Abstract
Introduction
It is generally recognized that a wide variety of morphogens and growth factors bind to the glycosaminoglycans (GAG) of proteoglycans (PG) to affect their bioavailability to ligands. Many growth factors involving in osteogenic differentiation require the GAG side chains to facilitate their interaction to the cell surface receptors and the biosynthesis of osteogenic proteins. The objective of this study is to investigate the secretion of GAG from MC3T3-E1 pre-osteoblasts of a murine bone calvaria during the osteogenic differentiation.
Methods
When MC3T3-E1 cells were cultured in the differentiation medium (DM) containing bone morphogenetic protein (BMP)-2, the alkaline phosphatase activity, calcium content and the amount of basic fibroblast growth factor (bFGF)- or BMP-2-bound sulfated GAG were determined. Moreover, the disaccharide analysis of the GAG was performed.
Results
When MC3T3-E1 cells were cultured in the differentiation medium (DM) containing bone morphogenetic protein (BMP)-2, the alkaline phosphatase activity and calcium content were significantly enhanced compared with those of the BMP-2-free DM and normal medium with or without BMP-2. Significantly higher amount of GAG secreted was detected for cells cultured in the DM containing BMP-2, in contrast to other culture conditions. The GAG secreted had an affinity for BMP-2 and basic fibroblast growth factor (bFGF). The disaccharide analysis of GAG demonstrated that the percentage of ΔHexA α1,4GlcNSO3 and ΔHexA (2-OSO3) α1,4GlcNSO3 increased, but that of ΔHexA α1,4GlcNSO3(6-OSO3) decreased (ΔHexA: unsaturated uronic acid residue, GlcNSO3: N-sulfated glucosamine, ΔHexA (2-OSO3): unsaturated uronic acid 2-sulfate residue, GlcNSO3(6-OSO3): N-sulfated glucosamine 6-sulfated).
Conclusion
It was found that the osteogenic differentiation allowed cells to enhance the secretion of GAG with an affinity for BMP-2 and bFGF.
Keywords: Osteogenic differentiation, Glycosaminoglycans, Basic fibroblast growth factor, Bone morphogenetic protein-2, Secretion, Disaccharide
Abbreviations: GAG, glycosaminoglycans; PG, proteoglycans; DM, differentiation medium; NM, normal medium; BMP, bone morphogenetic protein; bFGF, basic fibroblast growth factor; ALP, alkaline phosphatase; αMEM, α-Minimum Essential Medium; FCS, fetal calf serum; SDS, sodium dodecyl sulfate; DMMB, 1, 9-dimethylmethylene blue; DDW, double distilled water; PBS, phosphate buffer solution; HSPG, heparin sulfate proteoglycans; ΔHexA, unsaturated uronic acid residue; GlcNAc, N-acetyl glucosamine; GlcNAc(6-OSO3), N-acetyl glucosamine 6-sulfated; GlcNSO3, N-sulfated glucosamine; GlcNSO3(6-OSO3), N-sulfated glucosamine 6-sulfated; ΔHexA (2-OSO3), unsaturated uronic acid 2-sulfate residue
1. Introduction
Glycosaminoglycans (GAG) are linear and long-chain carbohydrates of repeating disaccharide units with different positioned sulfate groups. Proteoglycans (PG) are generally composed of a core protein and the GAG, which are covalently bound to the core protein via a tetrasaccharide linkage. There are different types of the GAG: chondroitin sulfate, dermatan sulfate, heparan sulfate, and heparin. It is generally recognized that a wide variety of morphogens and growth factors bind to the GAG of PG to affect their bioavailability to ligands [1], [2], [3], [4], [5], [6]. Many growth factors involving in osteogenic differentiation require the GAG side chains to facilitate their interaction to the cell surface receptors and the biosynthesis of osteogenic proteins [7], [8]. For example, basic fibroblast growth factor (bFGF) is secreted into the extracellular matrix by matured osteoblasts and acts to regulate the bone formation [9]. It stimulates the proliferation of various types of bone cells, including bone marrow stromal cells and osteoblast-like cell lines [10], [11]. Moreover, it is well known that bone morphogenetic protein (BMP)-2 of osteogenic factor and has a binding ability for GAG. It is demonstrated that heparan sulfate modulates the osteogenic bioactivity of BMP-2 by the sequestration from the cell surface receptor [8]. Such many papers have been reported on the GAG properties to modulate the functions of growth factors. However, little has been reported on the association between the GAG secretion from the cells and affinity of the osteogenic proteins with the GAG during the osteogenic differentiation from the immature stage.
