Abstract
Objectives:
To identify the key points for improving severe maternal morbidity by analyzing pregnancy-related ICU admissions in Beijing.
Design:
This was a retrospective, multicenter cohort study.
Setting:
Three ICUs in tertiary hospitals in Beijing.
Patients:
A total of 491 severe maternal cases in any trimester of pregnancy or within 42 days of delivery were reviewed between January 1, 2008, and December 31, 2016.
Interventions:
None.
Measurements and Main Results:
Among 491 obstetric ICU admissions (median Sequential Organ Failure Assessment score, 2) out of 87,850 hospital deliveries (a frequency of 5.6 admissions per 1,000 deliveries), the leading diagnoses were postpartum hemorrhage (170; 34.62%), hypertensive disorders of pregnancy (156; 31.77%), and cardio-cerebrovascular diseases (78; 15.9%). Comparing 2008–2011 to 2012–2016, the rates of maternal mortality (2.5% vs 1.9%; p = 0.991) and fetal loss (8.5% vs 8.6%; p = 0.977) did not decrease significantly, whereas the rates of ICU admission (3.05% vs 7.85%; p trends < 0.001) and postpartum hemorrhage (23% vs 38.5%; p = 0.002) increased. Hypertensive disorder (150/156; 96.2% transferred to the ICU postpartum, 24/28 women with fetal loss transferred from lower-level hospitals) was an independent maternal factor associated with fetal loss, and infections were the leading cause of maternal death (6/10) in the ICU.
Conclusions:
Our study highlights the increasing rate of intensive care admissions for postpartum hemorrhage. Improving prenatal care quality for pregnancy-induced hypertension and sepsis at lower-level hospitals may improve maternal and fetal outcomes. Specifically, providing more effective regional cooperation before transfer and shifting patients who require continuous surveillance but not necessarily intensive care to a transitional ward in a tertiary hospital would provide more ICU beds for more prenatal intensive care for the most complex medical conditions.
Keywords: fetal loss, intensive care units, maternal mortality, pregnancy-related intensive care unit admissions, prenatal care, severe maternal morbidity
Although the maternal mortality ratio (per 100,000 live births) in China decreased from 141.7 to 17.2 between 1990 and 2013 (1), severe maternal morbidity (SMM) still occurs in approximately 23 per 1,000 live births (2). Increases in maternal obesity (3), advanced maternal age due to the implementation of the two-child policy in China (4), multiple gestation pregnancies resulting from assisted reproductive technology (5), and the number of cesarean deliveries (6) make it likely that maternal mortality and SMM will continue to increase unless improvements to the perinatal care system are made.
Critical maternal care centers were established in Beijing before they were generalized to the whole country for the implementation of regional high-risk pregnancy care (7). Until recently, few relevant studies from China have focused on pregnancy-related ICU admissions (8–12); thus, we conducted a multicenter study that included three tertiary academic centers with critical maternal care centers in Beijing and analyzed maternal ICU admissions to make recommendations for improving maternal outcomes in China.
MATERIALS AND METHODS
This retrospective cohort study was conducted in the ICUs of three hospitals in Beijing City, China, including Peking University Third Hospital, Peking University First Hospital, and Capital Medical University Affiliated Beijing Chao-Yang Hospital. All three are academic hospitals with critical maternal care centers.
All women in any trimester of pregnancy or within 42 days of delivery who were admitted to any of the three ICUs for at least 24 hours between January 2008 and December 2016 were reviewed, regardless of their reason for hospitalization (data from Capital Medical University Affiliated Beijing Chao-Yang Hospital from 2008 to 2011 was missing and could not be counted).
The following data were recorded: demographic characteristics; number of previous pregnancies; cesarean section history; mode of delivery; multiple births; ante/postpartum admission; diagnosis upon ICU admission; total maximum Sequential Organ Failure Assessment (SOFA) (13) score over the duration of the patient’s stay in the ICU, using the most abnormal value for each variable recorded; length of stay in the ICU and hospital; maternal death; fetal losses (fetal loss was recorded at any time, excluding induced or therapeutic abortions and ectopic pregnancies); and the level of intervention provided in the ICU (mechanical ventilation [MV] and the number of days on MV, central venous catheterization, hemofiltration, and plasmapheresis). The complications recorded in the ICU were as follows: acute kidney injury (AKI) (14), heart failure (15), and respiratory failure, which was defined as a SOFA score of 3 or more points for respiration.
