Abstract
This study was performed for investigation the relationship between variants of MTP gene polymorphism and the development of NAFLD in patients with and without MS. The study was included 174 NAFLD patients (106 with MS and 68 without MS), and 141 healthy control subjects. The 493 G/T polymorphism of MTP gene was evaluated by PCR-RFLP method. The frequency of MTP TT genotype and T allele were significantly higher in NAFLD patients when compared to healthy controls. Moreover, a significant association in MTP gene polymorphism was observed in NAFLD patients with MS compared to NAFLD patients without MS and controls. Our study suggested that MTP 493 G/T gene polymorphism may act as susceptibility biomarker for NAFLD and MS.
Keywords: Genetic variants, Metabolic syndrome (MS), Microsomal triglyceride transfer protein (MTP), Non-alcoholic fatty liver disease (NAFLD), PCR-RFLP
Abbreviations: MS, Metabolic syndrome; MTP -493G > T, Microsomal triglyceride transfer protein; NAFLD, Non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a universal disorder which is considered as the most common liver disease worldwide; it is defined as the accumulation of excessive fat in the liver in the absence of excessive drinking of alcohol and any secondary cause.1 Numerous risk factors have been suggested in NAFLD pathogenesis, including advanced age, dietary habits, obesity, and some traits of metabolic syndrome (MS), such as insulin resistance and dyslipidemia.2, 3
Non-alcoholic fatty liver disease is no longer considered to be a primary liver disease, but rather a constituent of metabolic syndrome.4 Epidemiologic studies support belief to the relation between NAFLD and MS, the latter may be the etiologic agent that triggers the pathophysiological cascade of NAFLD.5 Therefore, the possibility of NAFLD increases proportionately with the number of metabolic syndrome factors present.6
In general, NAFLD is a multifactorial disease produced by complex interactions between nutritional factors, lifestyle choices, and genetic determinants.7 Previous studies suggested that genetic factors play an important role in NAFLD etiology by altering hepatic lipid metabolism.8, 9, 10, 11
Microsomal triglyceride transfer protein (MTP, or MTTP), a lipid transfer protein involved in apolipoprotein B (apoB) assembly, is localized to the endoplasmic reticulum in hepatocytes and enterocytes.12 Lower hepatic expression of MTP plays a crucial role in NAFLD development.13 Although a large number of single-nucleotide polymorphisms in the MTP gene have been identified, -493G > T (rs1800591) is one of the most common and widely investigated polymorphisms.14, 15 The data concerning the importance of the MTP -493G > T polymorphism in NAFLD development are inconsistent.16, 17, 18, 19, 20, 2 Therefore, we performed this study to investigate whether MTP -493G > T polymorphism contributes to the risk of NAFLD and to investigate its relation with metabolic syndrome in NAFLD patients. Additionally, we studied the relationship between gene variants and lipid profile of NAFLD patients with MS.
Subjects and methods
Subjects
A total of 174 patients with non-alcoholic fatty liver disease who were newly diagnosed by liver ultrasonography using established criteria21 were recruited from the liver clinic of the Medical Service Unit at the National Research Center, Egypt. The NAFLD patients were subdivided according to metabolic syndrome criteria22 into 106 patients with MS and 68 without MS. In addition, 141 control healthy subjects with no detectable fatty liver disease or metabolic syndrome were also recruited from the same center during the same study period. They were frequency matched with the NAFLD patients regarding sex, age, ethnicity, occupation and area of residence according to the propensity score matching method. This research was approved by the Human Ethics Committee of the National Research Center. Written informed consent was obtained from all participants before their participation in the study.
Diagnosis of NAFLD
The diagnosis of NAFLD was based on abdominal ultrasound examinations without including other causes of chronic liver disease (liver cirrhosis, hepatic carcinoma, hepatitis history, impaired hepatic function (alanine transaminase > 2.0 times upper limit of normal), hepatitis B, hepatitis C virus infection, drugs for liver damage, and excessive drinking (≥20 g/d)). Abdominal ultrasonographic examinations were performed by the same physician for all patients and controls using SonoAce R5 (6 MHz; Samsung).
Definition of the metabolic syndrome
Metabolic syndrome was defined using a previously published modification of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) guidelines as having 3 or more of the following risk determinants: (1) waist circumference (WC) ≥90 cm; (2) raised triglyceride (TG) ≥150 mg/dL; (3) reduced high-density lipoprotein (HDL) <40 mg/dL or lipid medication use; (4) raised blood pressure (BP), systolic ≥130 mmHg or diastolic ≥85 mmHg; (5) fasting blood glucose (FBG) ≥100 mg/dL.
Anthropometric measurements
Body mass index (BMI) was determined by dividing weight by square height (kg/m2). Waist circumference (WC) was obtained from each subject by measuring at the midpoint between the lower rib margin and the iliac crest using a conventional tape graduated in centimeters (cm). Hip circumference (HC) was measured as the greatest circumference at the level of greater trochanters. Waist-to-hip ratio was calculated by dividing the waist circumference by the hip circumference.
