Abstract
Introduction
Multipotent mesenchymal stem cells (MSCs) are widespread in adult organisms and are implicated in tissue maintenance and repair, regulation of hematopoiesis, and immunologic responses. Human (h)MSCs have applications in tissue engineering, cell-based therapy, and medical devices but it is unclear how they respond to unfavorable conditions such as hypoxia or inflammation after in vivo transplantation. Although endotoxin testing is a requirement for evaluating the quality and safety of transplanted MSCs, there have been no reports on the dose response to endotoxins to establish limits for in vitro MSC culture systems. The present study aimed to accurately quantify the risk of endotoxin contamination in cell culture systems in order to establish the acceptable endotoxin limit for hMSC proliferation.
Methods
Three types of bone marrow-derived hMSC (hMSC-1: 21 years, M/B; hMSC-2: 36 years, M/B; hMSC-3: 43 years, M/C) and adipose-derived stem cells (ADSCs; StemPro Human) were cultured in medium from commercial kits containing various concentrations of endotoxin (0.1–1000 ng/ml). The proliferative capacity of cells was estimated by cell counts using a hemocytometer. To clarify the molecular mechanism underlying the effect of endotoxin on hMSCs proliferation, cellular proteins were extracted from cultured cells and subjected to liquid chromatograph-tandem mass spectrometry shotgun proteomics analysis. The expression of Cu/Zn-type superoxide dismutase (SOD1) and Fe/Mn-type superoxide dismutase (SOD2) induced in hMSCs by endotoxin stimulation were evaluated by enzyme-linked immunosorbent assay (ELISA), and the effect of SOD2 on hMSC proliferation was also estimated.
Results
Although there was no change in cell morphology during the culture period, proliferative capacity increased with endotoxin concentration to over 0.1 ng/ml for ADSCs, 1 ng/ml for hMSC-1, and 100 ng/ml for hMSC-2; hMSC-3 proliferation was unaffected by the presence of endotoxin. A proteomic analysis of hMSC-1 revealed that various proteins related to the cell cycle, apoptosis, and host defense against infection were altered by endotoxin stimulation, whereas SOD2 expression was significantly and consistently upregulated during the culture period. The latter was also confirmed by ELISA. Moreover, recombinant SOD2 increased proliferative capacity in hMSC-1 cells in a manner similar to endotoxin. These results suggest that endotoxin protects MSCs from oxidative stress via upregulation of SOD2 to improve cell survival.
Conclusions
Since endotoxins can affect various cellular functions, an endotoxin limit should be set for in vitro MSC cultures. The lowest observed adverse effect level was determined to be 0.1 ng/ml based on the effect on MSC proliferation.
Keywords: Endotoxin limit, Regenerative medicine products, Human mesenchymal stem cells, Proliferative capacity, Superoxide dismutase
Abbreviations: CD, cluster of differentiation; ELISA, enzyme-linked immunosorbent assay; IGF, insulin-like growth factor; iPS cell, induced pluripotent stem cell; LC-MS/MS, liquid chromatograph-tandem mass spectrometry; LOAEL, lowest observed adverse effect level; (h)ADSC, (human) adipose-derived stem cell; (h)MSC, (human) mesenchymal stem cell; PBS, phosphate-buffered saline; SOD2, superoxide dismutase 2; TLR, Toll-like receptor
Highlights
-
•
Risk of endotoxin contamination in an hMSC culture system was quantified.
-
•
Endotoxin increased proliferative capacity of hMSCs.
-
•
SOD2 has similar effects of hMSC proliferation as endotoxin.
-
•
Lowest observed adverse effect level of endotoxin was determined as 0.1 ng/ml.
1. Introduction
Multipotent mesenchymal stem cells (MSCs) are widespread in adult organisms and are implicated in tissue maintenance and repair, regulation of hematopoiesis, and immunologic responses [1]. Human (h)MSCs have applications in tissue engineering, cell-based therapy, and medical devices, but it is unclear how they respond to unfavorable conditions such as hypoxia or inflammation after in vivo transplantation [2]. Toll-like receptors (TLRs) play an important role in the immune system by participating in the initial recognition of microbial pathogens and pathogen-associated components, and TLR agonists can affect the proliferation and differentiation of hMSCs, which express TLRs such as TLR-4 and endotoxin receptor [1], [3], [4], [5].
Naturally derived biomaterials such as collagen, gelatin, chitin, chitosan, hyaluronate, and alginate are commonly used in scaffolds for cell culture owing to their biocompatibility. Recent advances in tissue engineering have enabled the use of naturally derived biomaterials beyond the regulation of tissue response at the material interface, for example in the fabrication of three-dimensional culture matrices [6], [7], [8], [9], [10], [11], [12]. However, a major limitation of these materials is quality control; in particular, microbial safety has not been well characterized and is difficult to control.
Most TLR agonists are microbial components such as lipoprotein, glycoprotein, double-stranded RNA, non-methylated CpG DNA, flagellin, and mycetoma-polysaccharide as well as endotoxin, which induces the greatest biological effect at the lowest dose [13], [14]. Endotoxins are surface lipopolysaccharides of Gram-negative bacteria and typical pyrogens that elicit host immune responses even in trace amounts [13] and have various other biological activities in vitro and/or in vivo [4], [14].