Acute diseases leading to ICU admission were categorized as obstetric and nonobstetric. As proposed by Vasquez et al (16), obstetric disorders were defined as disorders occurring only during pregnancy or the postpartum period (17, 18).
The overall frequency of ICU utilization, the case fatality rate, the maternal ICU mortality rate, the hospital maternal mortality rate, and the fetal loss rate were all calculated.
The present study was approved by the ethics committee, and all information obtained was used only to describe the patient population and for data analysis. Categorical variables are presented as numbers (%), and continuous variables are presented as mean ± sd or median (interquartile range) according to their distribution. Normal and nonnormal continuously distributed variables were compared using Student t test and the Mann-Whitney U test, respectively. The categorical variables were compared using the chi-square test or Fisher exact test. A p value less than or equal to 0.05 was considered significant. The absolute rate difference (RD) with 95% CIs was estimated for diagnosis associated with ICU admission. The 95% CIs of the absolute RDs were calculated using the Newcombe-Wilson score method (19).
Bivariate analysis was performed to evaluate the maternal risk factors associated with fetal loss that were identified as significant (p of up to 0.05) in the univariate analysis using the chi-square test with continuity correction or Fisher exact test. The odds ratio (OR) and its respective 95% CI, defined as the exact range, were calculated. The multivariable models were built manually and included variables with a p value less than or equal to 0.05 on the Wald test. All statistical analyses were conducted using the statistical package SPSS, Version 18.0 (SPSS Inc.: Chicago, IL).
RESULTS
Frequency of ICU Utilization
Within the 9-year study period (2008–2016), 87,850 deliveries occurred, and 491 women were admitted to the ICU during or after pregnancy. The average pregnancy-related ICU admission rate was 5.6 per 1,000 deliveries, and it increased from 2008 to 2016 from 3.05 to 7.27 per 1,000 deliveries (p < 0.001). The rate peaked at 7.85 per 1,000 deliveries in 2012, decreased in 2013, and then increased in 2016 (Fig. 1).
Figure 1.
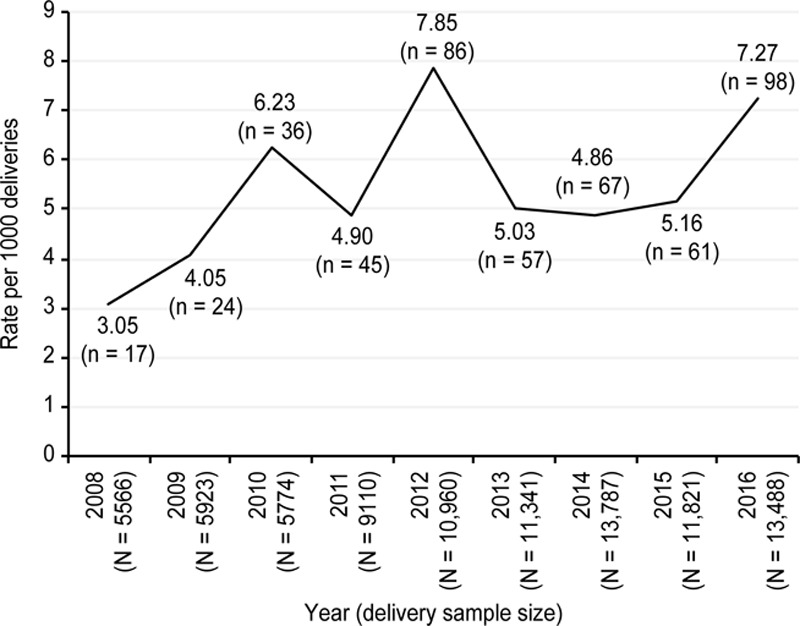
Rate of pregnancy-related ICU admissions, Beijing 2008–2016. There was a significant difference over the 9-yr study period. *Overall trend test: p < 0.001.
Demographics of Pregnancy-Related ICU Admissions
Approximately 60% of the patients in our study were originally from referral centers, 19.6% were transferred to wards directly from another healthcare facility, and 39.3% were admitted from the emergency department originally referred from another facility.
Overall, the median age of the women admitted to the ICUs was 30 years; 16.70% were older than 35 years (Table 1).The median gestation time was 35 weeks. Most of the patients were admitted during the postpartum period (92.26%), 20.8% had a history of cesarean section, and 86.8% had undergone cesarean deliveries. Nulliparous women comprised 44.6% of the ICU admissions, and women with a second child comprised 38.9% (Table 1). Forty-five cases of fetal loss in 43 women occurred in the study group. A total of 66.4% of the subjects were from urban areas. The median length of ICU stay was 2 days, and the median hospital stay was 11 days. The median SOFA score was 2, and 48.1% of the women had SOFA scores greater than or equal to 3.
