Abstract
In clinical practice, the important hygienic prevention of bacterial pathogen spread is disinfection of potentially contaminated area. Benzalkonium bromide and chlorhexidine acetate are commonly used disinfectants with a broad spectrum of anti-microbial effect. It is vital to inhibit the spread of pathogen in hospital. However, a large number of pathogens with the decreased antiseptic susceptibility have been isolated from clinical samples which showed an increased minimal inhibitory concentration (MIC) against those antiseptics. These resistant pathogens are the major causes for nosocomial cross-infections in hospital. The present study demonstrated the utility of Oxford plate assay system in determining the potential disinfectant resistance of bacteria. The microbiological assay is based on the inhibitory effect of tested disinfectants upon the strains of Staphylococcus aureus and Escherichia coli. Statistical analysis of the bioassay results indicated the linear correlation (r = 0.87–0.99, P < 0.01) between the diameter of growth inhibition zone and the log dosage of the tested disinfectants. Moreover, comparison of inhibitory efficacy of benzalkonium bromide upon 29 S. aureus strains isolated from clinical samples by both Oxford plate method and broth dilution method showed that the diameter of growth inhibition zone has significantly negative correlation with the minimal inhibitory concentration (MIC) (r = −0.574, P < 0.001). These results suggest that the Oxford plate is a simple and time-saving method in detecting potential clinical disinfectant resistance and its usefulness for routine surveillance of pathogenic resistance to disinfectants warrants further investigation.
Keywords: Clinical technology, Disinfectants resistance, Inhibitory efficacy, Oxford plate method, Staphylococcus aureus
Introduction
In clinical practice, the important hygienic prevention of bacterial pathogen spread is disinfection of potentially contaminated area, such as rooms, utensils and hands. Some antiseptics and disinfectants, sharing common characteristics,1 such as irritation, contact dermatitis, and urticaria, have to be carefully used at minimal bactericidal concentrations in controlling pathogen contamination. For example, povidone-iodine and chlorhexidine are important antiseptics for decreasing skin contaminations of Gram-positive and Gram-negative bacteria, especially those antibiotic-resistant strains, such as MRSA and CA-MRSA. Aldehydes and quaternary ammonium compounds, such as benzalkonium bromide and benzalkonium chloride, are wildly used as disinfectants for sterilization of non-living objects or surfaces.2, 3 The usage of those chemical antiseptics and disinfectants is the vital way to inhibit the spread of pathogen in hospital. However, a large number of pathogens with the decreased antiseptic susceptibility have been isolated from clinical samples which showed an increased minimal inhibitory concentration (MIC) against those antiseptics.4, 5, 6, 7, 8 These resistant pathogens are the major causes for nosocomial cross-infections in hospital. Sometimes, the infection is fatal. Since 1980s, Pseudomonas and Pseudomonas cepacia have been isolated in iodophor and polyvinylpyrrolidone-iodine complex (PVP-I) respectively. And the last one has resulted in pseudobacteremia.1 Similarly, Gram-positive bacteria resistance has also been reported in the past decades. The activity of three Enterococcus spp strains can last for 5 min in 100 ppm available chlorine, while only 2 min for non-resistant Enterococcus in 0.5 ppm chlorine.9 Based on these clinical observations it's important and crucial to monitor pathogen resistance against system antiseptics and disinfectants.
To closely monitor the bacterial adaptation and resistance to antiseptics and disinfectants, some microbiological assays used in evaluation of antibacterial activity of antibiotics, such as agar diffusion assay,10 cylinder plate assay,11 could be applied. Although less accurate and less precise than HPLC assays, the microbiological assays are still widely used for clinical practice because of their advantages of short turn-around time, simplicity, and low cost. One of the agar diffusion bioassay, Oxford plate assay, is commonly used for the measurements in antibiotics for antibacterial activities and cytotoxicity based on the correlation of the size of bacteriostatic ring and the dosage of antibiotics.12, 13, 14, 15, 16 However, the usefulness of the Oxford plate method in detecting the resistance of pathogen against chemical disinfectant is not well studied. To explore such potential Oxford plate method was used in the present study to determine the potential disinfectant resistance of Staphylococcus aureus and Escherichia coli against benzalkonium bromide and chlorhexidine acetate in dosage forms of MIC.
