Abstract
Background
Phospholipase A2 receptor (PLA2R) has been identified as a major autoantigen in primary membranous nephropathy (MN). We evaluated the association between anti-PLA2R antibodies and clinical outcome in Korean patients with primary MN.
Methods
A total of 66 patients with biopsy-proven MN were included. Serum level of anti-PLA2R antibodies was measured by enzyme-linked immunosorbent assay. Biochemical parameters were estimated initially and at follow-up.
Results
Anti-PLA2R antibodies were detected in 52.1% and 27.8% of patients with primary and secondary MN, respectively. Forty-eight patients with primary MN were grouped based on presence or absence of anti-PLA2R antibodies. Proteinuria was more severe in anti-PLA2R-positive patients than in anti-PLA2R-negative patients (urine protein/creatinine ratio 7.922 ± 3.985 g/g vs. 4.318 ± 3.304 g/g, P = 0.001), and anti-PLA2R antibody level was positively correlated with proteinuria. The incidence of chronic kidney disease stage ≥ 3 was higher in anti-PLA2R-positive patients compared with anti-PLA2R-negative patients (P = 0.004). The probabilities of spontaneous remission were higher in anti-PLA2R-negative patients compared with anti-PLA2R-positive patients (P < 0.001). Multivariate analysis demonstrated that anti-PLA2R antibodies are an independent risk factor for developing chronic kidney disease stage ≥ 3 and for not reaching spontaneous remission.
Conclusion
Detection of anti-PLA2R antibodies at diagnosis in patients with primary MN can predict prognosis and guide treatment decisions.
Keywords: Membranous nephropathy, Phospholipase A2 receptors, Prognosis
Introduction
Membranous nephropathy (MN) is a major cause of nephrotic syndrome in adults [1]. Approximately 20% of MN cases are associated with systemic conditions such as malignancy, infections, or autoimmune disease, while about 80% have no identifiable cause and are therefore classified as primary MN, an organ-specific autoimmune disease [2,3]. Diagnosis of primary MN is based on pathologic findings characterized by thickened capillary walls of glomeruli due to subepithelial immune deposits [4]. These immune deposits cause damage to the glomerular capillary wall, resulting in proteinuria, a major clinical manifestation of MN [5]. Proteinuria is generally used as a treatment standard in patients with primary MN, but is insufficient to predict the immunological activity of the disease and prognosis [4,5].
M-type phospholipase A2 receptor (PLA2R), a trans-membrane receptor that presents on human glomerular podocytes, was identified as the first autoantigen in primary MN, and circulating autoantibodies against PLA2R were demonstrated in about 70% of primary MN patients [6]. Genome-wide association studies in patients with primary MN showed an association between genetic variants of PLA2R1 and primary MN [7]. Assays to measure anti-PLA2R antibodies are available, and multiple cohort studies have been performed to determine the clinical relevance of anti-PLA2R antibodies in patients with primary MN [8–13]. Many investigations have suggested that anti-PLA2R antibodies are associated with disease activity and remission [14–18], but conflicting results have also been reported [19–22]. Additionally, few studies regarding the association between anti-PLA2R antibodies and outcome have been conducted in Korean patients with primary MN.
Thus, in this study, we measured anti-PLA2R antibodies using enzyme-linked immunosorbent assay (ELISA) in patients with primary MN and explored the association between anti-PLA2R antibodies and clinical outcome. We also evaluated the prognostic value of anti-PLA2R antibodies in Korean patients with primary MN.
Methods
Patients
Among consecutive patients who underwent renal biopsy and were diagnosed with MN between July 2005 and May 2012 in Kyungpook National University Hospital, 66 were included in the study, for whom a stored serum sample obtained at the time of renal biopsy was available. MN was considered as primary when no secondary cause was identified based on clinical and laboratory criteria. We reviewed each patient’s medical records retrospectively from the date of renal biopsy to July 31, 2017.
