Abstract
The human epidermal growth factor receptor 2 (HER2) is overexpressed in 25%–30% of breast cancer patients. Anti-HER2 therapies have changed the aggressive course of HER2+ breast cancer. In spite of the therapeutic benefits, their cardiotoxicities are major concerns, especially when used concurrently with anthracyclines.
Here we present an elderly patient with relapsed HER2+ breast cancer. Her presentation for relapsed disease was unusual for the physical finding as well as the history of trastuzumab-induced severe cardiotoxicity while requiring additional anti-HER2 therapy. She received neoadjuvant anti-HER2 treatment for stage III breast caner. Due to severe reduction of cardiac ejection fraction (EF), she only received five doses of adjuvant transtuzumab. Unfortunately her disease relapsed one year later with chest wall lesions and a persistent low EF. We treated the patient with lapatinib combined with capecitabine which resulted rapid resolution of her chest wall lesion. More importantly, the patient had one year of disease control without deterioration in her ejection fraction. We discussed the management of recurrent HER2+ breast cancer with chest wall disease and the choice of anti-HER2 therapy in patients with a history of transtuzumab-induced cardiac dysfunction.
Keywords: Diagnosis and management, HER2 positive breast cancer, Lapatinib, Trastuzumab-induced cardiac toxicity, Unusual presentation
Patient
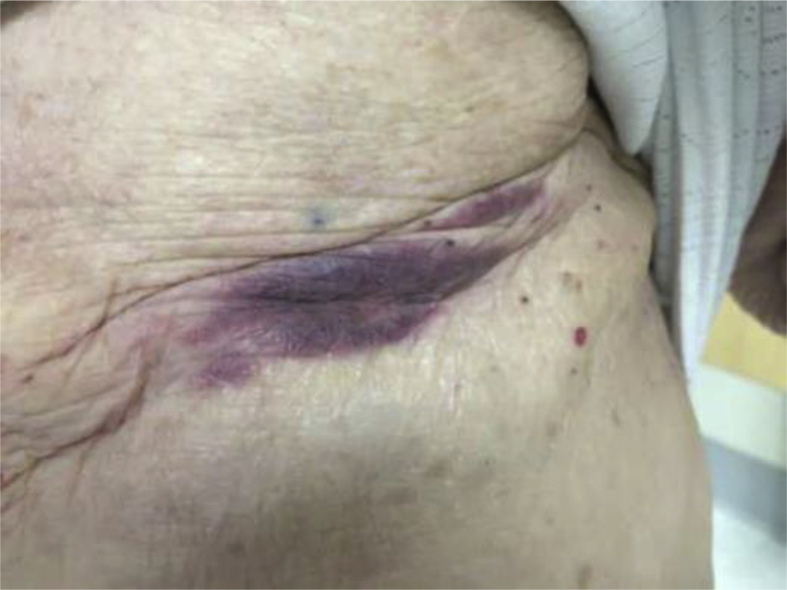
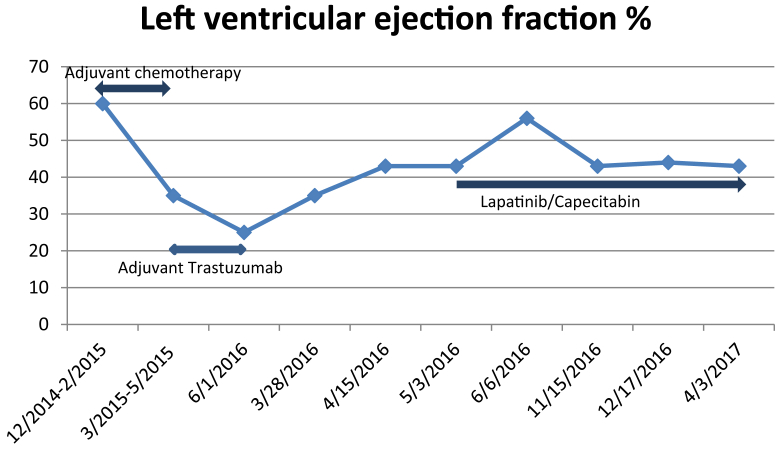
Our patient is an 82 year-old woman who was diagnosed at an outside facility with stage III, ER/PR−, HER2+ left breast cancer. She had no history of cardiac dysfunction, hypertension or diabetes. She completed only three out of the four planned cycles of neoadjuvant pertuzumab, docetaxel and pertuzumab (PTH) on October 7, 2014 because of grade 3 fatigue. Left ventricular ejection fraction was not monitored during her neoadjuvant therapy. She had left mastectomy and sentinel lymph node dissection on November 25, 2014. The pathology report showed twelve of the seventeen nodes were positive for invasive ductal adenocarcinoma. From December 29, 2014 to February 9, 2015, she received three cycles of adjuvant chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). On February 2, 2015, an echocardiogram showed an ejection fraction of 60%. Adjuvant trastuzumab every three weeks was started on March 2, 2015, ending in May 2015 due to a decline in ejection fraction to 35% which further dropped to 25% with severe fatigue in June 2015 without other symptoms or signs of congestive heart failure. She was evaluated by a cardiologist and was started on ACE inhibitor but couldn't tolerate this therapy because of cough. Lisinopril was replaced by valsartan and she continued on the beta-blocker carvedilol. The patient received adjuvant radiation therapy from March 24, 2015 to May 11, 2015. She received only 5 doses of adjuvant trastuzumab. Follow up PET scan on February 29, 2016 showed no evidence of recurrent disease. In March 2016, she developed a purplish discoloration on her left mastectomy skin flap along the incision which widened