Abstract
Endothelial damage and dysfunction are implicated in cardiovascular pathological changes and the development of vascular diseases. In view of the fact that the spontaneous endothelial cell (EC) regeneration is a slow and insufficient process, it is of great significance to explore alternative cell sources capable of generating functional ECs to repair damaged endothelium. Indeed, recent achievements of cell reprogramming to convert somatic cells to other cell types provide new powerful approaches to study endothelial regeneration. Based on progress in the research field, the present review aims to summarize the strategies and mechanisms of generating endothelial cells through reprogramming from somatic cells, and to examine what this means for the potential application of cell therapy in the clinic.
Keywords: Atherosclerosis, Cell reprogramming, Endothelial cells, Endothelial regeneration, iPS cells, Stem cells
Introduction
Vascular endothelial cells (ECs) array the most inner layer of the entire circulatory system, from the largest arteries and veins to the smallest capillaries and serve as a semi-selective and non-adherent interface between blood and the underlying cells of the vessel wall such as smooth muscle cells (SMCs), pericytes and connective tissues. ECs play a key role in critical vascular functions, such as permeability, interaction with circulating platelets and leukocytes, regulation of vascular tone and growth. EC dysfunction or damage precedes the development of many vascular pathological conditions such as peripheral vascular disease, hypertension and atherosclerosis.1 Chronic exposure to cardiovascular risk factors such as diabetes, hyperlipidaemia or smoking, compromises the integrity of the endothelium and atherosclerosis occurs in the vessel wall. When the disease progresses severely, a treatment with angioplasty is routinely used in clinic. However, there is a risk of thrombosis due to the loss-of ECs. The regeneration of endothelium in this case is a slow process and there is currently no effective drug or gene therapy to promote it. Recently, efforts to achieve endothelial repair have shifted to stem cell-based approaches. Endothelial progenitor cells (EPCs) and pluripotent embryonic stem cells (ESCs) can be isolated, easily expanded and then differentiated into EC to be used for stem cells-based cellular therapies.2, 3, 4, 5, 6, 7, 8
A third source of stem cell that has great potential for regenerative medicine is the induced pluripotent stem (iPS) cells. iPS cells are pluripotent cells engineered from terminally differentiated somatic cells through a process called reprogramming. iPS cells exhibit the cellular characteristics that are highly similar to ESCs.9, 10, 11 However, iPS cells avoid the ethical and immunological issues associated with the application of ESCs. Moreover, iPS cells can be generated and individualised for patient-specific therapies, disease modelling and drug screening. iPS cells are capable of differentiating into all cardiovascular cells including ECs, vascular mural cells, SMCs, and cardiomyocytes and therefore have a great potential for vascular regeneration. Nevertheless, this strategy is time consuming and raises tumour-forming hazards. To avoid issues associated with iPS cells, researchers start to explore direct cell fate conversion between two differentiated cell types without passing through the pluripotent state. This strategy called direct reprogramming opens up an exciting new area of research for cell-based therapy.
In the present review, we aim to summarize the recent progress in cell reprogramming technique for endothelial regeneration, to discuss the mechanisms involved, and to highlight the potential clinical application.
Vascular endothelial cells and endothelial functions
Blood vessels are composed of three layers: the innermost is the tunica intima, which is composed of a single continuous layer of endothelial cells and mediates the exchange of nutrients and cells with the circulation. Surrounding the intima is the thick layer of smooth muscle cells composing the tunica media, which is responsible for the maintenance of the vessel tone and elasticity. Finally, adventitia is the external layer mainly composed of fibroblasts and connective tissue and incorporating the vasa vasorum, the small network of vessels that provides oxygen and nutrients to the cells in the vessel wall. Vessels within the microvascular system (i.e. capillaries and venules) are formed by contractile cells called pericytes that wrap around the EC layer.12 ECs are key components for maintaining the function of the vessels. ECs are actively involved in regulating endothelium permeability, modulating vascular tone, regulating blood coagulation, and many other biological processes.
The integrity of the endothelium is the foundation of vascular homeostasis. The cell–cell junctional structures, which link EC with each other to form a continuous monolayer, profoundly contribute to the regulation of permeability and the maintenance of the endothelium integrity. Furthermore, EC junctions actively participate in transferring intercellular signals between adjacent cells. EC junctions are formed by transmembrane adhesive proteins linked to specific cytoplasmic and cytoskeletal molecules.13
Endothelium regulates vascular tone through secreting EC-derived vasodilators including NO, prostacyclin and other factors. These EC-derived factors mediate the relaxation of SMCs to achieve vasodilation. NO is recognised as the primary factor for vasodilation which is synthesised in ECs by eNOS and that is dependent on its cofactors including free calcium (Ca2+) and l-arginine.14 In addition to the modulation of vascular tone, NO also has other vessel-protective roles including regulating the growth of local cells, inhibiting the aggregation and adhesion of inflammatory cells and platelets to endothelial surface.15
Healthy ECs possess anticoagulant and antithrombotic functions to keep the vascular patency. Blood coagulation is prevented by ECs through synthesising and displaying inhibitors for tissue factor pathway and thrombin. ECs also express molecules for protein C activation which can demolish certain clotting factors and inhibits coagulation.16 ECs can physically separate the interaction of platelets with collagen which can activate platelets. ECs also secrete antiplatelet molecules like prostacyclin, NO and prostaglandin-E2 to inhibit the adhesion and activation of platelets on the endothelial surface.17 Under physiological condition, endothelium prevents the inflammatory cells adhesion by failing to express adhesion molecules that mediate leukocytes attachment.