Data collection and outcome definitions
Demographic and clinical data obtained at time of renal biopsy were as follows: age, gender, comorbidities, secondary cause of MN, and results of laboratory tests. In all patients, baseline data on serum creatinine and proteinuria could be obtained at the time of renal biopsy, and follow-up data and medical history of treatment were available in all patients except two with secondary MN who were transferred to another medical center after diagnosis. Common biochemical parameters such as creatinine, serum lipids, total protein, albumin, and spot urine protein to creatinine ratio (UPCR) were measured by routine clinical laboratory tests. Estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease (MDRD) study formula [23]. Clinical outcomes were assessed by evolution to chronic kidney disease (CKD) and remission of proteinuria. CKD stage 3 was defined as eGFR < 60mL/min/1.73 m2, and CKD stage 5 was defined as eGFR < 15 mL/min/1.73 m2 and need for dialysis. Based on the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines, partial remission was defined as UPCR < 3.5g/g with at least a 50% reduction from baseline and stable renal function, and complete remission was defined as UPCR < 0.3 g/g [24]. Remission that occurred without immunosuppressive therapy during follow-up was classified as spontaneous remission.
Detection of anti-PLA2R antibodies
Serum samples collected from each patient at the time of renal biopsy were stored at −80°C and thawed simultaneously for the measurement of anti-PLA2R antibodies. Serum level of anti-PLA2R antibodies was determined using commercially available ELISA kits (EUROIMMUN AG, Lubeck, Germany). Briefly, PLA2R-coated micro-plates were incubated with human sera diluted 1:101 in sample buffer for 30 minutes and were visualized after incubation with anti-human-IgG HRP conjugate for 30 minutes. The optical density was measured at 450 nm using a microplate absorbance reader (Model 550; Bio-Rad, Hercules, CA, USA). In accordance with the manufacturer’s guidelines, values ≥ 20 RU/mL were considered positive.
Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range), and percentage as appropriate. Student’s t test was used to evaluate differences in quantitative variables, and Pearson’s chi-square test was used for categorical variables. The relationship between anti-PLA2R antibody level and proteinuria was analyzed using Pearson’s correlation coefficient and multivariate logistic regression analysis. Cumulative probabilities of CKD and remission were estimated according to the Kaplan–Meier method and log-rank test. Multivariate Cox regression was performed to adjust for clinical parameters that might influence the outcome. All statistical analyses were performed using PASW Statistics version 18.0 (IBM Co., Armonk, NY, USA), and P < 0.05 was considered statistically significant.
Results
Prevalence of anti-PLA2R antibodies in MN
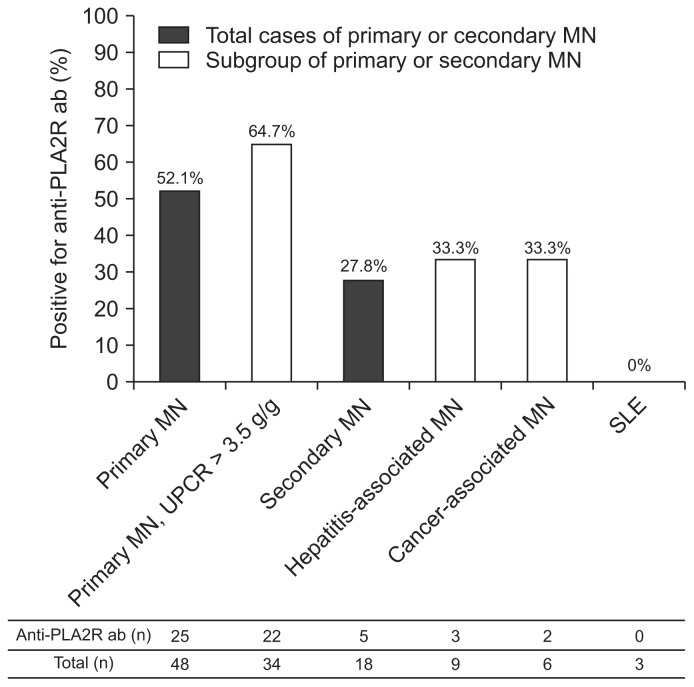
Sixty-six patients were included in analysis. According to clinical and renal pathological features, 48 patients were diagnosed with primary MN, and 18 patients were diagnosed with secondary MN having associated causes of hepatitis B (n = 8), hepatitis C (n = 1), malignancy (n = 6), and systemic lupus erythematosus (n = 3). Anti-PLA2R antibodies were detected in 52.1% (25 of 48) of patients with primary MN and 27.8% (5 of 18) of patients with secondary MN. Anti-PLA2R antibodies were present in 64.7% (22 of 34) of patients with primary MN having nephrotic-range proteinuria (Fig. 1). The prevalence of anti-PLA2R antibodies in primary MN was higher than that in secondary MN, but the difference was not statistically significant.
