Abstract
Irrespective of positive developments of cancer treatment, the mortality due to various cancers remains high and the mechanisms of cancer initiation and the development also remains mysterious. As we know that microRNAs are considered to be a short noncoding RNA molecules consisting of 21–25 nucleotides (nt) in length and they silence their target genes by inhibiting mRNA translation or degrading the mRNA molecules by binding to their 3′-untranslated (UTR) region and play a very important role in cancer biology. Recent evidences indicate that miR-21 is over expressed in cancer stem cells and plays a vital role in cell proliferation, apoptosis, and invasion. Even though an increased expression level of miR-21 has been observed in cancer stem cells, studies related to the role of miR-21 in cancer stem cells are limited. The main aim of this mini review is to explain the potency of miR-21 in various cancer stem cells (CSCs) and as a new target for therapeutic interventions of cancer progression.
Keywords: CSCs, Cancer stem cells, miR-21, miRNAs, RNAs, UTR
Introduction
Cancer stem cells (CSCs) are considering being an important cells and consistently reported in many malignancies. They are frequently associated with initiation and progression of various tumours. Interestingly, CSCs and normal somatic stem cells share many biological properties such as self-renewal and the nature of differentiation but they have differences in metastatic activity and other characters. Consistence evidences suggested that CSCs have potential clinical importance, but the regulation at the molecular level is not well-understood.1 As we know that CSCs are resistant to various drugs and they are considered to important cells clinical practice. It is important to know that characteristics of CSCs and the discovery of therapeutic agents that targeting CSCs are most valuable in cancer research.2, 3
MicroRNAs (miRNAs) are considered to be an endogenous non-coding RNAs played variety of role in several cancers. Recent evidences are shows that miRNAs can regulate the CSCs at a molecular level and are associated with cancer initiation and metastasis.4, 5 As we know that microRNAs are consider to be a short noncoding RNA molecules consisting of 21–25 nucleotides (nt) in length and they silence their target genes by inhibiting mRNA translation or degrading the mRNA molecules by binding to their 3′-untranslated (UTR) region and plays a very important role in cancer biology.4
microRNA 21, also known as hsa-mir-21 is encoded by the miR-21 gene located on chromosome 17q23.2 immediately downstream of the vacuole membrane protein-1 (VMP1) gene.4, 6 miR-21 is one of the common microRNA that is frequently upregulated in a variety of cancers including breast,7 ovaries,8 cervix,9 colon,10 lung11 and liver.12 miR-21 is also an oncogenic miRNA that can modulate the expression of multiple tumour suppressor genes such as Phosphatase and Tensin homolog (PTEN), Serpini1, and programmed cell death 4 protein (PDCD4).13, 14 Expectedly, inhibition of miR-21 through anti-miR-21 resulted in cell growth inhibition, increased apoptosis and decreased cell proliferation. Recent reports show that miR-21 and their networks play critical roles in regulating CSCs growth differentiation in the colon cancer and progression of chemo-resistance.15 Consistently, miR-21 plays an important role in regulating stemness by modulating TGFβR2 signalling in colon cancer cells.10 Inhibition of miR-21 can inhibit tumour growth through elevating PTEN, SNX1, and SGPP1 expression and inhibiting Akt phosphorylation in lung cancer like cells.10
Interestingly, aberrantly expressed miR-21 regulates CSCs apoptosis and proliferation partly through directly down-regulating FASLG protein expression in Glioblastoma Cancer Stem Cells (GSCs) and this may be a potential therapeutic target for glioblastoma.16 From the above points, we know that miR-21 is consistently involved in the various kinds of CSCs and Up to date, there is no review demonstrating the role of miR-21 in cancer stem cells and the number of studies related to miR-21 in CSCs is limited. Therefore, the main thrust of this mini review is to provide clinical evidence and significance of miR-21 in CSCs. We are also summarizing the important research findings surrounding the role of miR-21 in CSCs.
