joints, of immune response evasion, uncontrolled response for pro-inflammatory cytokine and cross reactivity with autoantigens [7,14,39,107,108].
Diagnosis and treatment
Clinical diagnosis of Chikungunya fever is very difficult, especially in regions where there is simultaneous circulation of other arboviruses such as Dengue viruses, Zika virus and Mayaro virus, which can cause very similar symptoms to those of the Chikungunya virus. Because of this, laboratory diagnosis becomes extremely necessary. Virus isolation and identification is considered the gold standard technique. However, its complexity, high cost and the delay in obtaining the result make it unsuitable for routine use.
Molecular biology techniques for detecting genetic material of the virus from conventional RT-PCR (reverse transcriptase-polymerase chain reaction) or real-time RT-PCR are more viable alternatives as they present high sensitivity and specificity and provide faster results than viral isolation, although they are still costly. The Centers for Disease Control and Prevention (CDC) in the United States provide the RNA-positive primers and control of the virus to be used in conventional RT-PCR, the RNA-positive primers and control of the virus, and the nucleotide sequences from the probes for real-time RT-PCR assays [109].
Serological tests for detecting IgM and IgG Anti-CHIKV are alternative diagnostic methods that can be used in both the acute and chronic phases of infection. It has been shown that the detection of CHIKV-specific IgM antibodies presents a sensitive test for samples collected after five days of the onset of symptoms. However, only three MAC-ELISA kits and an indirect immunofluorescence kit presented performance comparable to the reference assays and were recommended in an analysis carried out by the CDC of nine commercial assays available on the market. All the analyzed rapid tests did not show the desired sensitivity and thus were not recommended [109].
One of the limitations of molecular techniques is that the collection of material should be done in the acute phase of the disease in the period of greatest viremia up to five days after the onset of symptoms. The immunological tests do not have this limitation in relation to the collection time of the material for examination. However, they do not have the same sensitivity and specificity of the molecular methods and presented cross-reactions with other arboviruses, members of the Semliki forest virus (SFV) antigenic complex [7,110].
Regarding therapeutic treatment, there is still no specific treatment for patients suffering from chikungunya fever. Efforts are currently mainly being focused on preventive actions of combat to vectors, and actions aimed at eliminating mosquito breeding sites, as well as wearing long-sleeved clothing and repellents.
Therefore, the treatment that is being performed is only in support of individuals who suffer from intense pain in the joints. The use of non-steroidal anti-inflammatory drugs (NSAIDs), antipyretics and analgesics for the relief of symptoms is recommended. Other drugs such as codeine and tramadol may be used to treat refractory or severe allergic symptoms. The use of these resources associated with rest has shown variable efficiency, but does not solve the problem of artralgias. The use of aspirin should be avoided in the first weeks of the disease because of the difficulty in establishing the differential diagnosis in the acute phase between chikungunya fever and dengue fever, and aspirin would increase the risk of bleeding if the patient has dengue. In addition, salicylate should also be avoided because the use of this drug in acute viral infections in children may lead to Reye syndrome. In the chronic phase, an oral corticosteroid may be recommended for treating musculoskeletal and neuropathic complaints, using low doses (5 to 20 mg/day) of prednisone or prednisolone. The usage time may vary from six to eight weeks and then must be withdrawn. One study showed that the risk of reoccurring joint symptoms after treatment withdrawal was slow and gradual [111].
Currently, there are nor therapeutic products neither vaccines licensed to the infection CHIKV control, so it still depends on the use of personal protection measures and vector control, which are only minimally effective [112]. Therapy is mainly limited to supportive care because the available antiviral agents are still in different stages of testing or in development [113]. Several antivirals including ribavirin, IFN-α and chloroquine presented satisfactory effects on the viremia control from chikungunya virus. Some results have shown that chloroquine may be useful in treating chronic cases of the infection, but there are indications that chloroquine increases joint symptoms when given in the acute phase [15,33,111]. Doxycycline combined with ribavirin significantly reduces viral load and the extent of inflammation in mice [114]. Newly invented antivirals including polymerase inhibitors and protease such as favipiravir, protected mice from lethal infection by CHIKV, which may be a promising candidate [115]. Rest is considered one of the main recommendations. Physical activities should be stopped as they tend to aggravate the inflammatory process of the joints by prolonging the prodrome period of the disease [7].
