Abstract
Background
Puromycin aminonucleoside (PAN) is a known podocytotoxin. PAN-induced nephrosis is a widely used animal model for studying human idiopathic nephrotic syndrome. Abnormal protein accumulation associated with podocyte-specific endoplasmic reticulum (ER) stress damages cells structurally and functionally, which in turn induces apoptosis and severe proteinuria. In the present study, we investigated the effect of PAN on ER stress and apoptosis in podocytes in vitro.
Methods
Mouse podocytes were cultured and treated with various concentrations of PAN. ER stress markers were then evaluated by western blotting, and apoptosis was evaluated by fluorescence-activated cell sorting (FACS) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays.
Results
PAN treatment increased ER stress markers such as activating transcription factor (ATF) 6α and caspase-12 in a dose-dependent manner at 12 and 24 hours, respectively. These markers were reduced by chemical chaperones, such as sodium 4-phenylbutyric acid and tauroursodeoxycholic acid. PAN treatment also increased 78 kD glucose-regulated protein (GRP78)/binding immunoglobulin protein (BiP) at the earlier stage of 12 hours. PAN significantly induced podocyte apoptosis in concentration- and time-dependent manners, as seen using FACS and TUNEL assays. This result was improved by Nox4 siRNA, ATF6 siRNA, and chemical chaperones. LY294002, a PI3-kinase inhibitor, significantly boosted ER stress and apoptosis. PAN-induced ER stress increased oxidative stress and subsequently induced apoptosis, and could be mitigated by inhibition of PI3-kinase signaling.
Conclusion
Our findings suggest that PAN induces ER stress in podocytes mainly through the GRP78/BiP, ATF6α, and caspase-12 pathways, which trigger apoptosis via induction of oxidative stress. This stress is mitigated by inhibiting PI3-kinase signaling.
Keywords: Apoptosis, Endoplasmic reticulum stress, Podocyte, Proteinuria, Puromycin aminonucleoside
Introduction
Podocytes are highly specialized and terminally differentiated epithelial cells situated on the outer surface of the glomerular basement membrane (GBM) that play a crucial role in maintenance of the glomerular filtration barrier [1,2]. Podocytes are key sites of injury in a variety of renal diseases, particularly proteinuric glomerular diseases such as minimal change disease (MCD), focal segmental glomerulosclerosis, and diabetic nephropathy. Injured podocytes invariably respond with foot process effacement, an often reversible process thought to help injured podocytes remain attached to the GBM [3]. Sustained podocyte injury, however, is likely to cause podocyte cell detachment and/or death, which if extensive, leads to progressive glomerulosclerosis and end-stage kidney disease [4]. Podocytes are terminally differentiated with limited mitotic capability and can be replaced only rarely if lost by podocyte injury and subsequent apoptosis. Therefore, alleviating podocyte apoptosis is crucial in treating proteinuric and progressive glomerular diseases [5,6].
Puromycin aminonucleoside (PAN) is a podocytotoxin that is widely used to induce experimental nephrotic syndrome in rats [7,8]; it also causes foot process effacement and apoptosis in cultured podocytes [9,10]. Podocytes specifically injured by PAN show flattening of foot processes, focal detachment from GBM, actin cytoskeleton disorganization, and decreased expression and abnormal distribution of slit diaphragm (SD) proteins; these findings are consistent with those observed at the onset of proteinuria [9,10]. Morphologic changes include pronounced effacement and fusion of foot processes, consistent with the general morphological features of MCD. In-vivo and in-vitro studies have supported that PAN-induced glomerular injury is mediated via overproduction of reactive oxidative species, particularly by podocytes [11–14]. Reactive oxidative species may then interact with other biomolecules, leading to modification and potentially deleterious cellular consequences, including apoptosis [12,15,16].
The endoplasmic reticulum (ER) is a highly dynamic organelle responsible for multiple cellular functions wherein newly synthesized secretory and transmembrane proteins are assembled and folded into their correct tertiary structures; it plays a critical role in controlling cellular proteostasis and fate. Maintenance of protein metabolism is disturbed under various pathogenic stresses, resulting in the accumulation of unfolded proteins due to ER dysfunction, which in turn causes cell damage. This series of events is termed ER stress [17,18].
Because ER stress leads to accumulation of unfolded or misfolded proteins in the ER, it triggers an adaptive program called the unfolded protein response (UPR) [19–21]. The adaptive UPR pathway is regulated by three major ER resident transducers (i.e., ER stress sensors) in the ER lumen, namely, RNA-dependent pancreatic eukaryotic translation initiation factor 2α (eIF2α) kinase (PKR-like ER kinase; PERK), activating transcription factor 6 (ATF6), and inositol-requiring ER-to-nucleus signal kinase 1. These transducers are normally inactive due to binding of the ER chaperone GRP78 (78 kD glucose-regulated protein), also known as binding immunoglobulin protein (BiP). ER stress dissociates GRP78/BiP from the transducers, which then binds to unfolded proteins. Activation of PERK leads to phosphorylation of eIF2α, which causes general inhibition of protein synthesis and induction of ATF4, which binds to the amino acid response element [19,20]. ER stress is common under various pathogenic microenvironments and contributes to progression of various podocyte diseases. Abnormal protein accumulation associated with podocyte-specific ER stress induces structural and functional damage in cells, which in turn leads to apoptosis and severe proteinuria [21–23]. The ER stress mechanisms underlying the development of podocyte injury by PAN remain unclear.