Figure 1. Prevalence of anti-PLA2R ab in patients with membranous nephropathy (MN).
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; SLE, systemic lupus erythematosus; UPCR, urine protein/creatinine ratio.
Associations between anti-PLA2R antibodies, renal function, and proteinuria in primary MN
Baseline characteristics between anti-PLA2R-positive and -negative patients are presented in Table 1. The mean age of anti-PLA2R-positive and -negative patients was 56.6 ± 12.7 years and 54.0 ± 14.0 years, respectively. Gender ratio and prevalence of diabetes and hypertension were similar between the two groups. Renal function at the time of diagnosis did not differ between the two groups in terms of serum creatinine (0.78 ± 0.33 mg/dL vs. 0.85 ± 0.33 mg/dL) and eGFR (110.3 ± 50.3 mL/min/1.73 m2 vs. 95.5 ± 31.2 mL/min/1.73 m2). Proteinuria was more severe in the anti-PLA2R-positive group than in the anti-PLA2R-negative group (UPCR 7.922 ± 3.985 g/g vs. 4.318 ± 3.304 g/g, P = 0.001). Furthermore, the number of patients with proteinuria > 3.5 g/g was higher in the anti-PLA2R-positive group than in the anti-PLA2R-negative group (88% vs. 52.2%, P = 0.006). Serum albumin level was significantly lower in the anti-PLA2R-positive group (2.5 ± 0.6 g/dL vs. 3.3 ± 0.7 g/dL, P < 0.001), and serum total cholesterol level was higher in the anti-PLA2R-positive group, but there was no statistical significance (304.0 ± 68.9 mg/dL vs. 259.9 ± 91.5 mg/dL, P = 0.064).
Table 1.
Baseline characteristics of patients with primary membranous nephropathy according to the presence of serum anti-PLA2R ab
| Variable | Anti-PLA2R ab(+) | Anti-PLA2R ab(−) | P value |
|---|---|---|---|
| Patient (n) | 25 | 23 | |
| Age (yr) | 56.6 ± 12.7 | 54.0 ± 14.0 | 0.503 |
| Sex, male:female | 15:10 | 14:9 | 0.951 |
| Diabetes mellitus | 3 (12.0) | 4 (17.4) | 0.597 |
| Hypertension | 12 (48.0) | 9 (39.1) | 0.536 |
| Serum creatinine (mg/dL) | 0.78 ± 0.33 | 0.85 ± 0.33 | 0.469 |
| eGFR (mL/min/1.73 m2) | 110.3 ± 50.3 | 95.5 ± 31.2 | 0.230 |
| UPCR (g/g) | 7.922 ± 3.985 | 4.318 ± 3.304 | 0.001 |
| UPCR > 3.5 g/g | 22 (88.0) | 12 (52.2) | 0.006 |
| Serum albumin (g/dL) | 2.5 ± 0.6 | 3.3 ± 0.7 | < 0.001 |
| Serum total cholesterol (mg/dL) | 304.0 ± 68.9 | 259.9 ± 91.5 | 0.064 |
| Anti-PLA2R ab titer (RU/mL) | 219.59 ± 154.59 | 1.74 ± 4.00 | < 0.001 |
Data are presented as number only, mean ± standard deviation, or number (%). Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; eGFR, estimated glomerular filtration rate; UPCR, urine protein/creatinine ratio.