TABLE 1.
The Demographics of Pregnancy-Related ICU Admissions From 2008 to 2011 and From 2012 to 2016
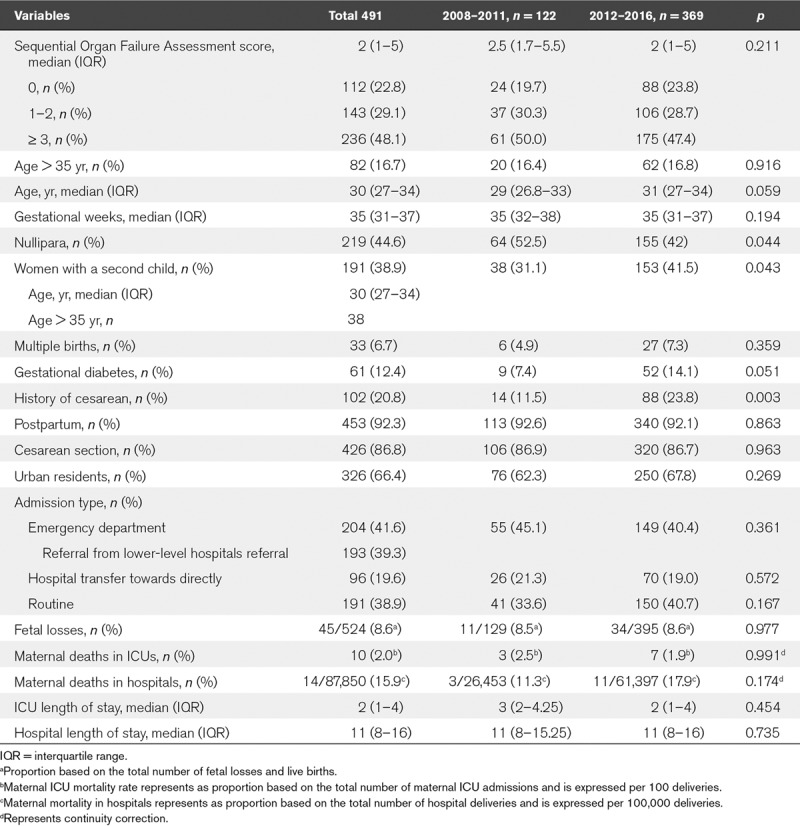
Comparing 2008–2011 to 2012–2016, the percentage of women with advanced age (16.4% vs 16.8%; p = 0.916) and multiple births (4.9% vs 7.3%; p = 0.359) did not increase significantly, whereas the rate of gestational diabetes increased to a near-significant degree (7.4% vs 14.1%; p = 0.051), and the proportion of women with a second child increased (31.1% vs 41.5%; p < 0.05).
Diagnoses Associated With ICU Admission
Over the 9-year study period, the leading diagnoses associated with ICU admission were postpartum hemorrhage (PPH) (170/491), hypertensive disorders of pregnancy (156/491), and cardio-cerebrovascular diseases (78/491). Diseases with median SOFA scores greater than or equal to 3 points included acute fatty liver of pregnancy (8), amniotic fluid embolism (8), PPH (4), hypertensive disorders (3), systemic lupus erythematosus (3), and cardiopulmonary arrest (3) (Table 2).
TABLE 2.