In this study, an in-vitro proteinuria model involving PAN was used to study its effect on ER stress and apoptosis in mouse podocytes.
Methods
Mouse podocyte culture
Conditionally immortalized mouse podocytes were kindly provided by Peter Mundel (University of Harvard, Boston, MA, USA) and were cultured and differentiated as described previously [24]. For proliferation, podocytes were cultured in collagen type I-coated flasks in the presence of 10 U/mL mouse recombinant γ-interferon (Roche, Mannheim, Germany) at 33°C (permissive conditions). For differentiation, they were maintained at 37°C without γ-interferon (non-permissive conditions) for at least 2 weeks.
Treatment conditions and antibody preparation
Podocytes were treated with 50 μg/mL of PAN (Sigma Chemical Co., St. Louis, MO, USA) dissolved in ethanol for 2, 6, 12, and 24 hours. A cytotoxic dose of PAN was used as determined previously [14]. The primary antibodies for anti-ATF6α, anti-caspase-12, anti-phospho-eIF2α, and anti-GRP78 were purchased from Cell Signaling (Danvers, MA, USA). Anti-β-tubulin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
To inhibit ER stress, podocytes were treated with 5 μM 4-phenylbutyric acid (PBA) and 100 μM tauroursodeoxycholic acid (TUDCA) (both from Sigma Chemical Co.). To inhibit phosphoinositide 3 (PI3)-kinase/Akt signaling, podocytes were exposed to 5 μM LY294002 (Cell Signaling Technology), a PI3-kinase inhibitor.
Small interference RNA (siRNA) for ATF6α, Nox4, and CD2AP transfection
The culture medium was removed one day before transfection, and differentiated podocytes were grown in antibiotic-free RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The cells were transfected with siRNA using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s introductions. In brief, ATF6α, Nox4, and CD2AP siRNAs along with control scrambled siRNA (all from Santa Cruz Biotechnology) were diluted with Transfection Medium (Opti-MEM, Invitrogen) and incubated in 6-well plates for 5 minutes. In parallel, Lipofectamine was diluted with Transfection Medium (Opti-MEM). The diluted Lipofectamine reagent and siRNA were mixed and incubated at room temperature (RT) for 20 minutes. The medium was replaced with Opti-MEM and the siRNA/Lipofectamine mixture was added to cells. The transfection mixture was replaced with RPMI 1640 medium supplemented with 10% FBS after 5 hours, and the inhibitory effect of siRNAs was confirmed by western blotting.
Western blotting
Confluently grown cell layers were incubated with additives for various durations and were extracted in protein extraction solution (PRO-PREP; Intron, Seongnam, Korea) according to the manufacturer’s instructions. Thirty micrograms of boiled extract was separated with 10% or 12% SDS-PAGE gels, and the separated proteins were transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Then, the membranes were air-dried and blocked in 3% fat-free milk before incubation with primary antibodies. After incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology), the bands were detected using an enhanced chemiluminescence system (Amersham Biotech Ltd., Bucks, UK). All experiments were repeated at least three times. In each experiment, the ratio of absorbance of each molecule to β-tubulin was calculated. Density values were expressed as percentage of control.
Immunofluorescence staining
Podocytes grown on type I collagen-coated glass cover slips incubated for 24 hours were fixed with 4% paraformaldehyde, permeabilized in 0.3% Triton X-100 for 10 minutes at RT, blocked with 10% normal goat serum, and labeled with monoclonal rabbit anti-ATF6α (Cell Signaling Technology) and anti-CD2AP (Santa Cruz Biotechnology) antibodies. Primary antibody-bound specimens were incubated with 1:500 (v/v) Alexa 488 for green and Alexa 594 for red (Invitrogen) conjugated secondary antibodies at RT for 1 hour. DAPI was used to stain nuclei. The coverslips were mounted in aqueous mounting medium and visualized under a fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Scanning electron microscopy (SEM)
SEM was used for mouse podocytes grown confluently on the surface of cellulose semi-permeable membranes (Millicell-HA; Millipore Corp., Burlington, MA, USA). After washing twice with phosphate buffer solution, the monolayered cells were fixed with 5% glutaraldehyde and post-fixed with 2% osmium tetroxide, followed by dehydration with progressive concentrations of ethanol. These samples were then passed through critical-point drying and gold coating before examination with SEM (Hitachi S-570; Hitachi Science Systems Ltd., Hitachinaka, Japan).
Fluorescence-activated cell sorting (FACS) assay
FACS was used to quantify apoptotic and necrotic podocytes after annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) labeling, as described in the manufacturer’s protocol (Annexin V-FITC Apoptosis Detection Kit I; BD Biosciences, San Jose, CA, USA). Equal numbers of podocytes were grown on 6-cm tissue culture plates in medium. After each treatment, the cell culture medium was collected and stored. Cells were washed once with 1.5 mL of PBS. PBS used for washing was combined mixed with the saved stored culture medium. The percentages of apoptotic and necrotic cells in the adherent-cell fraction were estimated by FACS (FACSCalibur-S System; Becton Dickinson Biosciences, San Jose, CA, USA) after annexin V-FITC and PI labeling. The cell number was calculated by multiplying the percentages of apoptotic and necrotic cells, as determined by FACS, to with the total number of adherent cells. The total number of cells was defined as the number of detached cells plus the number of adherent cells. All FACS analyses for apoptosis detection were performed in triplicate under each experimental condition.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Podocytes grown on type I collagen-coated glass cover slips incubated for 24 hours were fixed with 4% paraformaldehyde for 1 hour followed by permeabilization with 0.1% Triton X-100 for 10 minutes at RT. TUNEL assay was performed according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, AP; Roche). After coverslips were mounted, the samples were immediately evaluated using a fluorescence microscope. The TUNEL index (apoptotic podocytes) was determined by counting positively and negatively staining cells in 10 visual fields.