Sample collection
A venous blood sample of 6 mL was drawn from each subject after an overnight fast, 3 mL of which were collected in a glass tube for serum lipid determination, 2 mL were collected in EDTA containing tube for DNA extraction to determine MTP gene polymorphism, while the remaining 1 mL was transferred to a tube with a mixture of EDTA and fluoride for plasma separation to measure fasting plasma glucose.
Biochemical analyses
Fasting plasma glucose (FPG) was determined using a modified hexokinase technique; total cholesterol (TC), triglycerides (TGs) and high-density lipoprotein (HDL) were measured enzymatically with commercially available kits from STANBIO, USA; low-density lipoprotein (LDL) was calculated by the Friedewald equation.23
Genotyping
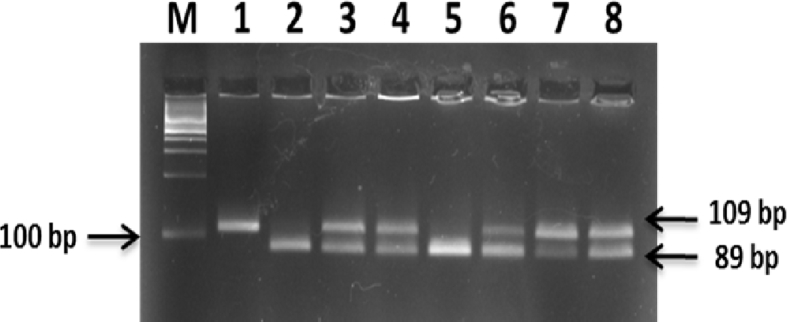
Genomic DNA was extracted and purified from whole peripheral blood samples using QIAamp DNA extraction kit (Qiagen Inc., Valencia, CA, USA). The MTP 493G/T polymorphism was genotyped by a polymerase chain reaction (PCR)-restriction fragment length polymorphism assay as described by Karpe et al.24 Briefly, a 109 bp fragment in the MTP gene was amplified by PCR using, the following primers: F: 5′-AGTTTCACACATAAGGACAATCATCTA-3′ and R: 5′ GGATTTAAATTTAAACTGTTAATTCATATCAC-3′ (New England Biolabs, USA). PCR was performed using Taq PCR Master Mix (Qiagen, Valencia, CA, USA) and T-Gradient thermal cycler (Biometra, Germany). The cycle profile was as follow: predenaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 7 min. PCR products (10 μL) were incubated with 2.5 U HphI (New England Biolabs, USA) at 37 °C for 1 h. Electrophoresis was then performed on 3% agarose gel stained with Red-Safe and then the electrophoretic products were visualized using ultra-violet light transillumination. A “G” at position -493 yielded bands of 89 and 20 bp, whereas a “T” at position -493 yielded a band of 109 bp, thus an individual with band(s) at 89 and 20 bp, at 109 bp only, and at 109, 89 and 20 bp was defined as GG homozygotic genotype, TT homozygotic genotype, and GT heterozygotic genotype, respectively (Fig. 1).
Figure 1.
Agarose gel electrophoresis of MTP 493G/T products after digestion with HphI restriction enzyme. M lane: DNA Ladder (100–1000 bp). Lanes 2 and 5: homozygous GG genotype yielded 2 bands of 89 bp and 20 bp. Lanes 3, 4, 6, 7, and 8: heterozygous GT genotype yielded 3 bands of 109 bp, 89 bp and 20 bp. Lane 1: homozygous TT genotype yielded 1 band of 109 bp.
Statistical analysis
Data are expressed as means ± standard deviation for quantitative variables, and as frequency for qualitative variables. Qualitative variables were compared using the Chi-square (χ2) test or Fischer's exact test. One-way ANOVA was used to compare the clinical and laboratory characteristics of patients divided according to genotypes. The Statistical Package for the Social Sciences software (SPSS 17.0, Chicago, IL, USA) was used. P ≤ 0.05 was considered significant. Sample size calculation was done using Stats Direct statistical software version 2.8 for MS Windows (Stats Direct Ltd., Cheshire, UK).
Results
Table 1 shows a significant difference in age distribution between NAFLD patients and controls according to MTP -493G/T genotype (TT = 39.35 ± 0.82, G/T = 37.6 ± 1.19, G/G = 40.65 ± 1.67, T/T = 37.8 ± 2.37; G/T = 35.68 ± 1.29; and G/G = 35.45 ± 1.22, years respectively). Additionally, fasting plasma glucose (FPG), insulin and homoeostasis model assessment-insulin resistance (HOMA-IR) index were significantly higher in NAFLD than healthy subjects at P value ≤ 0.05.
Table 1.