Diagnostic Conditions by Time of ICU Admission From 2008 to 2011 and From 2012 to 2016
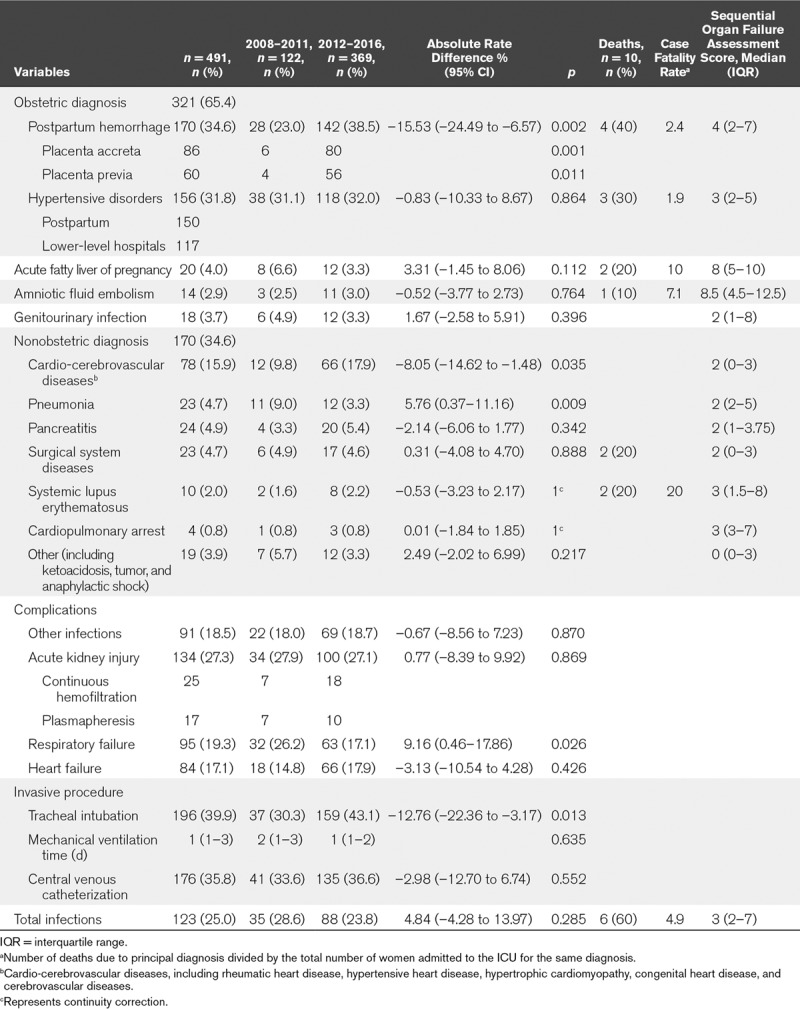
The cause-specific rate of pregnancy-related ICU admissions significantly increased for PPH (23.0–38.5%; p = 0.002) and cardio-cerebrovascular diseases (9.8–17.9%; p = 0.035) and decreased for pneumonia (9.0–3.3%; p = 0.009), but did not change significantly for other diagnoses (Table 2).
PPH (170/491; 34.62%) was the main reason for ICU admission (Table 2). Placenta accreta (86/170) and placenta previa (60/170) accounted for 50.6% and 35.29% of PPH cases, respectively, and occurred concurrently in 56 women. Over the 9-year study period, the number of PPH cases caused by placenta accreta and placenta previa increased significantly (80/142, 56.3% vs 6/28, 21.4%; p < 0.05 and 4/28, 39.4% vs 56/142, 14.3%; p < 0.05, respectively) (Table 2).
Complications and Invasive Operations Upon ICU Admission
The most common end-organ injuries among the obstetric patients who received intensive care were AKI (27.3%), with 25 cases of continuous hemofiltration and 17 cases of plasmapheresis; respiratory failure (19.3%); and heart failure (17.1%). The rate of tracheal intubation increased significantly over the study period (30.3% vs 43.1%; p = 0.013); in most cases, intubation was not performed because of respiratory failure but for general anesthesia in the operating room. Respiratory failure decreased (26.2–17.1%; p = 0.026) over the study period.
Maternal Mortality Rate and Fetal Loss
The overall maternal mortality rates were 2.0 per 100 obstetric patients admitted to the ICU and 15.9 per 100,000 hospital deliveries. The fetal loss rate was 8.6 per 100 births. During the periods from 2008 to 2011 and from 2012 to 2016, no significant differences were found regarding the ICU or hospital maternal mortality rates (2.5 per 100 deliveries to 1.9 per 100 deliveries in ICUs; p = 0.991 and 11.3 per 100,000 deliveries to 17.9 per 100,000 deliveries in hospitals; p = 0.174) (Table 1). There were 14 maternal deaths at the three hospitals. Ten women died in the ICU. Infections (6/10, including four nonobstetric severe sepsis cases sent by referral that were not unique to pregnancy or postpartum; these included one gastrointestinal perforation, one necrotic infection of the limbs, and two cases of severe pneumonia) and PPH (4/10) were the leading causes of maternal death. Another four women died outside the ICU; one died in the emergency ward due to pulmonary hypertension, two died in the obstetrics ward due to rupture associated with aortic dissection and amniotic fluid embolism, and one died in the neurology ward due to subarachnoid hemorrhage.