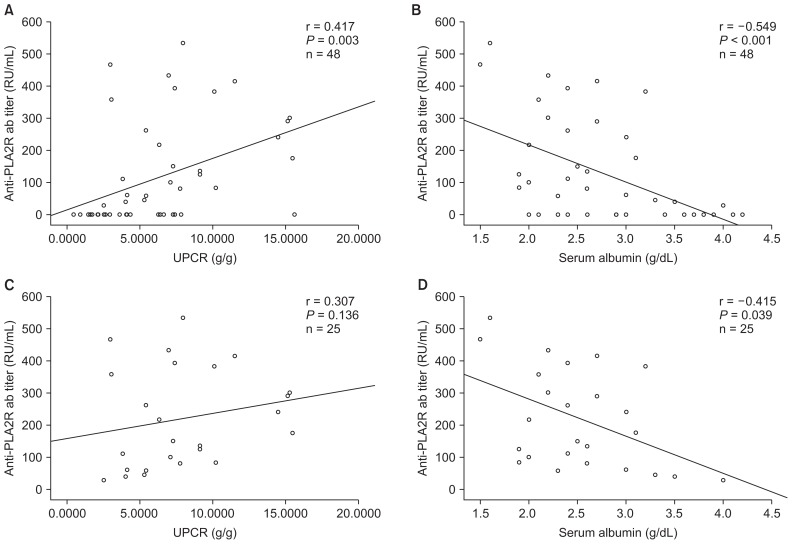
Additionally, we assessed the correlations between anti-PLA2R antibody level and baseline laboratory parameters such as proteinuria, eGFR, and serum albumin. Anti-PLA2R antibody level was positively correlated with UPCR (n = 48; r = 0.417, P = 0.003), but negatively correlated with serum albumin (n = 48; r = −0.549, P < 0.001) (Fig. 2). Multivariate logistic regression analysis showed that, after adjustment for age, sex, diabetes, hypertension, and eGFR, anti-PLA2R antibody level was significantly correlated with proteinuria (odds ratio, 1.006; 95% confidence interval [CI], 1.001 to 1.010; P = 0.023) (Table 2). In subgroup analysis obtained from anti-PLA2R-positive patients, a similar relationship was observed between anti-PLA2R antibody level and serum albumin (n = 25; r = −0.415, P = 0.039), but there was no statistically significant difference between UPCR and anti-PLA2R level.
Figure 2. Correlation analysis between anti-PLA2R ab level and proteinuria and serum albumin level of patients with primary membranous nephropathy (A and B) and of anti-PLA2R-positive patients (C and D).
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; UPCR, urine protein/creatinine ratio.
Table 2.
Multivariate logistic regression analysis of clinical parameters associated with proteinuria
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age (yr) | 1.005 (0.947–1.065) | 0.816 |
| Sex, male | 1.295 (0.316–5.297) | 0.719 |
| Diabetes mellitus | 0.235 (0.026–2.114) | 0.196 |
| Hypertension | 3.730 (0.732–19.020) | 0.113 |
| eGFR (mL/min/1.73 m2) | 1.014 (0.994–1.034) | 0.177 |
| Anti-PLA2R ab titer (RU/mL) | 1.006 (1.001–1.010) | 0.023 |
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
Associations between anti-PLA2R antibody level and clinical outcomes in primary MN
To assess clinical outcomes, we evaluated the prognosis of the disease status including progression of renal function and remission rate of proteinuria and checked whether immunosuppressive agents were prescribed in anti-PLA2R-positive and -negative patients. The median follow-up period was 65 months (range, 3 to 133 months). A similar proportion of patients in the two groups had been treated with renin-angiotensin system blockers (Table 2). All anti-PLA2R-positive patients had received a statin. Statin use was significantly more common in the anti-PLA2R-positive group than in the anti-PLA2R-negative group (100% vs. 73.9%, P = 0.006). During follow-up, 19 (76.0%) anti-PLA2R-positive patients received immunosuppressive treatment, which was remarkably higher than the percentage of anti-PLA2R-negative patients that received such treatment (76.0% vs. 17.4%, P < 0.001). Steroids were used in all patients who received immunosuppressive therapy. Fourteen (56.0%) anti-PLA2R-positive patients but only four (17.4%) anti-PLA2R-negative patients had received cyclosporin A; this difference was significant (P = 0.006). The five patients who received mycophenolate mofetil and two patients who received tacrolimus were anti-PLA2R-positive.