over the following 3 weeks (Fig. 1). Biopsy from left chest wall skin on March 21, 2016 confirmed the local recurrent of ER/PR-negative and HER2+ disease. Repeat echocardiogram revealed an ejection fraction of 40% on March 28, 2016. At that time she continued to be active with a Karnofsk performance status of 70%. The patient was referred to us to decide on the modality of therapy and the anti-HER2 agents to choose in the presence of persistent trastuzumab-induced cardiac toxicity. A restaging PET scan on April 21st 2016 showed no evidence of visceral disease.
Figure 1.
Skin lesions below prior mastectomy site.
Her case was discussed at our breast tumor board. Her skin lesions were considered difficult to resect or radiate due to the infiltrative nature of the skin involvement and prior chest wall radiation. Since her tumor was ER/PR−, the decision was made to start her on a systemic anti-HER2 agent with chemotherapy. After literature search and discussion with the patient, we offered her lapatinib combined with capecitabine. She received the first cycle on May 3rd 2016. Her purplish skin lesions disappeared three weeks later. Her ejection fraction increased from a baseline of 43%–56%, one month after starting lapatinib, which came down to 44% with no further drop afterward (Fig. 2). After three cycles, of drug treatment a PET scan showed significant improvement of the extensive soft tissue disease at the site of the left mastectomy. We reduced the capecitabine from 2 gm/m2 to 1.5 gm/m2 on cycle 4 because of fatigue and diarrhea. On November 10th, 2016 we further reduced the capecitabine dose to 1 gm/m2 because of hand/foot syndrome and worsening diarrhea. Her ejection fraction remained above 43% during treatment from April 2016 to April 2017 with no symptoms of heart failure. At the last visit on May 8th 2017, the patient was alive with no evidence of disease per last PET scan.
Figure 2.
Changes in left ventricular ejection fraction while the patient was on anti-HER2 therapy.
Discussion
The incidence of locoregional recurrent (LRR) breast cancer has become relatively decreased following advances in adequate treatment of primary disease. However LRR poses a therapeutic challenge in terms of how aggressive the therapy should be and the modality of therapy needed, especially in patients with HER2+ disease and trastuzumab-induced cardiomyopathy. One recent review suggests that prognosis for patients with LRR is not universally poor, and that aggressive locoregional and systemic therapy is warranted in certain patients who may achieve long-term survival. LRR treatments can include surgery and/or radiation therapy. Adjuvant systemic therapy including chemotherapy, hormonal therapy or anti-HER2 therapy also may offer these patient better survival and adequate long-term disease control.1 The purple skin discoloration from the recurrent HER2+ breast cancer in our patient fortuitously served as an indicator of the infiltrative nature of the chest wall relapse, for which local therapy was unlikely to be feasible. Since her tumor was ER/PR−, we offered this patient palliative systemic anti-HER2 therapy with chemotherapy.