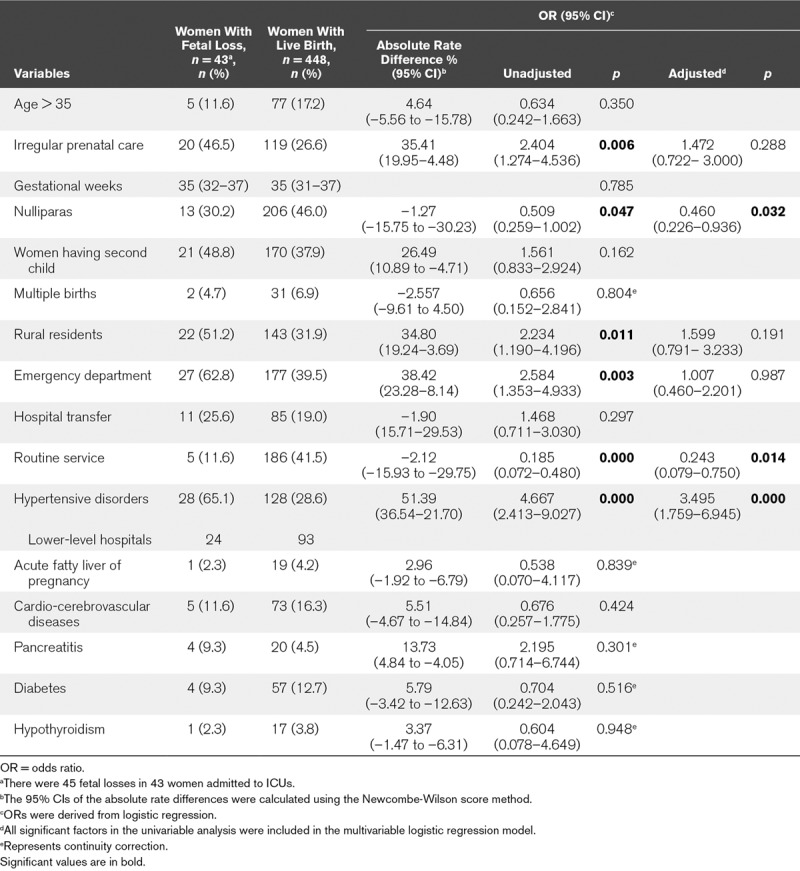
The rate of fetal loss did not decrease over the study period (8.5% vs 8.6%; p = 0.977) (Table 1). Binary logistic analysis showed that hypertensive disorders of pregnancy represented an independent maternal risk factor for fetal loss (Table 3). The adjusted OR values (< 1) of the nulliparous women and those admitted by the routine service indicated that women with these two characteristics had a lower risk of fetal loss.
TABLE 3.
Variables That Were Independently Associated With Fetal Loss in 491 Obstetric ICU Cases
DISCUSSION
The improvement of perinatal care worldwide has focused on decreasing maternal morbidity and mortality (20–22). In China, unlike the maternal care levels designated in the United States (23), maternal care has been provided among primary, secondary, and tertiary hospitals according to risk levels (indicated by the colors green, yellow, orange, red, and purple) since 2017 (24). ICU beds for critically ill obstetric patients have been required since 2017 at secondary and tertiary hospitals with critical maternal care centers, where physicians use the most advanced technology (7). Maternal ICU admission is recognized as an indicator of the SMM (25–27).
The ICU admissions of mothers with advanced age and multiple births did not increase significantly, possibly due to reduced enthusiasm for a second child among women of advance aged, economic burdens (28), and less access to assisted reproductive technology facilities per citizen (29). The nearly significant increase in the ICU admission of women with gestational diabetes is recognized as a risk related to obesity (30), indicating the urgent need to give more attention to controlling excessive weight gain.
We observed an increased frequency of maternal ICU admissions and a greater frequency of ICU utilization than other reports (5.6 vs international reports of 2.7 per 1,000 deliveries) (27), excluding the study by Oud (31) (39 per 1,000 pregnancy-associated hospitalization-years). This increasing frequency appeared to be due in part to the growth in the diagnosis of PPH cases, especially the increase in placenta accreta.
The increased frequency of PPH is consistent with the findings reported for high-resource countries (32–37). It is possible that the pursuit of admission for the advanced perioperative management of placenta accreta at Peking University Third Hospital led to the higher proportion of placenta accreta-related PPH compared with other reports (38).