In this paper, the GAG secretion from the MC3T3-E1 cells of a murine bone calvaria pre-osteoblast was investigated during their osteogenic differentiation. The cells were osteogenically differentiated by BMP-2 and the affinity of GAG secreted for BMP-2 and bFGF was evaluated. To further obtain the chemical structural information containing bonding positions of the sulfate groups, we examined the composition ratio of repeated disaccharide units of GAG secreted with a disaccharide analysis.
2. Materials and methods
2.1. Materials
bFGF was kindly supplied by Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). BMP-2 was purchased from R&D Systems, Inc. (Minneapolis, USA). Other chemicals were obtained from Nacalai Tesque, Inc., Kyoto, Japan and Wako Pure Chemical Industries, Ltd., Osaka, Japan, and used without further purification.
2.2. Cell culture and osteogenic differentiation
MC3T3-E1 cells of a murine bone calvaria pre-osteoblast were cultured in the normal medium (NM) consisting of α-Minimum Essential Medium (αMEM, Invitrogen Corp., California, USA) with 10 vol % fetal calf serum (FCS, HyClone Laboratories, Inc., Logan, USA) and antibiotics (100 U/ml penicillin G and 100 mg/ml streptomycin (Sigma Aldrich Corp., St. Louis, USA)) at 37 °C in a humidified atmosphere with 5% CO2 -95% air at an initial density of 6 × 103 cells per cm2. After 2 days culture, the medium was replaced with the differentiation medium (DM) which is the NM supplemented with 10 nM dexamethasone, 25 μg/ml l-ascorbic acid and 10 mM β-glycerophosphate (Sigma Aldrich Corp., St. Louis, USA). Then, the cell was cultured further for 7 days while the DM was changed every 3–4 days.
2.3. Alkaline phosphatase and calcium assay
Alkaline phosphatase (ALP) enzyme activity was assayed with a commercial kit (LabAssay ALP; Wako Pure Chemical Industries, Ltd., Osaka, Japan) on the basis of the absorbance measurement of a p-nitrophenol product. Briefly, MC3T3-E1 cells were rinsed twice with PBS, freeze-thawed, and finally incubated in 70 μl/cm2 of aqueous solution containing sodium dodecyl sulfate (SDS, 0.2 mg/ml), NaCl (3 M), and sodium citrate (0.3 M) for 1 h at 37 °C for cell lysis. Next, 20 μl of cell lysate was mixed with 100 μl of 6.7 mM p-nitrophenyl phosphate aqueous solution, followed by incubation for 15 min at 37 °C. After mixing 1 ml of 20 mM NaOH aqueous solution, the absorbance of the solution mixture was measured at 405 nm with a spectorophotometer (VERSAmax; Molecular Devices Japan KK, Tokyo, Japan) to assess the ALP activity.
Cells were incubated in 1 ml of 0.5 N HCl solution by shaking for 4 h at 4 °C. The amount of calcium in the HCl solution obtained was determined with a commercial kit (Calcium C-test Wako; Wako Pure Chemical Industries, Ltd., Osaka, Japan). Briefly, 10 μl of cell lysate was mixed with 1 ml of 0.88 M monoethanolamine aqueous solution. After mixing 100 mL of 69 mM 8-hydroxyquinoline and 0.63 mM o-cresolphthalein complexone aqueous solution, the solution mixture was allowed to stand for 5 min. The absorbance of the solution mixture was measured at 570 nm with the spectrometer to assess the calcium amount. Experiment was done independently 3 times for each sample.
