Abstract
Osteosarcoma is a primary bone tumor that affects children and young adults. The estrogen metabolite 2-methoxyestradiol (2-ME) induces cell death in osteosarcoma cells. To determine whether 2-ME actions involve the control of protein synthesis, we studied the effect of 2-ME on eukaryotic initiation factor 4E (eIF4E) and eIF4E-binding protein 1 (4E-BP1) in MG63 osteosarcoma cells. Our results show that 2-ME treatment increases the association of eIF4E with 4E-BP1 in osteosarcoma cells. Also, 2-ME decreases the binding of eIF4E protein to 7-methyl-guanosine cap structure, indicating that 2-ME treatment results in the inhibition of translational initiation. These findings are further supported by the inhibition of protein synthesis in 2-ME-treated osteosarcoma cells. Taken together, our studies show that 2-ME-mediated antitumor effects in osteosarcoma cells involve the regulation of protein synthesis, and translational machinery could serve as a target in the treatment of osteosarcoma.
Keywords: 2-Methoxyestradiol, 4E-BP, Estrogen metabolite, eIF4E, Osteosarcoma
Introduction
Osteosarcoma is a pediatric malignancy. Even though a combination of surgery and chemotherapy has improved the survival rate, the mortality rate remains high for this disease. 2-Methoxyestradiol (2-ME) is a metabolite of 17β-estradiol that exerts antitumor effects in a number of tumor cells, including osteosarcoma.1, 2, 3, 4, 5, 6, 7 We have previously shown that 2-ME induces apoptosis in osteosarcoma cells, but not in normal osteoblasts.5, 8
Protein synthesis regulation has been implicated in the control of tumor cell proliferation and apoptosis. Protein synthesis comprises three steps: initiation, elongation, and termination. The majority of the regulation takes place at the level of translation initiation involving eukaryotic translation initiation factor (eIF) 4E. eIF4E is a part of a multiprotein eIF4F complex, which consists of three proteins: eIF4E, which binds the 5′ cap structure of the mRNA; eIF4A, an ATP-dependent RNA helicase that is expected to unwind the secondary structures in mRNA; and eIF4G, a protein that interacts with eIF4E, eIF4A, and other initiation factors to form a scaffold.9, 10, 11 The eIF4E serves as a rate-limiting factor of the eIF4F complex and is required by almost all mRNAs to be translated into proteins. eIF4E binds to 7-methyl guanosine triphosphate cap, which is present in most mRNAs, and thereby brings them to ribosomes and regulates cap-dependent protein synthesis.12 The eIF4E activity is regulated by eIF4E-binding proteins (4E-BPs) that function as translational inhibitors.12, 13, 14, 15 The 4E-BP binding to eIF4E is determined by its phosphorylation status. The dephosphorylated 4E-BP binds to eIF4E and thereby blocks its activity.12, 13 RNA dependent protein kinase (PKR) is another protein which has been implicated in the regulation of protein synthesis and induction of apoptosis in a variety of different tumor cells.16, 17, 18 PKR has been shown to act through the phosphorylation of eIF-2α.8, 16, 17, 18 Previous studies demonstrate the involvement of translational control and the components of eIF4F complex in malignant transformation and progression.12, 19 The goal of this study was to investigate the role of initiation factors eIF4E and 4E-BP1 in 2-ME-mediated actions in osteosarcoma cells.
Materials and methods
Cell culture and metabolite treatment
MG63 osteosarcoma cells and normal human osteoblast (HOB) cells5 were maintained in Dulbecco's modified eagles medium (DMEM)/F12 containing 10% charcoal-stripped fetal bovine serum and supplemented with 100 units/mL penicillin and 100 μg/mL streptomycin and maintained at 37°.4, 20 Cells were treated with 10 μM 2-ME (Sigma Chemical Co., St. Louis, MO) or the vehicle (70% ethanol) for indicated periods of time.