Endothelial dysfunction in vascular diseases
The dysfunction of the endothelial monolayer is the key initiation event of vascular diseases caused by a variety of stimuli including hypertension, diabetes, dyslipidaemia, oxidative stress and others. Endothelial dysfunction is characterised by leukocytes recruitment and platelet aggregation, increased permeability, thrombus formation, and impaired endothelium-mediated vasodilation. The expression of surface adhesion molecules are changed within the injured ECs which initiate the recruitment of blood leukocytes and platelets. In parallel, the endothelium permeability and the sub-endothelial extracellular matrix composition are altered to permit the penetration and accumulation of leukocytes and oxidised LDL particles.18 The NO synthesis capacity of ECs is also disturbed during atherosclerosis which impairs the endothelium-dependent vasodilation. Reduced NO synthesis occurs simultaneously with the increased generation of reactive oxygen species (ROS). Increased ROS bioavailability, together with the dysregulated oxidative stress, further damages the endothelial homeostasis.19 To repair the injured endothelium and reconstruct normal endothelial physiological function is a major target for therapy against vascular disease.
After endothelial dysfunction and denudation during vascular disease or treatment with angioplasty, endogenous resident ECs tend to proliferate and replace the injured endothelium. A number of studies from early years showed that local ECs participate in the repair of small areas of endothelial damage through migration and the repair of larger areas of damage through both proliferation and migration.20 Recently, by transplanting wire-injured carotid artery segments from wild type mice into Tie2-GFP mice, Hagensen et al demonstrated the resident ECs from the transplanted graft contribute to the re-endothelialisation of the lesion.21
Although the proliferation and migration of resident ECs represent a straightforward way for endothelial regeneration, it is a relatively slow and inefficient process.22 In addition, with the effects of the cardiovascular risk factors, the adjacent ECs around injured endothelial area may also be in a dysfunctional state. The rejuvenation of ECs with normal function represents a significant target of vascular disease therapies. A number of different stem cell sources, that have been considered to contribute to EC regeneration, potentially provide promising methods for regenerative medicine. In this review, we will focus on the generation of ECs through reprogramming technique.
Induced pluripotent stem cell and endothelial regeneration
In 2006, Takahashi and Yamanaka first described the process of converting a lineage-committed somatic cell back to the pluripotent state by simultaneously overexpressing four transcription factors Oct4, Sox2, Klf4 and c-Myc using viral vectors.10 This Nobel Prize-winning hallmark study successfully reprogrammed mouse fibroblasts to a new type of pluripotent cell that highly resembled ESC in morphology, proliferation, gene expression and DNA methylation patterns. The newly generated cell population was termed “induced pluripotent stem cell” or iPS cell. Since then, iPS cells have been successfully generated from different somatic cell types with different combinations of reprogramming factors and various induction methods, which proved the universality of the concept of cell reprogramming.23
iPS cells have the potential to differentiate towards vascular cell lineages including ECs. ECs can be derived from iPS cells by using three approaches: embryoid body (EB) formation, coculture with feeder cells or defined chemical condition. In 2009, two groups first showed that ECs could be generated from human iPS cells. Choi et al cocultured different human iPS cell lines with OP9 feeder cells for 8 days and then selected CD34- and PECAM-1- double positive cell population which could give rise to functional ECs after 7 days under endothelial-promoting culture conditions.24 Using a similar approach, Taura et al cocultured human iPS cells with OP9 feeder cells for 10 days and observed the emergence of a VEGFR2-positive population with EC differentiation capacity.25 Endothelial lineage-committed cells could also be derived from EB formed by iPS cells.26 Most commonly, feeder-free culture systems with the combination of different culture substrates and chemical conditions have been successfully applied to induce ECs from iPS cells.27
iPS-ECs display similar features with mature ECs at the genetic and functional levels. A major advantage of using iPS cells as EC source is the abundant origins of iPS cells and the potential to generate patient individualised ECs that bypass the immunogenicity and ethical issues. iPS-ECs have been tested in peripheral vascular disease mouse model to show their neoangiogenic capacity that led to the improvement of blood perfusion of ischaemic tissue.26
In spite of the fact that iPS cells start a new era of regeneration medicine, the tumourigenesis risk jeopardises their further clinical applications. The fact that many reprogramming factor cocktails contain oncogenes and many gene delivery methods use viral vectors raise the risk of tumour formation in vivo.28 In addition, iPS cells exhibit more genetic and epigenetic instability compared to ESC due to the artificial reprogramming process.29 Therefore, there is still a long way to go before the mature utilisation of iPS cells at the bedside. Cell direct reprogramming techniques to convert cell fate between two differentiated cell types without passing through the pluripotent state provide new possibilities for endothelial rejuvenation.
Direct cell lineage reprogramming and endothelial regeneration
Presently, based on the use of transcription factors, there are two dominant reprogramming strategies to achieve direct cell lineage conversion. One is through introducing various combinations of target cell type-specific transcription factors to directly drive cell lineage switch. In 2008, a case of in vivo study demonstrated the direct conversion of pancreatic exocrine cell to functional β-cell by injecting adenoviruses encoding three transcription factors Nng3, Pdx1, and Mafa into adult mice pancreas.30 In 2010, via the overexpression of Gata4, Mef2c, and Tbx5, Srivastava's group directly reprogrammed cardiac fibroblasts into functional cardiomyocytes in vitro.31 The same group subsequently showed the in vivo reprogramming of murine cardiac fibroblasts into cardiomyocytes through intra-myocardial injection of the identical set of the three transcription factors.32 In addition, a variety of reports provided evidence of directly reprogramming fibroblasts into other cell types including neurons, hepatocytes, etc.33, 34
Another fast and efficient approach to modulate cell fate is based on the use of iPS-generating pluripotency factors such as Oct4, Sox2, Klf4, Nanog, etc to erase lineage particular signatures and reactivate repressed epigenetic network as a first step, but with shorter reprogramming time and different culture conditions to avoid the full induction of pluripotency. After this step, cells revert to an intermediate plastic state which permits further manipulations towards the desired cell types. Short term reactivation of reprogramming genes Oct4, Sox2, and Klf4 plus chemically defined media and cardio-inductive growth factor BMP4 converted embryonic and adult fibroblasts to functional cardiomyocytes.35 During the conversion, the role of reprogramming factors is to erase the original cell identity via epigenetic mechanisms, instead of directly activate cardiomyocyte-specific genes.