Role of miR-21 in different types of cancer stem cells (CSCs)
Recent reports suggested that miR-21 functions have been linked to cancer progression and chemo resistance.17 In the same study it has been reported that the role of miR-21 as an oncogenic regulator in stem/progenitor cell populations that is involved in the promotion of the cellular transformation process and chemotherapy resistance. It is very clear that there are several potential mechanisms by which miR-21 might promote cancer stem/progenitor populations, miR-21 in non-progenitor cancer cells could produce growth factors that enrich stem cell populations, secondly, miR-21 in the cancer progenitor cell niche might directly regulate progenitor cells to self-renew and finally miR-21 in certain non-progenitor cancer cells may trigger a dedifferentiation process, so enriching stem cell populations.17
Recent study reported that among other miRNAs, miR-21 is the most reported miRNAs in colon CSCs regulation. Additionally, it has been reported to act on processes involving CSCs through cell cycle regulation genes and epithelial–mesenchymal transition.18 Interestingly, in Anaplastic Thyroid carcinoma (ATC) therapy, the knockdown of miR-21 significantly changed the expression pattern certain genes such as of PDCD4, p21, Oct-4, ABCG2, and Mcl-1 suggesting that miR-21, as an oncomiR, controls stemness and tumour growth, differentiation, and apoptosis.19
Another study with CSCs showed that the expression of miR-21 to be greatly increased and miR-145 decreased in colon cancer cells that are highly enriched in CSC. They found that expression pattern of miR-145 in colon cancer cells greatly inhibits CSCs and tumour growth, whereas up-regulation of miR-21 induce CSCs and tumour growth. In addition, administration of mature miR-145 or antagomir-21 (anti-sense miR-21) greatly suppresses the growth of colon cancer cell xenografts in SCID mice and their in vitro studies also demonstrated that miR-21 negatively regulates miR-145 and vice versa, suggesting that miR-21, miR-145, and their networks play critical roles in regulating CSCs growth and/or differentiation in the colon cancer and progression of chemo-resistance.15
It has been revealed that miR-21 increased significantly in glioblastoma brain tissue, and it is up-regulated in glioblastoma cancer stem cells (GSCs) remarkably, suggesting that aberrantly expressed miR-21 regulates GSCs apoptosis and proliferation partly through directly down-regulating FASLG protein expression in GSCs and this might offer a new potential therapeutic stratagem for glioblastoma.16 We know that resistance of cancer stem/progenitor cells (CSPCs) to chemotherapy can lead to cancer relapse. Ovarian terato carcinoma (OVTC) arises from germ cells and comprises pluripotent cells that can be used to study cancer cell stemness. Knockdown of miR-21 resulted in a marked reduction in the CD133 + population and sphere formation of CSPCs. Contrastingly, overexpression of miR-21 resulted in a marked increase in the population of CD133 + cells as well as sphere formation of CSPCs. The above result clearly indicates that microRNA-21 plays a vital role in cancer growth by regulating stemness in cancer cells20 and Table 1 describes the role of miR-21 in different types of CSCs.
Table 1.
Represents the regulations of miR-21 in various cancer stem cells (CSCs).