MSCs can differentiate along several lineages via tightly regulated processes. Human adipose tissue contains cell populations that have characteristics similar to bone marrow stromal cells. Wnt proteins induced by stimulation with TLR agonists have been linked to the proliferation and differentiation of various cell types, including MSCs [15]. For example, endotoxin derived from Porphyromonas gingivalis was shown to inhibit osteoblast differentiation at doses greater than 100 ng/ml [16], whereas Escherichia coli endotoxin at concentrations between 50 and 500 ng/ml stimulated fibroblast proliferation after 6 days [17]. With the exception of CpG DNA, there are no known TLR agonists that affect the proliferation of human adipose-derived stem cell (hADSCs); endotoxin and peptidoglycans increase whereas CpG DNA decreases osteogenic differentiation [2]. In addition, double-stranded RNA analogs do not affect adipogenic or osteogenic differentiation, but act synergistically with endotoxin or peptidoglycan to induce osteogenic differentiation. Pam3Cys, a TLR-2 ligand, inhibited the differentiation of MSCs into osteogenic, adipogenic, and chondrogenic lineages while preserving their immunosuppressive function [1]. It was also reported that TLR ligand can antagonize MSC differentiation triggered by exogenous mediators and consequently maintain cells in an undifferentiated and proliferative state in vitro. Moreover, MSCs derived from myeloid factor 88-deficient mice lacked the capacity for differentiation into osteogenic and chondrogenic cells [1].
The above reports suggest that TLRs and their ligands are regulators of cell proliferation and differentiation and contribute to the maintenance of MSC multipotency; moreover, these effects differ according to the type of TLR agonist and source of cells. However, it remains unclear why endotoxin has different effects on the proliferative capacities of fibroblasts and hADSCs, since both cell types recognize endotoxin via TLR-4 and activate the same downstream signal transduction pathway. Furthermore, previous studies used a high concentration of TLR ligand; this is especially true of endotoxin, which can induce biological responses in the concentration range of pg/ml or ng/ml depending on cell type. Endotoxin testing is required to evaluate the quality and safety of pharmaceuticals and medical devices derived from the processing of autologous human somatic stem cells [18], but there have been no reports focused on the dose response of endotoxin to establish appropriate limits for in vitro MSC culture systems.
To address this issue, the present study investigated the lowest observed adverse effect level (LOAEL) in several types of MSC cultured with medium containing various concentrations of endotoxin. We examined the effect of endotoxin on proliferative capacity and the underlying mechanisms in order to empirically establish an in vitro endotoxin limit for MSC proliferation.
2. Materials and methods
2.1. Reagents and materials
Three types of bone marrow-derived hMSCs (hMSC-1: 21 years, M/B; hMSC-2: 36 years, M/B; hMSC-3: 43 years, M/C) and the MSCGM BulletKit were purchased from Lonza (Walkersville, MD, USA). hADSCs (StemPro Human) and the MesenPRO RS medium kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Recombinant human superoxide dismutase (SOD)2 protein was purchased from Ab FRONTIER (Seoul, Korea). Human Cu/Zn-type SOD1 and the SOD2 enzyme-linked immunosorbent assay (ELISA) kit were purchased from Ab FRONTIER. Other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan). All tools made of glass, metal, or Teflon were autoclaved at 250 °C for more than 16 h prior to use.
2.2. Preparation of bacterial endotoxin
E. coli strain O3:K2a,K2b:H3 (American Type Culture Collection, Manassas, VA, USA) was cultured in a 50-l fermenter at 37 °C for 16 h under gentle stirring with an air flow of 1 l/min in minimum nutrient broth containing 0.2% beef extract, 1% peptone, and 0.5% NaCl (pH 7.4). After pH neutralization of the culture medium and heat inactivation at 121 °C for 15 min, bacterial cells were collected by continuous centrifugation (7000 × g) and washed three times with distilled water followed by sequential extraction with ethanol, acetone, and diethyl ether to dehydrate the cells. Endotoxin was extracted from dried cells using the phenol-water method [19] and purified by repeated ultracentrifugation after deoxyribonuclease and ribonuclease treatments [20]. The activity of purified endotoxin was 27.5 EU/ng.
2.3. Cell culture and analysis of proliferation
Three types of bone marrow-derived hMSCs and hADSCs were cultured using the MSCGM BulletKit and MesenPRO RS medium kits, respectively at 37 °C in a humidified atmosphere containing 5% CO2, with a medium changed every 3 days. When cells had reached 80%–90% confluence they were trypsinized, counted, and passaged. Passage 3 or 4 cells free of contamination were used for experiments.
To evaluate the effects of endotoxin on proliferative capacity, hMSC-1, hMSC-2, hMSC-3, and hADSCs were cultured for 40 days in the presence of various concentrations of endotoxin (0.1–1000 ng/ml). Cells cultured without endotoxin served as a negative control. After morphological analysis on an inverted microscope (Leica DN IL; Leica Microsystems, Wetzlar, Germany), cell proliferation was evaluated by counting the number of cells using a Burker-Turk hemocytometer (Sunlead Glass, Tokyo, Japan). The total cell number during the culture period was sequentially integrated every passage according to proliferation ratio. For the proteomics analysis and ELISA, hMSC-1 was cultured in the presence or absence of endotoxin (1000 ng/ml) for 49 days followed by extraction and purification of cellular proteins, as described below. To estimate the effect of SOD2 on hMSC proliferation, hMSC-1 was cultured for 46 days with medium containing recombinant SOD2 (12.8 μg/ml), and the cells were also cultured with or without endotoxin (1000 ng/ml) as a positive or negative control, respectively.