2.4. Determination of sulfated GAG
The determination of sulfated GAG was performed according to the method reported [12]. Briefly, 16 mg of 1, 9-dimethylmethylene blue (DMMB) was dissolved in 5 ml ethanol. Then, 2 g of sodium formate and 2 ml of 98 vol % formic acid aqueous solution were added to the DMMB ethanol solution, and the final volume was adjusted to 100 ml with double distilled water (DDW). The culture supernatant of cells (25 μl) was mixed with 225 μl of DMMB dye solution and the absorbance of solution mixture was measured at 525 nm with a spectorophotometer (VERSAmax; Molecular Devices Japan KK, Tokyo, Japan). Shark chondroitin sulfate A sodium salt (Sigma Aldrich Corp., St. Louis, USA) was used to prepare the standard curve.
2.5. Determination of bFGF- or BMP-2-bound sulfated GAG
Heparin sepharose (5 μl, GE Healthcare UK Ltd., UK) was washed twice with ten-times volume of phosphate buffer solution (PBS, 10 mM Na2HPO4 and NaH2PO4, pH 7.0) in a tube and mixed with 200 μl PBS containing 10 μg of bFGF or BMP-2 for 10 min at room temperature. After washed twice with 200 μl of PBS, bFGF- or BMP-2-heparin sepharose was mixed with 200 μl of the culture medium obtained. Next 25 μl of the supernatant was mixed with 225 μl of the DMMB dye solution, the absorbance of solution mixture was measured at 525 nm with a spectorophotometer (VERSAmax; Molecular Devices Japan KK, Tokyo, Japan). Shark chondroitin sulfate A sodium salt (Sigma Aldrich Corp., St. Louis, USA) was used to prepare the standard curve. The GAG bound to bFGF or BMP-2 was determined by subtraction the absorbance of culture medium after treatment with bFGF- or BMP-2-heparin sepharose from the one before treatment with bFGF- or BMP-2-heparin sepharose.
2.6. Disaccharide analysis of sulfated GAG
The culture medium obtained was filtered with the centrifugal filter of 10 000 molecular weight cut-off, and then treated with heparin lyase I, II, and III (Heparinase and Heparitinase (Seikagaku Corporation, Tokyo, Japan) at 0.1 unit each in 30 μl of CH3COONa (20 mM, pH 7.0), Ca(CH3COO)2 (2 mM) and 0.2 wt% BSA aqueous solution) and incubated at 37 °C for 1 h. The products were purified with BlotGlyco® (Sumitomo Bakelite Co., Ltd, Tokyo, Japan) to prepare the sample of disaccharides. Disaccharide analysis was performed on a high performance liquid chromatography (HPLC) system (ELITE Lachrom; Hitachi High-Technologies Corporation, Tokyo, Japan). Solutions A and B for the HPLC separation contained 50 mM HCOONH4 (pH 4.4) and acetonitrile, respectively. Separation was performed on the column (TSK-GEL Amide-80; Tosoh Corporation, Yamaguchi, Japan) at 400 μl/min flow rate using 20 vol % solution A, followed by a linear gradient from 0 to 80 min of 20–41.7 vol % solution A.
2.7. Statistical analysis
All assays were performed in triplicate. All data were expressed as means ± standard deviation. The statistical analysis was performed by the independent sample t tests and p < 0.05 was accepted to be significant.
3. Results
3.1. Osteogenic differentiation of MC3T3-E1 cells
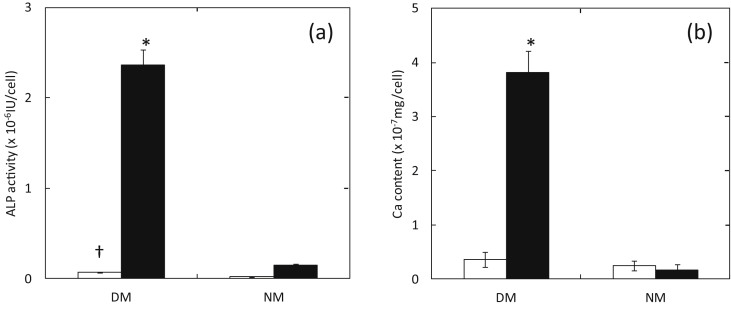
Fig. 1 shows the ALP activity and Ca content of MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 and 100 ng/ml of BMP-2. The MC3T3-E1 cells cultured in the DM containing 100 ng/ml of BMP-2 exhibited significantly higher levels of ALP activity and Ca content than those cultured in other culture conditions. Osteogenic differentiation culture without BMP-2 showed significantly higher levels of ALP activity than the normal culture.
