Abstract
Acute kidney injury is a clinical syndrome that can be caused by numerous diseases including acute tubular necrosis (ATN). ATN evolves in several phases, all of which are accompanied by different immune mechanisms as an integral component of the disease process. In the early injury phase, regulated necrosis, damage-associated molecular patterns, danger sensing, and neutrophil-driven sterile inflammation enhance each other and contribute to the crescendo of necroinflammation and tissue injury. In the late injury phase, renal dysfunction becomes clinically apparent, and M1 macrophage-driven sterile inflammation contributes to ongoing necroinflammation and renal dysfunction. In the recovery phase, M2-macrophages and anti-inflammatory mediators counteract the inflammatory process, and compensatory remnant nephron and cell hypertrophy promote an early functional recovery of renal function, while some tubules are still badly injured and necrotic material is removed by phagocytes. The resolution of inflammation is required to promote the intrinsic regenerative capacity of tubules to replace at least some of the necrotic cells. Several immune mechanisms support this wound-healing-like re-epithelialization process. Similar to wound healing, this response is associated with mesenchymal healing, with a profound immune cell contribution in terms of collagen production and secretion of profibrotic mediators. These and numerous other factors determine whether, in the chronic phase, persistent loss of nephrons and hyperfunction of remnant nephrons will result in stable renal function or progress to decline of renal function such as progressive chronic kidney disease.
Keywords: Acute kidney injury, Extracellular traps, Injury, Necroptosis, Therapeutics
Introduction
Acute kidney injury (AKI) is a term frequently used in clinical practice and kidney research; however, its meaning is not always clear. The current clinical classification defines AKI based on functional parameters such as urinary output and serum creatinine (SCr) and not on injury markers [1,2]. This definition of AKI does not cover important unilateral kidney injuries such as renal colic or unilateral renal embolism but instead defines transient renal hypoperfusion-related decline of glomerular filtration rate (GFR) as AKI despite the absence of nephron injury [3]. Because acute tubular necrosis (ATN) has been investigated in most studies addressing the role of the immune system in AKI, for scientific accuracy and to avoid further confusion, we reviewed the role of the immune system in ATN, whether unilateral or bilateral, and whether or not this results in reduced urinary output or increased SCr. We believe that all long-term sequelae arising from an ATN episode are associated with the irreversible loss of nephrons; thus, we are interested in the immune mechanisms that contribute to nephron loss in each of the ATN phases. First, we provide a short description of the role of the immune system in the healthy kidney during homeostasis before covering recent insights into the role of the immune system in the early and late injury phases of ATN. Then, we discuss the contribution of the immune system to the recovery and post-recovery phases of ATN. Finally, we discuss the obstacles to overcome before experimental knowledge is implemented into clinical practice.
The immune system of the healthy kidney during homeostasis
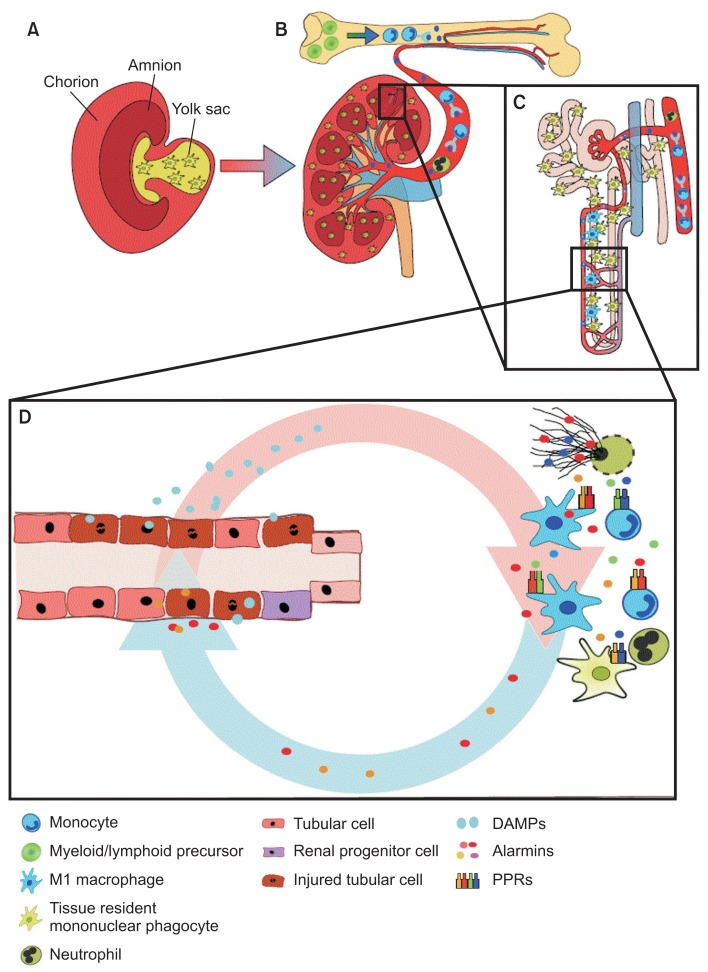
The healthy kidney harbors numerous immune cells in different compartments (Fig. 1).