Despite the lower ICU maternal mortality rate (2% vs 3.4%) (27) in our study, which, as Chantry et al (39) indicated, may reflect a lower threshold for ICU admission (considering that 112/491 women had a SOFA score = 0), the three academic hospitals included in this study had higher maternal mortality rates overall compared with developed countries (15.9 vs 12.1 per 100.000 deliveries) (1), which indicates that greater efforts must be made to improve maternal outcomes in China. Strengthening perinatal care for infections, which were the leading cause of maternal mortality in our study, is a challenge. Although sepsis does not rank among the top three causes of maternal death worldwide (which are major hemorrhage, abortion, and pregnancy-related hypertensive disorders) (1), the rate of growth in pregnancy-associated severe sepsis (236%) over the past decade (40) is alarming. The increasing mortality rate from sepsis (41–43) shows that obstetric patients with sepsis should be given medical advice quickly and should be subject to heightened clinician vigilance to facilitate early diagnosis and timely and effective intervention. Three out of four nonobstetric severe sepsis-related deaths in our study may have been preventable with intensive prenatal care at the hospitals where they were initially treated, more timely risk assessment, and more timely transfer to tertiary hospitals with the capacity to provide critical maternal care. More attention should be paid to the implementation of sepsis bundles before a referral, and effective regional cooperation between obstetricians at lower-level hospitals and experts at tertiary hospitals, including obstetricians, ICU physicians, surgeons, and anesthesiologists, should be strengthened.
We found that hypertensive disease of pregnancy was a risk factor for fetal loss, and Allen et al (44) mentioned it as a risk factor for stillbirth. Despite the increased rate of maternal admissions, the admission rate for hypertensive disease of pregnancy remained unchanged. The majority of women with fetal loss (24/28) related to hypertensive disorder were initially cared for at lower-level hospitals, and the lower admission rates during the antepartum period at the three tertiary hospitals compared with other studies (45, 46) hints at the need to improve the quality of prenatal care for hypertensive disease of pregnancy at lower-level hospitals and to address the paucity of ICU beds for obstetrical patients with SMM in tertiary hospitals. Deficiencies in the close follow-up of specific maternal conditions prior to admission for labor and birth, incompetence in caring for complicated conditions, and failure to identify the proper time for transfer to higher-level maternal care are pertinent issues in lower-level hospitals. A team of family physicians available as needed for consultation onsite, by phone (as Su et al [47] designed), or by telemedicine should be established to improve the quality of care before admission for labor and birth to strengthen continuous care for women with complicated conditions. As one of the top aims of intensified national healthcare reform in 2017 (48), reducing the differences between lower-level hospitals and tertiary hospitals by optimizing the distribution of medical resources is important to decrease SMM in China. Under these circumstances, tertiary hospitals with critical maternal care centers should provide educational and consultation functions for lower-level hospitals to a greater extent, even providing training for quality improvement initiatives as specified in the functions of level IV maternal centers in the United States (23). With the implementation of risk assessment and networks for SMM surveillance (49), early risk assessment (50, 51), and timely transfer to risk-appropriate hospitals (52–54) would play key roles in improving maternal and fetal outcomes. In tertiary hospitals, women who require continuous surveillance but not necessarily intensive care should be shifted to a transitional ward to provide more ICU beds for prenatal intensive care for the most complicated cases (i.e., women with hypertensive diseases of pregnancy and a SOFA score ≥ 2).
CONCLUSIONS
Our study highlights the increasing role of PPH in ICU admissions. Improving prenatal care quality for pregnancy-induced hypertension and sepsis at lower-level hospitals may improve maternal and fetal outcomes. Specifically, providing more effective regional cooperation before transfer and shifting patients who require continuous surveillance but not necessarily intensive care to a transitional ward in a tertiary hospital would provide more ICU beds for more prenatal intensive care for the most complex medical conditions.