| Types of CSCs | Status of miR-21 | Gene target | References |
|---|---|---|---|
| Glioblastoma Cancer Stem (GSCs) | Upregulated | FASLG | Shang C et al 2015 |
| Lung cancer stem like cells | Upregulated | PTEN, SNX1, and SGPP1 | Zhang J et al 2015 |
| Colon CSCs | Upregulated | Wnt/B-catenin and Notch signalling pathways | Mamoori A et al 2016 |
| Anaplastic Thyroid Carcinoma (ATC) | Upregulated | PDCD4, p21, Oct-4, ABCG2, and Mcl-1 | Haghpanah V et al 2015 |
| Colon CSCs | Upregulated | Yu Y et al 2015 | |
| Cancer Stem/Progenitor Cells (CSPCs) | Upregulated | CD133+ | Chung WM et al 2013 |
| Hepatocellular Carcinoma (HCC) | Upregulated | Zhou L et al 2013 | |
| Paediatric cancer (CSCs) | Upregulated | Sanchez-Diaz PC et al 2013 | |
| Glioblastoma Cancer Stem (GSCs) | Upregulated | Zhang S et al 2012 | |
| Cancer Stem Cells (CSCs) | Upregulated | EMT Markers (N-cadherin, Vimentin, alpha-smooth muscle actin and E-cadherin | Han M et al 2012 |
From the literature studies, we know that CSCs may be involved in metastasis formation and side population (SP) cells isolated from diverse cancer cells possess stem cell-like properties. Interestingly, the aberrant expression of miR-21 in SP cells showed that it regulates the expression of multiple target proteins that are associated with tumour dissemination. miR-21 is a pro-metastatic miRNA in SP cells and raises the possibility that therapy of hepatocellular carcinoma (HCC) may be improved by pharmaceutical strategies directed towards miR-21.21 Knocking down hsa-miR-21-5p using antisense oligonucleotides and small interfering RNA in cell lines led to the depletion of the CSCs fraction and impairment of sphere formation (CSC surrogate assays) suggesting that CSCs associated with miR-21 and the putative pathways may have potential therapeutic applications in paediatric cancers.22
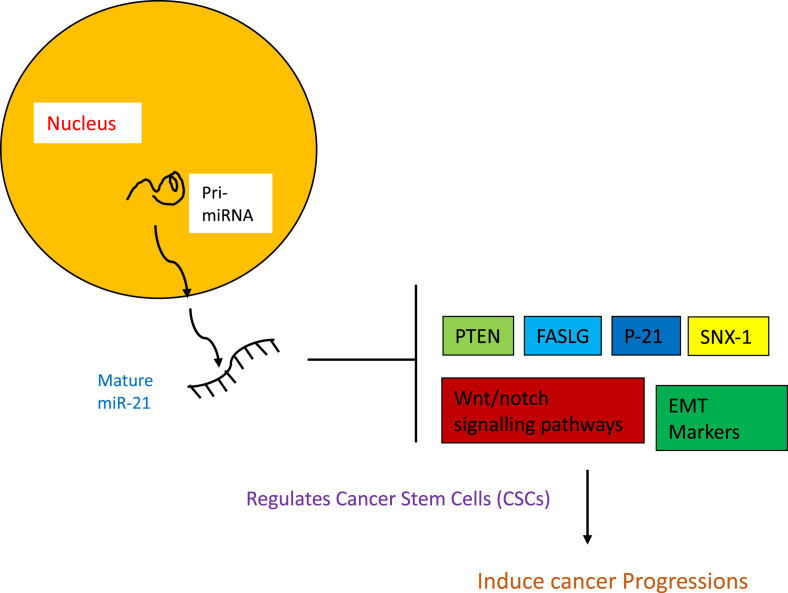
It has been known that several miRNAs recently reported in the modulation of glioma development, especially some upregulated miRNAs, such as miR-21, which has been found to function as an oncogene in cultured glioblastoma multiform cells. miR-21 inhibitor or TMZ could not induce apoptosis on GSCs. However, miR-21 inhibitor combined with TMZ significantly enhanced GSCs apoptosis suggesting that combination of miR-21 inhibitor and TMZ could be an effective therapeutic strategy for GSC apoptosis to prevent potential glioblastoma recurrence.23 Recent study shows that the induction of epithelial–mesenchymal transition (EMT) phenotype and acquisition of cancer stem cell (CSC) characteristics are highly interrelated, usually EMT was assessed by overexpression of mesenchymal cell markers (N-cadherin, Vimentin, alpha-smooth muscle actin [α-SMA]) and suppression of epithelial cell marker (E-cadherin), so they demonstrated that the formation of CSC-like cells usually undergo the process of EMT which are mainly regulated by miR-21.24 Interestingly, recent studies show that cancers may originate from special cells named cancer stem cells (CSCs) and miRNAs have a prominent role in regulating cellular activities. Real-time RT-PCR analysis demonstrated an increase in mir-21 expression level in CSCs relative to cancer cells and it may be promising objects for targeting CSCs specifically and efficiently.25 Fig. 1 represents regulation of miR-21 in CSCs.
Fig. 1.
Represents the regulation of miR-21 in cancer stem cells (CSCs).