Statistical analysis
The results are expressed as mean values ± standard deviation. Statistical significance was assessed by non-parametric Kruskal–Wallis ANOVA analysis or Student’s t test using the SPSS 9.0.0 (SPSS Inc., Chicago, IL, USA) software program. P values less than 0.05 were considered significant.
Results
PAN induced ER stress in mouse podocytes
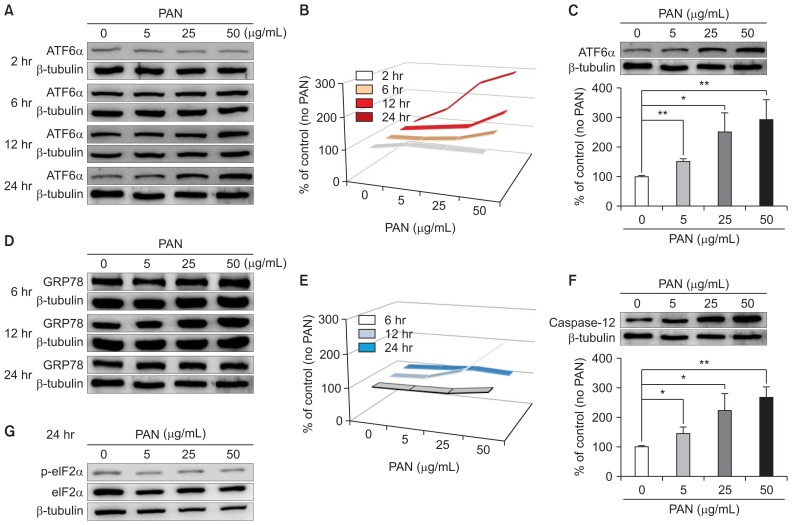
PAN treatment tended to increase levels of ATF6α, an ER stress marker, in time- and dose-dependent manners (Fig. 1A), as presented in Fig. 1B. In particular, a high dose of PAN (50 μg/mL) significantly increased ATF6α in a dose-dependent manner after incubation for 24 hours, correcting for β-tubulin levels (n = 3; Fig. 1C). PAN also increased GRP78/BiP at an earlier stage of 12 hours, which normalized at 24 hours (n = 3; Fig. 1D, E). In addition, PAN significantly increased the levels of another ER stress marker, caspase-12, in a dose-dependent manner after incubation for 24 hours (Fig. 1F); however, no differences in the levels of phospho-eIF2 were evident (Fig. 1G). These results suggested that PAN induces podocyte ER stress through GRP78/BiP at an early stage and through the ATF6α and caspase-12 pathways later.
Figure 1. PAN induces ER stress in podocytes, as determined by western blotting.
(A) PAN increases ATF6α, an ER stress marker, in time- and dose-dependent manners. (B) Time- and dose-dependent changes in ATF6α levels because of PAN are shown (arrows). (C) PAN (50 μg/mL) significantly upregulates ATF6α in a dose-dependent manner at 24 hours after correcting for β-tubulin levels (n = 3). (D) PAN also increases GRP78 at earlier stage of 12 hours, and is normalized at 24 hours (n = 3). (E) Time- and dose-dependent changes in GRP78 levels induced by PAN are shown (arrow). (F) PAN increases caspase-12, another ER stress marker, significantly in a dose-dependent manner after 24 hours of incubation (n = 3). (G) PAN does not apparently affect phospho-eIF2 levels. Data are presented as mean ± standard deviation. Control (100%); value without PAN. *P < 0.05 and **P < 0.01 versus control.
ATF, activating transcription factor; ER, endoplasmic reticulum; GRP78, 78 kD glucose-regulated protein; PAN, puromycin aminonucleoside.
ER stress inhibitors, PBA and TUDCA, reduced PAN-induced ER stress
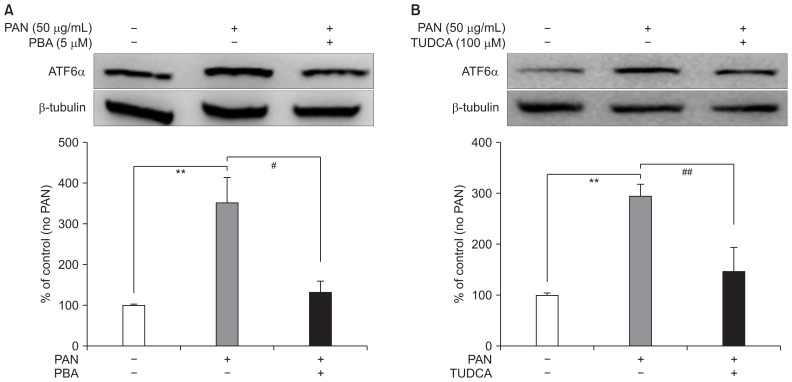
To confirm the activation of ER stress by PAN, we assessed whether 4-PBA and TUDCA, which are ER stress inhibitors, could alleviate the increased ER stress in PAN-treated mouse podocytes. Exposing the PAN-treated podocytes to 5 μM of 4-PBA and 100 μM of TUDCA significantly reduced ATF6α expression after incubation for 24 hours, correcting for β-tubulin levels, as expected (n = 3; Fig. 2A, B, respectively).