2.4. Proteomics analysis
Cultured hMSC-1 cells were recovered by conventional trypsin treatment followed by three washes with phosphate-buffered saline (PBS) at 37 °C. Cells were mixed with protein extraction reagent consisting of 7 M urea, 2 M thiourea, 4% CHAPS, and 30 mM Tris-HCl (pH 7.5) and maintained for 0.5 h at room temperature before centrifugation (10,000 × g for 10 min). Cellular protein was semi-purified from the supernatant using the 2D Clean-Up kit (GE Healthcare Japan, Tokyo, Japan), and the cell pellet was dissolved in protein extraction reagent. Protein concentration was measured with the 2D Quant kit (GE Healthcare Japan). Equal amounts of protein from each sample were transferred to Eppendorf tubes and then reduced with tributylphosphine for 1 h, alkylation with iodoacetamide for 1.5 h at room temperature, and digestion with Trypsin Gold (mass spectrometry grade; Promega, Tokyo, Japan) in the presence of ProteaseMAX surfactant/trypsin enhancer (Promega) for 5 h at 37 °C. The digest was cleaned up and desalted with an OMIX C18 chip (100 μl; Agilent Technologies, Santa Clara, CA, USA) and adsorbed peptide was eluted with 80% acetonitrile and dried in a Speed Vac (Thermo Fisher Scientific), then dissolved in the same volume of 2% acetonitrile containing 0.1% trifluoroacetic acid (TFA). Samples were analyzed by liquid chromatograph-tandem mass spectrometry (LC-MS/MS) using an LTQ-OrbiTrap-XL instrument (Thermo Fisher Scientific) equipped with a DiNa nano-LC system with electrospray ionization nanospray interface (KYA TECH Corporation, Tokyo, Japan), a C-18 trap cartridge, and C-18 capillary column (0.1 × 150 mm; Chemicals Evaluation and Research Institute, Saitama, Japan). Purified water containing 0.1% TFA (pump A) and acetonitrile (pump B) were used as eluents at a flow rate of 300 nl/min at 40 °C. The initial gradient condition of 2% B was maintained for 10 min, then linearly increased to 40% B in 150 min followed by a linear increase to 80% B in 5 min, where it was held constant for 15 min. MS/MS spectra were automatically acquired with the top three modes of Xcalibur software (Thermo Fisher Scientific). Protein identification was carried out with Proteome Discoverer software (Thermo Fisher Scientific) with Mascot (Matrix Science, Tokyo, Japan) and the UniProtKB/Swiss-Prot database. Multivariate analysis was performed with i-RUBY software (Medical ProteoScope, Tokyo, Japan). The multivariate value of each protein was calculated as an expression ratio relative to the negative control (1.00).
2.5. SOD2 assay
hMSC-1 cells cultured with or without endotoxin were recovered by conventional trypsin treatment followed by three washes with PBS. Cells (6 × 105) were lysed in 100 μl M-PER protein extraction reagent (Thermo Fisher Scientific) and the extract was assayed using commercial human SOD1 and SOD2 ELISA kits.
3. Results
3.1. Effect of endotoxin on hMSC and hADSC proliferation
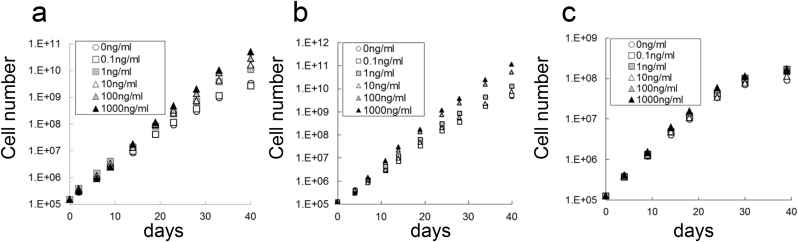
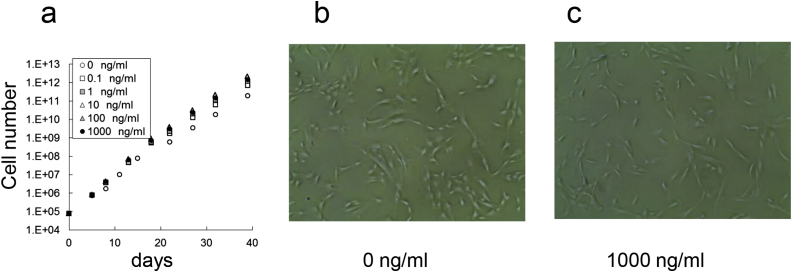
hMSCs and hADSC were cultured in the presence or absence of endotoxin for a total of 40 days, and the effect on proliferation was evaluated. The number of hMSC-1 cultured with 0 (negative control), 0.1, 1, 10, 100, or 1000 ng/ml endotoxin was 3.3 × 109, 2.6 × 109, 10.8 × 109, 16.4 × 109, 28.1 × 109, and 50.7 × 109, respectively (Fig. 1a). Similarly, the number of hMSC-2 cultured under these conditions was 4.5 × 109, 5.0 × 109, 12.8 × 109, 7.7 × 109, 51.6 × 109, and 114.0 × 109, respectively (Fig. 1b). Thus, proliferative capacity was enhanced at endotoxin concentrations over 1 ng/ml for hMSC-1 and 100 ng/ml for hMSC-2. In contrast, the number of hMSC-3 cells was 14.5 × 107, 16.7 × 107, 11.3 × 107, 16.9 × 107, and 15.6 × 107, respectively, which was almost identical to the negative control (9.0 × 107) (Fig. 1c). The number of hADSCs cultured with 0, 0.1, 1, 10, 100, or 1000 ng/ml of endotoxin were 1.9 × 1011, 6.9 × 1011, 12.1 × 1011, 20.9 × 1011, 23.0 × 1011, and 14.3 × 1011, respectively. Although there was no change in cell morphology over the culture period, hADSCs were more sensitive to endotoxin than hMSC-1 and proliferative capacity was increased at endotoxin concentrations over 0.1 ng/ml (Fig. 2).