Baseline characteristics of controls and patients with NAFLD according to -493G/T MTP polymorphism.
| controls |
NAFLD |
ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| GG (n = 60) |
GT (n = 66) |
TT (n = 15) |
GG (n = 34) |
GT (n = 78) |
TT (n = 62) |
P value | |
| Sex (M/F) | 36/24 | 42/24 | 3/12 | 14/20 | 24/54 | 22/40 | – |
| Age (years) | 35.45 ± 1.22 | 35.68 ± 1.29 | 37.8 ± 2.37 | 40.65 ± 1.67 | 37.60 ± 1.19 | 39.35 ± 0.82 | 0.033* |
| BMI (kg/m2) | 22.21 ± 0.23 | 23.02 ± 0.21 | 23.28 ± 0.21 | 40.31 ± 1.47 | 37.56 ± 1.21 | 38.37 ± 1.17 | 0.000* |
| Waist circumference (WC) (cm) | 80.4 ± 0.31 | 82.27 ± 1.52 | 83.0 ± 1.66 | 110.58 ± 2030 | 107.46 ± 1.31 | 106.58 ± 1.36 | 0.000* |
| Waist hip ratio | 0.81 ± 0.006 | 0.8 ± 0.006 | 0.81 ± 0.009 | 0.89 ± 0.012 | 0.89 ± 0.008 | 0.88 ± 0.008 | 0.000* |
| Liver size (cm bcm) | 13.29 ± 0.34 | 13.56 ± 0.27 | 14.26 ± 0.62 | 16.39 ± 0.32 | 16.22 ± 0.30 | 16.50 ± 0.28 | 0.000* |
| Spleen size (cm bcm) | 9.09 ± 0.26 | 9.02 ± 0.35 | 9.88 ± 0.38 | 10.97 ± 0.28 | 10.62 ± 0.21 | 10.62 ± 0.21 | 0.000* |
| Systolic BP (mmHg) | 69.25 ± 2.41 | 76.13 ± 2.22 | 74 ± 6.78 | 82.05 ± 2.27 | 84.07 ± 1.50 | 83.35 ± 2.37 | 0.000* |
| Diastolic BP (mmHg) | 107.00 ± 2.52 | 115.0 ± 2052 | 114 ± 6.78 | 125.88 ± 3.44 | 125.28 ± 3.43 | 128.09 ± 2.59 | 0.000* |
| Glucose (mg/dL) | 85.12 | 83.47 ± 1.47 | 80.86 ± 5.59 | 91.1 ± 2.86 | 98.32 ± 2.63 | 102.77 ± 4.59 | 0.001* |
| Insulin (μU/mL) | 6.89 ± 0.40 | 6.36 ± 0.34 | 6.12 ± 0.34 | 10.06 ± 1.02 | 10.15 ± 0.79 | 9.54 ± 0.90 | 0.000* |
| HOMA index | 1.43 ± 0.082 | 1.28 ± 0.071 | 1.28 ± 0.71 | 2.22 ± 0.21 | 2.27 ± 0.21 | 2.66 + 0.23 | 0.000* |
| Serum cholesterol (mg/dL) | 171.45 ± 5.65 | 184.51 ± 6.78 | 188.44 ± 6.57 | 267.48 ± 14.4 | 268.62 ± 8 | 263.9 + 8 | 0.000* |
| Serum triglycerides (mg/dL) | 106.81 ± 4.30 | 111.16 ± 3.95 | 87.74 ± 13.60 | 235.65 ± 15.49 | 221.85 ± 12.15 | 202 + 7.9 | 0.000* |
| LDL cholesterol (mg/dL) | 74.81 ± 7.55 | 90.62 ± 7.99 | 109.56 ± 17.49 | 175.91 ± 14.74 | 174.72 ± 7.93 | 187.3 + 9.3 | 0.000* |
| HDL cholesterol (mg/dL) | 75.27 ± 5.44 | 71.66 ± 4.38 | 61.34 ± 9.70 | 44.44 ± 2.81 | 49.54 ± 2.55 | 48.5 + 2.4 | 0.000* |
| VLDL (mg/dL) | 21.36 ± 4.38 | 22.23 ± 0.79 | 17.54 ± 2.72 | 47.12 ± 3.09 | 44.36 ± 2.42 | 40.8 + 1.6 | 0.000* |
| Subjects without MS (%) | 42.6 | 46.8 | 10.6 | 14.7 | 47.1 | 38.2 | 0.005* |
| Subjects with MS (%) | – | – | – | 22.6 | 43.4 | 34 | |
| Subjects without hypertension (%) | 42.6 | 46.8 | 10.6 | 19.4 | 45.2 | 35.5 | 0.002* |
| Subjects with hypertension (%) | – | – | – | 20 | 44 | 36 | |
Data are mean ± SD, ANOVA test was used for means comparison according MTP polymorphism.
*P values indicate significant difference.
P ≤ 0.05 was considered significant.
NAFLD: non-alcohol fatty liver disease.