Fig. 1.
(a) ALP activity and (b) Ca content of MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 (□) and 100 ng/ml of BMP-2 (■). *, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration. †, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration.
3.2. Secretion of GAG
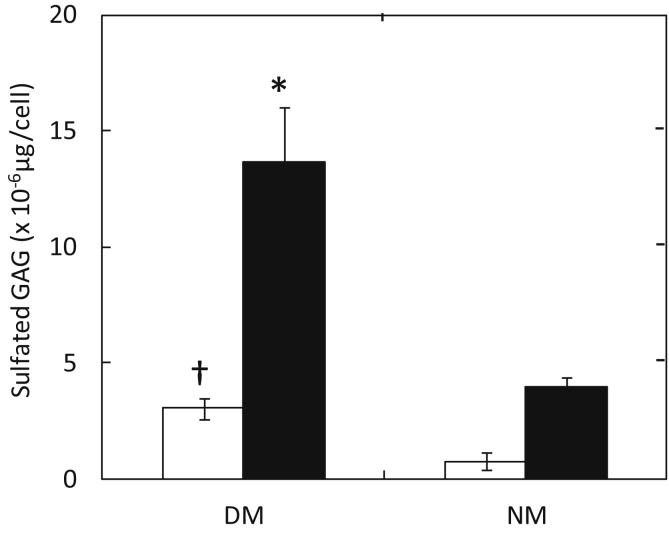
Fig. 2 shows GAG in conditioned medium of MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 and 100 ng/ml of BMP-2. MC3T3-E1 cells cultured in the DM with and without 100 ng/ml of BMP-2 exhibited significantly higher amount of GAG than those cultured in NM. Moreover, GAG in conditioned medium of MC3T3-E1 cells cultured in the DM increased over time (data not shown).
Fig. 2.
Amount of sulfated GAG secreted from MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 (□) and 100 ng/ml of BMP-2 (■). *, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration. †, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration.
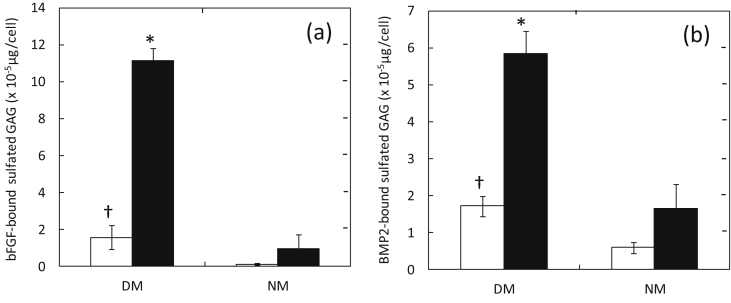
Fig. 3 shows bFGF- and BMP-2-bound GAG in conditioned medium of MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 and 100 ng/ml of BMP-2. The GAG secreted from MC3T3-E1 cells cultured in the DM with or without BMP-2 had an affinity for bFGF and BMP-2.
Fig. 3.
(a) Amount of bFGF-bound and (b) BMP-2-bound sulfated GAG secreted from MC3T3-E1 cells 7 days after culturing in DM and NM containing 0 (□) and 100 ng/ml of BMP-2 (■). *, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration. †, P < 0.05; significant against the value of cells cultured in NM at the corresponding BMP-2 concentration.
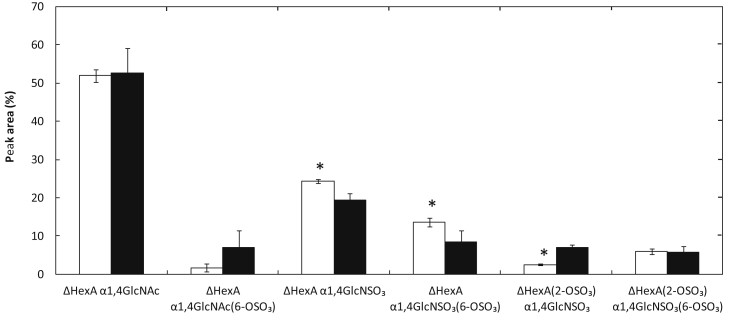
Fig. 4 shows the disaccharide analysis results of sulfated GAG secreted from MC3T3-E1 cells 7 days after culturing in DM containing 100 ng/ml of BMP-2 and NM. The disaccharide analysis of GAG demonstrated that the percentage of ΔHexA α1,4GlcNSO3 and ΔHexA (2-OSO3) α1,4GlcNSO3 increased, but that of ΔHexA α1,4GlcNSO3(6-OSO3) decreased (ΔHexA: unsaturated uronic acid residue, GlcNSO3: N-sulfated glucosamine, ΔHexA (2-OSO3): unsaturated uronic acid 2-sulfate residue, GlcNSO3(6-OSO3): N-sulfated glucosamine 6-sulfated).