Materials and methods
Bacteria strains
The S. aureus (ATCC6538) and E. coil (8099) were routine strains in chemical disinfectant and antibiotic-resistance detection. They were provided by Chinese PLA Center for Disease Control & Prevention, Academy of Military Medical Sciences. The 29 S. aureus clinical strains were automatically separated by VITEK 2 Compact (Biomérieux, France) from the Department of Infection Control, Air Force General Hospital.
Chemicals
The stainless steel Oxford plates with inner diameter (6.0 ± 0.1) mm, outer diameter (7.8 ± 0.1) mm, height (10.0 ± 0.1) mm were purchased from Shanghai Huake Labware Co., LTD. The gradient dilutions of benzalkonium bromide from 50 mg/mL stock solution (Nanchang Baiyun Pharmaceutical) were 32 000, 16 000, 8 000, 4 000, 2 000, 1 000, 500, 250, 125, 62.5, 31.25, 15.625, 7.812 5 μg/mL with ddH2O. And chlorhexidine acetate (Jiutai Pharmaceutical) was dissolved with ddH2O to a final concentration of 2 000, 1 000, 500, 250, 125, 62.5, 31.25, 15.625, 7.812 5, 3.906 μg/mL. The MUELLER-HINTON (MH) agar plates (90 mm) and MH broth powder were purchased from BIOMERIEUX and OXOID.
Microbiological assay
Oxford plate assay
3–4 bacteria colonies were picked up and resuspended in sterile PBS. The suspension was adjusted to 0.5 McF (equal to 1.5 × 108 cfu/mL) by turbidimeter and then diluted by 10 folds in which the concentration was around 1.5 × 107 cfu/mL. The suspension was homogeneously smeared on the MH plate using sterile cotton swab. 2 cylinders were placed on the surface of inoculated medium. The cylinders were filled with 250 μL containing the titrations of benzalkonium bromide or chlorhexidine acetate. After incubation for 24 h at 37 °C, the zone diameters of the growth inhibition were measured and compared. Same experiments were performed with the 29 clinically isolated S. aureus with 60 μg/mL benzalkonium bromide. Each concentration listed in 2.2 chemicals was tested for quadruplicates.
Suspension quantitative germicidal test
2.5 mL of each concentrations (as above) of benzalkonium bromide was added to MH broth tube to make a final concentration of 8, 6, 4, 3, 2, 1.5, 1, 0.5 μg/mL respectively. The suspension of S. aureus strains (named as sau + numbers) was adjusted to 0.5 McF. Adding 0.1 mL of those into the benzalkonium bromide MH broth medium, the bacteria were cultured at 37 °C for 48 h. The minimal inhibitory concentration (MIC) was determined as the highest dilution of medium in which none bacteria could grow. Each experiment was conducted in quadruplicates.
Statistical analysis
The data were analyzed by SPSS20.0. The correlation analysis of the diameter of growth inhibition zone and the minimal inhibitory concentration was performed by Sperman (α = 0.01). The linear correlation between the diameter of growth inhibition zone and the log concentration (μg/mL) of tested disinfectants was analyzed by Origin 7.5 software.
Results
Comparison of the disinfectant susceptibility of S. aureus and E. coli
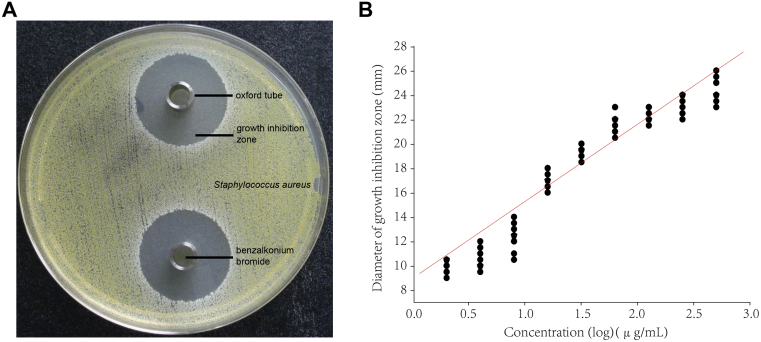
Generally, the Oxford plate assay is an agar diffusion bioassay which is used to detect the antibacterial activity of antibiotics. In order to explore if it's useful for evaluating the antibacterial effects of disinfectants, it was used in this study to detect the bactericidal activities of benzalkonium bromide and chlorhexidine acetate against S. aureus (ATCC6538) and E. coli (8099) respectively. Using the Oxford plate method, well-defined inhibition growth zones were formed allowing accurate measurements (Fig. 1A). The corresponding mean diameter of bacteriostatic zone was positively correlated with the logarithm of the applied doses of benzalkonium bromide (Table 1). Similar results were found with the chlorhexidine acetate (Table 2). We also found the bacteriostasis of tested disinfectants on S. aureus is similar. However, E. coli is more sensitive to chlorhexidine acetate than to benzalkonium bromide (Table 1, Table 2).