Serum concentrations of lipids in NAFLD subjects according to MTP -493G/T genotypes are shown in Table 1. Non-alcohol fatty liver homozygous subjects for the rare MTP -493T variant had lower serum triglyceride concentration compared to subjects carrying 1 or 2 copies of the common G allele (T/T, 204.63 ± 11.35; G/T, 221.85 ± 12.15; and G/G, 235.65 ± 15.49 mg/dL). Also in NAFLD patients, lipid profile parameters (total cholesterol, triacylglycerol, LDL-cholesterol and very low-density lipoprotein (VLDL)-cholesterol) were significantly higher when compared to controls, while there was significant decrement in HDL between NAFLD and controls (P = 0.000).
Twenty percent of NAFLD GG, 44% NAFLD GT and 36% NAFLD TT had hypertension (systolic/diastolic blood pressure >130/85 mm Hg), 22.6% of NAFLD GG, 43.4% of NAFLD GT and 34% of NAFLD TT had the whole picture of the metabolic syndrome.
The results regarding the relationship between the frequency of MTP -493G/T polymorphism and the risk of NAFLD are presented in Table 2.
Table 2.
MTP (-493G/T) genotype in Non-alcoholic fatty liver disease (NAFLD) and healthy controls.
| MTP (-493G/T) | NAFLD (n = 174) | Controls (n = 141) | Pf values | P values | OR | 95%CI |
|---|---|---|---|---|---|---|
| Additive model | ||||||
| GG/GT/TT | 34/78/62 | 60/66/15 | 0.000 | |||
| Co-dominant model | ||||||
| GG | 34 (19.6%) | 60 (42.6%) | 0.007 | |||
| GT | 78 (44.8%) | 66 (46.8%) | 0.317 | 0.006 | 0.479 | 0.281–0.817 |
| TT | 62 (35.6%) | 15 (10.6%) | 0.000 | 0.000 | 0.137 | 0.068–0.277 |
| Recessive model | ||||||
| GG | 34 (19.6%) | 60 (42.6%) | 0.007 | 1 | ||
| GT + TT | 140 (80.4%) | 81 (57.4%) | 0.000 | 0.000 | 0.328 | 0.198–0.542 |
| Dominant model | ||||||
| GG + GT | 112 (64.4%) | 126 (89.4%) | 0.364 | 1 | ||
| TT | 62 (35.6%) | 15 (10.6%) | 0.000 | 0.000 | 0.215 | 0.116–0.399 |
| Over-dominant model | ||||||
| GG + TT | 96 (55.2%) | 75 (53.2%) | 0.108 | |||
| GT | 78 (44.8%) | 66 (46.8%) | 0.317 | 0.726 | 1.083 | 0.694–1.691 |
| Allele model | ||||||
| G | 145 (41.7%) | 186 (66%) | 0.024 | |||
| T | 203 (58.3%) | 96 (34%) | 0.000 | 0.000 | 0.369 | 0.266–0.511 |
Data are number (%), variables were compared using chi square (χ2) test or Fischer's exact test.
Pf values for the frequency of each genotype within the studied groups.
P values for comparison between NAFLD and Controls; OR: odd ratio; Cl: confidence interval.
Bold values indicate significant difference.
P value ≤ 0.05 was considered significant.
MTP: Microsomal triglyceride transfer protein.
The results showed that the prevalence of -493 MTP G/G carriers was 42.6% in controls versus 19.6% in non-alcoholic fatty liver disease (P = 0.007), heterozygous G/T carriers were 46.8% in controls and 44.8% in NAFLD (P = 0.317), and homozygous TT carriers were 10.6% in controls versus 35.6% in NAFLD (P = 0.000). The results revealed that there was a significant difference in the genotype distribution of MTP -493G/T polymorphism between the NAFLD patients and healthy controls.
The results also revealed that there was an association between the presence of the T allele (58.3%) in the MTP -493G/T polymorphism and the incidence of NAFLD (P = 0.000). There was significant difference in -493 MTP allelic frequency (G allele versus T allele) between the NAFLD and control groups (OR = 0.369, 95%CI = 0.266–0.511, P = 0.000).
Additionally, the results demonstrated that the homozygous T/T carrier for -493 MTP genotype (adjusted odds ratio [OR]: 0.137; 95% confidence interval [CI]: 0.068–0.277; P = 0.000) was associated with increased risk of NAFLD, compared with the wild-type homozygous of -493 MTP G/G carriers genotype. These associations were further demonstrated using a dominant model: for -493G/T genotypes GT + TT versus GG, the OR: 0.328; 95%CI: 0.198–0.542 and P = 0.000. In addition there was significant difference in recessive model GG + GT versus TT with P value of 0.000; OR = 0.215; 95%CI = 0.116–0.399. However, there was no significant difference in over-dominant model, GG + TT versus GT at P-value = 0.726; OR = 1.083; 95%CI = 0.694–1.691 (Table 2).