Administration of anti-CHIKV human polyvalent immunoglobulins purified from plasma samples obtained from donors in the convalescent phase of the CHIKV infection, showed a high in vitro neutralizing activity and a potent prophylactic and therapeutic efficacy against CHIKV infection in vivo, including in newborns. Thus, the use of this product may constitute a safe and effective prevention strategy to be recommended in the treatment of individuals exposed to the virus who are at risk of serious infection such as newborns of viremic mothers and adults with underlying conditions including immunocompromised [116]. In addition, administration of human monoclonal antibodies in immunocompetent mice following CHIKV infection significantly reduced viral load in the joint tissues. Treatment of rhesus monkeys with this same antibody after CHIKV infection, showed a rapid elimination of viremia with reduction of joint infiltration making the disease less severe [117].
The search for solutions to prevent and treatment of CHIKV infection continues several products that have already been tested and some have shown promising results. However, due to the existence of numerous strains of the virus, the use of different animal models in which different vaccines and products have been tested with this purpose, it is difficult to establish direct comparisons between these products, to select one of them for the realization and more definitive clinical trials. Another challenge is to develop affordable products for a wide range of individuals living in endemic areas of the disease [112].
Currently there are several vaccine candidates being evaluated for their efficacy, including: an constituted by VLPs (Virus-Like Particles) generated from the CHIKV C-E3-E2-6K/TF-E1 polyprotein genes, using a cytomegalovirus CMV/R expression vector that was transfected into 293 human kidney cells [118]. The Measles virus-based chimeras (MV) vaccine has proven safety and efficacy [119]. Also, a CHIKV-IRES vaccine candidate that is based on a full-length clone of a wild-type strain from La Reunion (ECSA genotype), in which the natural sub genomic promoter was replaced by the encephalomyocarditis virus IRES [120]. This strain contains specific mutations in the genome of the parental virus that normally allow for greater specificity, better safety profiles and high levels of expression, allowing obtain protection with only a single dose of the vaccine [112].
The vaccine candidate of VLPs induced a strong immune response with the production of neutralizing antibodies against homologous and heterologous strains of CHIKV, when administered intramuscularly in BALB/c mice in a two-dose series. Non-human primates vaccinated with these VLPs produced neutralizing antibodies even after a single dose of the vaccine and the response was increased after a booster dose. This product appeared so promising that was submitted to the phase 1 clinic test in adult humans to assess safety, using a dose escalation format [121]. No fever or arthralgia and no serious adverse events were observed. All subjects developed neutralizing antibodies after the second dose of the vaccine, but at the final period of the study, antibodies titers fell approximately four-fold. Interestingly, the volunteers produced neutralizing antibodies that showed cross-protection against CHIKV strains of all genotypes [122].
Regarding the MV vaccine the main concern it was the possibility of a weak response due the fact that the vaccine vector is the measles vaccine virus for which large part of the populations have anti-MV immunity. However, this vaccine induced elevated titers of anti-CHIKV antibodies, both in virgin and pre-immunized mice with the measles virus [119]. Although no study in non-human primates with this candidate vaccine has been reported, phase 1 clinical trials have been completed, with encouraging results [112].
Finally, the vaccine candidate of live attenuated vaccine virus, CHIKV-IRES vaccine was tested in a mouse challenge model in which a group of 10 immunocompetent C57BL/6 mice, at three weeks of age they were vaccinated with 105 PFU subcutaneously and subsequently bled to determine antibody titers. All rats seroconverted with titers ≥20. On the same day, mice were given an intranasal challenge of 106 PFU using an ECSA genotype CHIKV strain. This same challenge was performed in a group of 10 unvaccinated mice. None of the previously vaccinated mice showed the disease, whereas 70% (7/10) of the sham-vaccinated mice died on the 10th day after challenge [120].
In this context, although several vaccine candidates are currently being developed and with very promising expectations, such as CHIKV is known to spread rapidly and generate high incidence of infection rates, the development of effective therapeutic products would also be of great value [112].