Direct endothelial reprogramming with EC-related transcription factors
Ectopic overexpression of endothelial related transcription factors has been applied to generate ECs from other somatic cell types. Ginsberg et al first reported the direct reprogramming of human amniotic fluid-derived cells into ECs by ETS transcription factors ETV2, FLI1, and ERG1 together with TGF-β suppression.36 ETS transcription factors are potent regulators for vascular development and angiogenesis and they regulate almost all typical endothelial markers.37 EC-specific genes can be switched on within 4 days of ectopic expression of ETV2, FLI1, and ERG1 with TFG-β suppression. However, to establish stably proliferative EC population, a more precise temporal control on gene overexpression is needed. Recently, there were two important studies published, relative to the direct conversion of fibroblasts into ECs through the overexpression of selected endothelial related transcription factors. Han et al converted mouse adult fibroblasts into ECs using a cocktail of five transcription factors: Foxo1, Etv2, Klf2, Tal1 and Lmo2.38 All of these five factors play crucial roles in vascular development and endothelial maturation. Interestingly, authors from this study tried to use Etv2, Erg and Fli1 to reprogram mouse adult fibroblasts as shown in Ginsberg's study. However, they did not observe any EC generation. On the contrary, including Erg or Fli1 into their reprogramming factor cocktail compromised EC reprogramming efficiency from fibroblasts. This finding indicates that for different cell types, specific optimisation of transcription factors combination and culture condition is required for successful endothelial reprogramming. Another study showed that solely overexpressing one ETS transcription factor ETV2 is sufficient to induce functional ECs from human adult fibroblasts.39 The ETV2 expression level needs to be carefully controlled. Too low or too high ETV2 expressions both jeopardise the endothelial reprogramming efficiency from fibroblasts. In addition to ETV2 overexpression, the endogenous FOXC2 expression in the human adult fibroblasts is essential for ETV2 to induce EC reprogramming.
Although using the target cell-specific transcription factors for direct cell lineage reprogramming represents a straightforward strategy, a major concern related to this method is that the original gene regulatory network of the starting cell type may be insufficiently inactivated. Indeed, a recent study provided evidence to support this concern by comparing the gene expression patterns of various directly reprogrammed cell types with cells derived from pluripotent stem cells using a computational network biology platform named CellNet. Comprehensive analysis showed that directly reprogrammed cells tend to inadequately silence the expression programs of the starting cell population.40 This suggests that using target cell-specific transcriptions factors to conduct the direct cell lineage conversion may fail to fully erase the identity of the starting cell type and result in the incomplete establishment of the gene regulatory networks of the target cell type.
Direct endothelial reprogramming using iPS-generating factors
Short term overexpression of iPS-generating pluripotency factors has been used to induce the plasticity of somatic cells which leads to further differentiation towards endothelial lineage. By overexpressing Oct4, Sox2, Klf4 and c-Myc for 8 days, fibroblasts were reverted to an intermediate CD34-positive mesodermal progenitor state which could be further differentiated towards endothelial or smooth muscle lineages under different stimulating conditions.41 The authors discussed that all four factors are requisite for the generation of CD34-positive cells. To remove any single factor leads to the failure of the mesodermal progenitor state induction. Converted EC is a mixed population of different endothelial subtypes including arterial, venous and lymphatic ECs. Studies from our lab showed that reprogramming human fibroblasts with OCT4, SOX2, KLF4, and c-MYC for 4 days generated partial-iPS (PiPS) cells with an upregulation of VEGFR2. PiPS cells have the ability to differentiate into both endothelial- and smooth muscle-like cells.42, 43 Functional ECs could be derived from PiPS cells after 6 days of differentiation in endothelial-inductive condition. Another study reduced the transcription factors to Oct4 and Klf4 to obtain functional endothelial cells transdifferentiated from human fibroblasts.44
To use reprogramming factors, especially the oncogene c-MYC, as part of the protocol still raises tumourigenesis concerns. Although teratoma formation has not been observed in the in vivo experiments of any studies, the long term effect of using reprogramming factors is difficult to predict. Two recent papers discussed the direct reprogramming strategy using pluripotent factors induced transient pluripotent state at certain stage of the protocol.45, 46 Further studies need to clarify the exact roles of pluripotent factors in direct reprogramming and the transitions of cell identity during the transdifferentiation.
Direct endothelial reprogramming using small molecules
Beyond the use of transcription factors, recent studies exploited novel approaches including microRNAs (miRNAs), epigenetic regulators, signal pathways modulators and other small molecules to drive cell lineage conversion. Conditionally adding these small molecules into the transcription factors cocktail can boost the efficiency of cell fate switching. Furthermore, some combinations of the small molecules alone could drive direct lineage conversion without the ectopic overexpression of transcription factors.47 A recent study demonstrated the transdifferentiation of human fibroblasts to endothelial cells using small molecule activators of toll-like receptor 3 (TLR3) combined with endothelial growth factors.48 TLR3 agonist Poly I:C activates innate immune signalling which leads to increased epigenetic plasticity for cell fate manipulation. Histone modifications at the promoter regions of PECAM-1 have been observed during the fibroblast to EC conversion.
Comparison of different stem cell-based strategies for EC generation
In the above sessions, we have discussed EC generation based on different reprogramming techniques (Fig. 1). Because of the critical role that ECs play in cardiovascular physiological and pathological conditions, it is of great importance to investigate and compare different cells sources and strategies that can regenerate functional ECs and further obtain therapeutic value. For now, three major stem cell types are regarded to be promising therapeutic options for endothelial regeneration: EPC, ESC, and iPS cell. In addition, the emergence of direct cell reprogramming provides novel powerful cell sources. The advantages and deficiencies of these cell types and their clinical application value are briefly summarised as follow (Table 1).