Fig. 1.
Effect of endotoxin on hMSC proliferation. (a–c) Number of hMSC-1 (a), hMSC-2 (b), and hMSC-3 (c) cultured for 40 days in medium containing 0, 0.1, 1, 10, 100, and 1000 ng/ml endotoxin.
Fig. 2.
Effect of endotoxin on hADSC proliferation and morphology. (a) Cell number of hADSCs cultured for 40 days in medium containing 0, 0.1, 1, 10, 100, and 1000 ng/ml endotoxin. (b, c) Morphology of hADSCs cultured in medium containing 0 ng/ml (b) and 1000 ng/ml (c) endotoxin for 35 days.
3.2. Proteomic analysis of cellular protein of endotoxin-stimulated hMSC
To identify the molecular mechanism by which endotoxin enhances the proliferative capacity MSCs, cellular proteins of hMSC-1 cultured in the presence or absence of 1000 ng/ml endotoxin for 49 days were extracted and subjected to LC-MS/MS shotgun proteomics analysis. A total of 4986 proteins (P < 0.05) were identified, including those related to apoptosis, cell cycling, and host defense against infection (Table 1).
Table 1.
Abbreviated list of proteins induced in hMSCs by endotoxin.
| Type | Protein ID |
Peptide count | Expression ratio [LPS(+)/control] |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | Name | Score | 3 days | 7 days | 14 days | 22 days | 36 days | 49 days | ||
| Apoptosis-induced proteins | PEF1 | Peflin | 102.9 | 4 | 0.36 | 1.09 | 0.94 | 0.96 | 0.79 | 0.62 |
| SUGT1 | Suppressor of G2 allele of SKP1 homolog | 56.9 | 4 | 0.48 | 0.86 | 0.64 | 1.37 | 0.49 | 0.18 | |
| RTL1 | Retrotransposon-like protein 1 | 30.9 | 2 | 0.36 | 0.98 | 0.58 | 1.28 | 0.49 | 0.66 | |
| PARVA | Alpha-parvin | 141.8 | 6 | 0.40 | 1.07 | 0.83 | 1.15 | 0.75 | 0.64 | |
| ADT3 | ADP/ATP translocase 3 | 302.3 | 11 | 0.43 | 1.10 | 0.77 | 1.04 | 0.82 | 0.67 | |
| TANC2 | Protein TANC2 | 34.1 | 2 | 0.33 | 0.87 | 0.47 | 0.95 | 0.34 | 0.57 | |
| APAF | Apoptotic protease-activating factor 1 | 34.5 | 2 | 0.40 | 0.95 | 0.57 | 1.21 | 0.41 | 0.67 | |
| G3P | Glyceraldehyde-3-phosphate dehydrogenase | 1395.0 | 30 | 0.38 | 1.10 | 1.06 | 1.12 | 1.09 | 0.38 | |
| Apoptosis-suppressive proteins | UBE2H | Ubiquitin-conjugating enzyme E2 H | 31.9 | 2 | 0.52 | 1.25 | – | 3.09 | – | – |
| TFIP8 | Tumor necrosis factor, alpha-induced protein 8 | 103.5 | 2 | 0.85 | 1.88 | 2.22 | 0.91 | 1.96 | 0.84 | |
| Infection protective proteins | UCRP | Interferon-induced 17 kDa protein | 66.3 | 2 | 9.61 | – | 2.75 | 4.79 | – | – |
| SIAS | Sialic acid synthase | 53.0 | 5 | 0.58 | 2.56 | 1.41 | 1.14 | 2.21 | 0.34 | |
| FYN | Proto-oncogene tyrosine-protein kinase Fyn | 25.7 | 2 | – | 2.17 | – | – | – | – | |
| BAT3 | Large proline-rich protein BAT3 | 64.9 | 2 | 0.60 | 1.34 | 1.25 | 3.18 | 1.74 | 0.67 | |
| UBE2H | Ubiquitin-conjugating enzyme E2 H | 31.9 | 2 | 0.52 | 1.25 | – | 3.09 | – | – | |
| Cell cycling proteins | FHL2 | Four and a half LIM domains protein 2 | 43.2 | 3 | 5.21 | 0.77 | 0.63 | 1.02 | 0.50 | 0.79 |
| SODM | Superoxide dismutase [Mn], mitochondrial | 243.8 | 8 | 2.76 | 7.74 | 5.38 | 12.19 | 7.56 | 3.43 | |
| FIGL1 | Fidgetin-like protein 1 | 24.0 | 2 | – | – | 7.44 | – | 0.51 | – | |
| M3K15 | Mitogen-activated protein kinase kinase kinase 15 | 25.5 | 2 | 0.35 | 1.03 | 0.64 | ∞ | – | – | |
| ERF3A | Eukaryotic peptide chain release factor GTP-binding subunit ERF3A | 95.3 | 4 | 0.56 | 1.04 | 1.00 | 6.67 | 0.48 | 0.33 | |
| UBP7 | Ubiquitin carboxyl-terminal hydrolase 7 | 50.0 | 3 | 0.73 | 1.20 | 1.03 | 2.66 | 1.16 | 0.79 | |
| HAP28 | 28 kDa heat- and acid-stable phosphoprotein | 48.0 | 2 | 0.29 | 1.37 | 0.53 | 4.69 | 0.34 | 0.26 | |
| REEP5 | Receptor expression-enhancing protein 5 | 50.9 | 2 | 0.10 | 0.96 | 1.67 | 3.38 | 0.71 | 0.14 | |
| AN32A | Acidic leucine-rich nuclear phosphoprotein 32 family member A | 125.0 | 5 | 0.78 | 1.47 | 1.02 | 3.36 | 0.62 | 0.71 | |
| ERF3B | Eukaryotic peptide chain release factor GTP-binding subunit ERF3B | 95.3 | 3 | 0.76 | 1.04 | 1.23 | 4.11 | 0.76 | 0.33 | |
The expression levels of apoptosis-related proteins induced by endotoxin stimulation varied, but in general, proteins that inhibit and induce apoptosis were up- and downregulated, respectively. For example, the expression of tetratricopeptide repeat, ankyrin repeat and coiled-coil-containing 2 which is involved in various processes such as stress-activated p38/mitogen-activated protein kinase signaling was downregulated during the culture period (Table 1). The expression ratios of endotoxin-stimulated groups relative to the negative control after culture for 3, 22, and 49 days were 0.33, 0.95, and 0.57, respectively.