Preparation and analysis of cytoplasmic extract
Cells were harvested after vehicle and 2-ME treatment and lysed in cell lysis buffer, as described.21, 22 Following centrifugation, the supernatant was collected, and protein concentration was determined by as described.21 Cytoplasmic extracts (60 μg protein) were analyzed by Western blot using anti-eIF4E, anti-eIF4E-BP1, antiphospho-eIF4E-BP1 (Thr37/46), anti-nonphospho-eIF4E-BP1 (Thr46) (Cell Signaling, Danvers, MA), and anti-actin antibodies (Sigma) as described in our earlier reports.21, 22
Immunoprecipitation analysis was carried out as described.22 Briefly, cytoplasmic extracts containing 60 μg protein were used for immunoprecipitation with anti-4E-BP1 antibodies. Bound proteins were purified using protein A Sepharose and analyzed by western blot hybridization using anti-4E antibodies. The levels of proteins on the western blots were quantitated using densitometer and Quantity one 4.5.2 software (BioRad, Hercules, CA).
Cap-binding assay
Cap-binding assay by m7GTP-Sepharose chromatography was performed as described.23 About 250 μL cell lysate (100 μg protein) was added to 20 μL of packed m7GTP-Sepharose beads to capture eIF4E and its binding partner proteins. Captured proteins were eluted with buffer containing 50 mM Tris (pH 8.0); 150 mM NaCl; 1mM EDTA; 0.1% IPEGAL and 0.2% gelatin and analyzed by western blot using anti-eIF4E directly or after immunoprecipitation with anti-eIF4E-BP1 antibodies.
Protein synthesis (3H-leucine incorporation)
The rate of protein synthesis was measured as described.15 Briefly, protein synthesis was studied following vehicle and 2-ME (10 μM) treatment and by pulse labeling of cells for 1 h with 10 μCi/mL of 3H-labled leucine. The cells were harvested and washed in 5 mL of cold phosphate buffered saline, and then the cells were lysed and precipitated with 10% trichloroacetic acid (TCA). The precipitate was filtered and washed with 5% TCA, and the TCA-insoluble radioactivity was determined by scintillation counting.
Statistical analysis
All values are expressed as means ± standard error. The data are representative of four independent experiments. Significant differences between groups were determined by Fisher's protected least significant difference post hoc test for multiple-group comparisons following detection of significance by one-way analysis of variance.
Results and discussion
Effect of 2-ME on initiation factors eIF4E and 4E-BP1 in osteosarcoma cells
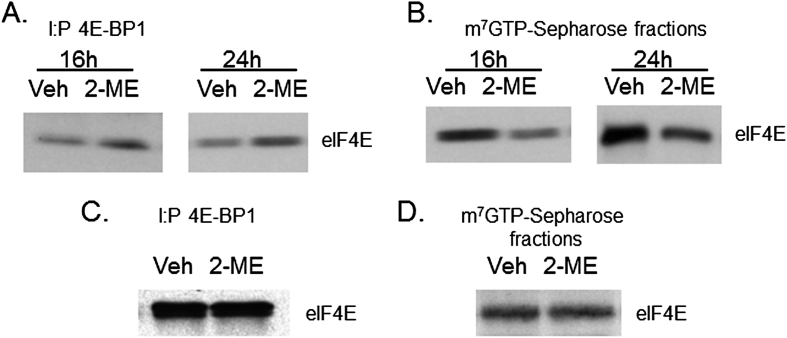
To determine the effect of 2-ME on protein synthesis, we have investigated whether 2-ME regulates the binding of protein synthesis initiation factor eIF4E to its inhibitor protein 4E-BP1. We have analyzed the cytoplasmic extracts from vehicle and 2-ME-treated MG63 cells by co-immunoprecipitation studies which show that 2-ME treatment increased the binding of 4E-BP1 to eIF4E at 16 and 24 h (Fig. 1A). Furthermore, the cap-binding assays carried out show that 2-ME treatment resulted in decreased binding of eIF4E to cap structure (Fig. 1B). In eukaryotic cells, eIF4E associates with ribosomes and facilitates cap-dependent mRNA translation.12, 14, 24, 25 Studies suggest that cancer cells depend more on cap-dependent translation than normal cells, and cap-dependent translation is essential for the synthesis of tumorigenic proteins.26 In addition, eIF4E is required for the transport of specific mRNAs. The eIF4E-mediated regulation of gene expression exhibits different gene specificities, as some genes are being regulated at the level of translation and others at the level of both transport and translation.12, 25, 27 Deregulated transport of mRNAs of oncogenes and growth regulatory genes appears to be the cause for the oncogenic functions of eIF4E.12, 27 Our current results show that an increased association of eIF4E and 4E-BP1 is accompanied by decreased binding of eIF4E to 7-methyl guanosine cap structure in the presence of 2-ME treatment. Furthermore, our results demonstrate that these effects are specific to osteosarcoma cells, and 2-ME treatment does not affect the binding of eIF4E to 4E-BP1 (Fig. 1C) and cap structure (Fig. 1D) in normal HOB cells.