The prevalence of MTP polymorphism in NAFLD patients, with and without metabolic syndrome, compared to healthy controls is shown in Table 3. MTP -493T/T genotype was significantly different in controls and NAFLD subjects, with and without metabolic syndrome, at P value = 0.000. Also, the allele frequencies were different among the studied groups and were respectively G: 66%, 44.3%, 38.2% and T: 34%; 55.7%; 61.8% (P = 0.000). Furthermore, using the recessive (GT + TT vs GG) and dominant model (GG + GT vs TT) a significant difference was found between the studied groups with P value = 0.000.
Table 3.
Co-dominant, dominant, recessive, and over-dominant models for MTP -493G > T gene polymorphism in NAFLD patients (with and without MS) and healthy controls.
| NAFLD with MS (n = 106) | NAFLD without MS (n = 68) | Controls (n = 141) | X2 | P value | ||
|---|---|---|---|---|---|---|
| MTP -493G > T | TT/GT/GG | 36/46/24 | 26/32/10 | 15/66/60 | 35.1 | 0.000 |
| Genotype | G | 94 (44.3%) | 52 (38.2%) | 186 (66%) | ||
| Allele | T | 118 (55.7%) | 84 (61.8%) | 96 (34%) | 37.2 | 0.000 |
| Co-dominant model | GG | 24 (22.6%) | 10 (14.7%) | 60 (42.6%) | ||
| GT | 46 (43.4%) | 32 (47.1%) | 66 (46.8%) | 8.6 | 0.014 | |
| TT | 36 (34%) | 26 (38.2%) | 15 (10.6%) | 35.2 | 0.000 | |
| Recessive model | GT + TT | 82 (77.4%) | 58 (85.3%) | 81 (57.4%) | 20.9 | 0.000 |
| GG | 24 (22.6%) | 10 (14.7%) | 60 (42.6%) | |||
| Dominant model | TT | 36 (34%) | 26 (38.2%) | 15 (10.6%) | 26.7 | 0.000 |
| GG + GT | 70 (66%) | 42 (61.8%) | 126 (89.4%) | |||
| Over-dominant model | GT | 46 (43.4%) | 32 (47.1%) | 66 (46.8%) | 0.35 | 0.84 |
| GG + TT | 60 (56.6%) | 36 (52.9%) | 75 (53.2%) | |||
Data are number (%), variables were compared using Chi square (χ2) test.
P values for comparison between the studied groups.
OR: odd ratio; Cl: confidence interval.
Bold values indicate significant difference at P ≤ 0.05.
As shown in Table 4, Table 5, we analyzed the influence of -493 MTP genotypes on lipid profile; the TG and VLDL levels of the MTP TT carriers were significantly lower than those of GT/GG carriers among NAFLD and NAFLD with MS groups (P = ≤0.05 for each).
Table 4.
Lipid profile of NAFLD patients according to the genotypes of MTP gene.
| TT (n = 62) |
GT + GG (n = 112) |
P value | |
|---|---|---|---|
| Serum cholesterol (mg/dL) | 263.9 ± 8 | 268.3 ± 4.9 | 0.708 |
| Serum triglycerides (mg/dL) | 202 ± 7.9 | 228 ± 6.8 | 0.05 |
| HDL cholesterol (mg/dL) | 48.5 ± 2.4 | 47.9 ± 1.4 | 0.833 |
| LDL cholesterol (mg/dL) | 175.4 ± 7.9 | 175 ± 4.9 | 0.967 |
| VLDL (mg/dL) | 40.8 ± 1.6 | 45.3 ± 1.3 | 0.05 |
Data are mean ± SE, ANOVA test was used for comparison.
Bold values indicate significant difference at P ≤ 0.05.
Table 5.
Lipid profile of NAFLD patients with MS according to the genotypes of MTP gene.
| TT (n = 36) |
GT + GG (n = 70) |
P value | |
|---|---|---|---|
| Serum cholesterol (mg/dL) | 274.2 ± 9.4 | 270.5 ± 6.3 | 0.74 |
| Serum triglycerides (mg/dL) | 216.5 ± 5.6 | 242.6 ± 7.4 | 0.022 |
| HDL cholesterol (mg/dL) | 43.6 ± 2 | 43.2 ± 1.3 | 0.882 |
| LDL cholesterol (mg/dL) | 187.3 ± 9.3 | 178.7 ± 6.5 | 0.452 |
| VLDL (mg/dL) | 43.3 ± 1.1 | 48.5 ± 1.5 | 0.021 |
Data are mean ± SE, ANOVA test was used for comparison.
Bold values indicate significant difference at P ≤ 0.05.