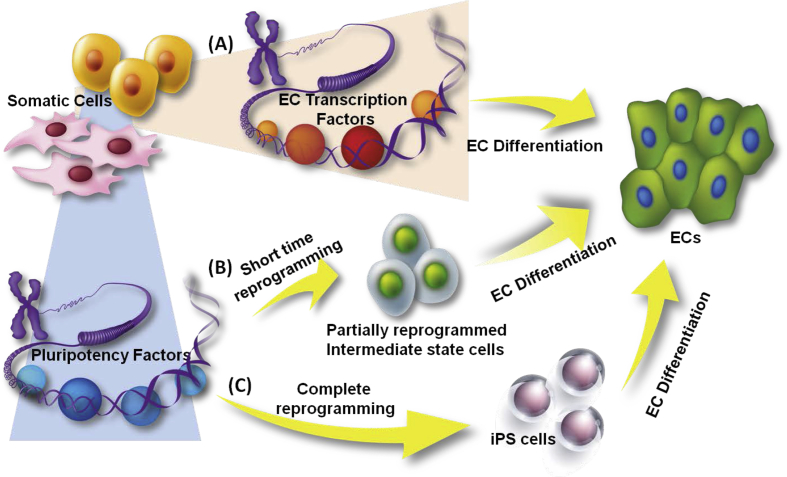
Fig. 1.
Reprogramming strategies for endothelial generation from other types of somatic cells. Somatic cells can be reprogrammed towards the endothelial lineage with or without passing through a pluripotent state. (A) Ectopic overexpression of endothelial-specific transcription factors with endothelial-inductive conditions can directly reprogram somatic cells into ECs. (B) Using iPS-generating pluripotency transcription factors for a short term can switch the differentiated somatic cells to a intermediate plastic state. Then the partially reprogrammed cells can be further differentiated towards ECs. (C) Somatic cells can also be fully reprogrammed into iPS cells and then be stimulated into the endothelial fate.
Table 1.
Comparison of different stem cell-based strategies for endothelial regeneration.
| Cell source | Adult stem cells | ESCs | iPS cells | Somatic cells |
|---|---|---|---|---|
| Origin | Circulation, bone marrow or resident tissue | Blastocyst of embryo | Generated by reprogramming of somatic cells, usually fibroblast | Many types of somatic cells: fibroblast, amniotic cell, etc |
| EC generation | Give rise to EC in response to specific stimulations and endothelial-promoting culture conditions | EB formation and subpopulation selection; culture with feeder cells or specific substrate under chemical defined endothelial-promoting condition | Culture under chemical defined endothelial-promoting conditions; EB formation and subpopulation selection | Reprogrammed by specific transcription factor with endothelial-promoting culture conditions |
| Main strengths |
|
|
|
|
| Main weaknesses |
|
|
|
|
| Clinical application | A number of clinical trials proved the therapeutic benefits for revascularisation and remodelling | No clinical trial data. | No clinical trial data | No clinical trial data |
Mechanisms involved in endothelial reprogramming
From the ectopic overexpression of different sets of transcription factors, to the following modulation of signalling pathways and endothelial-inductive conditions, mechanisms from many aspects have been implicated in the endothelial reprogramming process (Fig. 2). Signalling pathways regulating endothelial differentiation have been extensively reviewed before.27, 49, 50, 51 In this review, we focus on the mechanisms that are more related to the generation of ECs through reprogramming.
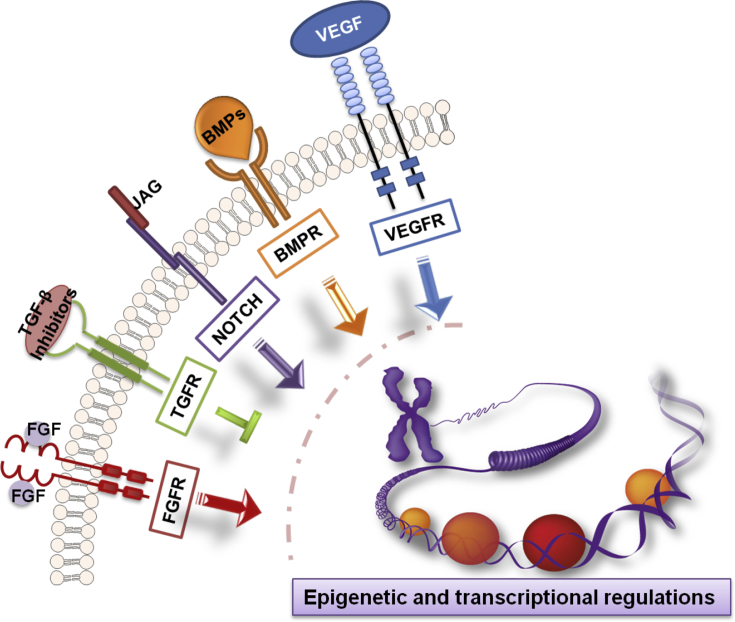
Fig. 2.
Mechanisms involved in endothelial reprogramming. Different signalling pathways together with epigenetic and transcriptional regulations comprehensively modulate the reprogramming towards the endothelial lineage. Relevant signalling pathways include VEGF, BMP, NOTCH, TGF-β, FGF signalling pathways. Epigenetic modulations include chromatin reorganisation, DNA demethylation, and post-translational histone modification. Transcriptional level is the wide-scale gene expression regulations induced by ectopically overexpressed transcription factors.
Epigenetic modulations during endothelial reprogramming
The conversion of cell type requires the fundamental resetting of the epigenome. The epigenetic signature of the starting cell type needs to be erased and a new epigenetic signature of the converted cell type needs to be established. The epigenetic modulations include chromatin reorganisation, DNA methylation changes, post-translational histone modification, etc. Many studies have shown the epigenetic changes along endothelial reprogramming. Furthermore, EC reprogramming can be achieved by targeting the epigenetic level instead of the transcriptional level. Using a small molecule of TLR3 agonist to active innate immunity could increase epigenetic plasticity and lead to the direct reprogramming of fibroblasts to ECs.48 However, the detailed mechanisms and regulatory factors involved in the process are not clear yet.