Figure. 1.
2-ME regulates eIF4E and 4E-BP1 functions in osteosarcoma cells. Cytoplasmic extracts were prepared from MG63 osteosarcoma cells (A, B) at 16 and 24 h and normal human osteoblast (HOB) cells (C, D) at 24 h following vehicle (Veh) and 2-ME treatment. The extracts were subjected to immunoprecipitation (IP) with anti-4E-BP1 antibodies (A, C) or cap-binding (B, D) and analyzed by western blot analysis using anti-eIF4E antibodies.
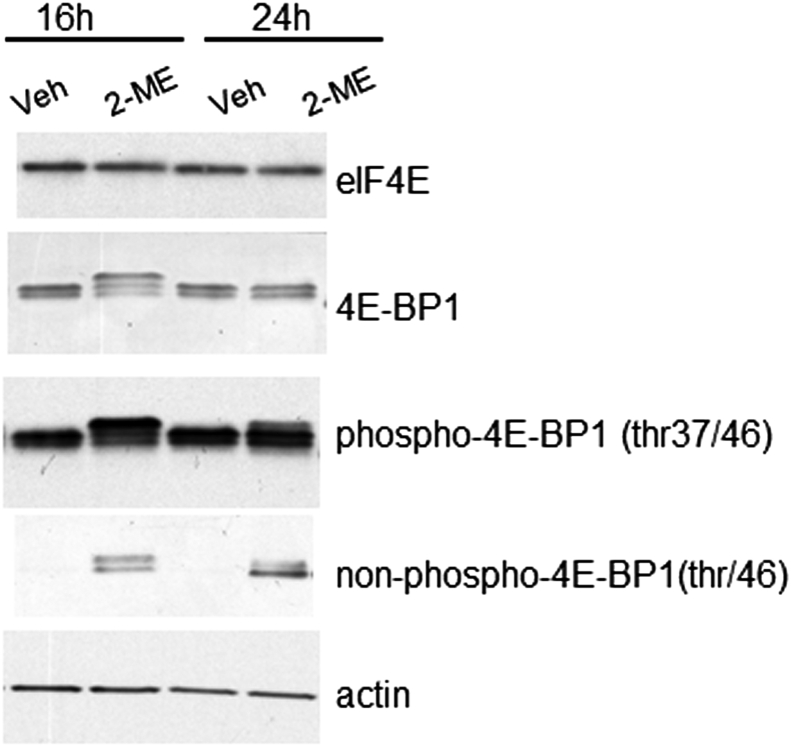
The western blot analysis shows that 2-ME treatment does not affect the levels of eIF4E and control actin, but modulates 4E-BP1 protein levels in MG63 cells (Fig. 2). The results show that overall band intensity ratio in vehicle and 2-ME-treated conditions at 16 h were 1.2:1.0; 1.0:1.5 and 1:20, respectively, when probed with the anti-4E-BP1, phospho-4E-BP1, and non-phospho-4E-BP1 antibodies. Similarly, the ratios at 24 h were 1.4:1.0; 1.0:1.6 and 1: 60 when probed with the anti-4E-BP1, phospho-4E-BP1, and non-phospho-4E-BP1 antibodies. Thus, these results point out that 2-ME treatment increases the non-phospho-4E-BP1 levels more compared to phospho-4E-BP1 protein levels. Published reports indicate that eIF4E and 4E-BP1 interaction is controlled by phosphorylation of the 4E-BPs.14, 15 There are 3 different types of 4E-BPs, and the hypophosphorylated forms of 4E-BPs interact with high affinity to competitively inhibit the binding of eIF4G to eIF4E.28 Conversely, phosphorylation of 4E-BPs decreases the affinity of the protein for eIF4E and facilitates the binding of eIF4E to cap structure and continuous protein synthesis in cells.14, 24 Current studies show that higher levels of non-phosphorylated or hypophosphorylated forms of 4E-BP1 are present in the presence of 2-ME treatment compared to vehicle controls. 4E-BPs could be phosphorylated at multiple sites, and hyperphosphorylation has been shown to be required for the dissociation of eIF4E and activation of eIF4E functions.13, 23 Also, a large number of studies report that protein kinase mTOR (mammalian target of rapamycin) is the major protein responsible for the 4E-BP phosphorylation.13, 25, 26, 27, 28 While our results clearly show an increase in non-phospho 4E-BP1 protein, additional investigations are required to establish the involvement of mTOR, and to delineate the various forms of the phosphorylated and non-phosphorylated forms of 4E-BP1 in 2-ME-treated osteosarcoma cells.