Fig. 1.
The correlation of benzalkonium bromide against S. aureus tested by Oxford plate assay. (A) An example photo of the Oxford plate assay. (B) Calibration curves of the log concentration (μg/mL) versus the zone diameter (mm) for the inhibition of benzalkonium bromide on S. aureus (ATCC6538).
Table 1.
Antibacterial susceptibility of benzalkonium bromide against S. aureus and E. coli.
| Concentration (μg/mL) |
S. aureus (ATCC6538) |
E. coli (8099) |
||
|---|---|---|---|---|
| Mean diameter of inhibition zone (SEM ± S) (mm) | Variation coefficients (%) | Mean diameter of inhibition zone (SEM ± S) (mm) | Variation coefficients (%) | |
| 7.81 | 9.88 ± 0.52 | 5.24 | Ns | Ns |
| 15.63 | 10.75 ± 0.89 | 8.25 | Ns | Ns |
| 31.25 | 12.31 ± 1.19 | 9.69 | Ns | Ns |
| 62.5 | 17.17 ± 0.82 | 4.76 | Ns | Ns |
| 125 | 19.38 ± 0.52 | 2.67 | Ns | Ns |
| 250 | 21.88 ± 0.88 | 4.01 | 9.06 ± 0.86 | 9.53 |
| 500 | 22.38 ± 0.58 | 2.6 | 11.69 ± 0.75 | 6.44 |
| 1000 | 23.17 ± 0.82 | 3.52 | 14.42 ± 0.58 | 4.05 |
| 2000 | 24.5 ± 1.04 | 4.22 | 15.88 ± 0.74 | 4.69 |
| 4000 | Ns | Ns | 17.13 ± 0.88 | 5.12 |
| 8000 | Ns | Ns | 18.00 ± 0.80 | 4.45 |
| 16,000 | Ns | Ns | 18.63 ± 0.44 | 2.38 |
| 32,000 | Ns | Ns | 19.44 ± 0.50 | 2.55 |
Table 2.
Antibacterial susceptibility of chlorhexidine acetate against S. aureus and E. coli.