Figure 1. The immune system during homeostasis and necroinflammation.
(A) Renal resident mononuclear phagocytes originate from the yolk sac during nephrogenesis. (B, C) These cells or the bone marrow scatter around the tubular basement membrane throughout the entire nephron. (B) Infiltrating mononuclear phagocytes originate from the bone marrow and follow chemokine tracks to the injured kidney. During necroinflammation (D), both resident and infiltrating immune cells react to danger-associated molecular patterns (DAMPs) released by dying tubular cells. By releasing more alarmins and DAMPs, further immunogenic cell death is induced, and more immune cells are attracted from the bone marrow via the circulation.
Resident intrarenal immune cells
Resident immune cells in the kidney belong to the myeloid lineage and originate from RUNX1, KIT+, and CSFR+ yolk sac precursors that express the surface markers CX3CR1, F4/80hi, and CD11blow (Fig. 1A) [4,5]. The cells reside in large numbers within the interstitial compartment in close proximity to the tubular basement membrane, the peritubular capillaries, and interstitial fibroblasts (Fig. 1B, C) [6]. Cells of the same origin appear as macrophages in many organs such as the liver or adipose tissue, but inside the kidney, they extend longer dendrites into the narrow spaces between the tubules and have therefore been referred to as intrarenal dendritic cells [7]. Because of the plasticity between functional properties typical for macrophages versus dendritic cells, these yolk sac-derived resident renal immune cells have also been termed mononuclear phagocytes [8].
The role of these cells during kidney homeostasis has not been rigorously explored. Assumedly, they continuously receive signals from the self, which maintains their inactive state and secretion of homeostatic signals such as anti-inflammatory lipid mediators and cytokines. Evidence is lacking regarding the hypothesis that these cells clear any bacterial products, an important function of cells in the liver (e.g., Kupffer cells), and the nephron’s ultrafiltrate is usually sterile [4]. Resident mononuclear phagocytes contribute to the turnover of extracellular matrix by releasing matrix metalloproteases and removing debris from apoptotic cells (Fig. 2A) [9–11]. The latter is an important homeostatic function, as the rapid clearance of cell debris from the extracellular space prevents neoepitopes from generating secondary modifications that could promote autoimmunity. In fact, people with genetic defects in phagocytic clearance mechanisms are prone to autoimmunity (e.g., systemic lupus erythematosus) [12].
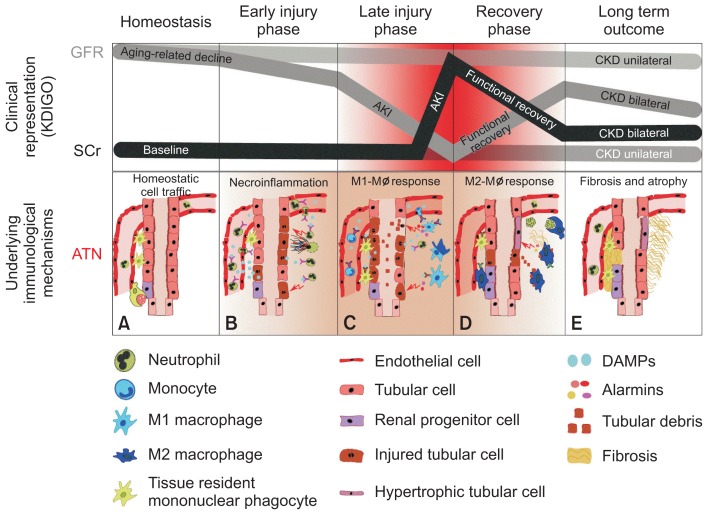
Figure 2. Acute kidney injury and renal inflammation.
A general misconception is that the current classification of acute kidney injury (AKI) is not based on markers of tubular necrosis but on markers of renal function. In fact, not the clinical representation of declined renal function, but only severity assessment of the underlying mechanisms of ATN serves as a reliable predictor of AKI-to-chronic kidney disease (CKD) progression. This becomes even more evident for unilateral forms of kidney injury, because serum creatinine (SCr) and urinary output usually remain unaffected in patients with normal baseline glomerular filtration rate (GFR). Nevertheless, some forms of unilateral acute kidney disease involve true kidney injury, renal inflammation, and necrosis such as unilateral renal embolism and thereby cover all phases of renal injury and regeneration. The concept of distinct phases of ATN is an extension of ideas by Sutton et al [10] and Okusa and Chertow [11]. The drop and recovery of GFR and the increase in SCr level are shown along the course of an AKI episode. The suggested course of AKI assumes that the trigger for injury was transient and not repetitive or persistent, either unilateral or bilateral. The immunological characterization of each underlying phase of acute tubular necrosis (ATN; A–E) is described in detail in the text.
KDIGO, Kidney Disease Improving Global Outcomes.
Circulatory blood compartment
Immune cells such as neutrophils, granulocytes, monocytes, and lymphocytes are also present inside the circulatory blood compartment of the kidney. These cells originate from myeloid and lymphoid precursors residing in the bone marrow, a lineage different from that giving rise to the resident immune cells of the kidney (Fig. 1B, C, and 2A) [5]. Circulating immune cells are immature effector cells of the innate and adaptive immune systems. They are equipped with integrins and chemokine receptors on their cell surface that allow them to be recruited to the kidney if kidney injury induces local expression of adhesion molecules and chemokines [5].