Figure. 2.
Effect of 2-ME treatment on eIF4E and 4E-BP1 expression levels. Cytoplasmic extracts were prepared following 16 and 24 h of vehicle and 2-ME treatment from MG63 cells and analyzed by western blot using anti-eIF4E, anti-4E-BP1, antiphospho-4E-BP1 (Thr37/46), anti-nonphospho-4E-BP1 (Thr46), and anti-actin antibodies.
Changes in the state of phosphorylation of the 4E-BP and in the extent of its association with eIF4E could occur in response to stimulation of cell death in cells. Previous reports show that the growth-inhibiting apoptotic activities of cytokines and drugs have an impact on the eIF4E/4E-BP system. TNFα has been shown to inhibit overall translation in breast cancer cells by mechanisms that involve decreased phosphorylation of 4E-BP1 and increased association of eIF4E and 4E-BP1.29 This observation is in agreement with the increased association of eIF4E with 4E-BP1 and decreased binding of eIF4E to cap structure observed in 2-ME-treated osteosarcoma cells. Hence, this indicates that the antitumor actions of 2-ME, in part, could be at the level of eIF4E-dependent translation initiation. Our result is corroborated by the absence of eIF4E regulation in HOB cells that are resistant to 2-ME-mediated antigrowth effects.
Effect of 2-ME on protein synthesis
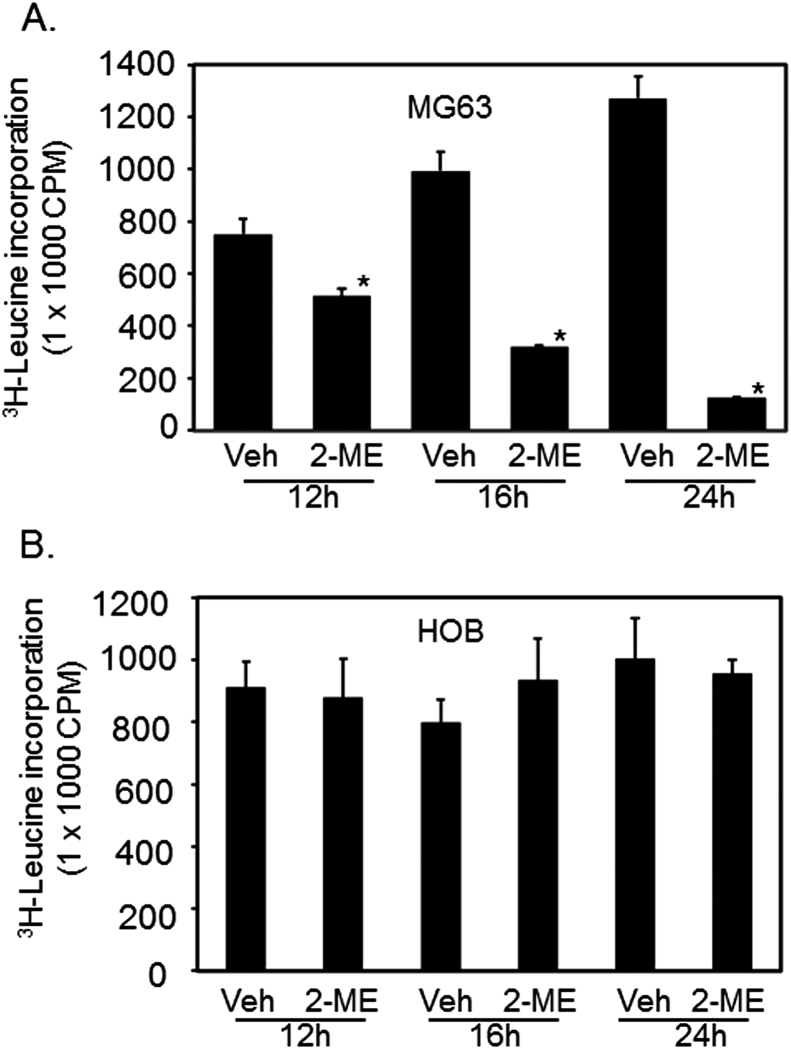
To determine whether 2-ME-mediated inhibition of eIF4E has any effect on protein synthesis, we have followed the rate of protein synthesis. Our findings from 3H-labeling studies show that the rate of protein synthesis is significantly (P < 0.1) reduced in MG63 cells to 68%, 32%, and 9.8%, respectively, at 12, 16, and 24 h in the presence of 2-ME compared to controls (Fig. 3A). However, 2-ME treatment does not affect protein synthesis in HOB cells (Fig. 3B) and it is in agreement with the absence of regulation of eIF4E and 4E-BP1 in HOB cells. Thus, the inhibition of eIF4E through sequestration by inhibitory protein 4E-BP1 leads to