DNA methylation profile is an important epigenetic signature of a committed cell type. Switching from one cell type to another requires the universal DNA demethylation towards the establishment of a new cell identity.52 ECs converted from fibroblasts through CD34-positive mesodermal progenitor state using OCT4, SOX2, KLF4, and c-MYC lost the DNA methylation profile of fibroblasts 33. The promoter regions of VE-Cadherin and Tie2 were demethylated in the induced ECs derived from fibroblasts by Foxo1, Etv2, Klf2, Tal1 and Lmo2.38
Histone modifications play fundamental roles in controlling the expression of the genes. Histone repressive marker H3K27me3 at PECAM-1 and VE-Cadherin promoter regions were significantly decreased during the conversion of fibroblasts towards ECs using Oct4 and Klf4.44 The histone active marker H3K4me3 was increased in the promoter regions of PECAM-1 together with the decrease of the H3K27me3 mark at the same regions during the reprogramming of fibroblasts towards ECs with TLR3 agonist.48
Cell plasticity induced by pluripotency reprogramming factors
Since the establishment of reprogramming technique to generate pluripotent iPS cells from lineage-committed somatic cells, researchers have endeavoured to exploit the underlying molecular mechanisms. In general, the complex transcriptional and epigenetic changes of the whole genome occur during the reprogramming to reverse the differentiated cells back to the pluripotent state. Genome-wide analyses of the cell populations at different time points during the reprogramming process revealed three phases for successful iPS cell generation: initiation, maturation, and stabilisation.53
Each phase is characterised by the expression of a distinct group of genes. The initial phase is marked by a mesenchymal-to-epithelial transition. Interestingly, the signature genes associated with the maturation and stabilisation phases are not pluripotency regulators, but rather the genes related to cell cycle, cytoskeletal dynamics and signalling pathways.54 Another study also used genome-wide analyses to show that there were two major gene expression changing waves. One occurred between 0 and 3 days, and the second wave happened between day 9 till the end.55 Genes responsible for proliferation and metabolic changes were activated and the genes related to fibroblast identity were suppressed during the first wave. The second wave was characterised by the expression of genes related to stem cell identity establishment and epigenetic remodelling. These cell population-based studies suggested that reprogramming is a multi-step process with transcriptome resetting. The existence of multiple phases along iPS cell generation, especially erasing the cell identity as a first step, provides more possibilities to adjust the iPS reprogramming strategy to apply on direct reprogramming by using iPS-generating factors.
In order to understand the molecular mechanisms regulating EC reprogramming from other cell types using iPS-generating factors, it is important to understand the roles of the key reprogramming factors Oct4, Sox2, Klf4, and c-Myc at early stage of iPS cell generation. The clear function of these four transcription factors during cell reprogramming has not been clarified. In general, these four factors act as pioneer factors for remodelling the epigenome. They open up chromatin regions and bind to the promoters of a wide range of genes to guide further epigenetic modification.56 Interestingly, in addition to the genes that they usually regulate in ESCs, they also bind to the genes that are not occupied by these factors in ESCs.57 This promiscuous binding phenomenon indicated that the roles of the reprogramming factors may be cell type dependent. Oct4, Sox2, and Klf4 collectively form a transcriptional network that associates with repressing somatic gene expression and upregulating pluripotent genes during reprogramming. c-Myc mainly acts as an transcriptional amplifier at all active promoters to enhance the kinetics and efficiency of reprogramming.58 The fact that Oct4, Sox2, Klf4, and c-Myc are able to open chromatin and induce cell plasticity early in reprogramming support the direct lineage conversion strategy of transiently expressing these four factors to dedifferentiate the somatic cell back to an intermediate state followed by further differentiating the cells towards another lineage.
KLF4 is particularly interesting when specifically considering the reprogramming towards vascular cell lineage. KLF4 has been shown to play a crucial role in regulating vascular cell development and function in addition to its reprogramming role and has been identified as an important transcription factor for both SMC and EC. KLF4 has been shown to directly bind to the promoter of VE-Cadherin in mature ECs to improve cell barrier function.59 Another study indicated that KLF4 plays a protective role in regulating the response of EC to inflammatory stimuli. Overexpressing KLF4 in ECs increases the expression of antiinflammatory and antithrombotic factors including eNOS and thrombomodulin.60 In c-Kit-positive vascular progenitor population derived from ESCs, Klf4 was shown to positively regulate their differentiation towards ECs. Overexpression of Klf4 in this population led to further upregulation of EC markers and knockdown of Klf4 resulted in an increase of SMC markers.61 The above studies suggest that KLF4 may play an additional favourable role in promoting reprogramming towards the endothelial lineage.
Mesenchymal-to-epithelial transition and cell reprogramming
Among the studies related to the different phase of cell reprogramming, a biological process named Mesenchymal-to-Epithelial Transition (MET) has emerged to be a key event for the initial stage of somatic cell reprogramming.
Based on the temporal changes of global gene expression, an important study in 2010 divided the reprogramming of mouse embryonic fibroblast (MEF) to iPS cell into three main phases: initiation, maturation and stabilisation. Among which, the initiation stage was characterised by the MET driven by bone morphogenetic proteins (BMPs) signalling.53 At the same time, another group demonstrated the requisite role of MET at early stage for successful mouse iPS cells generation. MET was regulated by the interactions with reprogramming transcription factors and TGF-β signalling.62
During the generation of human iPS cells from fibroblasts, the participation of MET was confirmed by a study that suggested that the reprogramming promoting function of miRNA-302 and miRNA-372 was partly acting through MET.63 Later on, a comprehensive proteomics analysis of the whole course of reprogramming confirmed the existence of MET during early phase at protein level.64 Till now, many studies have proved the occurrence of MET at early stage for successful somatic reprogramming in different cell systems.
In addition, recent studies indicated the involvement of MET during the early stage of direct cell lineage conversions, which suggested a more universal role of MET process in modulating cell identity plasticity. Ectopic introduction of transcription factors Nr5a1, Wt1 and Dmrt1 into fibroblasts could initiate MET as a first step and finally convert the cells towards embryonic sertoli-like cells.65 Another recent report on direct reprogramming of fibroblasts into induced cardiomyocytes demonstrated the involvement of MET.66 Promoting MET through the suppression of Snai1 by miR-133 could profoundly enhance the protocol efficiency and cell quality of converted cardiomyocytes.