| Concentration (μg/mL) |
S. aureus (ATCC6538) |
E. coli (8099) |
||
|---|---|---|---|---|
| Mean diameter of inhibition zone (SEM ± S) (mm) | Variation coefficients (%) | Mean diameter of inhibition zone (SEM ± S) (mm) | Variation coefficients (%) | |
| 3.91 | 10.25 ± 0.53 | 5.21 | Ns | Ns |
| 7.8125 | 12.13 ± 0.69 | 5.73 | 10.81 ± 0.80 | 7.39 |
| 15.625 | 15.00 ± 0.80 | 5.35 | 12.44 ± 0.56 | 4.53 |
| 31.25 | 16.69 ± 1.13 | 6.78 | 14.69 ± 0.84 | 5.74 |
| 62.5 | 18.00 ± 0.71 | 3.93 | 16.25 ± 0.76 | 4.65 |
| 125 | 19.19 ± 0.59 | 3.1 | 17.63 ± 0.58 | 3.3 |
| 250 | 21.13 ± 0.69 | 3.29 | 19.00 ± 0.80 | 4.22 |
| 500 | 21.63 ± 1.09 | 5.06 | 20.94 ± 0.68 | 3.24 |
| 1000 | 23.88 ± 0.69 | 2.91 | 21.83 ± 0.52 | 2.37 |
| 2000 | 25.38 ± 0.58 | 2.3 | 23.06 ± 0.62 | 2.7 |
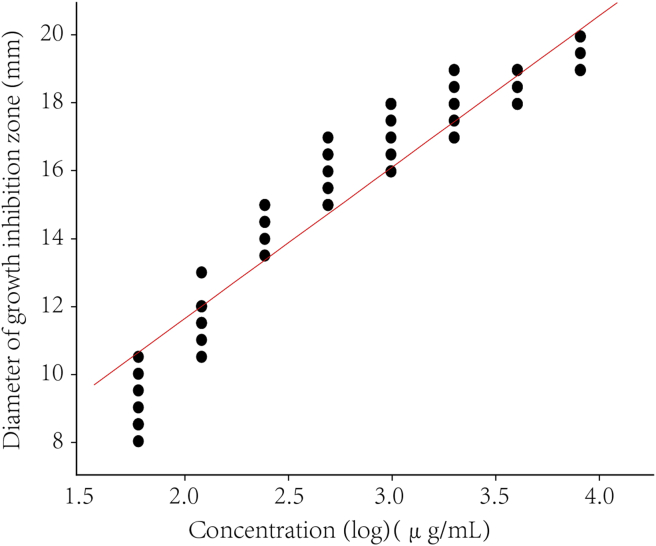
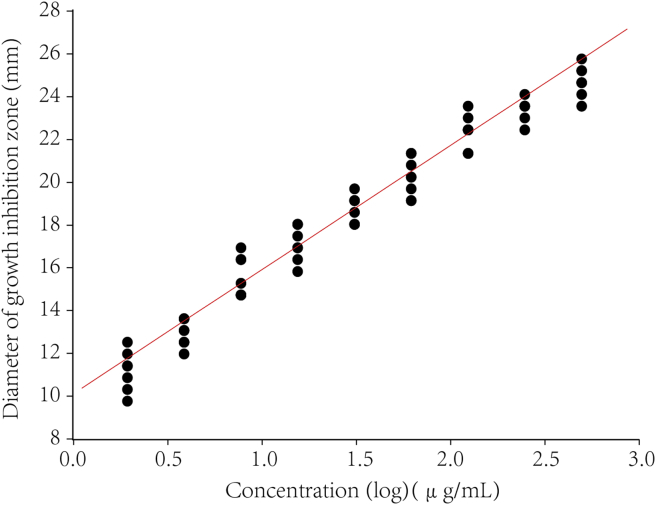
Further calculation assumed a linear relationship between the observed diameter of bacteriostatic zone and the logarithm of the applied dose. The calibration curves for disinfectants were constructed by the log concentration (μg/mL) versus the zone diameter (mm). The representative linear equation for benzalkonium bromide against S. aureus and E. coli was y = 9.05 + 6.28x (r = 0.92, P < 0.000 1) (Fig. 1B) and y = 2.47 + 4.54x (r = 0.87, P = 0.002) respectively (Fig. 2). For the activity of chlorhexidine acetate, the representative linear equation for S. aureus was y = 11.74 + 5.17x (r = 0.97, P < 0.000 1) (Fig. 3) and E. coli was y = 9.8 + 5.26x (r = 0.99, P < 0.000 1) (Fig. 4). All the correlation coefficient was statistically significant for the method.
Fig. 2.
Calibration curves of the log concentration (μg/mL) versus the zone diameter (mm) for the inhibition of benzalkonium bromide on E. coli (8099).
Fig. 3.
Calibration curves of the log concentration (μg/mL) versus the zone diameter (mm) for the inhibition of chlorhexidine acetate on S. aureus (ATCC6538).
Fig. 4.
Calibration curves of the log concentration (μg/mL) versus the zone diameter (mm) for the inhibition of chlorhexidine acetate on E. coli (8099).