a block in protein synthesis in the presence of 2-ME. We have also observed that 2-ME inhibits protein synthesis in a number of osteosarcoma cell lines (A. Maran, K. Shogren, M.J. Yaszemski, unpublished observations). Future investigations are necessary to identify the associated mechanism and the target mRNAs that undergo translational block in the presence of 2-ME treatment.
Figure. 3.
2-ME treatment downregulates protein synthesis in osteosarcoma cells. Protein synthesis was measured following vehicle and 2-ME treatment in MG63 (A) and HOB (B) cells at 12, 16, and 24 h through pulse labeling with 3H-leucine. *P < 0.01 vs vehicle.
Effect of 2-ME on MG63 cells expressing negative dominant mutant PKR protein
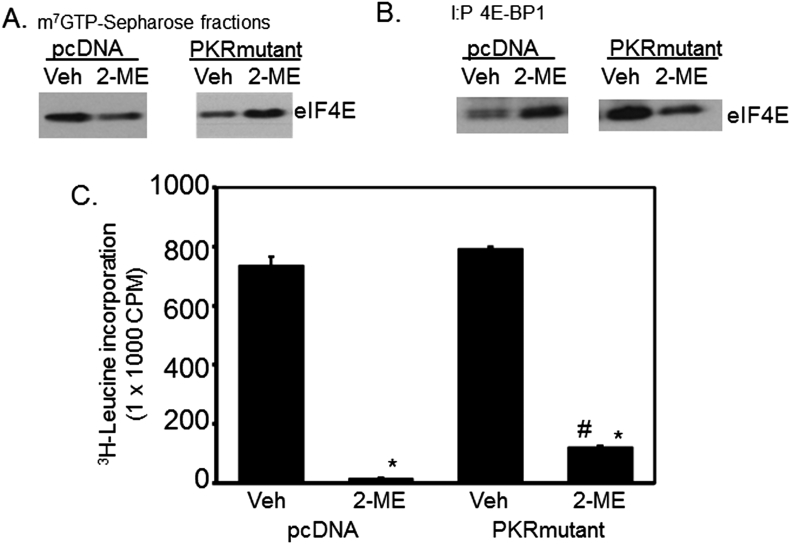
MG63 osteosarcoma cells expressing PKRmutant protein are resistant to 2-ME-mediated antigrowth effects.4, 8, 20 We have studied the effect of 2-ME on eIF4E binding to cap structure and to the inhibitory protein 4E-BP1 in cells expressing negative dominant mutant PKR (PKRmutant) and the control vector (pcDNA). Our results show that 2-ME treatment decreased the association of eIF4E with 4E-BP1 (Fig. 4A) and increased the binding of eIF4E to cap structure in control pcDNA cells but not in PKRmutant osteosarcoma cells (Fig. 4B). Furthermore, the studies reveal a partial reversal in 2-ME-mediated inhibition of protein synthesis in PKRmutant cells (Fig. 4C). Our results show that, compared to vehicle, 2-ME treatment decreases protein synthesis to 2% and 15% in MG63 cells expressing pcDNA and PKRmutant, respectively (Fig. 4). Our previous studies show that 2-ME treatment regulates the initiation factor, eIF2α, and induces phosphorylation of eIF2α through the activation of PKR.8 Current studies indicate that PKR could be involved in additional translational regulations in 2-ME-treated osteosarcoma cells involving eIF4E in addition to already demonstrated initiation factor eIF-2α-dependent mechanisms.