With the exception of SOD2, expression levels of cell cycle- and host defense-related proteins also varied (Table 1). For example, the relative expression ratios in endotoxin-stimulated groups at 3, 22, and 49 days of culture were 0.78, 3.36, and 0.71, respectively, for acidic nuclear phosphoprotein 32 family member A and 0.58, 1.14, and 0.34, respectively, for sialic acid synthase. In contrast, SOD2 expression was significantly and consistently upregulated during the culture period, with relative expression ratios of 2.76, 12.19, and 3.43, respectively.
3.3. Effect of SOD2 on hMSC proliferation
To verify the results of the proteomic analysis, the levels of SOD1 and SOD2 in hMSC-1 upon endotoxin stimulation were measured by ELISA. SOD1 expression was unaffected by endotoxin stimulation; the levels at 5, 12, 19, and 26 days of culture were 6.28, 4.19, 6.37, and 6.01 pg/cell, respectively, for endotoxin-stimulated groups as compared to 6.55, 5.98, 7.11, and 7.34 pg/cell, respectively, for the negative control (Table 2). On the other hand, SOD2 production was greatly enhanced by endotoxin stimulation relative to the negative control (15.93, 13.98, 13.18, and 10.60 pg/cell, respectively vs. 1.48, 1.04, 1.60, and 1.49 pg/cell, respectively).
Table 2.
Effect of endotoxin on SOD1 and SOD2 expression.
| Endotoxin | SOD1 (pg/cell) |
SOD2 (pg/cell) |
||
|---|---|---|---|---|
| − | + | − | + | |
| 5 days | 6.55 | 6.28 | 1.48 | 15.93 |
| 12 days | 5.98 | 4.19 | 1.04 | 13.98 |
| 19 days | 7.11 | 6.37 | 1.60 | 13.18 |
| 26 days | 7.34 | 6.01 | 1.49 | 10.60 |
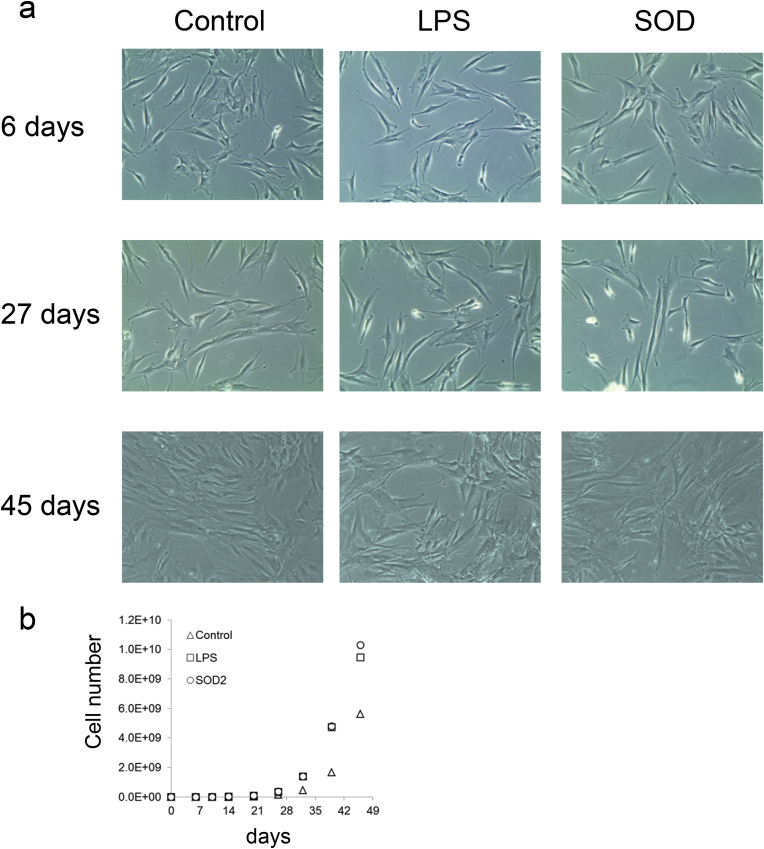
hMSC-1 was cultured with recombinant SOD2, endotoxin (positive control), or no additives (negative control) for 45 days. There was no change in cell morphology during the culture period (Fig. 3a). There were no significant differences in proliferative capacity in the early stages of culture, and the number of cells in each group after 6 days of culture 7.70 × 105, 8.62 × 105, and 7.80 × 105, respectively (Fig. 3b). Proliferation was slightly increased in the recombinant SOD2 and positive control groups at 26 days (3.53 × 108 and 3.42 × 108, respectively vs. 1.44 × 108 for the negative control). Compared to the negative control group, recombinant SOD2 increased the proliferative capacity of hMSC-1 after 32 days, with the number of cells at 45 days (10.3 × 109) almost the same as that of the positive control group (9.46 × 109).