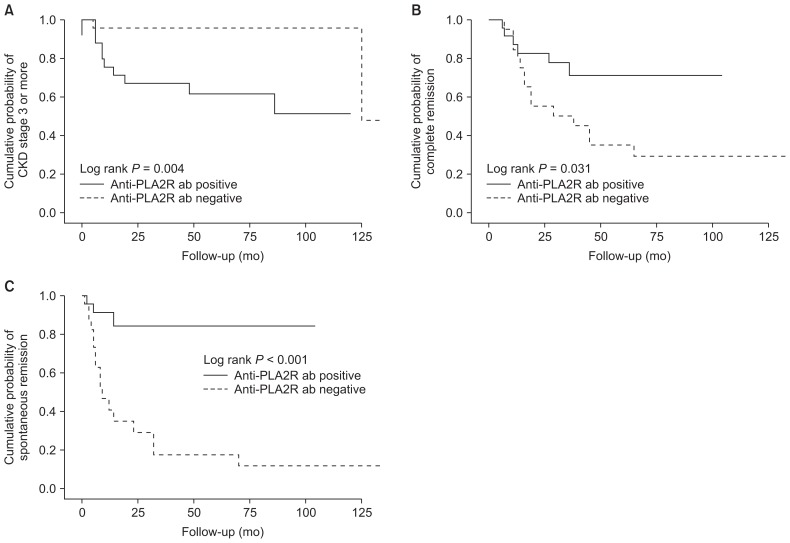
As shown in Table 3, during follow-up, 10 (40.0%) anti-PLA2R-positive patients and two (8.7%) anti-PLA2R-negative patients developed CKD stage 3 or higher (40.0% vs. 8.7%, P = 0.012). Using the Kaplan–Meier method and log-rank test, the cumulative incidence of CKD stage ≥ 3 was significantly higher in anti-PLA2R-positive patients than in anti-PLA2R-negative patients (P = 0.004) (Fig. 3). Three (12.0%) anti-PLA2R-positive patients progressed to CKD stage 5 and underwent dialysis, while one (4.3%) anti-PLA2R-negative patient progressed to renal failure. Remission rates of proteinuria were significantly higher in anti-PLA2R-negative patients than in anti-PLA2R-positive patients (91.3% vs. 60.0%, P = 0.012). Complete remission occurred in anti-PLA2R-negative patients significantly more frequently compared with their positive counterparts (60.9% vs. 24.0%, P = 0.010). Seventeen (73.9%) anti-PLA2R-negative patients and three (12.0%) anti-PLA2R-positive patients achieved remission without immunosuppressive treatment, and spontaneous remission rates were significantly higher in anti-PLA2R-negative patients (P < 0.001) compared with anti-PLA2R-positive patients (Table 4). The cumulative incidence of complete remission and spontaneous remission using the Kaplan–Meier method and log-rank test was significantly higher in anti-PLA2R-negative patients compared with anti-PLA2R-positive patients (P = 0.031 and < 0.001, respectively). To exclude influence of other baseline parameters, we performed multivariate Cox regression analysis (Table 5). Adjusted for age, male sex, diabetes, hypertension, baseline proteinuria, eGFR, and serum albumin, multivariate analysis revealed that anti-PLA2R antibodies are an independent risk factor for developing CKD stage 3 or more (hazard ratio [HR], 13.926; 95% CI, 1.385 to 140.034; P = 0.025) and for not achieving spontaneous remission (HR, 0.135; 95% CI, 0.026 to 0.689; P = 0.016).
Table 3.
Treatment modalities in patients with primary membranous nephropathy according to presence of anti-PLA2R ab
| Variable | Anti-PLA2R ab(+) | Anti-PLA2R ab(−) | P value |
|---|---|---|---|
| Patient (n) | 25 | 23 | |
| RAS blockers | 23 (92.0) | 22 (95.7) | 0.602 |
| Statin | 25 (100) | 17 (73.9) | 0.006 |
| Immunosuppression | 19 (76.0) | 4 (17.4) | < 0.001 |
| Steroid | 19 (76.0) | 4 (17.4) | < 0.001 |
| Cyclosporin | 14 (56.0) | 4 (17.4) | 0.006 |
| Mycophenolate mofetil | 5 (20.0) | 0 (0) | 0.023 |
| Tacrolimus | 2 (8.0) | 0 (0) | 0.166 |
Data are presented as number only or number (%).
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; RAS, renin-angiotensin system.
Figure 3. Kaplan–Meier analysis of anti-PLA2R ab level and cumulative probability of chronic kidney disease (CKD) stage 3 or more (A), complete remission (B), and spontaneous remission (C).