Figure. 4.
2-ME effect on protein synthesis is decreased in cells expressing PKRmutant protein. MG63 osteosarcoma cells expressing vector (pcDNA) and PKRmutant protein were treated with vehicle and 2-ME for 24 h. The cytoplasmic extracts prepared were subjected to cap-binding (A) or IP with anti-4E-BP1 antibodies (B) and analyzed by western blot analysis using anti-eIF4E antibodies. Protein synthesis was measured through pulse labeling with 3H-leucine following 24 h of vehicle and 2-ME treatment (C). *P < 0.01 vs Veh, #P < 0.01 vs pcDNA 2-ME.
Conclusion
In this report, we have shown a direct relationship between the assembly of the cap-dependent translation initiation apparatus and the antitumor effects of 2-ME. Our results show that 2-ME treatment in osteosarcoma cells resulted in a) increased association of eIF4E with eIF4E-BP1; b) decreased binding of eIF4E to 7-methyl guanosine cap structure; c) increased levels of hypophosphorylated 4E-BP1 protein; and d) decreased protein synthesis. Previous work demonstrates that 2-ME exerts antigrowth and antitumor effects in osteosarcoma cells but not in normal osteoblasts.4, 5, 30 Current findings reveal that 2-ME inhibits cap-dependent protein synthesis in cancerous MG63 cells without affecting protein synthesis in normal HOB. Thus, our studies suggest that eIF4E/4E-BP system could play a major regulatory role in 2-ME-mediated cell death, and could be further explored as a potential target in the treatment of osteosarcoma.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This work was supported by funding from the Riviera Foundation and Mayo Clinic.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Fotsis T., Zhang Y., Pepper M.S. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 2.Ganapathy M., Ghosh R., Jianping X. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clin Cancer Res. Mar 1 2009;15:1601–1611. doi: 10.1158/1078-0432.CCR-08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaVallee T.M., Zhan X.H., Johnson M.S. 2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003;63:468–475. [PubMed] [Google Scholar]
- 4.Maran A., Shogren K.L., Benedikt M., Sarkar G., Turner R.T., Yaszemski M.J. 2-methoxyestradiol-induced cell death in osteosarcoma cells is preceded by cell cycle arrest. J Cell Biochem. 2008;104:1937–1945. doi: 10.1002/jcb.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maran A., Zhang M., Kennedy A.M. 2-Methoxyestradiol induces interferon gene expression and apoptosis in osteosarcoma cells. Bone. 2002;30:393–398. doi: 10.1016/s8756-3282(01)00681-0. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay T., Roth J.A. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene. 1997;14:379–384. doi: 10.1038/sj.onc.1200835. [DOI] [PubMed] [Google Scholar]
- 7.Pribluda V.S., Gubish E.R., Jr., Lavallee T.M., Treston A., Swartz G.M., Green S.J. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Met Rev. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- 8.Shogren K.L., Turner R.T., Yaszemski M.J., Maran A. Double-stranded RNA-dependent protein kinase is involved in 2-methoxyestradiol-mediated cell death of osteosarcoma cells. J Bone Miner Res. Jan 2007;22:29–36. doi: 10.1359/JBMR.060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross J.D., Moerke N.J., von der Haar T. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. Dec 12 2003;115:739–750. doi: 10.1016/s0092-8674(03)00975-9. [DOI] [PubMed] [Google Scholar]
- 10.Rogers G.W., Jr., Komar A.A., Merrick W.C. eIF4A: the godfather of the DEAD box helicases. Prog Nucleic Acid Res Mol Biol. 2002;72:307–331. doi: 10.1016/s0079-6603(02)72073-4. [DOI] [PubMed] [Google Scholar]