. In conclusion, the chikungunya fever is a huge challenge to public health, especially in tropical regions, where exist high infestation levels of Ae. aegypti and Ae. albopictus, main transmitters of CHIKV as happens in the Northeast of Brazil, where the genotype ECSA circulates. After entering the skin through the bite of the infected mosquito's female, the virus spreads through the host's body, reaching multiple organs, including the brain. In addition to vector transmission, there is the possibility of transmission by blood transfusion and vertical transmission. Vertical and perinatal transmission presents a potential risk for severe disease in the newborn, presenting bullous cutaneous eruption and neurological manifestations, including encephalitis and intracerebral hemorrhage with risk of death. In adult immunocompetent individuals, the infection courses with abrupt onset high fever, rash and arthralgia, often accompanied by headache, conjunctivitis with photophobia and joint swelling. Atypical forms potentially fatal, also may occur with ocular manifestations, cardiovascular involvement, syndrome of shock with severe generalized purpuric lesions and bullous dermatoses, besides of neurological manifestations, including Guillain-Barré syndrome, especially in the elderly with some underlying disease and immunocompromised individuals. In this context, since measures to fight against vectors have been ineffective, the search for effective CHIKV infection treatment and prevention options becomes extremely important. Many advances have already been made in this area, so that, the futures prospects are positive.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond). 1956;54:177–191. 10.1017/S0022172400044442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Thiboutot MM, Kannan S, Kawalekar OU, et al. Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4(4):e623. 10.1371/journal.pntd.0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Halstead SB. Reappearance of chikungunya, formerly called Dengue, in the Americas. Emerg Infect Dis. 2015;21(4):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Powers AM, Brault Robert BAC, Tesh RB, et al. Re-emergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. 10.1099/0022-1317-81-2-471 [DOI] [PubMed] [Google Scholar]
- [5]. Volk SM, Chen R, Tsetsarkin KA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497–6504. 10.1128/JVI.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. 10.1016/j.antiviral.2015.04.016 [DOI] [PubMed] [Google Scholar]
- [7]. Cunha RVD, Trinta KS. Chikungunya virus: clinical aspects and treatment – a review. Mem Inst Oswaldo Cruz. 2017;112(8):523–531. 10.1590/0074-02760170044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Schuffenecker I, Iteman I, Michault A, et al. Genome microevolution of chikungunya viruses causing the indian ocean outbreak. PLoS Med. 2006;3(7):e263. 10.1371/journal.pmed.0030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491–500. 10.1038/nrmicro2368 [DOI] [PubMed] [Google Scholar]
- [10]. Burt FJ, Chen W, Miner JJ, et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis. 2017;17(4):e107–e117. 10.1016/S1473-3099(16)30385-1 [DOI] [PubMed] [Google Scholar]
- [11]. Honório NA, Câmara DCP, Calvet GA, et al. Chikungunya: an arbovirus in the process of establishment and expansion in Brazil. Cad Saude Publica. 2015;31(5):906–908. 10.1590/0102-311XPE020515 [DOI] [PubMed] [Google Scholar]
- [12]. Chen R, Wang E, Tsetsarkin KA, et al. Chikungunya virus 3′ untranslated region: adaptation to mosquitoes and a population bottleneck as major evolutionary forces. PLoS Pathog. 2013;9(8):e1003591. 10.1371/journal.ppat.1003591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Wahid B, Ali A, Rafique S, et al. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. 10.1016/j.ijid.2017.03.006 [DOI] [PubMed] [Google Scholar]
- [14]. Singh SK, Unni SK. Chikungunya virus: host pathogen interaction. Rev Med Virol. 2011;21(2):78–88. 10.1002/rmv.681 [DOI] [PubMed] [Google Scholar]