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody.
Table 4.
Clinical outcomes in patients with primary membranous nephropathy according to presence of anti-PLA2R ab
| Variable | Anti-PLA2R(+) | Anti-PLA2R(−) | P value |
|---|---|---|---|
| Number of patients | 25 | 23 | |
| CKD stage 3 or more | 10 (40.0) | 2 (8.7) | 0.012 |
| CKD stage 5 | 3 (12.0) | 1 (4.3) | 0.338 |
| Remission | 15 (60.0) | 21 (91.3) | 0.012 |
| Partial remission | 9 (36.0) | 7 (30.4) | 0.683 |
| Complete remission | 6 (24.0) | 14 (60.9) | 0.010 |
| Spontaneous remission | 3 (12.0) | 17 (73.9) | < 0.001 |
Data are presented as number only or number (%).
CKD stage 3, defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; CKD stage 5, defined as eGFR < 15 mL/min/1.73 m2 and the need for dialysis; partial remission, defined as proteinuria < 3.5 g/g with a decreased of 50% from baseline; complete remission, defined as proteinuria < 0.3 g/g; spontaneous remission, partial or complete remission without immunosuppressant.
Anti-PLA2R ab, anti-phospholipase A2 receptor antibody; CKD, chronic kidney disease.
Table 5.
Multivariate cox regression analysis of CKD stage 3 or higher and spontaneous remission
| Variable | CKD stage 3 or higher | Spontaneous remission | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.084 (0.987–1.191) | 0.091 | 1.069 (1.009–1.133) | 0.024 |
| Sex, male | 0.493 (0.065–3.752) | 0.494 | 0.470 (0.166–1.333) | 0.156 |
| Diabetes mellitus | 1.542 (0.203–11.709) | 0.676 | 3.217 (0.519–19.956) | 0.210 |
| Hypertension | 1.074 (0.145–7.951) | 0.944 | 0.202 (0.043–0.956) | 0.044 |
| eGFR | 0.975 (0.945–1.006) | 0.110 | 0.998 (0.979–1.018) | 0.844 |
| UPCR | 1.108 (0.914–1.344) | 0.298 | 1.054 (0.807–1.376) | 0.699 |
| Serum albumin | 0.412 (0.062–2.719) | 0.357 | 1.656 (0.488–5.617) | 0.418 |
| Anti-PLA2R ab(+) | 13.926 (1.385–140.034) | 0.025 | 0.135 (0.026–0.689) | 0.016 |
CKD stage 3, defined as eGFR < 60 mL/min/1.73 m2; spontaneous remission, partial or complete remission without immunosuppressant.
Anti-PLA2R ab(+), anti-phospholipase A2 receptor antibody; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; UPCR, urine protein/creatinine ratio.
Discussion
In this study, anti-PLA2R antibodies were detected in 52.1% of all patients with primary MN as measured by ELISA. The prevalence rose to 65% in patients with primary MN and nephrotic-range proteinuria. Prevalence of anti-PLA2R antibodies in primary MN has been reported as 44% to 89% in previous studies and tended to be higher in studies that enrolled patients with nephrotic-range proteinuria than in studies that included non-nephrotic-range proteinuria [6,8–18,20–22,25]. Several studies showed different positivity rates in patients with nephrotic-range proteinuria compared with those of the entire cohort; therefore, the prevalence of antibodies was influenced by the proportion of patients with active disease [10,11,14]. In two previous studies on Korean patients with primary MN, the prevalence of anti-PLA2R antibodies was reported to be 69% as measured by western blotting and 44.1% as measured by ELISA [10,16]. In these two studies, some patients who were tested for PLA2R-Ab using both ELISA and western blotting showed that the ELISA test was less sensitive than western blotting [16]. Even though serum samples for measurement were collected at the time of renal biopsy in the absence of the effect of immunosuppressive treatment in all these studies and the current study, differences in disease activity in the patients from each study and the different assays used for measurement might influence the observed prevalence. Anti-PLA2R antibodies were also detected in 27.8% of patients with secondary MN in this study, whereas Beck et al [6] reported that anti-PLA2R was not present in secondary MN. Several studies reported that anti-PLA2R antibodies were positive in 10–30% of secondary MN cases [9,12,18,22], and one of them suggested the possibility of coincidental occurrence of primary MN and systemic disease [9]. Further studies are needed to discover if the prevalence of anti-PLA2R antibodies is affected by ethnicity or other unknown factors. In a study by Yeo et al [26], histologic detection of PLA2R showed a sensitivity of 83% and a specificity of 88% in identifying primary MN, and a meta-analysis including about 600 patients with MN showed similar results. Detection of PLA2R in glomeruli and anti-PLA2R antibodies in serum is expected to be more sensitive and specific in discriminating between primary and secondary MN.