- 11.Sonenberg N. eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem Cell Biol. Apr 2008;86:178–183. doi: 10.1139/O08-034. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui N., Sonenberg N. Signalling to eIF4E in cancer. Biochem Soc Trans. Oct 1 2015;43:763–772. doi: 10.1042/BST20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras A.C., Kennedy S.G., O'Leary M.A., Sonenberg N., Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. Feb 15 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens M.J. Translational regulation in cell stress and apoptosis. Roles of the eIF4E binding proteins. J Cell Mol Med. Jul–Sep 2001;5:221–239. doi: 10.1111/j.1582-4934.2001.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constantinou C., Clemens M.J. Regulation of translation factors eIF4GI and 4E-BP1 during recovery of protein synthesis from inhibition by p53. Cell Death Differ. Mar 2007;14:576–585. doi: 10.1038/sj.cdd.4402045. [DOI] [PubMed] [Google Scholar]
- 16.Barber G.N. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–570. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- 17.Stark G.R., Kerr I.M., Williams B.R., Silverman R.H., Schreiber R.D. How cells respond to interferons. Ann Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 18.Garcia M.A., Meurs E.F., Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Mamane Y., Petroulakis E., Rong L., Yoshida K., Ler L.W., Sonenberg N. eIF4E–from translation to transformation. Oncogene. Apr 19 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 20.Yang C., Shogren K.L., Goyal R., Bravo D., Yaszemski M.J., Maran A. RNA-dependent protein kinase is essential for 2-methoxyestradiol-induced autophagy in osteosarcoma cells. PLoS One. 2013;8:e59406. doi: 10.1371/journal.pone.0059406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedikt M.B., Mahlum E.W., Shogren K.L. 2-methoxyestradiol-mediated anti-tumor effect increases osteoprotegerin expression in osteosarcoma cells. J Cell Biochem. Apr 1 2010;109:950–956. doi: 10.1002/jcb.22473. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy A.M., Shogren K.L., Zhang M., Turner R.T., Spelsberg T.C., Maran A. 17beta-estradiol-dependent activation of signal transducer and activator of transcription-1 in human fetal osteoblasts is dependent on Src kinase activity. Endocrinology. Jan 2005;146:201–207. doi: 10.1210/en.2004-0486. [DOI] [PubMed] [Google Scholar]
- 23.Constantinou C., Clemens M.J. Regulation of the phosphorylation and integrity of protein synthesis initiation factor eIF4GI and the translational repressor 4E-BP1 by p53. Oncogene. Jul 14 2005;24:4839–4850. doi: 10.1038/sj.onc.1208648. [DOI] [PubMed] [Google Scholar]
- 24.Gingras A.C., Raught B., Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. Apr 1 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 25.Karaki S., Andrieu C., Ziouziou H., Rocchi P. The eukaryotic translation initiation factor 4E (eIF4E) as a therapeutic target for cancer. Adv Protein Chem Struct Biol. 2015;101:1–26. doi: 10.1016/bs.apcsb.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y., Polunovsky V., Bitterman P.B., Wagner C.R. Cap-dependent translation initiation factor eIF4E: an emerging anticancer drug target. Med Res Rev. Jul 2012;32:786–814. doi: 10.1002/med.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemens M.J. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. Apr 19 2004;23:3180–3188. doi: 10.1038/sj.onc.1207544. [DOI] [PubMed] [Google Scholar]
- 28.Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey I.W., Bushell M., Tilleray V.J., Morley S., Clemens M.J. Inhibition of protein synthesis in apoptosis: differential requirements by the tumor necrosis factor alpha family and a DNA-damaging agent for caspases and the double-stranded RNA-dependent protein kinase. Cancer Res. Apr 15 2002;62:2272–2280. [PubMed] [Google Scholar]
- 30.Wimbauer F., Yang C., Shogren K.L. Regulation of interferon pathway in 2-methoxyestradiol-treated osteosarcoma cells. BMC Cancer. 2012;12:93. doi: 10.1186/1471-2407-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]