Discussion
Non-alcoholic fatty liver disease (NAFLD) is becoming a worldwide epidemic and one of the most important global health issues.25 NAFLD is associated with metabolic syndrome (MS), including obesity, hyperlipidemia, hypertension, and diabetes. Therefore, NAFLD is considered part of MS.26, 27, 28 Several genes have been identified as potential candidates in the pathogenesis and progression of fatty liver, genes involved in mechanisms of liver injury and genes influencing lipid metabolism like microsomal triglyceride transfer protein (MTP).29 Many previous studies have reported that a common polymorphism (-493G > T, rs1800591 G/T) in the MTP gene may be implicated in the susceptibility to NAFLD, but individually published data are inconclusive.17, 30, 31, 32, 14, 20, 33, 13, 34, 15, 2 Although the exact function of MTP genetic polymorphism in the development of NAFLD is not fully elucidated yet, a possible cause might be due to low hepatic expression of MTP induced by inherited variants in the MTP gene, which are associated with changes in hepatic lipid metabolism resulting in its low plasma level, thereby possibly explaining inter-individual differences in disease incidence of NAFLD.35
The finding of our study suggested that MTP -493G > T polymorphism might contribute to an increased risk of NAFLD, implying that MTP -493G > T polymorphism may be an important causative factor for the incidence of NAFLD. Besides, MTP -493G > T polymorphism could be associated with metabolic syndrome in non-alcoholic fatty liver disease. Our finding is in agreement with Bernard et al; Hussain et al; Peng et al; Zheng et al; Miyaaki and Nakao who reported that MTP -493G/T polymorphism may be used for early detection of NAFLD.16, 12, 2, 36, 37 This might appear in contrast with a study in a Brazilian population which showed that there was no significant association between the MTP -493G/T polymorphism and NAFLD,20 as well as Marra who demonstrated that, the frequency of the G allele or of the G/G genotype for MTP was not different from that of controls without fatty liver.38
Additionally, in the current study, the TT individuals in both NAFLD and NAFLD with MS had lower TG and VLDL levels, similar to investigators who demonstrated an association between the MTP -493T allele and hepatic lipids39, 40, 41; however, another investigator detected no relationship between this polymorphism and any lipid phenotype.42 Since it is widely accepted that hepatic lipid and lipoprotein abnormalities accompanied by chronic inflammation may play a vital role in the progression of NAFLD,43 excessive accumulation of triglycerides in hepatocytes is the hallmark of NAFLD.44 In general, hepatic triglycerides are exported from the liver in the form of VLDL particles mediated through plasma apoB-lipoprotein and MTP.45 Further, lipid transfer activity of MTP is required for the assembly and secretion of apoB-lipoproteins.46 However, MTP -493G > T polymorphism may result in a decrease in its protein and aberrant alterations of MTP synthesis and secretion, influence the capacity for lipid export, and induce dysregulation of hepatic lipid metabolism, thus contributing to the development of NAFLD.13, 47 Lipid metabolism in the liver and peripheral tissues plays a critical role in NAFLD onset and progression. Thus, there are many reports of gene polymorphisms involved in lipid metabolism that are associated with NAFLD and NAFLD progression.37 Microsomal triglyceride transfer protein has a pivotal role in metabolizing hepatic TG and VLDL for lipid export from the liver.48 Therefore, MTP plays an important role in lipid metabolism.
In conclusion, our findings demonstrate that MTP -493G > T polymorphism might be correlated with the risk of NAFLD and MS. Thus, MTP -493G > T polymorphism could be used as a valuable and practical biomarker for early diagnosis of NAFLD. However, further studies in larger samples of different populations are required to elucidate the participation of MTP polymorphisms in NAFLD susceptibility.
Author contributions
-
-
Conceived and designed the study: Yehia M. Shaker, Esmat Ashour and Wafaa Ezzat.
-
-
Diagnosis and selection all participants in the study: Wafaa Ezzat.
-
-
Contributed reagents/materials/analysis tools: Weaam Gouda and Esmat Ashour
-
-
Weaam Gouda and Esmat Ashour Contributed to the analysis of the results and to the writing of the manuscript.