It has been revealed that miR-21 is known to play a key role in the development and progression of breast cancer. Re-expression of miR-21 induced the acquisition of EMT phenotype by activation of mesenchymal cell markers (N-cadherin, Vimentin, α-SMA) and inhibition of epithelial cell marker (E-cadherin) in MCF-7/miR-21 cells, which consistent with increased cell subpopulation expressing CSCs surface markers (ALDH1(+) and CD44(+)/CD24(-/low)) and the capacity of sphere forming (mammospheres).26 MicroRNA-21 (miR-21) and its upstream regulator activator Protein-I (AP-1), composed of c-Jun and c-Fos family transcription factors, were found to be frequently upregulated in SP cells which harbours many cancer stem cell (CSC)-like properties. Specific inhibition of miR-21 by an anti-miR-21 locked nucleic acid increased drug sensitivity and decreased colony forming ability in CSCs.27 Recently, it has been suggesting that miR-21 plays an important role in regulating stemness by modulating TGFβR2 signalling in colon cancer cells.10
Clinical significance and future perspectives
Accumulating evidence suggesting that miRNAs are a broad class of small non-coding RNAs that control expression of complementary target messenger RNAs28 and dysregulation of miRNAs has been described in various diseases including cancer. From the literature it has been known that a particular miRNA that was consistently reported to be upregulated in various kind of cancer is miR-21. miR-21 exerts oncogenic activity in various cancer cells including CSCs and therefore is considered as an oncomir. Consistently, from the above evidence suggesting that miR-21 is an important oncogene, which plays a significant role in the regulation of CSCs. There are so many ways to inhibit miR-21 such as antisense-mediated inhibition of miRNAs or anti miR-21 and it has been reported that at synthetic anti-miRNA oligonucleotides (AMOs) deliver an effective inhibition of miRNAs in cell culture as well as in xenograft mouse models. Additionally, it has been demonstrated that targeting miR-21 by AMOs in breast cancer and glioblastoma has been achieved for in vitro and xenograft mice model.14, 29, 30 Although there is an evidence to stop miR-21 function but still, we need to have future validation for the above studies in order to prove that AMOs can function as therapeutic agents against CSCs, therefore it is important to have future functional studies on miR-21 and its regulatory elements/regulators in relation to various kinds of CSCs.
Conclusion
Cancer stem cells (CSCs) are considered to be a cancer cells that possess characteristics associated with normal stem cells and consistent evidence suggesting that miRNAs and CSCs have huge relationship to induce or repress cancer cells. MicroRNAs regulate various CSCs, through oncogenic and tumour suppressive proteins with signalling pathways. miR-21 considered to be an important miRNA frequently involved in wide varieties of CSCs. Inhibition of miR-21 decreased cell proliferation induced apoptosis and inhibited tumour growth in a number of CSCs indicated that miR-21 plays an important role in cancer progressions through CSCs. From the above evidences, high expression level of miR-21 is widely believed to be associated with CSCs and tumour growth however further investigations is still need in animal models and patient samples. Further studies are also required to elucidate the mechanism by which miR-21, its emerging targets impact and validate its usefulness as prognostic and diagnostic biomarker for various kinds of CSCs.
Conflicts of interest
There is no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Takahashi R.U., Miyazaki H., Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colmont C.S., Harding K.G., Piguet V., Patel G.K. Human skin cancer stem cells: a tale of mice and men. Exp Dermatol. 2012;21:576–580. doi: 10.1111/j.1600-0625.2012.01533.x. [DOI] [PubMed] [Google Scholar]
- 3.Karsten U., Goletz S. What makes cancer stem cell markers different? SpringerPlus. 2013;2:301. doi: 10.1186/2193-1801-2-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekar D., Hairul Islam V.I., Thirugnanasambantham K., Saravanan S. Relevance of miR-21 in HIV and non-HIV-related lymphomas. Tumour Biol. 2014;35:8387–8393. doi: 10.1007/s13277-014-2068-9. [DOI] [PubMed] [Google Scholar]
- 5.Sekar D., Saravanan S., Karikalan K. Role of microRNA 21 in mesenchymal stem cell (MSC) differentiation: a powerful biomarker in MSCs derived cells. Curr Pharm Biotechnol. 2015;16:43–48. doi: 10.2174/138920101601150105100851. [DOI] [PubMed] [Google Scholar]
- 6.Venturutti L., Romero L.V., Urtreger A.J. Stat3 regulates ErbB-2 expression and co-opts ErbB-2 nuclear function to induce miR-21 expression, PDCD4 downregulation and breast cancer metastasis. Oncogene. 2015;35:2208–2222. doi: 10.1038/onc.2015.281. [DOI] [PubMed] [Google Scholar]