Notch signalling mediating endothelial differentiation
The Notch signalling pathway participates in the regulation of diverse vascular cell function during embryonic and postnatal development.67 The Notch signals usually function to drive the differentiation of the precursors between two alternative fates.68 For example, Notch signals determine the arterial-venous fate of ECs and the tip or stalk cells selection of ECs during angiogenesis.
JAG1 is a Serrate/Jagged family transmembrane ligand for the Notch pathway containing multiple epidermal growth factor-like repeats.69 JAG1, as the upstream ligand of the transmembrane receptors in the Notch pathway, plays a complicated role in orchestrating cell fate. The homozygous mutation of the Jag1 gene in mice causes early embryonic lethality due to extensive embryonic and yolk sac vascular defects.70 The mutation of the JAG1 gene in humans leads to Alagille syndrome characterised by abnormal development of multiple systems during childhood.71, 72 Vascular anomalies including pulmonary artery abnormalities, intracranial haemorrhages and other events, frequently occur in Alagille syndrome patients and account for a large portion of mortality, which reflects the important role of JAG1 during human vascular development.73
The pro-angiogenic role of Jag1 has been shown using EC-specific and inducible knockout or overexpression in mice. Jag1 loss-of-function mutants exhibited reduced sprouting angiogenesis while Jag1 overexpression promotes sprouting angiogenesis.74 Kwon et al showed that Jag1-induced signals from the bone marrow microenvironment are critical for the development of angiogenic ability of endothelial progenitor cells.75 An interesting study recently demonstrated that JAG1 could subsequently activate KLF4 which induced the transdifferentiation of tumour cells into endothelial cells.76 Nevertheless, there are reports of the opposite effect in other cell models. A recent study emphasised the role of JAG1 in promoting haematopoietic lineage over endothelial lineage specification during pluripotent stem cell differentiation.77 It is conceivable that in different cell types and in response to different environmental cues, the JAG1 activated Notch pathway may delicately control a distinct regulation network which leads to altered consequences.
HES5 is a common downstream target of the Notch pathway which belongs to the basic helix-loop-helix transcription factor family and is usually associated with neural cell differentiation.78 One study suggested a role for HES5 in vascular development, as it might be a key positive mediator for the statin-induced differentiation of bone marrow stromal cells into ECs.79 Another study has suggested that HES5 plays a part in promoting endothelial proliferation in response to endothelial injury during atherosclerosis.80 This study also demonstrated that MicroRNA-126-5p promotes endothelial regeneration and limits atherosclerosis by suppressing the Notch inhibitor delta-like 1 homologue (Dlk1), which leads to the release of HES5 that was suppressed by Dlk1, allowing HES5 to play its role in endothelial repair.
Potential applications of EC generated through reprogramming
One big advantage of using iPS cell or direct cell conversion technique to generate ECs is the use for patient-specific disease modelling and drug screening. Moreover, an exceptional advantage of direct lineage conversion over iPS cell is the potential application for direct in vivo lineage reprogramming for cell replacement therapy, which avoids the unstable long term of in vitro cell culture, tumour-forming risks and the technical obstacles for cell transplantation. For example, direct injection of transcription factors cocktail Gata4, Mef2c and Tbx5 into the infracted cardiac area could reprogram resident non-myocytes into cardiomyocyte-like cells with improved cardiac function.32 The in vivo regeneration of functional insulin-producing pancreatic β-cells from other types of pancreatic cells has been reported by several studies for their potential benefits for treating diabetes.30, 81, 82
Cell-based therapeutic angiogenesis is a recently arising approach to restore the blood perfusion in ischaemic tissue. Successful therapeutic angiogenesis depends on the transplanted cells to directly incorporate into the neovasculature as well as to secrete angiogenic growth factors.83 This therapy is of special significance in treating peripheral artery disease (PAD) since current pharmacological and interventional revascularisation therapies are not beneficial enough.84 However, researchers are still investigating the optimal starting cells to generate functional angiogenic cells. In the murine ischaemia model of PAD, several studies demonstrated that direct reprogrammed ECs could efficiently engraft into local vasculogenesis of ischaemic tissue and profoundly improve the tissue perfusion.38, 39, 42, 44, 48 Moreover, direct reprogramming without reversing to a pluripotent state prevents the risk of tumour formation.
Reprogrammed ECs have the potential to become tissue-specific ECs which provide interesting therapeutic value to target precise pathological conditions. This could be achieved through culturing the cells under in vitro or in vivo tissue-specific microenvironment. In vitro co-differentiating ECs and neural cells from human iPS cells facilitates the reprogrammed ECs to acquire blood–brain barrier EC specification.85 Neural cells provide relevant cues including Wnt/β-catenin signalling to specify the ECs towards a blood–brain barrier phenotype. Ginsberg et al demonstrated that amniotic cells-derived ECs can be specifically educated into sinusoidal ECs to participate liver vasculature regeneration by intrasplenic transplantation.29 Recent studies further clarify the molecular signatures to define tissue-specific ECs, which provide us more information to achieve EC specification through introducing transcription factor that regulates tissue-specific EC identity.86
Tissue engineered vascular graft represents another promising direction for vascular regenerative medicine. In addition to direct transplantation to replace injured vessels, tissue engineered graft can also serve as a useful ex vivo model to study the mechanisms related to vascular cell or ECM behaviours. Based on a previous established protocol from our laboratory, functional vascular-resembling conduits can be generated by seeding the decellularised mouse aorta with human origin cells using an ex vivo bioreactor circulation system.43, 87 Human fibroblast-derived ECs exhibited good ability to reendothelialise the decellularised graft.42
The proliferation and accumulation of SMC and fibroblasts following endothelial denudation/dysfunction profoundly contributes to the development of atherosclerosis and restenosis. Protocols for the direct conversion of fibroblasts into functional ECs may provide promising tools for in situ endothelial regeneration. However, the main obstacle to this future application is the lack of proper gene delivery technique aiming at the specific type of cell for in vivo reprogramming. A recent study demonstrated an in situ virus delivery method to specifically target vascular SMCs without effecting ECs by constructing a designated gene with EC enriched microRNA target sequences within the same vector.88 By employing a similar strategy, it is possible to develop a method to specifically switch local fibroblasts or SMCs into the endothelial lineage to achieve autologous endothelium repair.