Fig. 3.
Effect of endotoxin or SOD2 on hMSC-1 proliferation and morphology. (a) Morphology of hMSC-1 cultured for 6, 27, and 45 days in medium containing 0 or 1000 ng/ml endotoxin or 12.8 μg/ml SOD2. (b) Number of hMSC-1 cultured for 45 days in medium containing 0 or 1000 ng/ml endotoxin or 12.8 μg/ml SOD2.
4. Discussion
The present study investigated the effect of endotoxin on MSC proliferation in an in vitro culture system and the underlying mechanism in order to accurately quantify the risks associated with endotoxin contamination in culture systems used for tissue engineering and to establish endotoxin limits based on empirical evidence. Endotoxin contamination in a culture system always indicates the presence of live or dead Gram-negative bacteria, but can also indicate the presence of other microbes such as Gram-positive bacteria and fungi. Although the effects of endotoxins on cell cultures should be estimated by bacterial cell counts, the detection of endotoxin activity in a system should be interpreted as a reflection of contamination level. It is therefore important to establish limits for endotoxin levels in order to assure the safety and quality of MSC-based products.
It should be noted that all items used for cell culture such as medium, serum, reagent, enzyme, scaffold, and plastic products (culture plate, pipet, and chip) may be contaminated with endotoxin even if they are labeled as endotoxin free. It may not be possible to estimate endotoxin levels when selecting serum lots, and scaffolds made from natural products—even those of high quality—may contain trace amounts; there has been at least one reported case of commercial enzymes such as collagenase contaminated with endotoxin [21]. SEIKAGAKU Corporation (Tokyo, Japan), a company that produces limulus amebocyte lysate reagents for endotoxin testing, has alerted consumers via their home page that commercially available plastic products labeled endotoxin free are occasionally contaminated with endotoxin. Therefore, endotoxin contamination should be estimated for all cell culture-related items before use.
In contrast to a previous report [2], the proliferative capacity of ADSCs was increased by the presence of endotoxin at concentrations >0.1 ng/ml hMSC proliferation was also increased at >1 ng/ml, but this effect was diminished or disappeared with cellular aging. It has been reported that various stressors including endotoxin induce the expression of growth factors such as vascular endothelial growth factor, fibroblast growth factor 2, hepatocyte growth factor, and insulin-like growth factor (IGF)-1 in hMSCs [22], but this was not detected in our proteomics approach without up-regulation of IGF-binding protein-7 and IGF-2 mRNA-binding proteins. On the other hand, endotoxin preconditioning (1.0 μg/ml) protected mouse MSCs from H2O2/serum deprivation-induced apoptosis and improved cell survival via activation of TLR4 and phosphoinositide 3-kinase/Akt signaling [4]. Similar results regarding the levels of apoptosis-related proteins induced by endotoxin were obtained in the present study. In addition, SOD2 expression was significantly and consistently upregulated during the culture period, whereas p16, a marker for cellular aging, was downregulated by endotoxin stimulation (data not shown). These results suggest that endotoxin rescues MSCs from oxidative stress via upregulation of SOD2, thereby improving cell survival. This is supported by the results obtained by gene expression profiling, which will be presented elsewhere.
On the other hand, the dose of endotoxin that increased MSC proliferation in this study was lower than those previously reported [4], [5]. Endotoxin has distinct biological activities depending on the chemical structure of the lipid A portion comprising the active center of the molecule; this structure is different for each species of Gram-negative bacterium [13], [14]. It has been reported that P. gingivalis endotoxin has a unique structure that has lower biological activity than that of E. coli [13], [14]; it is therefore reasonable that a relatively high dose (100 ng/ml) was required to inhibit osteoblast differentiation [16]. Coli-type lipid A has the same basic structure across E. coli serotypes (O-antigen), although the degree of saturation of acyl and phosphate groups varies depending on the culture conditions. Commercially available E. coli endotoxin often has a heterogeneous lipid A structure that is associated with a lower biological activity than typical coli-type lipid A, which consists of a β(1–6)-linked glucosamine disaccharide substituted with six acyl and two phosphate groups [13], [14]. The endotoxin used in this study had low heterogeneity, and most of the molecules were fully acylated (data not shown), since E. coli cells from which it was extracted were cultured under strict conditions in order to decrease the proliferation rate and promote complete endotoxin biosynthesis. It may be for this reason that a relatively large amount of endotoxin was needed to alter the behavior of MSCs as compared to previous studies using commercially available E. coli endotoxin. The stability of endotoxin may have also contributed to this discrepancy. Endotoxin is a heat-stable somatic antigen of Gram-negative bacteria, and the polysaccharide portion determining species serotype is highly stable. However, endotoxin was found to be unstable in aqueous solution, even in PBS, with the activity decreasing over time [23], [24], [25]. We therefore added fresh endotoxin to the culture medium that was replaced three times in a week during the culture period, since we observed that the activity in medium containing 10% fetal bovine serum was reduced to 20% after 2 days at 37 °C (data not shown).