Antibacterial effect of benzalkonium bromide on clinically isolated S. aureus
To determine the clinical application in screening disinfectant resistant bacteria strain, the Oxford plate assay was used to detect the potency of disinfectants by comparing the growth inhibition zone among 29 clinically isolated S. aureus and reference standard (ATCC6538) induced by 60 μg/mL benzalkonium bromide. After incubating at 37 °C for 24 h, the diameter of inhibition growth zone is ranging from 11.5 mm to 19.5 mm for the clinical strains compared with 17.0–19.5 mm for standard strain. The minimal inhibitory concentration (MIC) was evaluated using broth dilution method. The results showed that the MIC of the reference strain (ATCC6538) was around 1.5 μg/mL (Table 3). The results of the statistical analysis indicated that there is significant negative correlation between the diameter of growth inhibition zone from Oxford plate assay and the MIC value (r = −0.574, Ptwo-tailed = 0.000).
Table 3.
Antibacterial activities of benzalkonium bromide against clinically isolated S. aureus strains.
| Clinically isolated strain | Oxford plate assay |
Broth dilution method |
|
|---|---|---|---|
| Mean diameter of inhibition zone (SEM ± S) (mm) | Variation coefficients (%) | ||
| MICmedian (μg/mL) | |||
| ATCC6538 (control) | 18.19 ± 0.90 | 4.94 | 1.5 |
| sau33 | 17.94 ± 0.58 | 3.25 | 1.5 |
| sau34 | 12.38 ± 0.60 | 4.84 | 3 |
| Sau41 | 14.56 ± 0.63 | 4.36 | 3 |
| Sau46 | 18.19 ± 0.50 | 2.73 | 1.5 |
| Sau52 | 16.56 ± 0.39 | 2.36 | 2 |
| Sau53 | 13.25 ± 0.43 | 3.27 | 2 |
| Sau54 | 17.94 ± 0.58 | 3.25 | 1.5 |
| Sau55 | 17.81 ± 0.50 | 2.79 | 1.5 |
| Sau56 | 14.81 ± 0.66 | 4.45 | 2 |
| Sau70 | 17.44 ± 0.53 | 3.02 | 1.5 |
| Sau76 | 15.31 ± 0.56 | 3.63 | 1.5 |
| Sau77 | 16.94 ± 0.53 | 3.11 | 2 |
| Sau78 | 16.56 ± 0.46 | 2.8 | 1.5 |
| Sau80 | 16.83 ± 0.47 | 2.8 | 1.5 |
| Sau81 | 14.75 ± 0.56 | 3.79 | 1.5 |
| Sau82 | 14.94 ± 0.58 | 3.9 | 2 |
| Sau83 | 15.00 ± 0.61 | 4.08 | 2 |
| Sau133 | 14.56 ± 0.63 | 4.36 | 2 |
| Sau135 | 14.75 ± 0.43 | 2.94 | 2 |
| Sau142 | 15.19 ± 0.66 | 4.34 | 2 |
| Sau143 | 17.50 ± 0.71 | 4.04 | 1.5 |
| Sau149 | 18.06 ± 0.77 | 4.25 | 1.5 |
| Sau155 | 13.50 ± 0.50 | 3.7 | 3 |
| Sau157 | 17.00 ± 0.50 | 2.94 | 1.5 |
| Sau159 | 16.56 ± 0.46 | 2.8 | 1.5 |
| Sau161 | 16.06 ± 0.63 | 3.95 | 1.5 |
| Sau162 | 18.31 ± 0.61 | 3.33 | 1.5 |
| Sau165 | 14.94 ± 0.68 | 4.56 | 1.5 |
| Sau167 | 16.00 ± 0.61 | 3.83 | 1.5 |
Antibacterial coefficient of variation of disinfectants on S. aureus and E. coli
Generally, the coefficient variation of disinfectant vs. bacteria was determined by Oxford plate method in the current study. The data was summarized and calculated by SPSS20.0 in Table 4. And the index of disinfectant against 29 clinical isolated S. aureus was (3.61 ± 0.71)%, indicating a good reproducibility of Oxford plate assay.
Table 4.