The immune system in the early injury phase of ATN
Regulated necrosis
ATN involves 2 types of necrosis, unregulated and regulated. For many years, necrosis was considered merely an accidental, passive, and uncontrollable form of cell death. However, the discovery of signaling pathways of regulated necrosis facilitated the recognition of regulated necrosis and implies a therapeutic potential in ATN. Several forms of regulated necrosis with specific signaling pathways have been discovered: Necroptosis (receptor-interacting protein kinase [RIPK] 1-RIPK3-mixed lineage kinase domain-like [MLKL]-related [13]), ferroptosis (glutathione peroxidase 4-related [14]), regulated necrosis mediated by mitochondrial permeability transition (MPT-RN, cyclophilin D-related [15]), pyroptosis (caspase-1/-11-related [16]), parthanatos (poly [adenosine diphosphate-ribose] polymerase-1-related [17]), and NETosis (RIPK3-MLKL-related [18]). Necroptosis, ferroptosis, MPT-RN, and parthanatos have been reported to contribute to experimental ATN. Meanwhile, pyroptosis and NETosis evolve as cell type-specific forms of regulated necrosis. Necroptosis contributes to ischemia reperfusion injury (IRI)- [19], cisplatin- [20], contrast media- [21], rhabdomyolysis- [22], and crystal-induced ATN [23]. Ferroptosis contributes to IRI-, oxalate-, and folic acid-induced ATN [24]. MPT-RN contributes to IRI-induced ATN and possibly cisplatin-induced ATN [19]. Parthanatos contributes to IRI- and cisplatin-induced ATN [25,26]. Pyroptosis occurs mainly in macrophages and dendritic cells [27,28]. The occurrence of pyroptosis in tubule cells remains unclear to date. NETosis occurs in neutrophils; although recently, extracellular traps generated by macrophages have also been reported to contribute to ATN [29].
Damage-associated molecular patterns (DAMPs)
Innate immunity is highly conserved in all species to prevent spread of infection. Toll-like receptors (TLRs) and membrane-bound pattern recognition receptors (PRRs) expressed in innate immune cells control infection. However, TLRs and other PRRs also recognize DAMPs or alarmins released from damaged tubule cells [30]. The molecular nature of necrosis-related DAMPs and their receptors in kidney disease has been reviewed elsewhere [31]. DAMPs released from dying tubule cells activate DAMP receptors on immune and parenchymal cells. Proximal tubular cells highly express scavenger receptors as a response to tissue injury (e.g., phosphatidylserine receptor kidney injury molecule-1 [KIM-1]), which effectively transforms them into transient ‘semi-professional phagocytes’ [32,33]. Consequently, DAMPs are also released from tubular cells undergoing ferroptosis and MPT-RN. DAMP recognition activates cells to secrete mature proinflammatory cytokines, which in turn induces further necrosis. This vicious cycle is termed necroinflammation and is a hallmark of the early injury phase (Fig. 1D) [34,35]. Necroptosis is induced by tumor necrosis factor (TNF)-α and interferon-γ [36]. Neutrophil extracellular trap (NET) formation can be induced by TNF-α and interleukin (IL)-18 [37]. Several DAMPs activate NOD-like receptor protein 3 (NLRP3), resulting in formation of a caspase-1-activating complex, the inflammasome [38]. The activated inflammasome in macrophages or dendric cells releases the mature proinflammatory cytokines IL-18 and IL-1β; the inflammasome can also be activated by kidney-specific DAMPs such as uromodulin [39]. IL-1 activates IL-1R/MyD88 signaling, which activates NF-κB-dependent transcription of proinflammatory cytokines and chemokines, a process under control of p53/MDM2 [40]. DAMP-induced NLRP3 activation may also trigger pyroptosis [16]. Certain DAMPs directly promote NET formation. For example, expelled histone, a main component of extracellular chromatin, directly damages renal tubule cells and induces TLR/NLRP3-related inflammation [41,42]. DAMPs can also activate dendric cells [43].
Neutrophil-driven sterile inflammation
During ATN, neutrophils and natural killer T (NKT) cells first emerge at injured sites (Fig. 2B) [44]. Whether NKT cells directly damage tubule cells remains under debate [45]. In contrast, neutrophils play a central role in driving sterile inflammation. After IRI, neutrophils appear in the peritubular capillaries as early as 30 minutes. Neutrophils adhere to the vascular endothelium by binding to intercellular adhesion molecule-1, E-selectin, L-selectin, and integrin [46] and migrate into the interstitium, where further tubular injury is promoted by release of proinflammatory cytokines/chemokines and reactive oxygen species [47]. Neutrophils also release DAMPs, such as histones, and form NETs. NETs and NET-derived DAMPs mediate renal necroinflammation and induce remote organ injury (Fig. 1D and 2B) [41].