The expression of cluster of differentiation (CD)80, CD86, major histocompatibility complex-II, TLR-4, and tumor necrosis factor-α in MSCs were found to be most effectively increased by endotoxin at a concentration of 10 μg/ml [3]. However, the dose seems to be too high for increasing the expression at a molecular level, because proliferation ability of MSCs was perceptibly increased by endotoxin at the concentration more than 0.1 ng/ml in this study, and the change of related gene and protein expression levels in MSCs may be induced less than the dosage. The ability of endotoxin to enhance MSC proliferation is beneficial for in vitro cell cultures; however, since it can also affect other cellular functions, a concentration limit should be set for MSC cultures to assure safety and quality. Although the precise amount of endotoxin affecting MSCs at the molecular level remains unclear, an LOAEL of 0.1 ng/ml was established in this study based on the effect on MSC proliferation.
Little is known about the effect of endotoxin on MSCs in vivo. There have been several studies on the host response to biomaterials spiked with bacterial components such as endotoxin [26], [27], [28], [29], [30], but none have focused on their effect on MSCs and dose response to establish endotoxin limits at specific sites of the body. In the only quantitative analysis to date, we reported that a collagen sheet containing dried E. coli cells implanted into a cranial or femoral defect in rats caused a dose-dependent delay in osteoanagenesis with a no-observed-adverse-effect level of 9.6 EU/mg, which did not occur with an untreated collagen sheet or one containing Staphylococcus aureus cells [21]. These results suggest that endotoxin affects the process of osteoanagenesis and that the delayed formation of new bone was caused by dried cells that suppressed the development of connective tissue covering the defective parts and the proliferation and differentiation of MSCs (intramembranous ossification), since a pathological analysis did not detect any osteoclasts or inflammation. As described above, several reports suggest that TLR ligands including endotoxin may influence the differentiation capacity of MSCs in vitro [1], [2], [3], [4], [5], [15], [16], but details such as the dose response and underlying mechanism remain unknown. We also evaluated the effect of endotoxin on the differentiation potential of hMSCs in vitro and found that endotoxin affected differentiation of hMSCs into osteoblasts but not adipocytes. These results will appear elsewhere as a report on the establishment of specifications for the endotoxin limit for biomaterials used to induce osteoanagenesis. In addition, quantitative analyses to determine the limits for in vitro proliferation and differentiation capacity of induced pluripotent stem cells, which are another cell source for regenerative medicine, are now underway in our laboratory.
5. Conclusions
This is the first report describing endotoxin specifications for MSCs that can be used for tissue engineering products. The LOAEL for enhancement of proliferative capacity observed in an in vitro culture system was 0.1 ng/ml (2.75 EU/ml). Future research should focus on establishing limits for the proliferation of induced pluripotent stem (iPS) cells or for the differentiation capacity of MSCs and iPS cells.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Pevsner-Fischer M., Morad V., Cohen-Sfady M., Rousso-Noori L., Zanin-Zhorov A., Cohen S. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 2.Hwa Cho H., Bae Y.C., Jung J.S. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 3.Shi L., Liu X.M., Hu X.Y., Wang J.S., Fang Q. [The effect of lipopolysaccharide on the expression and activity of Toll-like receptor 4 in mesenchymal stem cells] Zhonghua Xue Ye Xue Za Zhi. 2007;28:828–831. [PubMed] [Google Scholar]
- 4.Wang Z.J., Zhang F.M., Wang L.S., Yao Y.W., Zhao Q., Gao X. Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int. 2009;33:665–674. doi: 10.1016/j.cellbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 5.He X., Wang H., Jin T., Xu Y., Mei L., Yang J. TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via Wnt3a and Wnt5a signaling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arahira T., Todo M. Effects of proliferation and differentiation of mesenchymal stem cells on compressive mechanical behavior of collagen/beta-TCP composite scaffold. J Mech Behav Biomed Mater. 2014;39:218–230. doi: 10.1016/j.jmbbm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Chen G., Lv Y., Dong C., Yang L. Effect of internal structure of collagen/hydroxyapatite scaffold on the osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2015;10:99–108. doi: 10.2174/1574888x09666140812112631. [DOI] [PubMed] [Google Scholar]
- 8.Sun K., Li H., Li R., Nian Z., Li D., Xu C. Silk fibroin/collagen and silk fibroin/chitosan blended three-dimensional scaffolds for tissue engineering. Eur J Orthop Surg Traumatol. 2015;25:243–249. doi: 10.1007/s00590-014-1515-z. [DOI] [PubMed] [Google Scholar]