Figure 2. ER stress inhibitors reduce PAN-induced ER stress.
(A) 4-PBA (5 μM) significantly abrogates ATF6α expression upregulated by PAN after 24 hours (n = 3). (B) TUDCA (100 μM) also significantly improves ATF6α expression upregulated by PAN 24 hours after correcting for β-tubulin levels (n = 3). Data are presented as mean ± standard deviation. Control (100%); value of PAN (−) group. **P < 0.01 versus control. #P < 0.05 and ##P < 0.01 versus PAN only group.
ATF, activating transcription factor; ER, endoplasmic reticulum; PAN, puromycin aminonucleoside; PBA, phenylbutyric acid; TUDCA, tauroursodeoxycholic acid.
LY294002, a PI3-K inhibitor, exaggerates podocyte ER stress
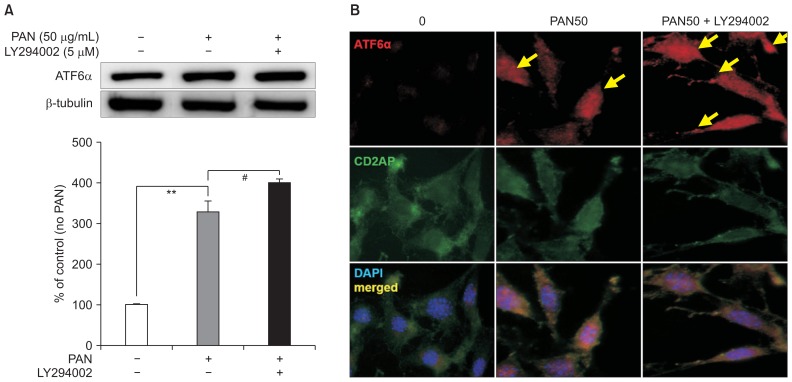
ATF6α upregulated by PAN was significantly augmented by 5 μM of LY294002 after incubation for 24 hours, correcting for β-tubulin levels (n = 3; Fig. 3A). Confocal imaging with CD2AP showed that PAN led to increased levels and accumulation of ATF6α, which was also further augmented by LY294002 (Fig. 3B).
Figure 3. LY294002 increases podocyte ER stress.
(A) LY294002 (5 μM) further augments PAN-increased ATF6α levels significantly after 24 hours after correcting for β-tubulin levels (n = 3). Data are presented as mean ± standard deviation. Control (100%); value of PAN (−) group. **P < 0.01 versus control. #P < 0.05 versus PAN only group. (B) In confocal imaging with CD2AP, PAN increases and concentrates ATF6α protein (arrows), which is also further augmented by LY294002.
ATF, activating transcription factor; ER, endoplasmic reticulum; PAN, puromycin aminonucleoside.
siRNAs modulate podocyte ER stress
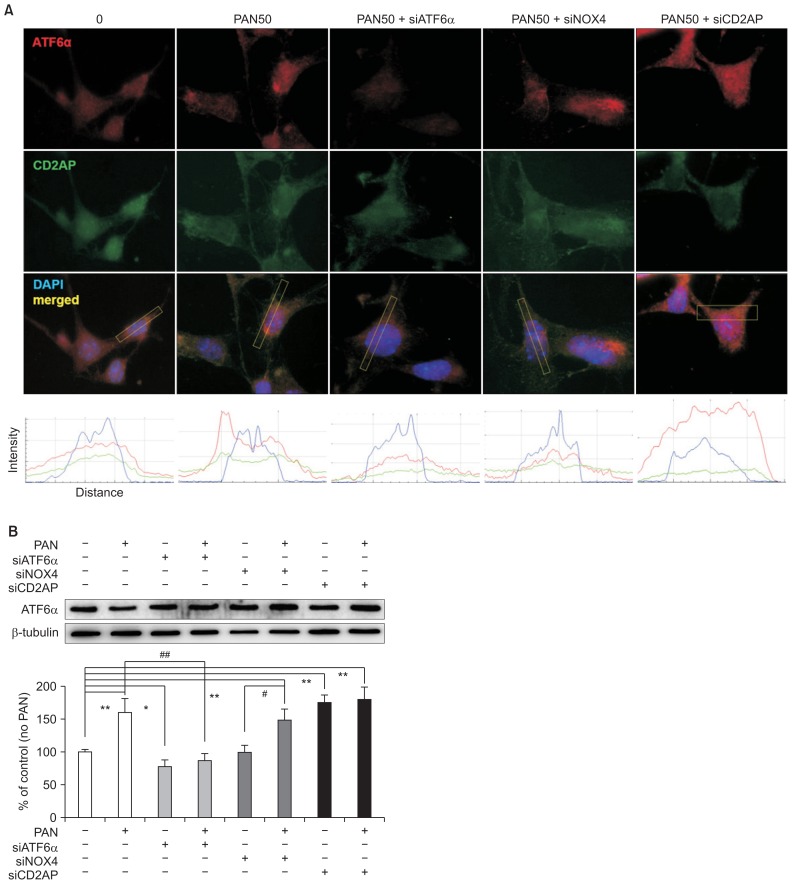
Confocal microscopy showed that although PAN increased ATF6α, it reduced CD2AP. ATF6α upregulated by PAN was modulated by each of the siRNAs that were tested. As expected, the siRNA that suppresses ER stress (siATF6α) countered the ATF6α increase induced by PAN. Although siNOX4 did not show any additive difference, siCD2AP further increased ATF6α. The densities of fluorescence staining analyzed using ImageJ image processing and analysis software (National Institutes of Health, Bethesda, MD, USA) are shown in the lower panel (Fig. 4A). These changes were confirmed by western blotting, as shown in Fig. 4B (n = 3). Because siNOX4 did not show any effect, the oxidative stress induced by PAN was downstream to ER stress; therefore, anti-oxidative stress might not inhibit the established ER stress.