The immune system in the late injury phase of ATN
In the late injury phase of ATN, different immune cells such as infiltrating bone marrow monocyte-derived M1 macrophages enter the process (Fig. 2C) [48]. Damaged tubular cells as well as resident mononuclear phagocytes attract circulatory immune cells such as CCR2+ monocytes by releasing DAMPs and chemokines [49]. Due to the proinflammatory microenvironment, these cells develop a M1 phenotype, which has been shown to contribute to kidney damage in the late injury phases of ATN [50,51]. Accordingly, depletion or reduction of M1 macrophages within the first 48 hours after renal injury is associated with less functional and structural damage in experimental models of ATN [46,52]. The proinflammatory environment in the damaged area is augmented by the ongoing auto-amplification loop of necroinflammation. Parenchymal cells undergoing necroptosis, ferroptosis, parthanatos, or MPT-RN continue to release DAMPs such as adenosine triphosphate (ATP), histones, heat shock proteins, and alarmins, which have both direct cytotoxic effects and immunostimulatory effects via PRRs. DAMPs and alarmins further drive regulated cell death. This can be a synchronized process, breaking down whole functional units of an organ (e.g., nephrons) and finally leading to organ failure or even sepsis and death [53]. However, when and how this auto-amplification loop terminates remain unanswered. The long-term outcome of ATN depends at least partially on the predominant macrophage phenotype present at the site of inflammation [49]. The macrophage capacity to undergo phenotype shifts appears to influence all subsequent phases of ATN by affecting the intrarenal microenvironment [54]. During the injury phase, DAMPs, interferon-γ, and TNF, produced by NK-cells and CD4+ TH1 and CD8+ T cells, prime the incoming M0 monocytes toward the proinflammatory M1 macrophage phenotype that contributes to the ongoing necroinflammation and kidney injury [50,55]. Over the course of events, the macrophage phenotype changes toward an anti-inflammatory effector function. It is not fully understood how this conversion occurs on the molecular level. Most of the knowledge is gained through in-vitro experiments and cannot be used to adequately explain the correlations within the much more complex and permanently adapting microenvironments in vivo. The M1-M2 shift is a complex interaction of outside-in signalling of different cytokines with the help of specific T-helper and regulatory T cells and delayed negative signaling of TLR within the macrophage [56]. During the process of wound healing, the clearing of neutrophils that underwent NET formation has been suggested to likely be associated with the shift in macrophage polarization (Fig. 2D) [57]. Adenosine promotes the shift to anti-inflammatory and angiogenic M2 macrophages by binding A2 receptors [58]. In addition, colony stimulating factor-1 (CSF-1) secreted by renal tubular cells enhances the expansion of M2 macrophages [59].
The alternatively activated M2 macrophages establish a predominantly anti-inflammatory micromilieu secreting IL-1R2, IL-10, and transforming growth factor-β (TGF-β) among others. Notably, the detailed characterization of in vitro classically activated M1 and alternatively activated M2 macrophages represent only the extremes of a potentially more mixed population of transient activation states in vivo [60]. The changing microenvironment and the macrophage phenotype switch mark the transition from injury to the recovery phase; epithelial and tubular repair mechanisms, proliferation, and fibrosis begin to predominate [61]. Without this change toward an anti-inflammatory micromilieu, necroinflammation leads to cell death and tissue damage [62].
The immune system in the recovery phase of ATN
Role of DAMPs and TLRs
Blocking TLRs in the context of ATN shows a mixed phenomenon; in the early injury phase, blocking TLRs prevents necroinflammation, whereas late blocking impairs recovery and renal outcome [63]. DAMPs, such as ATP, histone, and high mobility group box 1 protein, are strong proinflammatory triggers in sterile necroinflammation by activating TLRs, thereby initiating the expression and release of cytokines and chemokines in phagocytes [64–68]. However, DAMP-mediated TLR signaling also leads to the secretion of immunoregulatory mediators such as IL-22, a cytokine with pro-proliferative and pro-regenerative properties [69–71]. Phagocytes secrete IL-22, especially during inflammation [72]. IL-22 signaling is important for maintaining the integrity of the epithelial barrier and its recovery after injury in many different organs. Therefore, as expected, IL-22 receptors are mostly expressed on epithelial cells [73]. IL-22 signaling supports cell proliferation via the PI3K/Akt/mTOR pathway [74] and upregulation of cyclin D1 and CDKN4 [71]. Conversely, IL-22 enhances cell survival by downregulating pro-apoptotic factors including Bax and Bad and upregulating anti-apoptotic factors such as Bcl-2 via induction of STAT3 and ERK1/2 phosphorylation [69]. TLR signaling shows that activation of one biological mechanism, in this case necroinflammation, is closely associated with its counterregulatory mechanisms.