- [15]. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99(3):345–370. 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. van Duijl-Richter M, Hoornweg T, Rodenhuis-Zybert I, et al. Early events in Chikungunya virus infection from virus cell binding to membrane fusion. Viruses. 2015;7(7):3647–3674. 10.3390/v7072792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Das PK, Merits A, Lulla A. Functional cross-talk between distant domains of chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J Biol Chem. 2014;289(9):5635–5653. 10.1074/jbc.M113.503433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Utt A, Das PK, Varjak M, et al. Mutations conferring a noncytotoxic phenotype on chikungunya virus replicons compromise enzymatic properties of nonstructural protein 2. J Virol. 2015;89(6):3145–3162. 10.1128/JVI.03213-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Karpe YA, Aher PP, Lole KS. NTPase and 5′-RNA triphosphatase activities of chikungunya virus nsP2 protein. PLoS One. 2011;6(7):e22336. 10.1371/journal.pone.0022336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Akhrymuk I, Kulemzin SV, Frolova EI. Evasion of the innate immune response: the old world alphavirus nsP2 protein induces rapid degradation of Rpb1, a catalytic subunit of RNA polymerase II. J Virol. 2012;86(13):7180–7191. 10.1128/JVI.00541-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Fros JJ, Liu WJ, Prow NA, et al. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol. 2010;84(20):10877–10887. 10.1128/JVI.00949-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Fros JJ, van der Maten E, Vlak JM, et al. The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling. J Virol. 2013;87(18):10394–10400. 10.1128/JVI.00884-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Judith D, Mostowy S, Bourai M, et al. Species-specific impact of the autophagy machinery on Chikungunya virus infection. EMBO Rep. 2013;14(6):534–544. 10.1038/embor.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Malet H, Coutard B, Jamal S, et al. The crystal structures of chikungunya and venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol. 2009;83(13):6534–6545. 10.1128/JVI.00189-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Panas MD, Ahola T, McInerney GM. The C-terminal repeat domains of nsP3 from the old world alphaviruses bind directly to G3BP. J Virol. 2014;88(10):5888–5893. 10.1128/JVI.00439-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Neuvonen M, Kazlauskas A, Martikainen M, et al. SH3 domain-mediated recruitment of host cell amphiphysins by alphavirus nsP3 promotes viral RNA replication. PLoS Pathog. 2011;7(11):e1002383. 10.1371/journal.ppat.1002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Chen MW, Tan YB, Zheng J, et al. Chikungunya virus nsP4 RNA-dependent RNA polymerase core domain displays detergent-sensitive primer extension and terminal adenylyltransferase activities. Antiviral Res. 2017;143:38–47. 10.1016/j.antiviral.2017.04.001 [DOI] [PubMed] [Google Scholar]
- [28]. Leung JY, Ng MM, Chu JJ. Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol. 2011;2011:249640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Tang BL. The cell biology of Chikungunya virus infection. Cell Microbiol. 2012;14(9):1354–1363. 10.1111/j.1462-5822.2012.01825.x [DOI] [PubMed] [Google Scholar]
- [30]. Lum FM, Ng LFP. Cellular and molecular mechanisms of chikungunya pathogenesis. Antiviral Res. 2015;120:165–174. 10.1016/j.antiviral.2015.06.009 [DOI] [PubMed] [Google Scholar]
- [31]. Vega-Rúa A, Schmitt C, Bonne I, et al. Chikungunya virus replication in salivary glands of the mosquito aedes albopictus. Viruses. 2015;7(11):5902–5907. 10.3390/v7112917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379(9816):662–671. 10.1016/S0140-6736(11)60281-X [DOI] [PubMed] [Google Scholar]
- [33]. Teng T-S, Kam Y-W, Tan JJ, et al. Host response to chikungunya virus and perspectives for immune-based therapies. Future Virol. 2011;6(8):975–984. 10.2217/fvl.11.67 [DOI] [Google Scholar]
- [34]. Nunes MRT, Faria NR, de Vasconcelos JM, et al. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med. 2015;13:102. 10.1186/s12916-015-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Lourenço-de-Oliveira R, Failloux AB. High risk for chikungunya virus to initiate an enzootic sylvatic cycle in the tropical Americas. PLoS Negl Trop Dis. 2017;11(6):e0005698. 10.1371/journal.pntd.0005698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Vega-Rúa A, Zouache K, Girod R, et al. High level of vector competence of aedes aegypti and aedes albopictus from ten american countries as a crucial factor in the spread of chikungunya virus. J Virol. 2014;88(11):6294–6306. 10.1128/JVI.00370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. van Genderen FT, Krishnadath I, Sno R, et al. First chikungunya outbreak in suriname; clinical and epidemiological features. PLoS Negl Trop Dis. 2016;10(4):e0004625. 10.1371/journal.pntd.0004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re‐emerging infectious disease. Clin Infect Dis. 2009;49(6):942–948. 10.1086/599189 [DOI] [PubMed] [Google Scholar]