-
-
All authors provided critical feedback and helped shape the research, analysis and approved the final version submitted for manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by grant from National Research Centre, Egypt (Project Number 10010205).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.peng X.E., Wu Y.L., Lu Q.Q., Hu Z.J., Lin X. MTTP polymorphisms and susceptibility to non-alcoholic fatty liver disease in a Han Chinese population. Liver Int. 2014;34:118–128. doi: 10.1111/liv.12220. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed A.A., Gohary K.E., Mashad G.M. Interleukin 10, thyroid status and ferritin are non-invasive prognostic biomarkers for diagnosis of fatty liver disease in children. J Int Res Med Pharm Sci. 2016;8:85–93. [Google Scholar]
- 4.Watanabe S., Hashimoto E., Ikejima K. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364–377. doi: 10.1007/s00535-015-1050-7. [DOI] [PubMed] [Google Scholar]
- 5.Sherif Z.A., Saeed A., Ghavimi S. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig Dis Sci. 2016;61:1214–1225. doi: 10.1007/s10620-016-4143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Than N., Newsome P. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Malaguarnera M., Di Rosa M., Nicoletti F., Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med. 2009;87:679–695. doi: 10.1007/s00109-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 8.Day C.P. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 9.Duvnjak M., Barsić N., Tomasić V., Lerotić I. Genetic polymorphisms in non-alcoholic fatty liver disease: clues to pathogenesis and disease progression. World J Gastroenterol. 2009;15:6023–6027. doi: 10.3748/wjg.15.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dongiovanni P., Valenti L., Rametta R. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut. 2010;59:267–273. doi: 10.1136/gut.2009.190801. [DOI] [PubMed] [Google Scholar]
- 11.Alisi A., Cianfarani S., Manco M., Agostoni C., Nobili V. Non-alcoholic fatty liver disease and metabolic syndrome in adolescents: pathogenetic role of genetic background and intrauterine environment. Annals Med. 2012;44:29–40. doi: 10.3109/07853890.2010.547869. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M.M., Rava P., Walsh M., Rana M., Iqbal J. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab. 2012;9:14. doi: 10.1186/1743-7075-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowman J.K., Tomlinson J.W., Newsome P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirandola S., Osterreicher C.H., Marcolongo M. Microsomal triglyceride transfer protein polymorphism (-493G/T) is associated with hepatic steatosis in patients with chronic hepatitis C. Liver Int. 2009;29:557–565. doi: 10.1111/j.1478-3231.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 15.Siqueira E.R., Oliveira C.P., Correa-Giannella M.L. MTP -493G/T gene polymorphism is associated with steatosis in hepatitis C-infected patients. Braz J Med Biol Res. 2012;45:72–77. doi: 10.1590/S0100-879X2011007500160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard S., Touzet S., Personne I. Association between microsomal triglyceride transfer protein gene polymorphism and the biological features of liver steatosis in patients with type II diabetes. Diabetologia. 2000;43:995–999. doi: 10.1007/s001250051481. [DOI] [PubMed] [Google Scholar]
- 17.Namikawa C., Shu-Ping Z., Vyselaar J.R. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781–786. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Petit J.M., Masson D., Minello A. Lack of association between microsomal triglyceride transfer protein gene polymorphism and liver steatosis in HCV-infected patients. Mol Genet Metab. 2006;88:196–198. doi: 10.1016/j.ymgme.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Carulli L., Canedi I., Rondinella S. Genetic polymorphisms in non-alcoholic fatty liver disease: interleukin-6-174G/C polymorphism is associated with non-alcoholic steatohepatitis. Dig Liver Dis. 2009;41:823–828. doi: 10.1016/j.dld.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira C.P., Stefano J.T., Cavaleiro A.M. Association of polymorphisms of glutamate-cystein ligase and microsomal triglyceride transfer protein genes in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2010;25:357–361. doi: 10.1111/j.1440-1746.2009.06001.x. [DOI] [PubMed] [Google Scholar]
- 21.Kakrani A., Sharma Z., Thind S., Gokhale V. Correlation of NAFLD fibrosis score and BARD score with ultrasonographic evidence of nonalcoholic fatty liver disease in overweight patients: a prospective study. Int J Med Public Health. 2013;3:111. [Google Scholar]
- 22.Lin M.S., Shih S.R., Li H.Y. Serum C-reactive protein levels correlates better to metabolic syndrome defined by International Diabetes Federation than by NCEP ATP III in men. Diabetes Res Clin Pract. 2007;77:286–292. doi: 10.1016/j.diabres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Karpe F., Lundahl B., Ehrenborg E., Eriksson P., Hamsten A. A common functional polymorphism in the promoter region of the microsomal triglyceride transfer protein gene influences plasma LDL levels. Arterioscler Thromb Vasc Biol. 1998;18:756–761. doi: 10.1161/01.atv.18.5.756. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y., Chen J., Yang L. The value of neck circumference as a predictor of non-alcoholic fatty liver disease (NAFLD) J Clin Transl Endocrinol. 2014;1:133–139. doi: 10.1016/j.jcte.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchesini G., Brizi M., Bianchi G. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 27.Miyaaki H., Ichikawa T., Nakao K. Clinicopathological study of nonalcoholic fatty liver disease in Japan: the risk factors for fibrosis. Liver Int. 2008;28:519–524. doi: 10.1111/j.1478-3231.2007.01614.x. [DOI] [PubMed] [Google Scholar]