Resolution of inflammation
The fundamental condition that allows regeneration in any organ is resolution of the inflammatory milieu [75]. This is necessary for regeneration of the epithelium and restoration of a new host defense barrier [62]. The importance of this process can be seen in chronic dermal ulcers, which do not heal unless the inflammatory signal disappears, when comparing recovery after necroinflammation with dermal wound healing after injury. Resolution of the inflammatory milieu indicates the final eradication of pathogens and sterile wound conditions. Mononuclear phagocytes play an important role in changing the inflammatory milieu in the kidney. After phagocytosis of necrotic cell debris, these cells initiate the regenerative process of the nephron by removing apoptotic cells (Fig. 2D). Apoptosis, in contrast to necrotic forms of cell death, is a balanced physiological mechanism, which is not immunogenic but enhances epithelial healing [76,77]. Hence, apoptosis of immune cells, such as neutrophils and T-cells, represents a regulated physiological mechanism that limits an inappropriate immune response. Humoral factors such as C1q, mannose-binding lectin, and pentraxins support this process by opsonizing apoptotic cell debris [78–80]. The phagocytosis-related clearance of apoptotic cells further drives the polarization of mononuclear phagocytes from an M1- to an M2-like phenotype that favors changes in the inflammatory milieu toward anti-inflammatory conditions by secreting IL-10 and TGF-β [81,82]. Phagocytosis of dead cells and the release of an anti-inflammatory cytokine-cocktail enables immune cells, especially phagocytes, to dissolve the inflammatory signal in the nephron and allow epithelial and vascular healing. M2 macrophages also directly support parenchymal healing by secreting growth factors such as TGF-β, hepatocyte growth factor, epidermal growth factor, vascular endothelial growth factor-C, and platelet-derived growth factor [75]. These trophic functions of alternatively activated macrophages that also greatly contribute to embryonic nephrogenesis by increasing overall kidney growth and ureteric bud branching [83] can be additionally triggered in postnatal kidney repair. CSF-1 treatment rapidly accelerated renal repair after ischemic injury in vivo by altering the intrarenal macrophage population [84]. These results emphasize the crucial contribution of immune cells not only to collateral tissue damage, but also to solid organ regeneration.
Cell cycle activation in differentiated tubular cells versus progenitor cells
As an immediate response to tubular cell loss during ATN, the surviving differentiated tubular cells rapidly undergo aberrant cell cycles (endoreplication cycles or endocycles) to increase size and function, presenting as cell hypertrophy (Fig. 2D) [85]. True regeneration, i.e., division of surviving cells to replace lost tubular cells by daughter cells as a result of a completed G2/M phase of the cell cycle (mitosis) occurs rarely and is limited to scattered intrarenal progenitor cells residing mostly in the S3 segment of the proximal tubule and the thick ascending limb of the loop of Henle (Fig. 2D) [85,86]. Clonal proliferation of surviving progenitors in each single nephron determines the regenerative capacity of the kidney after ATN as humans and mammals cannot produce nephrons de novo; approximately only 50% of the irreversibly lost tubular cells during the injury phase are replaced by regeneration [85]. When differentiated tubular cells are forced to enter the cell cycle during the DNA damage and uncompleted DNA repair phase, this may lead to mitotic catastrophe, detachment, and further tubular cell losses beyond the initial injury and collateral damage caused by sterile inflammation. Currently, this mode of cell loss after injury is well described for podocytes [87–90], and although direct proof of mitotic catastrophe in renal tubular cells is lacking, evidence indicates that inhibition of cell cycle progression can be beneficial in models of ATN [91–94].
Mesenchymal healing
Mesenchymal healing is required to mechanically stabilize the remnant nephrons during the regeneration phase and fill the gaps of irreversibly lost nephrons. Thus, during the regeneration phase, fibrotic and vascular growth factors are simultaneously released with epithelial growth factors, a response that may be transient until epithelial healing is completed [95]. Insufficient epithelial healing leads to a persistent release of mesenchymal growth factors that induce a second line of defense, mesenchymal healing (Fig. 2E) [96]. Eventually, this also can lead to fibrosis with impaired renal function because interstitial fibrosis promotes vascular rarefication, tubular ischemia, and tubular atrophy [97–99]. Non-inflammatory M2 macrophages and bone marrow-derived fibrocytes promote the formation of fibrosis by releasing TGF-β and collagen matrix. Cell cycle arrest of epithelial cells in the G2/M phase leads to persistent TGF-β secretion and interstitial fibrogenesis [100], but this interpretation was based on the assumption that cell cycle marker p-histone H3 only indicates the G2/M phase. However, new data indicate that polyploid tubular cells undergoing endocycles and resting in the G1 cells also express p-histone H3. The tubular cells secrete TGF-β along with numerous other proteins and mediators as a sign of the hypersecretory state of compensatory cell hypertrophy [85]. This mechanism may explain why ATN can be followed by CKD as a result of pathomechanisms that ensure survival in the acute phase but evolve into maladaptive repair over time.
The intrarenal immune system in the post-recovery phase of ATN
The outcome after ATN is influenced by the number of lost nephrons during the injury phase and reconstitution of the remnant nephrons during the repair phase. When tubular regeneration fails, patients develop CKD with impaired renal function and structural changes in the kidney such as tubular atrophy and interstitial fibrosis (Fig. 2E) [101]. Reduced renal function causes alterations in immunity, observed both in persistent systemic sterile inflammation and in acquired immunosuppression [102]. Accordingly, patients suffering from CKD fail to generate an appropriate immune response to infections [103], a condition that can be largely explained by uremic toxin-induced immune suppression. Uremia triggers neutrophil apoptosis and oxidative burst in mononuclear phagocytes [104]. Furthermore, patients with CKD have higher blood level of the plasma protein fibroblast growth factor 23, which is associated with impaired neutrophil recruitment during infection [105]. These two factors, persistent systemic inflammation and immunosuppression, contribute to the mortality of CKD patients.