- 7.Cappellesso R., Tinazzi A., Giurici T. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122:685–693. doi: 10.1002/cncy.21442. [DOI] [PubMed] [Google Scholar]
- 8.Deftereos G., Corrie S.R., Feng Q. Expression of mir-21 and mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS One. 2011;6:e28423. doi: 10.1371/journal.pone.0028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Y.J., Li Y.J., Zheng W. Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int. 2015;15:77. doi: 10.1186/s12935-015-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Zhang C., Hu L. Abnormal expression of miR-21 and miR-95 in cancer stem-like cells is associated with radioresistance of lung cancer. Cancer Invest. 2015;33:165–171. doi: 10.3109/07357907.2015.1019676. [DOI] [PubMed] [Google Scholar]
- 11.Mao B., Xiao H., Zhang Z. MicroRNA21 regulates the expression of BTG2 in HepG2 liver cancer cells. Mol Med Rep. 2015;12:4917–4924. doi: 10.3892/mmr.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Zhou L., Li Y. MicroRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J BUON. 2014;19:228–236. [PubMed] [Google Scholar]
- 13.Yamanaka S., Olaru A.V., An F. MicroRNA-21 inhibits Serpini1, a gene with novel tumour suppressive effects in gastric cancer. Dig Liver Dis. 2012;44:589–596. doi: 10.1016/j.dld.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Si M.L., Zhu S., Wu H. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y., Nangia-Makker P., Farhana L. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015;14:98. doi: 10.1186/s12943-015-0372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang C., Guo Y., Hong Y., Liu Y.H., Xue Y.X. MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Mol Biol Rep. 2015;42:721–727. doi: 10.1007/s11033-014-3820-3. [DOI] [PubMed] [Google Scholar]
- 17.Kang H.Y. MicroRNA-21 regulates stemness in cancer cells. Stem Cell Res Ther. 2013;4:110. doi: 10.1186/scrt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamoori A., Gopalan V., Smith R.A., Lam A.K. Modulatory roles of microRNAs in the regulation of different signalling pathways in large bowel cancer stem cells. Biol Cell. 2016;108:51–64. doi: 10.1111/boc.201500062. [DOI] [PubMed] [Google Scholar]
- 19.Haghpanah V., Fallah P., Tavakoli R. Antisense-miR-21 enhances differentiation/apoptosis and reduces cancer stemness state on anaplastic thyroid cancer. Tumour Biol. 2015;37:1299–1308. doi: 10.1007/s13277-015-3923-z. [DOI] [PubMed] [Google Scholar]
- 20.Chung W.M., Chang W.C., Chen L. MicroRNA-21 promotes the ovarian teratocarcinoma PA1 cell line by sustaining cancer stem/progenitor populations in vitro. Stem Cell Res Ther. 2013;4:88. doi: 10.1186/scrt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L., Yang Z.X., Song W.J. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol. 2013;43:661–669. doi: 10.3892/ijo.2013.1965. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Diaz P.C., Hsiao T.H., Chang J.C. De-regulated microRNAs in pediatric cancer stem cells target pathways involved in cell proliferation, cell cycle and development. PLoS One. 2013;8:e61622. doi: 10.1371/journal.pone.0061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S., Wan Y., Pan T. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47:346–356. doi: 10.1007/s12031-012-9759-8. [DOI] [PubMed] [Google Scholar]
- 24.Han M., Wang Y., Liu M. MiR-21 regulates epithelial-mesenchymal transition phenotype and hypoxia-inducible factor-1α expression in third-sphere forming breast cancer stem cell-like cells. Cancer Sci. 2012;103:1058–1064. doi: 10.1111/j.1349-7006.2012.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golestaneh A.F., Atashi A., Langroudi L., Shafiee A., Ghaemi N., Soleimani M. miRNAs expressed differently in cancer stem cells and cancer cells of human gastric cancer cell line MKN-45. Cell Biochem Funct. 2012;30:411–418. doi: 10.1002/cbf.2815. [DOI] [PubMed] [Google Scholar]
- 26.Han M., Liu M., Wang Y. Re-expression of miR-21 contributes to migration and invasion by inducing epithelial-mesenchymal transition consistent with cancer stem cell characteristics in MCF-7 cells. Mol Cell Biochem. 2012;363:427–436. doi: 10.1007/s11010-011-1195-5. [DOI] [PubMed] [Google Scholar]
- 27.Misawa A., Katayama R., Koike S., Tomida A., Watanabe T., Fujita N. AP-1-Dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells. Oncol Res. 2010;19:23–33. doi: 10.3727/096504010x12828372551759. [DOI] [PubMed] [Google Scholar]
- 28.Sekar D., Venugopal B., Sekar P., Ramalingam K. Role of microRNA 21 in diabetes and associated/related diseases. Gene. 2016;582:14–18. doi: 10.1016/j.gene.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 29.Trang P., Medina P.P., Wiggins J.F. Regression of murine lung tumours by the let-7 microRNA. Oncogene. 2009;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]