Figure 4. Podocyte ER stress is modulated by siRNAs.
(A) In confocal images, PAN seems to upregulate ATF6α but downregulate CD2AP. siATF6α suppresses ATF6α induced by PAN, as expected. siNOX4 does not show any effect, although siCD2AP further increases ATF6α. Such changes are shown in lower panel as quantitative analysis of fluorescent staining densities using ImageJ image. (B) Changes induced by siRNAs were confirmed by western blotting (n = 3). Values are expressed as mean ± standard deviation. Control (100%); value of PAN (−)/siRNAs (−) group. *P < 0.05 and **P < 0.01 versus control. #P < 0.05 and ##P < 0.01 versus PAN (+) groups.
ATF, activating transcription factor; ER, endoplasmic reticulum; PAN, puromycin aminonucleoside; si, small interference.
PAN induces fusion of surface villi on podocytes
After 24 hours of incubation, surface villi were identified on podocytes in normal physiologic conditions without PAN. However, PAN (50 μg/mL) treatment induced fusion and effacement of surface villi on podocytes, which was aggravated by LY294002 and attenuated by 4-PBA and TUDCA (Fig. 5). These results suggest that ultrastructural changes of podocytes induced by PAN were mediated by ER stress activation and aggravated by PI3-kinase inhibition.
Figure 5. PAN induces fusion of surface villi on podocytes.
After 24 hours of incubation, surface villi (arrows) were identified on podocytes in normal conditions without PAN. However, PAN (50 μg/mL) induced fusion and effacement of surface villi (arrowheads) on podocytes, which was aggravated by LY294002 and attenuated by 4-PBA and TUDCA (×60,000).
PAN, puromycin aminonucleoside; PBA, phenylbutyric acid; TUDCA, tauroursodeoxycholic acid.
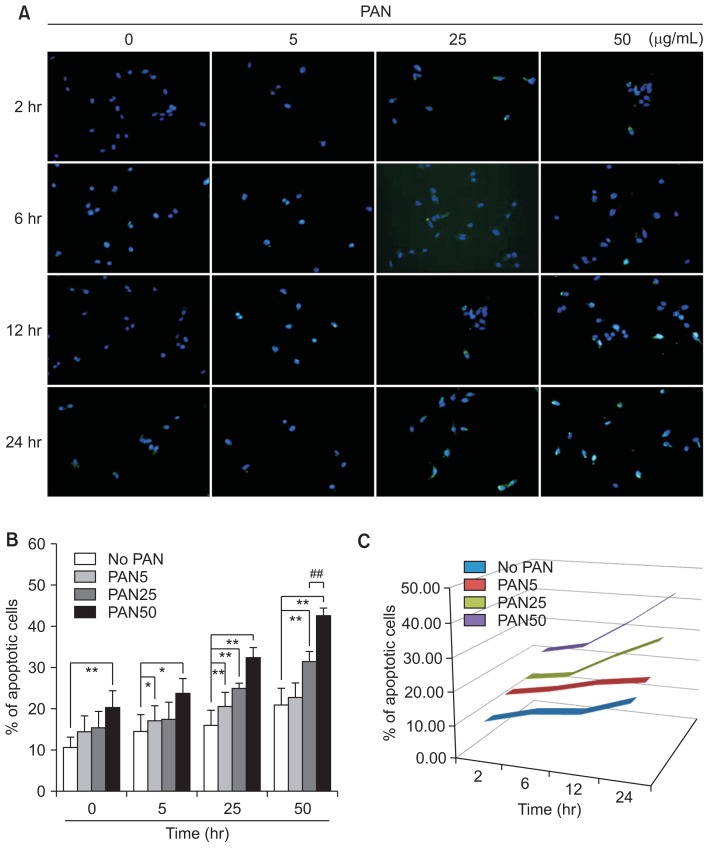
PAN induces podocyte apoptosis
PAN induced significant apoptosis in concentration- and time-dependent manners, as seen using the TUNEL assay. Apoptotic podocytes were stained and their concentration- and time-dependent increases are demonstrated in Fig. 6. These changes, particularly after incubation for 24 hours, were similar to those of ATF6α, as seen in Fig. 1A and B. Thus, PAN induced podocyte apoptosis, which was promoted by ER stress at high doses and longer exposure times.