Our data showed a significant difference in the severity of proteinuria, hypoalbuminemia, and hypercholesterolemia according to anti-PLA2R antibody status. Compared with anti-PLA2R-positive patients, anti-PLA2R-positive patients had significantly higher level of proteinuria and lower level of serum albumin, which generally indicates disease activity in primary MN, in agreement with previous studies [8,10,12,13]. Baseline renal function did not significantly differ between the two groups, suggesting that renal biopsy and diagnosis occurred before the development of renal function impairment in most patients. Notably, few patients showed renal impairment at the time of biopsy in this study. The titer of anti-PLA2R antibodies as determined by ELISA significantly correlated with proteinuria and hypoalbuminemia, although it was not consistent in the subgroup of anti-PLA2R-positive patients, which showed weak statistical significance because of the smaller number of patients. Consistent with these results, several previous studies showed a significant correlation between the level of anti-PLA2R antibodies and the amount of proteinuria [8,9,12,14–17,20]. This correlation between proteinuria and anti-PLA2R antibodies may occur because a higher level of circulating anti-PLA2R antibodies may lead to increased binding to PLA2R on podocytes; subsequently, more severe damage of the filtration barrier induces heavy proteinuria.
Regarding the clinical outcomes, the natural course of primary MN varies. Approximately one third of patients have spontaneous remission, whereas another third progress to end-stage renal disease (ESRD) [27,28]. Therefore, selection of patients with high risk for progression to ESRD, who are required to receive immunosuppressive therapy, is crucial when treating primary MN. So far, implementation of immunosuppressive therapy has been based on follow-up measurements of proteinuria and renal function, but recent studies have suggested measuring anti-PLA2R antibodies to guide decision making in primary MN [16–18]. Similarly, in this study, we found a significant difference in the clinical outcome according to anti-PLA2R reactivity. First, immunosuppressive treatment was required more frequently in anti-PLA2R-positive patients than in anti-PLA2R-negative patients during follow-up. Generally, immunosuppressive treatment is recommended when active proteinuria persists despite anti-proteinuric therapy [24]. Anti-PLA2R-positive patients showed more severe proteinuria than anti-PLA2R-negative patients at the time of diagnosis, and this was linked to higher rate of immunosuppressive treatment. This suggests a higher rate of persistently active proteinuria in anti-PLA2R-positive patients during follow-up.
Second, we found that the risk of developing CKD stage 3 or more was significantly higher in anti-PLA2R-positive patients than in anti-PLA2R-negative patients, and progression to CKD stage ≥ 3 was faster in the presence of anti-PLA2R antibodies. The influence of anti-PLA2R antibodies on progression to CKD appeared to be valid in multivariate analysis adjusted for baseline proteinuria and eGFR. The association between anti-PLA2R antibodies and deterioration of renal function has been assessed in several studies [14,17,20–22,25]. Kanigicherla et al [14] showed that the risk of doubling of serum creatinine increased significantly in patients with a high level of anti-PLA2R antibodies, while others did not show a significant difference in terms of developing various stages of CKD according to anti-PLA2R titer [17,20–22]. In this study, we found that anti-PLA2R antibodies were an independent risk factor of progression to CKD in patients with primary MN; however, we were unable to find a significant correlation with risk for ESRD or RRT.