Our patient's severe cardiac dysfunction before relapsed breast cancer was likely caused by trastuzumab, as evidenced by her normal cardiac EF after three cycles of FEC and before the adjuvant trastuzumab. However, trastuzumab usually causes reversible cardiac dysfunction, while anthracyclines may result in chronic congestive heart failure. Our patient's prolonged cardiac dysfunction of almost one year after trastuzumab exposure suggests that both anthracycline and trastuzumab may have been involved even though she received only 300 mg/m2 cumulative dose of epirubicin. Her advanced age and adjuvant radiation to the left chest wall after epirubicin and concurrently with trastuzumab also may have contributed to the development of cardiac dysfunction, although concurrent radiation and trastuzumab was deemed safe without increased cardiac toxicity.2 We cannot rule out that she had unrecognized coronary artery disease at the time of her breast cancer diagnosis.
The need for systemic anti-HER2 therapy in the presence of trastuzumab-induced myocardial dysfunction posed another dilemma in the management of our patient. All currently approved ant-HER2 therapies, including trastuzumab, pertuzumab, ado-trastuzumab emtansine (TDM-1) and lapatinib cause cardiac toxicity.3 HER2 expression in the myocardium plays an important compensatory role in dealing with stress. HER2 upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity.4 Trastuzumab is known to cause an asymptomatic drop in the left ventricular ejection fraction (LVEF) or, less frequently, congestive heart failure (CHF). This trastuzumab-associated cardiotoxicity results from blocking neuregulin-1 (NRG-1)-mediated activation of HER2, downregulation of the anti-apoptotic protein BCL-XL, and upregulation of the pro-apoptotic protein BCL-XS which affects mitochondria function.5 Through oxidative stress trastuzumab also upregulates angiotensin II which induces apoptosis through the AT1 receptor. It also activates NADPH oxidase leading to cell death through mitochondrial dysfunction.6 The incidence of cardiac dysfunction from single agent trastuzumab is 3%–7%, which goes up to 27% with concomitant trastuzumab and anthracycline plus cyclophosphamide.7 Pertuzumab is a monoclonal antibody that inhibits HER2–HER3 hetero-dimerization through binding to domain II of HER2 receptor.8 The combination of trastuzumab with pertuzumab in the metastatic setting does not seem to result in increased cardiac toxicity, when compared to trastuzumab treatment, alone.9 T-DM1 is a trastuzumab-emtansine conjugate. It has been found to have favorable efficacy in a clinical trial comparing T-DM-1 to a combination of lapatinib and capecitabine in metastatic breast cancer patients who had progressed on trastuzumab (EMILIA).10 Lapatinib is a dual tyrosine kinase inhibitor (TKI) of EGFR and HER2.11 It binds to the intracellular HER2 domain, compared to trastuzumab, pertuzumab and TD-M1, all of which bind to the extracellular HER2 domain. Preclinical data have shown that lapatinib does not have the same cardiotoxic effects as those induced by the other anti-HER2-targeted agents.12, 13 Currently, there is no direct comparison of the cardiac safety among various anti-HER2 therapies based on clinical trials. Lapatinib has shown a slightly lower incidence of cardiac events numerically compared to trastuzumab14, 15,16 or T-DM110, respectively. Clinical data on the use of anti-HER2 therapy in elderly patients (more than 70 years old) is limited, since most studies have exclude them from study entry. Available data from phase III clinical trials suggest a higher risk of cardiotoxicity in patients above 60 years of age when given trastuzumab.17
Based on our literature review and discussion with the patient, we offered our patient lapatinib with capecitabine to treat her HER2+ chest wall recurrent breast cancer, with close monitoring of her symptoms and regular monitoring of her ejection fraction every 3 months. Her chest disease responded rapidly with resolution of the skin discoloration within three weeks, without any clinical symptoms of congestive heart failure. More importantly, her ejection fraction actually increased after one month then stabilized for one year on lapatinib treatment. Our case indicates that lapatinib and capecitabine are safe and effective in patients with trastuzumab-induced cardiomyopathy, and in elderly patients with HER2+ chest recurrent disease.