- [39]. Versteeg L, Febres MEC, Beaumier CM. The role of cellular immune responses on chikungunya virus infection-induced arthritis. Curr Trop Med Rep. 2016;3(2):60–66. 10.1007/s40475-016-0074-2 [DOI] [Google Scholar]
- [40]. Chikungunya [internet]. World Health Organization; http://www.who.int/mediacentre/factsheets/fs327/en/. [Google Scholar]
- [41]. Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. 10.1099/vir.0.82858-0 [DOI] [PubMed] [Google Scholar]
- [42]. Cassadou S, Boucau S, Huc MP, et al. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Euro Surveill. 2014;19(13):20752. 10.2807/1560-7917.ES2014.19.13.20752 [DOI] [PubMed] [Google Scholar]
- [43]. Sá PKO, Nunes MM, Leite IR, et al. Chikungunya virus infection with severe neurologic manifestations: report of four fatal cases. Rev Soc Bras Med Trop. 2017;50(2):265–268. [DOI] [PubMed] [Google Scholar]
- [44]. Rodrigues NCP, Lino VTS, Daumas RP, et al. Temporal and spatial evolution of dengue incidence in Brazil, 2001–2012. PLoS One. 2016;11(11):e0165945. 10.1371/journal.pone.0165945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Marques CDL, Duarte ALBP, Ranzolin A, et al. Recommendations of the Brazilian Society of Rheumatology for diagnosis and treatment of chikungunya fever. Part 1 – Diagnosis and special situations. Rev Bras Reumatol. 2017;57(Suppl 2):421–437. 10.1016/j.rbr.2017.05.004 [DOI] [PubMed] [Google Scholar]
- [46]. Fares RCG, Souza KPR, Añez G, et al. Epidemiological scenario of Dengue in Brazil. BioMed Res Int. 2015;2015:321873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Monitoramento dos casos de dengue e febre de chickungunya até a Semana Epidemiológia 12 , 2015 [internet]. Secretaria de Vigilância em Saúde - Ministério da Saúde - Brasil. Boletim Epidemiológico 2015;46(14):1–7. http://portalarquivos.saude.gov.br/images/pdf/2015/maio/04/2015-016–Boletim-Dengue-SE15-2015.pdf. [Google Scholar]
- [48]. Guia de vigilância em saúde [internet]. Brasília (Brazil): Secretaria de Vigilância em Saúde - Ministério da Saúde; 2017. http://portalarquivos.saude.gov.br/images/pdf/2017/outubro/06/Volume-Unico-2017.pdf. [Google Scholar]
- [49]. Peterson LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98(4):495–503. 10.1111/vox.2010.98.issue-4 [DOI] [PubMed] [Google Scholar]
- [50]. Brouard C, Bernillon P, Quatresous I, et al. Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. 2008;48:1333–1341. 10.1111/trf.2008.48.issue-7 [DOI] [PubMed] [Google Scholar]
- [51]. Robillard PY, Boumahni B, Gérardin P, et al. Vertical maternal fetal transmission of the chikungunya virus. Ten cases among 84 pregnant women. Presse Med. 2006;35(5):785–788. 10.1016/S0755-4982(06)74690-5 [DOI] [PubMed] [Google Scholar]
- [52]. Gérardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the Island of La Réunion. PLoS Med. 2008;5(3):e60. 10.1371/journal.pmed.0050060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Torres JR, Falleiros-Arlant LH, Dueñas L, et al. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis. 2016;51:85–88. 10.1016/j.ijid.2016.09.009 [DOI] [PubMed] [Google Scholar]
- [54]. Bandeira AC, Campos GS, Sardi SI, et al. Neonatal encephalitis due to Chikungunya vertical transmission – First report in Brazil. IDCases. 2016;5:57–59. 10.1016/j.idcr.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Tandale BV, Sathe PS, Arankalle VA, et al. Systemic involvements and fatalities during chikungunya epidemic in India, 2006. J Clin Virol. 2009;46(2):145–149. 10.1016/j.jcv.2009.06.027 [DOI] [PubMed] [Google Scholar]
- [56]. Cardona-Correa SE, Castaño-Jaramillo LM, Quevedo-Vélez A. Vertical transmission of chikungunya virus infection – case report. Rev Chil Pediatr. 2017;88(2):285–288. 10.4067/S0370-41062017000200015 [DOI] [PubMed] [Google Scholar]