Finally, we demonstrated that complete remission rates were significantly higher in anti-PLA2R-negative patients than in anti-PLA2R-positive patients. Furthermore, anti-PLA2R-negative patients entered remission without immunosuppressive treatment more frequently than anti-PLA2R-positive patients. Moreover, anti-PLA2R-negative patients achieved either complete remission or spontaneous remission significantly faster than anti-PLA2R-positive patients. Previous studies have shown similar results regarding the association between anti-PLA2R antibodies and spontaneous remission [16,17,21,22]. In this study, the spontaneous remission rate of anti-PLA2R-negative patients was 73.9% (17 out of 23), consistent with the 88% (7 out of 8) observed in a study by Timmermans et al [17]. The rate of immunosuppressive treatment in anti-PLA2R-negative patients was low, at 17.4%. In multivariate analysis, anti-PLA2R antibodies predicted lower expectations of spontaneous remission, whereas baseline proteinuria did not. This suggests that patients with primary MN who do not show anti-PLA2R antibodies at diagnosis are highly expected to enter remission spontaneously and thus could be recommended for prolonged conservative treatment instead of immediate immunosuppressive treatment.
In summary, we evaluated the relationship between anti-PLA2R antibodies and clinical outcome of patients with primary MN. We found that the level of anti-PLA2R antibodies was correlated with the severity of proteinuria. Compared with anti-PLA2R-negative patients with primary MN, anti-PLA2R-positive patients with primary MN received immunosuppressive treatment more frequently and showed a higher risk of progressing to CKD. On the contrary, anti-PLA2R-negative patients with primary MN achieved spontaneous remission faster and more frequently than anti-PLA2R-positive patients. Therefore, screening for anti-PLA2R antibodies at diagnosis in patients with primary MN can assist in prediction of prognosis and decisions about immunosuppressive treatment.
In conclusion, serum anti-PLA2R antibodies may be a valuable prognostic marker for guiding treatment decisions in patients with primary MN.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HC15C1129).
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287. [PubMed] [Google Scholar]
- 2.Glassock RJ. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56:157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Makker SP, Tramontano A. Idiopathic membranous nephropathy: an autoimmune disease. Semin Nephrol. 2011;31:333–340. doi: 10.1016/j.semnephrol.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324–332. doi: 10.1016/S0270-9295(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 5.Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 6.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanescu HC, Arcos-Burgos M, Medlar A, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 8.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A– receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin W, Beck LH, Jr, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One. 2013;8:e62151. doi: 10.1371/journal.pone.0062151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19:653–660. doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wei D, Zhou Z, et al. Anti-PLA2R antibodies in Chinese patients with membranous nephropathy. Med Sci Monit. 2016;22:1630–1636. doi: 10.12659/MSM.896090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang L, Zhang AM, Li HX, et al. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy: A cross-sectional study. Medicine (Baltimore) 2017;96:e7218. doi: 10.1097/MD.0000000000007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 15.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YG, Choi YW, Kim SY, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol. 2015;42:250–257. doi: 10.1159/000440983. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, Damoiseaux JG, van Paassen P. Limburg Renal Registry. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol. 2015;42:70–77. doi: 10.1159/000437236. [DOI] [PubMed] [Google Scholar]
- 18.Wei SY, Wang YX, Li JS, et al. Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol. 2016;43:129–140. doi: 10.1159/000445361. [DOI] [PubMed] [Google Scholar]
- 19.Hoxha E, Kneißler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 20.Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jullien P, Seitz Polski B, Maillard N, et al. Anti-phospho-lipase A2 receptor antibody levels at diagnosis predicts spontaneous remission of idiopathic membranous nephropathy. Clin Kidney J. 2017;10:209–214. doi: 10.1093/ckj/sfw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourcine F, Dahan K, Mihout F, et al. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: A single-centre study over 14 years. PLoS One. 2017;12:e0173201. doi: 10.1371/journal.pone.0173201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 24.Beck L, Bomback AS, Choi MJ, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–441. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One. 2014;9:e110681. doi: 10.1371/journal.pone.0110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo MK, Kim YH, Choi DE, Choi SY, Kim KH, Suh KS. The usefulness of phospholipase A2 receptor and IgG4 detection in differentiation primary membranous nephropathy from secondary membranous nephropathy in renal biopsy. Appl Immunohistochem Mol Morphol. 2017 doi: 10.1097/PAI.0000000000000460. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Schieppati A, Mosconi L, Perna A, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 28.Polanco N, Gutiérrez E, Covarsí A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]