Obstacles to overcome before translating experimental findings into clinical practice
Late diagnosis
The definition of AKI currently does not rely on early injury markers but on kidney excretory dysfunction markers that increase only in the late injury phase [2]. However, drugs interfering with the inflammatory phase of ATN should have already been administered during the early injury phase. Implementing routine screening using kidney injury markers (e.g., tissue inhibitor of metalloproteinases 2 and insulin-like growth factor binding protein-7) in high-risk patients to identify ATN within a window of opportunity represents the most promising option for effective targeting of necroinflammation [106,107].
Animal models
Although cisplatin nephropathy is similar in rodents and humans, other forms of human ATN encompass a complex pathophysiology difficult to mimic in rodent models [108–110]. For example, renal pedicle clamping is frequently used in rodents but is an infrequent cause of ATN in humans. In addition, also other rodent study settings do not match the clinical setting [111]. Young, inbred rodents of the same sex do not mimic the genetically heterogenous, gender-mixed elderly population frequently affected by ATN. In addition, rodents cannot be dialyzed to survive severe AKI like humans. Hence, sublethal AKI episodes in rodents hardly mimic clinically relevant severe ATN episodes.
Human AKI often represents underlying CKD
In epidemiological studies, AKI was reported as a disease of the elderly [112], possibly an artifact due to the SCr criterion of the current AKI definition. Injury to 50% of nephrons will not increase SCr level in patients with a normal baseline GFR, while minor nephron injuries would increase SCr level in patients with impaired baseline GFR. Therefore, the clinical diagnosis of AKI is largely confounded by underlying CKD, which is ignored by animal experimentation, clinical trial design, and target selection. In addition, ‘CKD upon AKI,’ which finally includes the concept of AKI-related nephron loss, largely depends on baseline GFR, underlying CKD, and age [101].
Nephron number
The current functional definition of AKI integrates too many variables especially renal reserve as well as excess nephrons and hypertrophy. Demonstrating the effects of anti-inflammatory drugs would require preventing immunopathology-related nephron loss; however, no biomarker for nephron number is available at present [107]. In addition, the best time point for evaluation of AKI-related nephron loss after AKI is unclear.
Summary
The immune system is involved in all phases of ATN. During homeostasis, resident yolk sac-derived mono-nuclear phagocytes contribute to immune tolerance and turnover of interstitial matrix. Upon injury, these cells employ TLRs, inflammasomes, and other PRRs to translate the presence of DAMPs released from necrotic parenchymal cells into a release of extracellular cytokines and chemokines. Neutrophils are the first blood-derived immune cells to enter the site of injury and often undergo NET formation, a process setting off the crescendo of renal necroinflammation. In the late injury phase, the recruitment of CCR2+ M1 macrophages further enhances necroinflammation and renal dysfunction. In the recovery phase, M2-macrophages and the CSF-1-driven proliferation of resident myeloid cells counteract the inflammatory process by phagocytic clearance of dead pa-renchymal cells and NET-related chromatin and proteins. These cells secrete numerous immunoregulatory mediators that promote the resolution of necroinflammation unless repetitive episodes or persistent kidney injury prolong the injury phase. Healing processes include re-epithelialization, angiogenesis, and a transient fibrotic response. Over time, resident and infiltrating immune cells can reconstitute homeostasis once nephron regeneration is completed. Defect healing upon irreversible nephron loss implies persistent scar formation to which these cells also contribute by secreting collagen I and profibrotic mediators. Hence, the ultimate outcome after an ATN episode depends on the extent of irreversible nephron loss during the injury phase. The capacity of remnant nephrons to undergo compensatory hypertrophy supports functional recovery of renal function. Conversely, GFR recovery frequently leads to underestimation of true nephron loss during an ATN episode.