Figure 6. PAN induces podocyte apoptosis in concentration- and time-dependent manners, as seen using TUNEL assays (n = 3).
Values are expressed as mean ± standard deviation. Control (100%); value without PAN. *P < 0.05 and **P < 0.01 versus control. #P < 0.05 and ##P < 0.01 versus respective values with PAN.
PAN, puromycin aminonucleoside; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
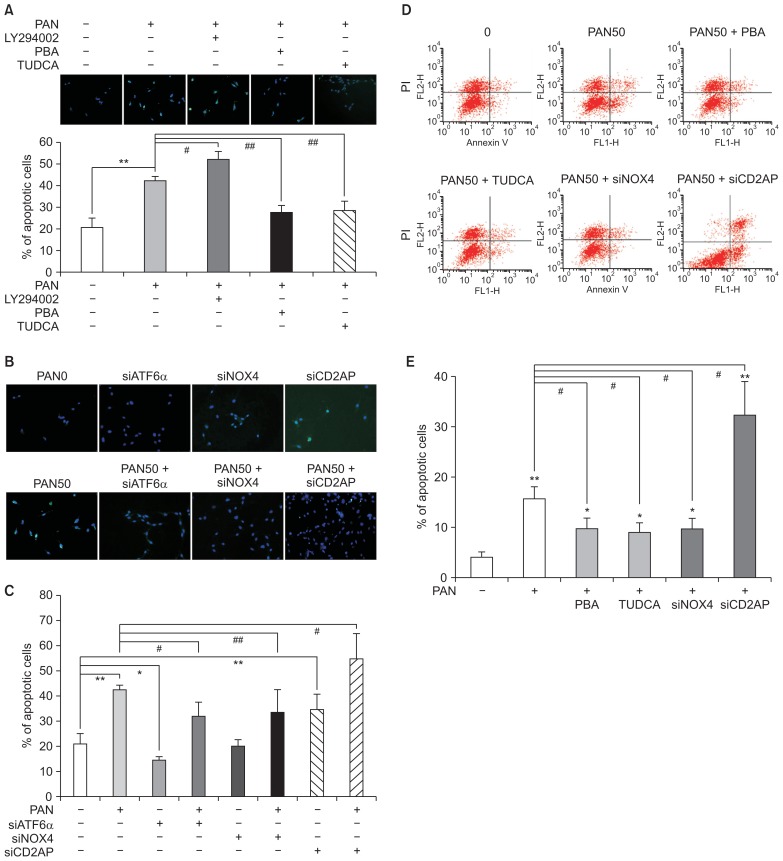
Podocyte apoptosis is modulated by LY294002, 4-PBA, TUDCA, and siRNAs
In addition to the siRNAs, podocyte apoptosis induced by PAN (50 μg/mL) was also modulated by LY294002, a PI3-kinase inhibitor, along with 4-PBA and TUDCA, which are ER stress inhibitors. LY294002 increased podocyte apoptosis, whereas 4-PBA and TUDCA significantly reduced it, as seen by TUNEL assay (n = 3; Fig. 7A). siATF6α, which suppressed ER stress, and siNox4, which inhibited oxidative stress, reduced PAN-induced apoptosis. In contrast, siCD2AP itself induced apoptosis and further increased the apoptosis induced by PAN (n = 3; Fig. 7B, C). These changes were confirmed by FACS assay (n = 3; Fig. 7D, E).
Figure 7. Podocyte apoptosis is modulated by LY294002, 4-PBA, TUDCA, and siRNAs.
(A) Podocyte apoptosis induced by PAN (50 μg/ mL) is exaggerated by LY294002, whereas it is reduced by 4-PBA and TUDCA, as seen using TUNEL assay (n = 3). (B and C) siRNA for ATF6α and Nox4 may reduce apoptosis. Whereas, siRNA for CD2AP itself induces apoptosis and further increases that induced by PAN (n = 3) (D and E). Podocyte apoptosis is induced by PAN (50 μg/mL) and modulated by 4-PBA, TUDCA, and siRNAs, as confirmed by FACS assay (n = 3). Values are expressed as mean ± standard deviation. Control (100%); value without PAN. *P < 0.05 and **P < 0.01 versus control. #P < 0.05 and ##P < 0.01 versus respective values with PAN.
ATF, activating transcription factor; FACS, fluorescence-activated cell sorting; PAN, puromycin aminonucleoside; PBA, phenylbutyric acid; si, small interference; TUDCA, tauroursodeoxycholic acid; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
One consequence of podocyte injury is apoptosis, which leads to podocyte depletion and subsequent glomerulosclerosis in both experimental and human glomerular disease [4–6]. PAN-induced nephropathy is characterized by podocyte apoptosis [7,8], and PAN also causes apoptosis in podocytes in vitro[16,25,26].