Summary and perspective
Generating functional ECs from other somatic cell types with or without passing through a pluripotent state provides intriguing prospects for therapeutic application of vascular regeneration, especially to generate patient individualised cells that bypass the immunogenicity and ethical issues. However, the existing reprogramming methods to produce ECs are of various efficiencies. Therefore, further optimisation and standardisation of the methods are required to be able to produce ECs at clinical grade and scale. In addition, the underlying mechanisms of endothelial reprogramming need to be elucidated to facilitate the optimisation of the technique.
The fast development in the field of computational biology provides new tools to analyse transcription factor combinations for efficient direct reprogramming.89, 90, 91 A recently developed computational platform, Mogrify, predicted the sets of reprogramming factors to successfully convert keratinocytes into microvascular ECs based on the combined calculation of gene expression data and regulatory network information.90 Novel bioinformatics approaches largely facilitate the development of cell lineage conversion protocols.
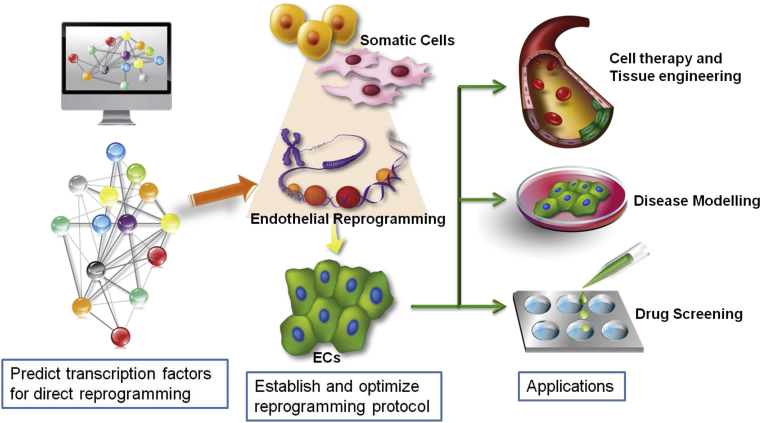
In the future, an efficient workflow could be to firstly use computational platform to predict the possible reprogramming strategy. Then to validate and optimise the reprogramming protocol at the bench to efficiently generate well characterised endothelial population. Finally the individualised endothelial population could be applied in downstream applications including vascular regenerative therapies, vascular disease modelling and drug screening (Fig. 3).
Fig. 3.
An efficient workflow for endothelial reprogramming and applications. Based on the fast development in the field of computational biology, an efficient workflow for endothelial reprogramming can start with using computational platform to calculate the possible sets of transcription factors to achieve efficient reprogramming. Then the protocol can be verified and optimised at the bench. Finally, ECs generated through reprogramming from patients can be used for individualised cell therapy and tissue engineering, disease modelling and drug screening.
Disclosures
None.
Acknowledgement
Vascular stem cell research in the Xu lab is supported by the British Heart Foundation (RG/14/6/31144) and Oak Foundation. The authors thank Dr. Siying Ma for composing the figures.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Fadini G.P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita J., Itoh H., Hirashima M. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira L.S., Gerecht S., Shieh H.F. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S., Ferreira L.S., Chen-Konak L., Kraehenbuehl T.P., Langer R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Protoc. 2010;5:1115–1126. doi: 10.1038/nprot.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho S.W., Moon S.H., Lee S.H. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 8.Prado-Lopez S., Conesa A., Arminan A. Hypoxia promotes efficient differentiation of human embryonic stem cells to functional endothelium. Stem Cells. 2010;28:407–418. doi: 10.1002/stem.295. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Yu J., Vodyanik M.A., Smuga-Otto K. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P. Developmental biology. One cell, two fates. Nature. 2000;408 doi: 10.1038/35040684. 43, 45. [DOI] [PubMed] [Google Scholar]
- 13.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 14.Michel T., Vanhoutte P.M. Cellular signaling and NO production. Pflugers Arch Eur J Physiol. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tousoulis D., Kampoli A.M., Tentolouris C., Papageorgiou N., Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 16.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 17.van Hinsbergh V.W. Endothelium – role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landmesser U., Hornig B., Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21 suppl 1):II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 19.Endemann D.H., Schiffrin E.L. Endothelial dysfunction. J Am Soc Nephrol JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 20.Hagensen M.K., Vanhoutte P.M., Bentzon J.F. Arterial endothelial cells: still the craftsmen of regenerated endothelium. Cardiovasc Res. 2012;95:281–289. doi: 10.1093/cvr/cvs182. [DOI] [PubMed] [Google Scholar]
- 21.Hagensen M.K., Raarup M.K., Mortensen M.B. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–231. doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 22.Hirase T., Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012;302:H499–H505. doi: 10.1152/ajpheart.00325.2011. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Choi K.D., Yu J., Smuga-Otto K. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taura D., Sone M., Homma K. Induction and isolation of vascular cells from human induced pluripotent stem cells – brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 26.Rufaihah A.J., Huang N.F., Jame S. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong W.T., Huang N.F., Botham C.M., Sayed N., Cooke J.P. Endothelial cells derived from nuclear reprogramming. Circ Res. 2012;111:1363–1375. doi: 10.1161/CIRCRESAHA.111.247213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabel L. Prospects for pluripotent stem cell therapies: into the clinic and back to the bench. J Cell Biochem. 2012;113:381–387. doi: 10.1002/jcb.23364. [DOI] [PubMed] [Google Scholar]
- 29.Pera M.F. Stem cells: the dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ieda M., Fu J.D., Delgado-Olguin P. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian L., Huang Y., Spencer C.I. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Sudhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang P., Zhang L., Gao Y. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Efe J.A., Hilcove S., Kim J. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg M., James D., Ding B.S. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dejana E., Taddei A., Randi A.M. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Han J.K., Chang S.H., Cho H.J. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–1178. doi: 10.1161/CIRCULATIONAHA.113.007727. [DOI] [PubMed] [Google Scholar]