Ongoing clinical research in the anti-HER2-induced cardiac dysfunction centers on the development of novel anti-HER2 TKI's such as neratinib and ONT-380.18 The use of cardioprotective agents such as ACE inhibitors or beta blockers is being explored, especially in high risk patients.19
In summary, our case was indeed a clinical challenge in terms of diagnosis, treatment options for locally recurrent HER2+ breast cancer, especially with a need for continued anti-HER2 therapy in the presence of prior trastuzumab-induced severe cardiomyopathy. Systemic therapy is effective for LRR with chest wall disease when local therapy is not feasible, even for HER2+ recurrent breast cancer. In addition, continued anti-HER2 therapy can be safely administered if agents with lower cardiotoxicity, such as lapatinib, are chosen. The fact that our patient's cardiac function was maintained throughout one year of lapatinib treatment and was disease-free following this treatment, support our treatment choice. Future clinical trials will be required to determine the effectiveness of newer generation TKI's as well as the potential benefit of administration of cardioprotective agents in the treatment of HER2+ breast cancer.
Conflicts of interest
The authors declare no conflict of interest in the publication of this manuscript.
Acknowledgement
We thank Dr. Edward Yeh from the University of Missouri for his kind comments and suggestions during the preparation of this manuscript. This work was supported by grants from the NIH (K12HD085817) and the Susan G. Komen Foundation (KG111460) to NJM.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Chand A.R., Ziauddin M.F., Tang S.C. Can locoregionally recurrent breast cancer be cured? Clin Breast Cancer. 2017 Mar 2 doi: 10.1016/j.clbc.2017.02.007. pii: S1526–8209(17)30113-1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Doyle J.J., Neugut A.I., Jacobson J.S. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. Epub 2007 Mar 2. [DOI] [PubMed] [Google Scholar]
- 3.Pondé Noam F., Lambertini Matteo, de Azambuja Evandro. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1(4) doi: 10.1136/esmoopen-2016-000073. Published online 2016 Jul 21 PMCID: PMC5070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupert C.E., Coulombe K.L. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights. 2015;10(suppl 1):1–9. doi: 10.4137/BMI.S20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spector N.L., Blackwell K.L. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 6.Kuramochi Y., Guo X., Sawyer D.B. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghigo A., Li M., Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim Biophys Acta. 2016;1863:1916–1925. doi: 10.1016/j.bbamcr.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Capelan M., Pugliano L., De Azambuja E. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24:273–282. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 9.Swain S.M., Ewer M.S., Cortés J. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krop I.E., Lin N.U., Blackwell K. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015 Jan;26(1):113–119. doi: 10.1093/annonc/mdu486. Epub 2014 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer C.E., Forster J., Lindquist D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Di Leo A., Gomez H.L., Aziz Z. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackwell K.L., Burstein H.J., Storniolo A.M. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 14.Azim Hamdy, Azim Hatem A., Jr., Escudier Bernard. Trastuzumab versus lapatinib: the cardiac side of the story. Cancer Treat Rev. November 2009;35(7):633–638. doi: 10.1016/j.ctrv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Piccart-Gebhart Martine, Holmes Eileen, Baselga José. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2–positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. April 2016;34(10):1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Untch Michael, Loibl Sibylle, Bischoff Joachim. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet. February 2012;13(2):135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 17.Hurria A., Mohile S.G., Dale W. Research priorities in geriatric oncology: addressing the needs of an aging population. J Natl Compr Canc Netw. 2012;10:286–288. doi: 10.6004/jnccn.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin M., López-Tarruella S. Emerging therapeutic options for HER2-positive breast cancer. Am Soc Clin Oncol Educ Book. 2016;35:e64–e70. doi: 10.1200/EDBK_159167. [DOI] [PubMed] [Google Scholar]
- 19.The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]