The ER performs multiple cellular functions, such as regulating protein synthesis, folding, and trafficking as well as modulating cellular responses to stress. Because ER homeostasis is maintained by UPR [19–21], which may also serve as an adaptive response, severe or prolonged ER stress will lead to apoptotic cell death. Severe podocyte injury with abnormal protein accumulation is associated with ER stress, which produces structural and functional damage to lead to apoptosis and severe proteinuria [21–23,27]. Nevertheless, the mechanisms of ER stress and apoptosis induced by PAN in podocytes remain unclear. Our data demonstrate that PAN can trigger ER stress in mouse podocytes through GRP78/BiP at an early stage and induce podocyte ER stress mainly through the ATF6α and caspase-12 pathways at a later stage. A report by Ito et al [28] revealed that eIF2α and GRP78 were already activated on day 1 and remained at their highest levels until day 3. eIF2α and GRP78 then decreased in the glomeruli of the PAN nephrosis model and were upregulated for up to 48 hours in-vitro experiments. However, their applied dose of PAN (5 μg/mL) in vitro was much lower than ours (50 μg/mL) and they did not observe changes in ATF6α and caspase-12. We assume that PAN could trigger ER stress in mouse podocytes through the GRP78/BiP at an early stage and PAN may induce podocyte ER stress through the ATF6α and caspase-12 pathways at a later stage.
We also unraveled the critical role of PI3-kinase survival signaling during podocyte apoptosis. PI3-kinase is involved in many fundamental cellular functions via activation of protein kinase B (PKB also known as Akt), including cell growth, proliferation, differentiation, motility, survival, and adhesion [29,30]. Akt is a central regulator of PI3-K-mediated cell survival, and activation of PI3-K results in phosphorylation of Akt, which presumably activates the Akt-dependent signaling processes in podocytes. PAN-induced podocyte injury models showed decreased Akt activity, and dexamethasone was found to inhibit apoptosis by stabilizing the PI3K/Akt signaling pathway in vitro [31]. Similarly, PAN reduces Akt phosphorylation levels of GSK3β in cultured podocytes, and LY294002 could further promote podocyte apoptosis induced by PAN [32]. In rats with PAN-induced nephropathy, 1,25(OH)2D3 significantly prevented loss of nephrin, foot process retraction, and podocyte apoptosis by stimulating Akt phosphorylation and suppressing the transforming growth factor-β/Smad-signaling pathway [26]. Furthermore, the PI3K/Akt pathway mediates podocyte survival during the early stage of high glucose nerve stimulation. Activation of the PI3K/Akt pathway also prevents apoptosis due to inhibition of Notch [33]. Our findings showed that PI3-kinase inhibition augmented Ang II-induced ER stress via the PERK-eIF2α pathway, suggesting that PI3-kinase inhibition is related to Ang II-induced ER stress in podocyte injury [23].
CD2AP serves as an adaptor protein by anchoring SD proteins, such as nephrin and podocin, to the actin-based cytoskeleton of podocytes, thus maintaining the filtration structure and sending signals inside or outside [34–37]. CD2AP and nephrin also interact with the p85 subunit of PI3-K to stimulate the anti-apoptotic intracellular Akt kinase (PI3-K/Akt) pathway, which plays essential roles in cell survival, actin reorganization, and endocytic machinery of podocytes [38,39]. CD2AP siRNA itself induced apoptosis in podocytes and further increased PAN-induced apoptosis. Recently, Saurus et al [40] reported that PAN increased the activity of SHIP2, a negative regulator of the PI3K/Akt signaling pathway. PAN reduced CD2AP expression in cultured human podocytes, thereby increasing oxidative stress and aggravating podocyte apoptosis. We previously found that PAN treatment significantly increased the oxidative stress level of podocytes with induction of Nox4 [14]. In this study, we also found that siRNA inhibits oxidative stress, and siNox4 could reduce apoptosis.
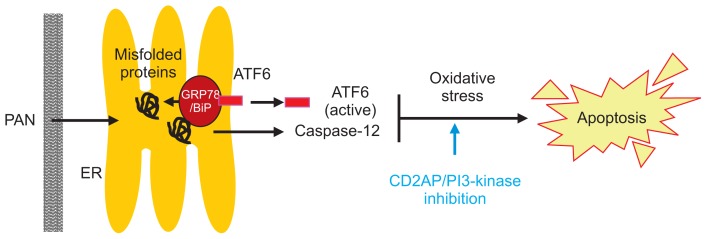
In conclusion, we demonstrated that PAN triggers podocyte ER stress in mouse podocytes through GRP78/ BiP at an early stage. Thereafter, PAN mainly triggers podocyte ER stress through the ATF6α and caspase-12 pathways, which promotes podocyte apoptosis due to oxidative stress and inhibits PI3-kinase survival signaling (Fig. 8).
Figure 8. Schematic representation of ER stress pathway induced by PAN in mouse podocytes.
PAN induces podocyte ER stress mainly via ATF6α and caspase-12 pathways, which promotes apoptosis by oxidative stress and inhibits PI3-kinase anti-apoptotic signaling.
ATF, activating transcription factor; ER, endoplasmic reticulum; GRP78, 78 kD glucose-regulated protein; PAN, puromycin aminonucleoside.