- 39.Morita R., Suzuki M., Kasahara H. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U. S. A. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahan P., Li H., Morris S.A., Lummertz da Rocha E., Daley G.Q., Collins J.J. CellNet: network biology applied to stem cell engineering. Cell. Aug 14 2014;158:903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurian L., Sancho-Martinez I., Nivet E. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods. 2013;10:77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margariti A., Winkler B., Karamariti E. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U. S. A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karamariti E., Margariti A., Winkler B. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ Res. 2013;112:1433–1443. doi: 10.1161/CIRCRESAHA.111.300415. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Huang N.F., Zou J. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Nur O., Verheul C., Sommer A.G. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotechnol. 2015;33:761–768. doi: 10.1038/nbt.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maza I., Caspi I., Zviran A. Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol. 2015;33:769–774. doi: 10.1038/nbt.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J., Du Y., Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Sayed N., Wong W.T., Ospino F. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131:300–309. doi: 10.1161/CIRCULATIONAHA.113.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campagnolo P., Wong M.M., Xu Q. Progenitor cells in arteriosclerosis: good or bad guys? Antioxid Redox Signal. 2011;15:1013–1027. doi: 10.1089/ars.2010.3506. [DOI] [PubMed] [Google Scholar]
- 50.Le Bras A., Vijayaraj P., Oettgen P. Molecular mechanisms of endothelial differentiation. Vasc Med. 2010;15:321–331. doi: 10.1177/1358863X10371685. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson J.E., 3rd, Kelley R.W., Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2246–2254. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- 52.Hochedlinger K., Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samavarchi-Tehrani P., Golipour A., David L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Golipour A., David L., Liu Y. A late transition in somatic cell reprogramming requires regulators distinct from the pluripotency network. Cell Stem Cell. 2012;11:769–782. doi: 10.1016/j.stem.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Polo J.M., Anderssen E., Walsh R.M. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buganim Y., Faddah D.A., Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soufi A., Donahue G., Zaret K.S. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papp B., Plath K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 2011;21:486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cowan C.E., Kohler E.E., Dugan T.A., Mirza M.K., Malik A.B., Wary K.K. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamik A., Lin Z., Kumar A. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. May 4 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 61.Campagnolo P., Tsai T.N., Hong X. c-Kit+ progenitors generate vascular cells for tissue-engineered grafts through modulation of the Wnt/Klf4 pathway. Biomaterials. 2015;60:53–61. doi: 10.1016/j.biomaterials.2015.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li R., Liang J., Ni S. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Liao B., Bao X., Liu L. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansson J., Rafiee M.R., Reiland S. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2012;2:1579–1592. doi: 10.1016/j.celrep.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buganim Y., Itskovich E., Hu Y.C. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell. 2012;11:373–386. doi: 10.1016/j.stem.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muraoka N., Yamakawa H., Miyamoto K. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565–1581. doi: 10.15252/embj.201387605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bray S.J. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 68.Artavanis-Tsakonas S., Muskavitch M.A. Notch: the past, the present, and the future. Curr Top Dev Biol. 2010;92:1–29. doi: 10.1016/S0070-2153(10)92001-2. [DOI] [PubMed] [Google Scholar]
- 69.Fleming R.J. Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol. 1998;9:599–607. doi: 10.1006/scdb.1998.0260. [DOI] [PubMed] [Google Scholar]
- 70.Xue Y., Gao X., Lindsell C.E. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 71.Li L., Krantz I.D., Deng Y. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 72.Oda T., Elkahloun A.G., Pike B.L. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 73.Kamath B.M., Spinner N.B., Emerick K.M. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004;109:1354–1358. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 74.Benedito R., Roca C., Sorensen I. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 75.Kwon S.M., Eguchi M., Wada M. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118:157–165. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 76.Chen H.F., Huang C.H., Liu C.J. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 77.Lee J.B., Werbowetski-Ogilvie T.E., Lee J.H. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells. Blood. 2013;122:1162–1173. doi: 10.1182/blood-2012-12-471649. [DOI] [PubMed] [Google Scholar]
- 78.Iso T., Kedes L., Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 79.Xu J., Liu X., Chen J. Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via notch signaling pathway. Am J Physiology Cell Physiol. 2009;296:C535–C543. doi: 10.1152/ajpcell.00310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schober A., Nazari-Jahantigh M., Wei Y. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Hasani K., Pfeifer A., Courtney M. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26:86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 82.Baeyens L., Lemper M., Leuckx G. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Raval Z., Losordo D.W. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112:1288–1302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Annex B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 85.Lippmann E.S., Azarin S.M., Kay J.E. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nolan D.J., Ginsberg M., Israely E. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong M.M., Hong X., Karamariti E., Hu Y., Xu Q. Generation and grafting of tissue-engineered vessels in a mouse model. J Vis Exp. 2015:e52565. doi: 10.3791/52565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santulli G., Wronska A., Uryu K. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest. 2014;124:4102–4114. doi: 10.1172/JCI76069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris S.A., Cahan P., Li H. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell. 2014;158:889–902. doi: 10.1016/j.cell.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rackham O.J., Firas J., Fang H. A predictive computational framework for direct reprogramming between human cell types. Nat Genet. 2016;48:331–335. doi: 10.1038/ng.3487. [DOI] [PubMed] [Google Scholar]
- 91.D'Alessio A.C., Fan Z.P., Wert K.J. A systematic approach to identify candidate transcription factors that control cell identity. Stem Cell Rep. 2015;5:763–775. doi: 10.1016/j.stemcr.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]