- [57]. Miner JJ, Aw-Yeang HX, Fox JM, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67(5):1214–1220. 10.1002/art.39027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Mahendradas P, Avadhani K, Shetty R. Chikungunya and the eye: a review. J Ophthalmic Inflamm Infect. 2013;3(1):35. 10.1186/1869-5760-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Simon F, Javelle E, Oliver M, et al. Chikungunya virus infection. Curr Infect Dis Rep. 2011;13(3):218–228. 10.1007/s11908-011-0180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Martínez-Pulgarín DF, Chowdhury FR, Villamil-Gomez WE, et al. Ophthalmologic aspects of chikungunya infection. Travel Med Infect Dis. 2016;14(5):451–457. 10.1016/j.tmaid.2016.05.008 [DOI] [PubMed] [Google Scholar]
- [61]. Alvarez MF, Bolívar-Mejía A, Rodriguez-Morales AJ, et al. Cardiovascular involvement and manifestations of systemic Chikungunya virus infection – a systematic review. F1000Res. 2017;6:390 10.12688/f1000research [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Torres JR, Codova Códova GLG, Castro JS, et al. Chikungunya fever: atypical and lethal cases in the Western hemisphere – a Venezuelan experience. IDCases. 2015;2(1):6–10. 10.1016/j.idcr.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Brizzi K. Neurologic manifestation of chikungunya virus. Curr Infect Dis Rep. 2017;19(2):6. 10.1007/s11908-017-0561-1 [DOI] [PubMed] [Google Scholar]
- [64]. Mohan A, Kiran DHN, Manohar ChiranjeeviIIC, et al. Epidemiology, clinical manifestations, and diagnosis of chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol. 2010;55(1):54–63. 10.4103/0019-5154.60355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Hochedez P, Jaureguiberry S, Debruyne M, et al. Chikungunya infection in travelers. Emerg Infect Dis. 2006;12(10):1565–1567. 10.3201/eid1210.060495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Marimoutou C, Vivier E, Oliver M, et al. Morbidity and impaired quality of life 30 months after chikungunya infection: comparative cohort of infected and uninfected French military policemen in Reunion Island. Medicine (Baltimore). 2012;91(4):212–219. 10.1097/MD.0b013e318260b604 [DOI] [PubMed] [Google Scholar]
- [67]. Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137. 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Phuklia W, Kasisith J, Modhiran N, et al. Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: a possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Res. 2013;177(2):179–188. 10.1016/j.virusres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- [69]. Gérardin P, Couderc T, Binter M, et al. Chikungunya virus-associated encephalitis - A cohort study on La Réunion Island, 2005-2009. Neurology. 2016;86(1):94–102. 10.1212/WNL.0000000000002234 [DOI] [PubMed] [Google Scholar]
- [70]. Rajapakse S, Rodrigo C, Rajapakse A. Atypical manifestations of chikungunya infection. Trans R Soc Trop Med Hyg. 2010;104(2):89–96. 10.1016/j.trstmh.2009.07.031 [DOI] [PubMed] [Google Scholar]
- [71]. Ramful D, Carbonnier M, Pasquet M, et al. Mother-to-child transmission of chikungunya virus infection. Pediatr Infect Dis J. 2007;26(9):811–815. 10.1097/INF.0b013e3180616d4f [DOI] [PubMed] [Google Scholar]
- [72]. Joob B, Wiwanitkit V, Joob B, et al. Neurological manifestations of Chikungunya. Arq Neuropsiquiatr. 2017;75(5):326. 10.1590/0004-282x20170029 [DOI] [PubMed] [Google Scholar]
- [73]. Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–5927. 10.4049/jimmunol.0900255 [DOI] [PubMed] [Google Scholar]
- [74]. Sudeep AB, Parashar D. Chikungunya : an overview. J Biosci 2008;33(4):443–449. arthritis and bone pathology. Trends Microbiol. 2015;23(1):35–43. [DOI] [PubMed] [Google Scholar]
- [75]. Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008;36(9):2536–2541. 10.1097/CCM.0b013e318183f2d2 [DOI] [PubMed] [Google Scholar]
- [76]. Kam YW, Ong EKS, Rénia L, et al. Immuno-biology of Chikungunya and implications for disease intervention. Microbes Infect. 2009;11:1186–1196. 10.1016/j.micinf.2009.09.003 [DOI] [PubMed] [Google Scholar]
- [77]. Kosasih H, de Mast Q, Widjaja S, et al. Evidence for endemic chikungunya virus infections in Bandung, Indonesia. PLos Negl Trop Dis. 2013;7(10):e2483. 10.1371/journal.pntd.0002483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Sourisseau M, Schilte C, Casartelli N, et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007;3(6):e89. 10.1371/journal.ppat.0030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Couderc T, Chrétien F, Schilte C, et al. A Mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4(2):e29. 10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Prashant S, Kumar AS, Basheeruddin DDM, et al. Cutaneous manifestations in patients suspected of chikungunya disease. Indian J Dermatol. 2009;54(2):128–131. 10.4103/0019-5154.53186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Puiprom O, Morales Vargas RE, Potiwat R, et al. Characterization of chikungunya virus infection of a human keratinocyte cell line: role of mosquito salivary gland protein in suppressing the host immune response. Infect Genet Evol. 2013;17:210–215. 10.1016/j.meegid.2013.04.005 [DOI] [PubMed] [Google Scholar]