Antibacterial coefficient of variation of disinfectant against S. aureus and E. coli (SEM ± S, %).
| Antiseptic | S. aureus | E. coli |
|---|---|---|
| Benzalkonium bromide | 5.00 ± 2.44 | 4.90 ± 2.29 |
| Chlorhexidine acetate | 4.36 ± 1.46 | 4.24 ± 1.59 |
Discussion
In healthcare facilities, antiseptics and disinfectants are used to reduce the cross-transmission of pathogenic microbes. Numerous studies demonstrated the resistance of the pathogenic microbes to antiseptics and disinfectants.4, 7, 8 Standardized methods of susceptibility testing and minimum inhibitory concentrations (MICs) were routinely applied in defining bacterial resistance to antibiotics. However, fewer reports demonstrated the utility of those methods in susceptibility testing for antiseptics and disinfectants. The use-dilution and agar-dilution methods have been used to evaluate the disinfectant activity and minimum inhibitory concentration (MIC).17 Alternatively it could be approximately determined by calculating the viable colonies in agar plates before and after exposure to a disinfectant.18 But these methods are less cost-effective and time-consuming. The emerging challenges of bacterial resistance to antiseptics and disinfectants in healthcare facilities call for more frequent susceptibility testing.8, 19, 20, 21 For routine quality control in hospitals, validation of time-saving and less expensive methods to monitor resistance to antiseptics and disinfectants is highly warranted.
In this study, the Oxford plate assay, a standard microbiological method for evaluating the susceptibility and resistance to antibiotics,22 was used to evaluate the disinfectant susceptibility of S. aureus and E. coli. The results obtained in this study showed a significant correlation between the applied dosages of disinfectants, benzalkonium bromide and chlorhexidine acetate, and the diameter of growth inhibition zone for S. aureus (ATCC6538) and E. coli (8099). These results confirmed that the Oxford plate assay is suitable for its application in detecting bactericidal activities of disinfectants. However, we also noticed the limitations of the Oxford plate assay in the cases of o-phthalaldehyde and chlorine. Specifically we were not able to get a well-defined growth inhibition zone when using o-phthalaldehyde and chlorine against S. aureus (ATCC6538) and E. coli (8099). It might be due to the chemical properties of tested disinfectants as alcohols or aldehyde.
While the pathogen resistance to antibiotics has been known for decades, pathogen strains resistant to disinfectants are relatively recent findings. The spread of disinfectant-resistant pathogens has resulted in serious secondary cross-infections and deaths in hospitals. In the present study, both Oxford plate assay and broth dilution method were used to determine disinfectant susceptibility of 29 clinically isolated S. aureus strains to benzalkonium bromide. The results showed increased MICs using broth dilution method, indicating an acquired resistance in S. aureus, which corresponded to shrinked sizes of growth inhibition zones in Oxford plates. Our results also showed that MICs from broth dilution method have a negative correlation with the size of growth zone in Oxford plate. These comparisons gave further validation on the merit of Oxford plate assay in detecting disinfectant resistance, raising a promising prospect for this method to be used in the surveillance of bacterial resistance in hospitals.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
All authors would like to thank Prof. Fei Li for helpful comments and revision on this manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Craven D.E., Moody B., Connolly M.G., Kollisch N.R., Stottmeier K.D., McCabe W.R. Pseudobacteremia caused by povidone-iodine solution contaminated with Pseudomonas cepacia. N Engl J Med. 1981;305(11):621–623. doi: 10.1056/NEJM198109103051106. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J.M., Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3–S40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinkunmi E.O., Lamikanra A. Susceptibility of community associated methicillin resistant Staphylococcus aureus isolated from faeces to antiseptics. J Infect Dev Ctries. 2012;6(4):317–323. doi: 10.3855/jidc.1609. [DOI] [PubMed] [Google Scholar]