- 9.Snyder T.N., Madhavan K., Intrator M., Dregalla R.C., Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng. 2014;8:10. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiwatashi N., Hirano S., Mizuta M., Tateya I., Kanemaru S., Nakamura T. Biocompatibility and efficacy of collagen/gelatin sponge scaffold with sustained release of basic fibroblast growth factor on vocal fold fibroblasts in 3-dimensional culture. Ann Otol Rhinol Laryngol. 2015;124:116–125. doi: 10.1177/0003489414546396. [DOI] [PubMed] [Google Scholar]
- 11.Sapir Y., Ruvinov E., Polyak B., Cohen S. Magnetically actuated alginate scaffold: a novel platform for promoting tissue organization and vascularization. Methods Mol Biol. 2014;1181:83–95. doi: 10.1007/978-1-4939-1047-2_8. [DOI] [PubMed] [Google Scholar]
- 12.Curtin C.M., Tierney E.G., McSorley K., Cryan S.A., Duffy G.P., O'Brien F.J. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv Healthc Mater. 2015;4:223–227. doi: 10.1002/adhm.201400397. [DOI] [PubMed] [Google Scholar]
- 13.Rietschel E.T.B.L., Schade U., Seydel U., Zähringer U., Lindner B., Morgan A.P. Chemical structure and biological activity of lipopolysaccharides. In: Baumgartner J.D., Calandra T., Carlet J., editors. Endotoxin from pathophysiology to therapeutic approaches. Flammarion Medicine-Sciences; Paris: 1990. pp. 5–18. [Google Scholar]
- 14.Rietschel E.T.M.H., Wollenweber H.W., Zähringer U., Lüderitz O., Westphal O., Brade H. Bacterial lipopolysaccharides and their lipid A component. In: Homma J.Y., Kanegasaki S., Lüderitz O., Shiba T., Westphal O., editors. Bacterial endotoxin; Chemical, biological and clinical aspects. Wiley-VCH Verlag GmbH; Weinheim: 1984. pp. 11–22. [Google Scholar]
- 15.Cho H.H., Kim Y.J., Kim S.J., Kim J.H., Bae Y.C., Ba B. Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng. 2006;12:111–121. doi: 10.1089/ten.2006.12.111. [DOI] [PubMed] [Google Scholar]
- 16.Kadono H., Kido J., Kataoka M., Yamauchi N., Nagata T. Inhibition of osteoblastic cell differentiation by lipopolysaccharide extract from Porphyromonas gingivalis. Infect Immun. 1999;67:2841–2846. doi: 10.1128/iai.67.6.2841-2846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H., Kaneko M., He C., Hughes M.A., Cherry G.W. Effect of a lipopolysaccharide from E. coli on the proliferation of fibroblasts and keratinocytes in vitro. Phytother Res. 2002;16:43–47. doi: 10.1002/ptr.912. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa T., Aoi T., Umezawa A., Ozawa K., Sato Y., Sawa Y. A study on ensuring the quality and safety of pharmaceuticals and medical devices derived from the processing of autologous human somatic stem cells. Regen Ther. 2015;2:57–69. doi: 10.1016/j.reth.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphal O., Jann K. Bacterial lipopolysaccharides. extraction with phenol-water and further applications of the procedure. Methods carbohydra Chem. 1965;5:83–91. [Google Scholar]
- 20.Haishima Y., Murai T., Nakagawa Y., Hirata M., Yagami T., Nakamura A. Chemical and biological evaluation of endotoxin contamination on natural rubber latex products. J Biomed Mater Res. 2001;55:424–432. doi: 10.1002/1097-4636(20010605)55:3<424::aid-jbm1032>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Haishima Y., Hasegawa C., Todoki K., Sasaki K., Niimi S., Ozono S. A biological study establishing the endotoxin limit of biomaterials for bone regeneration in cranial and femoral implantation of rats. J Biomed Mater Res B Appl Biomater. 2016;105:1514–1524. doi: 10.1002/jbm.b.33692. [DOI] [PubMed] [Google Scholar]
- 22.Crisostomo P.R., Wang Y., Markel T.A., Wang M., Lahm T., Meldrum D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kano S., Mochida K., Ogawa Y. Studies on heat-inactivation of pyrogen from Escherichia coli. Biken J. 1970;13:233–239. [PubMed] [Google Scholar]
- 24.Miyamoto T., Okano S., Kasai N. Inactivation of Escherichia coli endotoxin by soft hydrothermal processing. Appl Environ Microbiol. 2009;75:5058–5063. doi: 10.1128/AEM.00122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa Y., Murai T., Kawasaki H. Endotoxin test for medical devices: the correlation of the LAL test with the pyrogen test. J Antibact Antifung Agents. 1991;19:561–566. [Google Scholar]
- 26.Daly K.A., Liu S., Agrawal V., Brown B.N., Huber A., Johnson S.A. The host response to endotoxin-contaminated dermal matrix. Tissue Eng Part A. 2012;18:1293–1303. doi: 10.1089/ten.TEA.2011.0597. [DOI] [PubMed] [Google Scholar]
- 27.Ho T.Y., Chen Y.S., Hsiang C.Y. Noninvasive nuclear factor-kappaB bioluminescence imaging for the assessment of host-biomaterial interaction in transgenic mice. Biomaterials. 2007;28:4370–4377. doi: 10.1016/j.biomaterials.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Martinez Avila H., Schwarz S., Feldmann E.M., Mantas A., von Bomhard A., Gatenholm P. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol. 2014;98:7423–7435. doi: 10.1007/s00253-014-5819-z. [DOI] [PubMed] [Google Scholar]
- 29.Schutte R.J., Xie L., Klitzman B., Reichert W.M. In vivo cytokine-associated responses to biomaterials. Biomaterials. 2009;30:160–168. doi: 10.1016/j.biomaterials.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Putten S.M., Wubben M., Plantinga J.A., Hennink W.E., van Luyn M.J., Harmsen M.C. Endotoxin contamination delays the foreign body reaction. J Biomed Mater Res A. 2011;98:527–534. doi: 10.1002/jbm.a.33144. [DOI] [PubMed] [Google Scholar]