Fig. 4.
Disaccharide analysis of sulfated GAG secreted from MC3T3-E1 cells 7 days after culturing in DM containing 100 ng/ml of BMP-2 (□) and NM (■). *, P < 0.05; significant against the value of cells cultured in NM at the corresponding disaccharide. ΔHexA: unsaturated uronic acid residue, GlcNAc: N-acetyl glucosamine, GlcNAc(6-OSO3): N-acetyl glucosamine 6-sulfated, GlcNSO3: N-sulfated glucosamine, GlcNSO3(6-OSO3): N-sulfated glucosamine 6-sulfated, ΔHexA (2-OSO3): unsaturated uronic acid 2-sulfate residue.
4. Discussion
The present study demonstrates that MC3T3-E1 cells secreted GAG accompanied with the osteogenic differentiation. The GAG secreted from cells cultured in the DM containing BMP-2 had a higher affinity for BMP-2 and bFGF than those cultured in the BMP-2-free DM. The affinity was significantly high compared with that of cells cultured in the NM with or without BMP-2.
Song et al. demonstrate that the proliferation and mineralization of primary rat calvarial osteoblasts were enhanced by the exogenous treatment of bFGF, and syndecan-4, a type of heparin sulfate proteoglycans (HSPG), was upregulated by the bFGF treatment [13]. Zehe et al. also report that HSPG are required for the bFGF secretion from mammalian cells [14]. Secretion of PG bearing GAG is normally accompanied with the growth factor secretion from cells. The researches indicate that GAG is generally involved in growth factor. In addition, it is well recognized that bFGF and BMP-2 involving the osteogenic differentiation can bind with heparin and heparan sulfate to stabilize the biological activities [15], [16], [17], [18], [19], [20]. Levenstein et al. report that HSPG secreted from human embryonic stem (ES) cells stabilizes bFGF [21]. Moreover, the binding needs the subsequent intracellular signaling [22]. Considering the in vivo stability of growth factors secreted into the body tissue, it will greatly depend on their inherent nature susceptible to the enzymatic degradation. Therefore, it is likely that the living body should provide some manners to increase the in vivo stability. As a natural stabilizer, the sulfated GAG must be suitable. With the differentiation process of cells, growth factors are secreted. In addition, it is highly conceivable that the cells simultaneously produce GAG to stabilize or enhance the biological functions of growth factors, resulting in promoted the growth factor-based cell differentiation.
The disaccharide analysis was performed to examine the chemical structure of GAG secreted from the cells. The analysis demonstrated that the percentage of ΔHexA α1,4GlcNSO3 and ΔHexA (2-OSO3) α1,4GlcNSO3 increased, whereas that of ΔHexA α1,4GlcNSO3(6-OSO3) decreased.