Acknowledgments
This research was supported by the Korean Society of Nephrology (2015) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03931888). We thank J-Y Choi and Park HY for their technical assistances.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 2.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 4.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 5.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 6.D’Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17:271–281. doi: 10.1097/MNH.0b013e3282f94a96. [DOI] [PubMed] [Google Scholar]
- 7.Shiiki H, Sasaki Y, Nishino T, et al. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology. 1998;66:221–229. doi: 10.1159/000028027. [DOI] [PubMed] [Google Scholar]
- 8.Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 9.Löwenborg EK, Jaremko G, Berg UB. Glomerular function and morphology in puromycin aminonucleoside nephropathy in rats. Nephrol Dial Transplant. 2000;15:1547–1555. doi: 10.1093/ndt/15.10.1547. [DOI] [PubMed] [Google Scholar]
- 10.Guan N, Ding J, Deng J, Zhang J, Yang J. Key molecular events in puromycin aminonucleoside nephrosis rats. Pathol Int. 2004;54:703–711. doi: 10.1111/j.1440-1827.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- 11.Gwinner W, Landmesser U, Brandes RP, et al. Reactive oxygen species and antioxidant defense in puromycin aminonucleoside glomerulopathy. J Am Soc Nephrol. 1997;8:1722–1731. doi: 10.1681/ASN.V8111722. [DOI] [PubMed] [Google Scholar]
- 12.Marshall CB, Pippin JW, Krofft RD, Shankland SJ. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 2006;70:1962–1973. doi: 10.1038/sj.ki.5001965. [DOI] [PubMed] [Google Scholar]
- 13.Kinugasa S, Tojo A, Sakai T, et al. Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int. 2011;80:1328–1338. doi: 10.1038/ki.2011.282. [DOI] [PubMed] [Google Scholar]
- 14.Ha TS, Park HY, Seong SB, Ahn HY. Puromycin aminonucleoside increases podocyte permeability by modulating ZO-1 in an oxidative stress-dependent manner. Exp Cell Res. 2016;340:139–149. doi: 10.1016/j.yexcr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Takemura H, Noiri E, et al. Puromycin aminonucleoside induces apoptosis and increases HNE in cultured glomerular epithelial cells(1) Free Radic Biol Med. 2001;31:615–623. doi: 10.1016/S0891-5849(01)00641-4. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum: new mechanisms in kidney disease. Nat Rev Nephrol. 2014;10:369–378. doi: 10.1038/nrneph.2014.67. [DOI] [PubMed] [Google Scholar]
- 22.Cybulsky AV. The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 2013;84:25–33. doi: 10.1038/ki.2012.390. [DOI] [PubMed] [Google Scholar]
- 23.Ha TS, Park HY, Seong SB, Ahn HY. Angiotensin II induces endoplasmic reticulum stress in podocyte, which would be further augmented by PI3-kinase inhibition. Clin Hypertens. 2015;21:13. doi: 10.1186/s40885-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mundel P, Reiser J, Zúñiga Mejía Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 25.Gong W, Yu J, Wang Q, et al. Estrogen-related receptor (ERR) γ protects against puromycin aminonucleoside-induced podocyte apoptosis by targeting PI3K/Akt signaling. Int J Biochem Cell Biol. 2016;78:75–86. doi: 10.1016/j.biocel.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Xiao H, Shi W, Liu S, et al. 1.25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol. 2009;30:34–43. doi: 10.1159/000200769. [DOI] [PubMed] [Google Scholar]
- 27.He F, Chen S, Wang H, et al. Regulation of CD2-associated protein influences podocyte endoplasmic reticulum stress-mediated apoptosis induced by albumin overload. Gene. 2011;484:18–25. doi: 10.1016/j.gene.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Ito N, Nishibori Y, Ito Y, et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Invest. 2011;91:1584–1595. doi: 10.1038/labinvest.2011.135. [DOI] [PubMed] [Google Scholar]
- 29.Braccini L, Ciraolo E, Martini M, et al. PI3K keeps the balance between metabolism and cancer. Adv Biol Regul. 2012;52:389–405. doi: 10.1016/j.jbior.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu-Shengyou, Li Y. Dexamethasone inhibits podocyte apoptosis by stabilizing the PI3K/Akt signal pathway. Biomed Res Int. 2013;2013:326986. doi: 10.1155/2013/326986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Q, You Yu S. CD2-associated protein participates in podocyte apoptosis via PI3K/Akt signaling pathway. J Recept Signal Transduct Res. 2016;36:288–291. doi: 10.3109/10799893.2015.1101137. [DOI] [PubMed] [Google Scholar]
- 33.Wang XM, Yao M, Liu SX, Hao J, Liu QJ, Gao F. Interplay between the Notch and PI3K/Akt pathways in high glucose-induced podocyte apoptosis. Am J Physiol Renal Physiol. 2014;306:F205–F213. doi: 10.1152/ajprenal.90005.2013. [DOI] [PubMed] [Google Scholar]
- 34.Ha TS. Roles of adaptor proteins in podocyte biology. World J Nephrol. 2013;2:1–10. doi: 10.5527/wjn.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw AS, Miner JH. CD2-associated protein and the kidney. Curr Opin Nephrol Hypertens. 2001;10:19–22. doi: 10.1097/00041552-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shawa AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Sun N, Aoudjit L, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 38.Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HY, Seong SB, Min SY, Ha TS. CD2-associated protein/ phosphoinositide 3-kinase signaling has a preventive role in angiotensin II-induced podocyte apoptosis. Int J Biochem Cell Biol. 2016;79:370–381. doi: 10.1016/j.biocel.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 40.Saurus P, Tolvanen TA, Lindfors S, et al. Inhibition of SHIP2 in CD2AP-deficient podocytes ameliorates reactive oxygen species generation but aggravates apoptosis. Sci Rep. 2017;7:10731. doi: 10.1038/s41598-017-10512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]