- [82]. Thon-Hon VG, Denizot M, Li-Pat-Yuen G, et al. Deciphering the differential response of two human fibroblast cell lines following chikungunya virus infection. Virol J. 2012;9:213. 10.1186/1743-422X-9-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Thangamani S, Higgs S, Ziegler S, et al. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS One. 2010;5(8):e12137. 10.1371/journal.pone.0012137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Couderc T, Gangneux N, Chrétien F, et al. Chikungunya virus infection of corneal grafts. J Infect Dis. 2012;206(6):851–859. 10.1093/infdis/jis296 [DOI] [PubMed] [Google Scholar]
- [85]. Nhan T-X, Fallevoz T, De Pina J-J, et al. Chikungunya virus uveitis during French Polynesia outbreak, 2014-2015. J Clin Case Rep. 2016;6:1000682. [Google Scholar]
- [86]. Cerny T, Schwarz M, Schwarz U, et al. The range of neurological complications in chikungunya fever. Neurocrit Care. 2017;27(3):447–457. 10.1007/s12028-017-0413-8 [DOI] [PubMed] [Google Scholar]
- [87]. Chiam CW, Chan YF, Ong KC, et al. Neurovirulence comparison of Chikungunya virus isolates of the Asian and East/Central/South/African genotypes from Malaysia. J Gen Virol. 2015;96(11):3243–3254. 10.1099/jgv.0.000263 [DOI] [PubMed] [Google Scholar]
- [88]. Inglis FM, Lee KM, Chiu KB, et al. Neuropathogenesis of chikungunya infection: astrogliosis and innate immune activation. 2016;22(2):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Brito CA, Sohsten AK, Leitão CC, et al. Pharmacologic management of pain in patients with Chikungunya: a guideline. Rev Soc Bras Med Trop. 2016;49(6):668–679. 10.1590/0037-8682-0279-2016 [DOI] [PubMed] [Google Scholar]
- [90]. Hawman DW, Stoermer KA, Montgomery SA, et al. Chronic joint disease caused by persistent chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87(24):13878–13888. 10.1128/JVI.02666-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Reddy V, Mani RS, Desai A, et al. Correlation of plasma viral loads and presence of chikungunya IgM antibodies with cytokine/chemokine levels during acute chikungunya virus infection. J Med Virol. 2014;86(8):1393–1401. 10.1002/jmv.v86.8 [DOI] [PubMed] [Google Scholar]
- [92]. Pakran J, George M, Riyaz N, et al. Purpuric macules with vesiculobullous lesions: a novel manifestation of Chikungunya. Int J Dermatol. 2011;50(1):61–69. 10.1111/ijd.2010.50.issue-1 [DOI] [PubMed] [Google Scholar]
- [93]. Peiris JS, Porterfield JS. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature. 1979;282(5738):509–511. 10.1038/282509a0 [DOI] [PubMed] [Google Scholar]
- [94]. Lum FM, Couderc T, Chia BS, et al. Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity. Sci Rep. 2018;8(1):1860. 10.1038/s41598-018-20305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Her Z, Malleret B, Chan M, et al. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol. 2010;184(10):5903–5913. 10.4049/jimmunol.0904181 [DOI] [PubMed] [Google Scholar]
- [96]. Gasque P, Couderc T, Lecuit M, et al. Chikungunya virus pathogenesis and immunity. Vector Borne Zoonotic Dis. 2015;15(4):241–249. 10.1089/vbz.2014.1710 [DOI] [PubMed] [Google Scholar]
- [97]. Schilte C, Couderc T, Chretien F, et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207(2):429–442. 10.1084/jem.20090851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Ng LFP, Chow A, Sun YJ, et al. IL-1β, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS One. 2009;4(1):e4261. 10.1371/journal.pone.0004261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Wauquier N, Becquart P, Nkoghe D, et al. The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204(1):115–123. 10.1093/infdis/jiq006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Kelvin AA, Banner D, Silvi G, et al. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl Trop Dis. 2011;5(8):e1279. 10.1371/journal.pntd.0001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Teng TS, Kam YW, Lee B, et al. A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J Infect Dis. 2015;211(12):1925–1935. 10.1093/infdis/jiv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Chow A, Her Z, Ong EKS, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203(2):149–157. 10.1093/infdis/jiq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. 10.1056/NEJMra0707449 [DOI] [PubMed] [Google Scholar]