ACKNOWLEDGMENTS
We thank Xiaoyue Guo, PhD, and Yueming Sun, BS, who collected data throughout the study. They are from the Department of Gynecology and Obstetrics, Peking University Third Hospital, and the Department of Surgical ICU, Peking University First Hospital, respectively. They received no compensation for their contributions.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan J, Chen M, Li Y, et al. Modeling to predict severe maternal morbidity based on 33993 deliveries of registered study in China. Value Health 2014; 17:A750. [DOI] [PubMed] [Google Scholar]
- 3.Leung TY, Leung TN, Sahota DS, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. BJOG 2008; 115:1529–1537. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HX, Zhao YY, Wang YQ. Analysis of the characteristics of pregnancy and delivery before and after implementation of the two-child policy. Chin Med J (Engl) 2018; 131:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Li Y, Li C, et al. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril 2014; 101:385–391. [DOI] [PubMed] [Google Scholar]
- 6.Li HT, Luo S, Trasande L, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008–2014. JAMA 2017; 317:69–76. [DOI] [PubMed] [Google Scholar]
- 7.National Health and Family Planning Commission of the People’s Republic of China: Guidelines for the construction and management of critical maternal centers. J Dev Med 2018; 1:1–6. [Google Scholar]
- 8.Yuqi L, Tan G, Chengming S, et al. The ICU is becoming a main battlefield for severe maternal rescue in China: An 8-year single-center clinical experience. Crit Care Med 2017; 45:e1106–e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang LC, Kwok AC, Wong AY, et al. Critical care in obstetrical patients: An eight-year review. Chin Med J (Engl) 1997; 110:936–941. [PubMed] [Google Scholar]
- 10.Chu Y, Yuan Z, Meng M, et al. Red blood cell distribution width as a risk factor for inhospital mortality in obstetric patients admitted to an intensive care unit: A single centre retrospective cohort study. BMJ Open 2017; 7:e012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YQ, Ge QG, Wang J, et al. The WHO near miss criteria are appropriate for admission of critically ill pregnant women to intensive care units in China. Chin Med J (Engl) 2013; 126:895–898. [PubMed] [Google Scholar]
- 12.Hou L, Wang X, Li G, et al. Cross sectional study in China: Fetal gender has adverse perinatal outcomes in mainland China. BMC Pregnancy Childbirth 2014; 14:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working group on sepsis related problems of the ESICM. Intensive Care Med 1999; 25:686–696. [DOI] [PubMed] [Google Scholar]
- 14.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez DN, Estenssoro E, Canales HS, et al. Clinical characteristics and outcomes of obstetric patients requiring ICU admission. Chest 2007; 131:718–724. [DOI] [PubMed] [Google Scholar]
- 17.Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: Case-control study. BMJ 2001; 322:1089–1093; discussion 10931094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham W, Wagaarachchi P, Penney G, et al. Criteria for clinical audit of the quality of hospital-based obstetric care in developing countries. Bull World Health Organ 2000; 78:614–620. [PMC free article] [PubMed] [Google Scholar]
- 19.Newcombe RG. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat Med 1998; 17:873–890. [DOI] [PubMed] [Google Scholar]
- 20.Brantley MD, Davis NL, Goodman DA, et al. Perinatal regionalization: A geospatial view of perinatal critical care, United States, 2010–2013. Am J Obstet Gynecol 2017; 216:185.e1–185.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayburn WF, Richards ME, Elwell EC. Drive times to hospitals with perinatal care in the United States. Obstet Gynecol 2012; 119:611–616. [DOI] [PubMed] [Google Scholar]
- 22.Knight M, Acosta C, Brocklehurst P, et al. Beyond maternal death: Improving the quality of maternal care through national studies of ‘near-miss’ maternal morbidity. Programme Grants Appl Res 2016; 4:1–180. [PubMed] [Google Scholar]
- 23.Menard MK, Kilpatrick S, Saade G, et al. ; American College of Obstetricians and Gynecologists and Society for Maternal–Fetal Medicine: Levels of maternal care. Am J Obstet Gynecol 2015; 212:259–271. [DOI] [PubMed] [Google Scholar]
- 24.National Health and Family Planning Commission of the People’s Republic of China: Pregnancy risk assessment and management work rules. Chin Pract J Rural Doct 2017; 12:5–7. [Google Scholar]
- 25.Zeeman GG. Obstetric critical care: A blueprint for improved outcomes. Crit Care Med 2006; 34:S208–S214. [DOI] [PubMed] [Google Scholar]
- 26.Zwart JJ, Dupuis JR, Richters A, et al. Obstetric intensive care unit admission: A 2-year nationwide population-based cohort study. Intensive Care Med 2010; 36:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock W, Rose L, Dennis CL. Pregnant and postpartum admissions to the intensive care unit: A systematic review. Intensive Care Med 2010; 36:1465–1474. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Cai Y, Feng W, et al. Below replacement fertility and childbearing intention in Jiangsu Province, China. Asian Popul Stud 2009; 5:329–347. [Google Scholar]