Acknowledgments
Hans-Joachim Anders is supported by the Deutsche Forschungsgemeinschaft (AN372/14-3, 16-2, 17-1, 23-1, 24-1) and the Bundesministerium für Bildung und Forschung (BMBF, 31L0071). Zhibo Zhao received support from the Chinese Scholarship Council.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Mulay SR, Holderied A, Kumar SV, Anders HJ. Targeting inflammation in so-called acute kidney injury. Semin Nephrol. 2016;36:17–30. doi: 10.1016/j.semnephrol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol. 2014;10:625–643. doi: 10.1038/nrneph.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol. 2017;79:449–469. doi: 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- 6.Soos TJ, Sims TN, Barisoni L, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 7.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun. 2012;4:463–477. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanmarco LM, Eberhardt N, Ponce NE, Cano RC, Bonacci G, Aoki MP. New insights into the immunobiology of mononuclear phagocytic cells and their relevance to the pathogenesis of cardiovascular diseases. Front Immunol. 2018;8:1921. doi: 10.3389/fimmu.2017.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 11.Okusa MD, Chertow GM Acute Kidney Injury Advisory Group of the American Society of Nephrology. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4:520–522. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaipl US, Munoz LE, Grossmayer G, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Linkermann A, Kunzendorf U, Krautwald S. Phosphorylated MLKL causes plasma membrane rupture. Mol Cell Oncol. 2014;1:e29915. doi: 10.4161/mco.29915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 16.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devalaraja-Narashimha K, Padanilam BJ. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol. 2009;20:95–103. doi: 10.1681/ASN.2008030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai J, Kumar SV, Mulay SR, et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol. 2016;46:223–229. doi: 10.1002/eji.201545605. [DOI] [PubMed] [Google Scholar]
- 19.Linkermann A, Bräsen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Ma H, Shao J, et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015;26:2647–2658. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linkermann A, Heller JO, Prókai A, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Homsi E, Andreazzi DD, Faria JB, Janino P. TNF-α-mediated cardiorenal injury after rhabdomyolysis in rats. Am J Physiol Renal Physiol. 2015;308:F1259–F1267. doi: 10.1152/ajprenal.00311.2014. [DOI] [PubMed] [Google Scholar]
- 23.Mulay SR, Desai J, Kumar SV, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1834–R1840. doi: 10.1152/ajpregu.2000.279.5.R1834. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F387–F398. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 27.Sanz AB, Sanchez-Niño MD, Izquierdo MC, et al. Macrophages and recently identified forms of cell death. Int Rev Immunol. 2014;33:9–22. doi: 10.3109/08830185.2013.771183. [DOI] [PubMed] [Google Scholar]
- 28.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo K, Kurosawa M, Kamiya M, et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med. 2018;24:232–238. doi: 10.1038/nm.4462. [DOI] [PubMed] [Google Scholar]
- 30.Vallés PG, Lorenzo AG, Bocanegra V, Vallés R. Acute kidney injury: what part do toll-like receptors play? Int J Nephrol Renovasc Dis. 2014;7:241–251. doi: 10.2147/IJNRD.S37891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage-associated molecular patterns (DAMPs) Am J Transplant. 2016;16:3338–3361. doi: 10.1111/ajt.13963. [DOI] [PubMed] [Google Scholar]
- 32.Hato T, El-Achkar TM, Dagher PC. Sisters in arms: myeloid and tubular epithelial cells shape renal innate immunity. Am J Physiol Renal Physiol. 2013;304:F1243–F1251. doi: 10.1152/ajprenal.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulay SR, Linkermann A, Anders HJ. Necroinflammation in kidney disease. J Am Soc Nephrol. 2016;27:27–39. doi: 10.1681/ASN.2015040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulay SR, Kumar SV, Lech M, Desai J, Anders HJ. How kidney cell death induces renal necroinflammation. Semin Nephrol. 2016;36:162–173. doi: 10.1016/j.semnephrol.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keshari RS, Jyoti A, Dubey M, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7:e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Darisipudi MN, Thomasova D, Mulay SR, et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol. 2012;23:1783–1789. doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulay SR, Thomasova D, Ryu M, Anders HJ. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012;81:1199–1211. doi: 10.1038/ki.2011.482. [DOI] [PubMed] [Google Scholar]
- 41.Nakazawa D, Kumar SV, Marschner J, et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol. 2017;28:1753–1768. doi: 10.1681/ASN.2016080925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allam R, Darisipudi MN, Tschopp J, Anders HJ. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol. 2013;43:3336–3342. doi: 10.1002/eji.201243224. [DOI] [PubMed] [Google Scholar]
- 43.Okusa MD, Li L. Dendritic cells in acute kidney injury: cues from the microenvironment. Trans Am Clin Climatol Assoc. 2012;123:54–62. discussion 62–63. [PMC free article] [PubMed] [Google Scholar]
- 44.Weller S, Varrier M, Ostermann M. Lymphocyte function in human acute kidney injury. Nephron. 2017;137:287–293. doi: 10.1159/000478538. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZX, Wang S, Huang X, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008;181:7489–7498. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 46.Kelly KJ, Williams WW, Jr, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolisetty S, Agarwal A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int. 2009;75:674–676. doi: 10.1038/ki.2008.689. [DOI] [PubMed] [Google Scholar]
- 48.Ysebaert DK, De Greef KE, Vercauteren SR, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 51.Swaminathan S, Griffin MD. First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int. 2008;74:1509–1511. doi: 10.1038/ki.2008.555. [DOI] [PubMed] [Google Scholar]