- [104]. Chen W, Foo SS, Sims NA, et al. Arthritogenic alphaviruses: new insights into arthritis and bone pathology. Trends Microbiol. 2015. Jan;23(1):35–43. 10.1016/j.tim.2014.09.005 [DOI] [PubMed] [Google Scholar]
- [105]. Chaaithanya IK, Muruganandam N, Raghuraj U, et al. Chronic inflammatory arthritis with persisting bony erosions in patients following chikungunya infection. Indian J Med Res. 2014;140(1):142–145. [PMC free article] [PubMed] [Google Scholar]
- [106]. Teo TH, Lum FM, Claser C, et al. A pathogenic role for CD4+ T cells during chikungunya virus infection in mice. J Immunol. 2013;190(1):259–269. 10.4049/jimmunol.1202177 [DOI] [PubMed] [Google Scholar]
- [107]. Das T, Jaffar-Bandjee MC, Hoarau JJ, et al. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog Neurobiol. 2010;91(2):121–129. 10.1016/j.pneurobio.2009.12.006 [DOI] [PubMed] [Google Scholar]
- [108]. Goupil BA, Mores CN. A review of Chikungunya virus-induced arthralgia: clinical manifestations, therapeutics, and pathogenesis. Open Rheumatol J. 2016;10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis. 2016;214(Suppl 5):S471–S474. 10.1093/infdis/jiw274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Lo Presti A, Lai A, Cella E, et al. Chikungunya virus, epidemiology, clinics and phylogenesis: a review. Asian Pac J Trop Med. 2014;7(12):925–932. 10.1016/S1995-7645(14)60164-4 [DOI] [PubMed] [Google Scholar]
- [111]. Waymouth HE, Zoutman DE, Towheed TE. Chikungunya-related arthritis: case report and review of the literature. Semin Arthritis Rheum. 2013;43(2):273–278. 10.1016/j.semarthrit.2013.03.003 [DOI] [PubMed] [Google Scholar]
- [112]. Powers AM. Vaccine and therapeutic options to control Chikungunya virus. Clin Microbiol Rev. 2018;31(1):e00104–e00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Parashar D, Cherian S. Antiviral perspectives for chikungunya virus. Biomed Res Int. 2014;2014:631642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Rothan HA, Bahrani H, Mohamed Z, et al. A combination of doxycycline and robaravin alleviated chikungunya infection. PLoS One. 2015;10(5):e0126360. 10.1371/journal.pone.0126360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Abdelnabi R, Jochmans D, Verbeken E, et al. Antiviral treatment efficiently inhibits chikungunya virus infection in the joints of mice during the acute but not during the chronic phase of the infection. Antiviral Res. 2018;149:113–117. 10.1016/j.antiviral.2017.09.016 [DOI] [PubMed] [Google Scholar]
- [116]. Couderc T, Khandoudi N, Grandadam M, et al. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis. 2009;200(4):516–523. 10.1086/599176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Broeckel R, Fox JM, Haese N, et al. Therapeutic administration of a recombinant human monoclonal antibody reduces the severity of chikungunya virus disease in rhesus macaques. PLoS Negl Trop Dis. 2017;11(6):e0005637. 10.1371/journal.pntd.0005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Akahata W, Yang ZY, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. 10.1038/nm.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Brandler S, Tangy F. Recombinant vector derived from live attenuated measles virus: potential for flavivirus vaccines. Comp Immunol Microbiol Infect Dis. 2008;31(2–3):271–291. 10.1016/j.cimid.2007.07.012 [DOI] [PubMed] [Google Scholar]
- [120]. Plante K, Wang E, Partidos CD, et al. Novel chikungunya vaccine candidate with an ires-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7(7):e1002142. 10.1371/journal.ppat.1002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Chang LJ, Dowd KA, Mendoza FH, et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384(9959):2046–2052. 10.1016/S0140-6736(14)61185-5 [DOI] [PubMed] [Google Scholar]
- [122]. Goo L, Dowd KA, Lin TY, et al. A virus-like particle vaccine elicits broad neutralizing antibody responses in humans to all chikungunya virus genotypes. J Infect Dis. 2016;214(10):1487–1491. 10.1093/infdis/jiw431 [DOI] [PMC free article] [PubMed] [Google Scholar]