- 29.Qiao J, Feng HL. Assisted reproductive technology in China: Compliance and non-compliance. Transl Pediatr 2014; 3:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding XX, Xu SJ, Hao JH, et al. Maternal pre-pregnancy BMI and adverse pregnancy outcomes among Chinese women: Results from the C-ABCS. J Obstet Gynaecol 2016; 36:328–332. [DOI] [PubMed] [Google Scholar]
- 31.Oud L. Epidemiology of pregnancy-associated ICU utilization in Texas: 2001 - 2010. J Clin Med Res 2017; 9:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph KS, Rouleau J, Kramer MS, et al. ; Maternal Health Study Group of the Canadian Perinatal Surveillance System: Investigation of an increase in postpartum haemorrhage in Canada. BJOG 2007; 114:751–759. [DOI] [PubMed] [Google Scholar]
- 33.Ford JB, Roberts CL, Simpson JM, et al. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet 2007; 98:237–243. [DOI] [PubMed] [Google Scholar]
- 34.Lutomski JE, Byrne BM, Devane D, et al. Increasing trends in atonic postpartum haemorrhage in Ireland: An 11-year population-based cohort study. BJOG 2012; 119:306–314. [DOI] [PubMed] [Google Scholar]
- 35.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013; 209:449.e1–449.e7. [DOI] [PubMed] [Google Scholar]
- 36.Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: A review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth 2009; 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 2010; 202:353.e1–353.e6. [DOI] [PubMed] [Google Scholar]
- 38.Mehrabadi A, Hutcheon JA, Liu S, et al. Contribution of placenta accreta to the incidence of postpartum hemorrhage and severe postpartum hemorrhage. Obstet Gynecol 2015; 125:814–821. [DOI] [PubMed] [Google Scholar]
- 39.Chantry AA, Deneux-Tharaux C, Bonnet MP, et al. Pregnancy-related ICU admissions in France: Trends in rate and severity, 2006–2009. Crit Care Med 2015; 43:78–86. [DOI] [PubMed] [Google Scholar]
- 40.Oud L, Watkins P. Evolving trends in the epidemiology, resource utilization, and outcomes of pregnancy-associated severe sepsis: A population-based cohort study. J Clin Med Res 2015; 7:400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent JL, Marshall JC, Namendys-Silva SA, et al. ; ICON Investigators: Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med 2014; 2:380–386. [DOI] [PubMed] [Google Scholar]
- 42.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272. [DOI] [PubMed] [Google Scholar]
- 43.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011; 140:1223–1231. [DOI] [PubMed] [Google Scholar]
- 44.Allen VM, Joseph K, Murphy KE, et al. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: A population based study. BMC Pregnancy Childbirth 2004; 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashraf N, Mishra SK, Kundra P, et al. Obstetric patients requiring intensive care: A one year retrospective study in a tertiary care institute in India. Anesthesiol Res Pract 2014; 2014:789450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanderer JP, Leffert LR, Mhyre JM, et al. Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008*. Crit Care Med 2013; 41:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su Y, Yuan C, Zhou Z, et al. Impact of an SMS advice programme on maternal and newborn health in rural China: Study protocol for a quasi-randomised controlled trial. BMJ Open 2016; 6:e011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The State Council of the People’s Republic of China: State Council Rolls Out Plan to Intensify Medical Reform. 2017. Available at: http://english.gov.cn/policies/latest_releases/2017/01/09/content_281475537307114.htm. Accessed July 24, 2018
- 49.Gan XL, Hao CL, Dong XJ, et al. Provincial maternal mortality surveillance systems in China. Biomed Res Int 2014; 2014:187896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields LE, Wiesner S, Klein C, et al. Use of maternal early warning trigger tool reduces maternal morbidity. Am J Obstet Gynecol 2016; 214:527.e1–527.e6. [DOI] [PubMed] [Google Scholar]
- 51.Martin JN, Jr, May WL, Magann EF, et al. Early risk assessment of severe preeclampsia: Admission battery of symptoms and laboratory tests to predict likelihood of subsequent significant maternal morbidity. Am J Obstet Gynecol 1999; 180:1407–1414. [DOI] [PubMed] [Google Scholar]
- 52.Paneth N, Kiely JL, Wallenstein S, et al. Newborn intensive care and neonatal mortality in low-birth-weight infants: A population study. N Engl J Med 1982; 307:149–155. [DOI] [PubMed] [Google Scholar]
- 53.Gortmaker S, Sobol A, Clark C, et al. The survival of very low-birth weight infants by level of hospital of birth: A population study of perinatal systems in four states. Am J Obstet Gynecol 1985; 152:517–524. [DOI] [PubMed] [Google Scholar]
- 54.Clapp MA, James KE, Kaimal AJ. The effect of hospital acuity on severe maternal morbidity in high-risk patients. Am J Obstet Gynecol 2018; 219:111.e1–111.e7. [DOI] [PubMed] [Google Scholar]