- 5.Horner C., Mawer D., Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother. 2012;67(11):2547–2559. doi: 10.1093/jac/dks284. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov I.B., Gritsenko V.A., Kuzmin M.D. The effect of brief exposure to sub-therapeutic concentrations of chlorhexidine digluconate on the susceptibility of staphylococci to platelet microbicidal protein. Surg Infect (Larchmt) 2015;16(3):263–266. doi: 10.1089/sur.2013.173. [DOI] [PubMed] [Google Scholar]
- 7.Russell A.D. Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J Hosp Infect. 2004;57(2):97–104. doi: 10.1016/j.jhin.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura-Sato K., Wachino J., Kondo T., Ito H., Arakawa Y. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J Antimicrob Chemother. 2008;61(3):568–576. doi: 10.1093/jac/dkm498. [DOI] [PubMed] [Google Scholar]
- 9.Kearns A.M., Freeman R., Lightfoot N.F. Nosocomial enterococci: resistance to heat and sodium hypochlorite. J Hosp Infect. 1995;30(3):193–199. doi: 10.1016/s0195-6701(95)90314-3. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt C.A., Carazzo M., Laporta L.V., Bittencourt C.F., Santos M.R., Friedrich M. Development and validation of an agar diffusion assay for determination of ceftazidime in pharmaceutical preparations. J AOAC Int. 2008;91(1):59–66. [PubMed] [Google Scholar]
- 11.Souza M.J., Kulmann R.R., Silva L.M., Nogueira D.R., Zimmermann E.S., Schmidt C.A. Development and in-house validation of a microbiological assay for determination of cefepime in injectable preparations. J AOAC Int. 2006;89(5):1367–1372. [PubMed] [Google Scholar]
- 12.Costa M.C., Barden A.T., Andrade J.M., Oppe T.P., Schapoval E.E. Quantitative evaluation of besifloxacin ophthalmic suspension by HPLC, application to bioassay method and cytotoxicity studies. Talanta. 2014;119:367–374. doi: 10.1016/j.talanta.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 13.Paim C.S., Fuhr F., Barth A.B. Gemifloxacin mesylate (GFM) stability evaluation applying a validated bioassay method and in vitro cytotoxic study. Talanta. 2011;83(5):1774–1779. doi: 10.1016/j.talanta.2010.11.069. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoudi A., Fourar R.E., Boukhechem M.S., Zarkout S. Microbiological assay for analysis of certain macrolides in pharmaceutical dosage forms. Int J Pharm. 2015;491(1-2):285–291. doi: 10.1016/j.ijpharm.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Lopes C.C., Salgado H.R. Development and validation of a stability-indicative agar diffusion assay to determine the potency of linezolid in tablets in the presence of photodegradation products. Talanta. 2010;82(3):918–922. doi: 10.1016/j.talanta.2010.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Wagner R.D., Johnson S.J., Cerniglia C.E., Erickson B.D. Bovine intestinal bacteria inactivate and degrade ceftiofur and ceftriaxone with multiple beta-lactamases. Antimicrob Agents Chemother. 2011;55(11):4990–4998. doi: 10.1128/AAC.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekadu S., Merid Y., Beyene H., Teshome W., Gebre-Selassie S. Assessment of antibiotic- and disinfectant-resistant bacteria in hospital wastewater, south Ethiopia: a cross-sectional study. J Infect Dev Ctries. 2015;9(2):149–156. doi: 10.3855/jidc.4808. [DOI] [PubMed] [Google Scholar]
- 18.Reichel M., Schlicht A., Ostermeyer C., Kampf G. Efficacy of surface disinfectant cleaners against emerging highly resistant gram-negative bacteria. BMC Infect Dis. 2014;14:292. doi: 10.1186/1471-2334-14-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbarth S., Tuan Soh S., Horner C., Wilcox M.H. Is reduced susceptibility to disinfectants and antiseptics a risk in healthcare settings? A point/counterpoint review. J Hosp Infect. 2014;87(4):194–202. doi: 10.1016/j.jhin.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Ledder R.G., Gilbert P., Willis C., McBain A.J. Effects of chronic triclosan exposure upon the antimicrobial susceptibility of 40 ex-situ environmental and human isolates. J Appl Microbiol. 2006;100:1132–1140. doi: 10.1111/j.1365-2672.2006.02811.x. [DOI] [PubMed] [Google Scholar]
- 21.Sasatsu M., Shimizu K., Noguchi N., Kono M. Triclosan-resistant Staphylococcus aureus. Lancet. 1993;341(8847):756. doi: 10.1016/0140-6736(93)90526-m. [DOI] [PubMed] [Google Scholar]
- 22.Commission C.P. vol. II. China Medical Science Press; Beijing: 2010. Pharmacopoeia of the People's Republic of China. (China Medical Science Press). Appendix 93–130 [in Chineses version] [Google Scholar]