- 52.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/S0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 54.Günthner R, Anders HJ. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 58.Leibovich SJ, Chen JF, Pinhal-Enfield G, et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosín-Roger J, Ortiz-Masiá D, Calatayud S, et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One. 2013;8:e78128. doi: 10.1371/journal.pone.0078128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinsey GR, Sharma R, Huang L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders HJ. Immune system modulation of kidney regeneration: mechanisms and implications. Nat Rev Nephrol. 2014;10:347–358. doi: 10.1038/nrneph.2014.68. [DOI] [PubMed] [Google Scholar]
- 63.Kulkarni OP, Hartter I, Mulay SR, et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol. 2014;25:978–989. doi: 10.1681/ASN.2013050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 65.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol. 2010;22:717–728. doi: 10.1093/intimm/dxq058. [DOI] [PubMed] [Google Scholar]
- 67.Lech M, Susanti HE, Römmele C, Gröbmayr R, Günthner R, Anders HJ. Quantitative expression of C-type lectin receptors in humans and mice. Int J Mol Sci. 2012;13:10113–10131. doi: 10.3390/ijms130810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 69.Xu MJ, Feng D, Wang H, Guan Y, Yan X, Gao B. IL-22 ameliorates renal ischemia-reperfusion injury by targeting proximal tubule epithelium. J Am Soc Nephrol. 2014;25:967–977. doi: 10.1681/ASN.2013060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng D, Kong X, Weng H, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–198.e7. doi: 10.1053/j.gastro.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 72.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Tachiiri A, Imamura R, Wang Y, Fukui M, Umemura M, Suda T. Genomic structure and inducible expression of the IL-22 receptor alpha chain in mice. Genes Immun. 2003;4:153–159. doi: 10.1038/sj.gene.6363934. [DOI] [PubMed] [Google Scholar]
- 74.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 75.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 18322013:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 77.Savill J, Gregory C, Haslett C. Cell biology. Eat me or die. Science. 2003;302:1516–1517. doi: 10.1126/science.1092533. [DOI] [PubMed] [Google Scholar]
- 78.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nauta AJ, Castellano G, Xu W, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173:3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 80.Lech M, Rommele C, Anders HJ. Pentraxins in nephrology: C-reactive protein, serum amyloid P and pentrax-in-3. Nephrol Dial Transplant. 2013;28:803–811. doi: 10.1093/ndt/gfs448. [DOI] [PubMed] [Google Scholar]
- 81.Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 82.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rae F, Woods K, Sasmono T, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308:232–246. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Alikhan MA, Jones CV, Williams TM, et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. 2011;179:1243–1256. doi: 10.1016/j.ajpath.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazzeri E, Angelotti ML, Peired A, et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun. 2018;9:1344. doi: 10.1038/s41467-018-03753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sallustio F, Costantino V, Cox SN, et al. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-driven inhibin-A and microvesicle-shuttled decorin. Kidney Int. 2013;83:392–403. doi: 10.1038/ki.2012.413. [DOI] [PubMed] [Google Scholar]
- 87.Wiggins JE, Goyal M, Sanden SK, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 88.Mulay SR, Thomasova D, Ryu M, et al. Podocyte loss involves MDM2-driven mitotic catastrophe. J Pathol. 2013;230:322–335. doi: 10.1002/path.4193. [DOI] [PubMed] [Google Scholar]
- 89.Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 91.Price PM, Yu F, Kaldis P, et al. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol. 2006;17:2434–2442. doi: 10.1681/ASN.2006020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodeify R, Megyesi J, Tarcsafalvi A, Safirstein RL, Price PM. Protection of cisplatin cytotoxicity by an inactive cyclin-dependent kinase. Am J Physiol Renal Physiol. 2010;299:F112–F120. doi: 10.1152/ajprenal.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120:2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiRocco DP, Bisi J, Roberts P, et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2014;306:F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 97.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 100.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens. 2012;21:587–592. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JU, Kim M, Kim S, et al. Dendritic cell dysfunction in patients with end-stage renal disease. Immune Netw. 2017;17:152–162. doi: 10.4110/in.2017.17.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen G, Hörl WH. Immune dysfunction in uremia—an update. Toxins (Basel) 2012;4:962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossaint J, Oehmichen J, Van Aken H, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126:962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Endre ZH, Pickering JW. Acute kidney injury: cell cycle arrest biomarkers win race for AKI diagnosis. Nat Rev Nephrol. 2014;10:683–685. doi: 10.1038/nrneph.2014.198. [DOI] [PubMed] [Google Scholar]
- 107.Endre ZH, Pickering JW. Biomarkers and creatinine in AKI: the trough of disillusionment or the slope of enlightenment? Kidney Int. 2013;84:644–647. doi: 10.1038/ki.2013.168. [DOI] [PubMed] [Google Scholar]
- 108.Heyman SN, Rosen S, Rosenberger C. Animal models of renal dysfunction: acute kidney injury. Expert Opin Drug Discov. 2009;4:629–641. doi: 10.1517/17460440902946389. [DOI] [PubMed] [Google Scholar]
- 109.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 110.Heyman SN, Rosenberger C, Rosen S. Acute kidney injury: lessons from experimental models. Contrib Nephrol. 2011;169:286–296. doi: 10.1159/000313957. [DOI] [PubMed] [Google Scholar]
- 111.Ortiz A, Sanchez-Niño MD, Izquierdo MC, et al. Translational value of animal models of kidney failure. Eur J Pharmacol. 2015;759:205–220. doi: 10.1016/j.ejphar.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 112.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]