Turnbull et al. demonstrate that oligosaccharides derived from heparin sulfate with a high affinity for bFGF contain ΔHexA (2-OSO3) αl,4GlcNSO3 more than those with medium and low affinity for bFGF. Oppositely, oligosaccharides derived from heparin sulfate with a low affinity for bFGF contain ΔHexA α1,4GlcNAc(6-OSO3) and ΔHexA α1,4GlcNSO3(6-OSO3) more than those with medium and high affinity for bFGF [16]. In addition, bFGF binds to heparin and to selectively glucosaminyl 6-O-desulfated heparin, but poorly to iduronosyl 2-O-desulfated heparin, and 2-O-sulfate groups thus are essential to the interaction, whereas 6-O-sulfates are not required nor do they interfere with the bFGF binding [17]. Moreover, it is well recognized that the sulfate sequences of heparin and heparan sulfate require iduronosyl 2-O-sulfate groups to bind bFGF and both iduronosyl 2-O-sulfate groups and glucosaminyl 6-O-sulfate groups to form trimeric signaling complex with bFGF and FGF receptor, resulting in activating mitogenicity and angiogenesis of Swiss mouse 3T3 fibroblasts and Chinese hamster ovary cell line 677 (CHO677) [22], [23]. It is demonstrated that a high content of 2-O-sulfate groups in uronate residues and 6-O-sulfate groups in N-sulfated glucosamine residues of heparin are required for activation of both FGF-1 and FGF-2 [24], [25]. In contrast, 6-O-desulfated heparins had no effect on the proliferation of human neural stem/progenitor cells [26]. This implies that different types of cells might require the different sulfation patterns of heparin necessary for their proliferation. Considering the research findings, it is likely that the GAG secreted from MC3T3-E1 cells cultured in the DM to bind and stabilize growth factors rather than to directly promote their biological activity. It is apparent that the GAG secreted from MC3T3-E1 cells cultured in the DM had more 2-O-sulfate groups in uronate residues and less 6-O-sulfate groups in glucosamine than those cultured in the NM. Taken together, we can say with certainty that upon secreting growth factors for differentiation, cells simultaneously produce sulfated GAG to allow the factors to enhance the in vivo stability for promotion of their biological functions.
5. Conclusion
This study has investigated the secretion of glycosaminoglycans (GAG) from MC3T3-E1 pre-osteoblasts of a murine bone calvaria during the osteogenic differentiation. When MC3T3-E1 cells were cultured in the differentiation medium (DM) containing bone morphogenetic protein (BMP)-2, significantly higher amount of GAG secreted was detected for the cells compared with those cultured in the BMP-2-free DM and normal medium with or without BMP-2. The GAG secreted had an affinity for BMP-2 and basic fibroblast growth factor (bFGF). The disaccharide analysis of GAG demonstrated that the percentage of ΔHexA α1,4GlcNSO3 and ΔHexA (2-OSO3) α1,4GlcNSO3 increased, but that of ΔHexA α1,4GlcNSO3(6-OSO3) decreased. It was found that the osteogenic differentiation allowed cells to enhance the secretion of GAG with an affinity for BMP-2 and bFGF.
Conflict of interest
There is no conflict of interest to disclose.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Nurcombe V., Ford M.D., Wildschut J.A., Bartlett P.F. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993;260:103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 2.Brickman Y.G., Ford M.D., Gallagher J.T., Nurcombe V., Bartlett P.F., Turnbull J.E. Structural modification of fibroblast growth factor-binding heparan sulfate at a determinative stage of neural development. J Biol Chem. 1998;273:4350–4359. doi: 10.1074/jbc.273.8.4350. [DOI] [PubMed] [Google Scholar]
- 3.Yeo T.K., Yeo K.T., Wight T.N. Differential transport kinetics of chondroitin sulfate and dermatan sulfate proteoglycan by monkey aorta smooth muscle cells. Arch Biochem Biophys. 1992;294:9–16. doi: 10.1016/0003-9861(92)90129-k. [DOI] [PubMed] [Google Scholar]
- 4.Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991;16:268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher J.T. Heparan sulfate: growth control with a restricted sequence menu. J Clin Invest. 2001;108:357–361. doi: 10.1172/JCI13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling L., Murali S., Dombrowski C., Haupt L.M., Stein G.S., Van Wijnen A.J. Sulfated glycosaminoglycans mediate the effects of FGF2 on the osteogenic potential of rat calvarial osteoprogenitor cells. J Cell Physiol. 2006;209:811–825. doi: 10.1002/jcp.20760. [DOI] [PubMed] [Google Scholar]