- 28.Sugihara T., Koda M., Kishina M. Fatty liver Shionogi-ob/ob mouse: a new candidate for a non-alcoholic steatohepatitis model. Hepatol Res. 2013;43:547–556. doi: 10.1111/j.1872-034X.2012.01101.x. [DOI] [PubMed] [Google Scholar]
- 29.Macaluso F.S., Maida M., Petta S. Genetic background in nonalcoholic fatty liver disease: a comprehensive review. World J Gastroenterol. 2015;21:11088–11111. doi: 10.3748/wjg.v21.i39.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musso G., Gambino R., De Michieli F. Nitrosative stress predicts the presence and severity of nonalcoholic fatty liver at different stages of the development of insulin resistance and metabolic syndrome: possible role of vitamin A intake. Am J Clin Nutr. 2007;86:661–671. doi: 10.1093/ajcn/86.3.661. [DOI] [PubMed] [Google Scholar]
- 31.Gambino R., Cassader M., Pagano G., Durazzo M., Musso G. Polymorphism in microsomal triglyceride transfer protein: a link between liver disease and atherogenic postprandial lipid profile in NASH? Hepatology. 2007;45:1097–1107. doi: 10.1002/hep.21631. [DOI] [PubMed] [Google Scholar]
- 32.Zampino R., Ingrosso D., Durante-Mangoni E. Microsomal triglyceride transfer protein (MTP) -493G/T gene polymorphism contributes to fat liver accumulation in HCV genotype 3 infected patients. J Viral Hepat. 2008;15:740–746. doi: 10.1111/j.1365-2893.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 33.Musso G., Gambino R., Cassader M. Lipoprotein metabolism mediates the association of MTP polymorphism with beta-cell dysfunction in healthy subjects and in nondiabetic normolipidemic patients with nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21:834–840. doi: 10.1016/j.jnutbio.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 34.El-Koofy N.M., El-Karaksy H.M., Mandour I.M., Anwar G.M., El-Raziky M.S., El-Hennawy A.M. Genetic polymorphisms in non-alcoholic fatty liver disease in obese Egyptian children. Saudi J Gastroenterol. 2011;17:265–270. doi: 10.4103/1319-3767.82582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Filippo M., Crehalet H., Samson-Bouma M.E. Molecular and functional analysis of two new MTTP gene mutations in an atypical case of abetalipoproteinemia. J Lipid Res. 2012;53:548–555. doi: 10.1194/jlr.M020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng W., Wang L., Su X., Hu X.F. MTP -493G > T polymorphism and susceptibility to nonalcoholic fatty liver disease: a meta-analysis. DNA Cell Biol. 2014;33:361–369. doi: 10.1089/dna.2013.2238. [DOI] [PubMed] [Google Scholar]
- 37.Miyaaki H., Nakao K. Significance of genetic polymorphisms in patients with nonalcoholic fatty liver disease. Clin J Gastroenterol. 2017;10:201–207. doi: 10.1007/s12328-017-0732-5. [DOI] [PubMed] [Google Scholar]
- 38.Marra F. NASH: are genes blowing the hits? Comment on polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:853–856. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Juo S.H., Han Z., Smith J.D., Colangelo L., Liu K. Common polymorphism in promoter of microsomal triglyceride transfer protein gene influences cholesterol, ApoB, and triglyceride levels in young African American men: results from the coronary artery risk development in young adults (CARDIA) study. Arterioscler Thromb Vasc Biol. 2000;20:1316–1322. doi: 10.1161/01.atv.20.5.1316. [DOI] [PubMed] [Google Scholar]
- 40.Yin R.X., Li R.S., Lin W.X., Yang D.Z., Pan S.L. Effect of the MTP -493 G/T polymorphism on the lipid profiles of the Guangxi Hei Yi Zhuang and Han populations. Eur J Lipid Sci Technol. 2006;108:561–568. [Google Scholar]
- 41.Zák A., Jáchymová M., Tvrzická E. The influence of polymorphism of -493G/T MTP gene promoter and metabolic syndrome on lipids, fatty acids and oxidative stress. J Nutr Biochem. 2008;19:634–641. doi: 10.1016/j.jnutbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y.C., Chang P.F., Hu F.C. A common variant in the PNPLA3 gene is a risk factor for non-alcoholic fatty liver disease in obese Taiwanese children. J Pediatr. 2011;158:740–744. doi: 10.1016/j.jpeds.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Fon Tacer K., Rozman D. Nonalcoholic fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids. 2011:783976. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postic C., Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y., Lian Z., Jiang C., Wang Y., Zhu H. Beneficial metabolic effects of 2′,3′,5′-tri-acetyl-N6- (3-hydroxylaniline) adenosine in the liver and plasma of hyperlipidemic hamsters. PLoS One. 2012;7:e32115. doi: 10.1371/journal.pone.0032115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Q., Bengmark S., Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD) Lipids Health Dis. 2010;9:42. doi: 10.1186/1476-511X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira I.V., Stefano J.T., Oliveira C.P. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2011;5:245–251. doi: 10.1586/egh.11.22. [DOI] [PubMed] [Google Scholar]
- 48.Levy E., Stan S., Delvin E. Localization of microsomal triglyceride transfer protein in the golgi: possible role in the assembly of chylomicrons. J Biol Chem. 2002;277:16470–16477. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]