- 8.Jiao X., Billings P.C., O'Connell M.P., Kaplan F.S., Shore E.M., Glaser D.L. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282:1080–1086. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- 9.Globus R.K., Plouet J., Gospodarowicz D. Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellularmatrix. Endocrinology. 1989;124(3):1539–1547. doi: 10.1210/endo-124-3-1539. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D., Bab I., Nevo Z. Osteogenic growth peptide regulates proliferation and osteogenic maturation of human and rabbit bone marrow stromal cells. J Bone Miner Res. 1995;10(5):690–696. doi: 10.1002/jbmr.5650100504. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A., Shinoda J., Kanda S., Oiso Y., Kozawa O. Basic fibroblast growth factor stimulates phosphatidylcholine-hydrolyzing phospholipase D in osteoblast-like cells. J Cell Biochem. 1996;63(4):491–499. doi: 10.1002/(sici)1097-4644(19961215)63:4<491::aid-jcb10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.de Jong J.G.N., Wevers R.A., Laarakkers C., Poorthuis B.J.H.M. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- 13.Song S.J., Cool S.M., Nurcombe V. Regulated expression of Syndecan-4 in rat calvaria osteoblasts induced by fibroblast growth Factor-2. J Cell Biochem. 2007;100:402–411. doi: 10.1002/jcb.21068. [DOI] [PubMed] [Google Scholar]
- 14.Zehe C., Engling A., Wegehingel S., Schafer T., Nickel W. Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc Natl Acad Sci USA. 2006;103:15479–15484. doi: 10.1073/pnas.0605997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habuchi H., Suzuki S., Saito T., Tamura T., Harada T., Yoshida K. Structure of a heparan sulphate oligosaccharide that binds to basic fibroblast growth factor. Biochem J. 1992;285:805–813. doi: 10.1042/bj2850805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull J.E., Fernig D.G., Ke Y., Wilkinson M.C., Gallagherg J.T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992;267:10337–10341. [PubMed] [Google Scholar]
- 17.Maccarana M., Casu B., Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993;268:23898–23905. [PubMed] [Google Scholar]
- 18.Guglieri S., Hricovı´ni M., Raman R., Polito L., Torri G., Casu B.S. Minimum FGF2 binding structural requirements of heparin and heparan sulfate oligosaccharides as determined by NMR spectroscopy. Biochemistry. 2008;47:13862–13869. doi: 10.1021/bi801007p. [DOI] [PubMed] [Google Scholar]
- 19.Guerrini M., Hricovíni M., Torri G. Interaction of heparins with fibroblast growth factors: conformational aspects. Curr Pharmaceut Des. 2007;13:2045–2056. doi: 10.2174/138161207781039733. [DOI] [PubMed] [Google Scholar]
- 20.Ruppert R., Hoffmann E., Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 21.Levenstein M.E., Berggren W.T., Lee J.E., Konard K.R., Llanas R.A., Wagner R.J. Secreted proteoglycans directly mediate human embryonic stem cell-FGF2 interactions critical for proliferation. Stem Cell. 2008;26(12):3099–3107. doi: 10.1634/stemcells.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guimond S., Maccarana M., Olwin B.B., Lindahl U., Rapraeger A.C. Activating and inhibitory HeparinS equences for FGF-2( Basic FGF) J Biol Chem. 1993;268:23906–23914. [PubMed] [Google Scholar]
- 23.Lundin L., Larsson H., Kreuger J., Kanda S., Lindahl U., Salmivirta M. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem. 2000;275(32):24653–24660. doi: 10.1074/jbc.M908930199. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara M., Kariya Y., Kikuchi H., Minamisawa T., Yoshida K. Importance of 2-O-sulfate groups of uronate residues in heparin for activation of FGF-1 and FGF-2. J Biochem. 1997;121(2):345–349. doi: 10.1093/oxfordjournals.jbchem.a021593. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara M., Takano R., Kanda T., Hayashi K., Hara S., Kikuchi H. Importance of 6-O-sulfate groups of glucosamine residues in heparin for activation of FGF-1 and FGF-2. J Biochem. 1995;118(6):1255–1260. doi: 10.1093/oxfordjournals.jbchem.a125015. [DOI] [PubMed] [Google Scholar]
- 26.Mori H., Kanemura Y., Onaya J., Hara M., Miyake J., Yamasaki M. Effect of heparin and 6-O- and 2-O-desulfated derivatives with low anticoagulant activity on proliferation of human neural stem/progenitor cells. J Biosci Bioeng. 2005;100(1):54–61. doi: 10.1263/jbb.100.54. [DOI] [PubMed] [Google Scholar]