Abstract
Objective
The aim of this study was to investigate the impact of COPD on the outcomes of patients with advanced chronic kidney disease (CKD).
Patients and methods
All patients with advanced CKD from 2000 to 2010 were identified from the Taiwanese National Health Insurance Research Database. Associations between COPD and the risk of long-term dialysis and all-cause mortality were assessed.
Results
A total of 33,399 advanced CKD patients were enrolled, of whom 31,536 did not have COPD (non-COPD group) and 1,863 had COPD (COPD group). The incidence of end-stage renal disease (ESRD) was higher for those with COPD than those without COPD (744.2 per 1,000 person-years vs 724.6 per 1,000 person-years, adjusted HR [aHR] 1.04; 95% CI 0.96–1.12). The cumulative incidence rates of ESRD were similar between the COPD and non-COPD groups (log-rank test, P=0.356). Overall, the patients with COPD had a higher risk of death than those without COPD (151.7 per 1,000 person-years vs 125.5 per 1,000 person-years, aHR 1.22; 95% CI 1.11–1.33). The cumulative mortality rate was higher in the COPD group than in the non-COPD group (log-rank test, P<0.001).
Conclusion
COPD increased the risk of mortality among the advanced CKD patients in this study, especially the elderly and male patients. In contrast, COPD did not increase the risk of ESRD among the advanced CKD patients.
Keywords: COPD, CKD, ESRD, mortality
Introduction
COPD population is gradually increasing worldwide,1,2 and comorbidities including heart failure, ischemic heart disease, lung cancer, depression/anxiety, arrhythmia, sleep apnea, hypertension, and diabetes mellitus often coexist with COPD.3–5 In addition, a recent meta-analysis6 showed that COPD patients carry a higher risk of developing chronic kidney disease (CKD) (OR 2.20; 95% CI 1.83–2.65). Furthermore, a nationwide case cohort study7 using the Taiwan National Health Insurance Research Database (NHIRD) reported similar findings in which the overall incidence of CKD was higher among patients with COPD (470.9 per 10,000 person-years) than in those without COPD (287.52 per 10,000 person-years), with an adjusted HR (aHR) of 1.61 (95% CI 1.52–1.72). Conversely, a Korean population-based study8 reported that CKD could be one of the risk factors for COPD. Moreover, another study reported a 41% (95% CI 1.31–1.52) increased risk of mortality among COPD patients.9
Advanced CKD is the most severe form of CKD, which involves a severe reduction in glomerular filtration rate. Early detection and appropriate management of advanced CKD can help improve long-term morbidity and slow the deterioration of renal function. Despite the complicated association between COPD and CKD that has been reported, information regarding the impact of COPD on advanced CKD is limited. Thus, this study investigated the impact of COPD on two major outcomes: the risk of end-stage renal disease (ESRD) and mortality of advanced CKD patients.
Patients and methods
Data sources
The Taiwan NHIRD is established by the National Health Research Institute (NHRI) and includes detailed medical care records of more than 97% of the Taiwanese residents. NHIRD included all claims data for outpatient visits, prescriptions, inpatient care records, and disease. This study obtained ethical approval from the institutional review board (IRB) of National Taiwan University Hospital. The used data from NHIRD were de-identified; therefore, the IRB waived informed consent from the enrolled patients.
Study group
All patients aged ≥40 years who were diagnosed with CKD and received erythropoiesis-stimulating agent (ESA) treatment from January 1, 2000, to December 31, 2010, were identified from NHIRD. Due to the reimbursement regulations of the National Health Insurance (NHI), only anemic patients with advanced CKD with a hematocrit level of ≤28% and a serum creatinine level of >6 mg/dL can be prescribed ESAs. In this study, we defined advanced CKD as the presence of CKD coding and concomitant reimbursements for prescriptions of ESA like the previous study.10 The index date was defined as the date of the first ESA. Patients were excluded if they commenced dialysis before the index date, received dialysis, or died within 90 days after the index date, or if they received a renal transplant.
The COPD group comprised patients with advanced CKD and incident COPD within 1 year after the index hospitalization. To ensure accuracy, the diagnosis of COPD was validated based on one inpatient or three outpatient records of International Classification of Diseases (ICD), ninth revision, Clinical Modification (ICD-9-CM) codes 491, 492, and 496 and the prescription of at least one bronchodilator during the follow-up period.11–13 Patients without COPD and no history of asthma or COPD medications were included in the control (non-COPD) group.
Initially, 78,551 eligible patients with advanced CKD were identified between January 1, 2000, and December 31, 2010. Then, 41,361 patients who had a history of dialysis in the previous year, 2,040 patients aged <40 years or >100 years, 669 patients who received a kidney transplantation, and 1,082 patients who died within 3 months were excluded. The remaining 33,399 patients with advanced CKD were included for further analysis.
Research variables
Baseline underlying diseases/conditions including CKD were defined as at least three outpatient visits or one inpatient claim within 1 year preceding the first ESA prescription like previous studies.14,15 We collected the Charlson comorbidity index using baseline comorbidities as previously described.16 The prescriptions that might be associated with the risks of dialysis or all-cause mortality were identified if they were used within 30 days before the events, including alpha-blocker, beta-blocker, calcium channel blocker, diuretics, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), aspirin, clopidogrel, ticlopidine, dipyridamole, nitrate, statin, and proton pump inhibitor.
Outcome measures
The follow-up period started from the index date to the date of commencing long-term dialysis, death, or December 31, 2010, whichever occurred first. The primary end point was the long-term dialysis,17 and the secondary end point was all-cause mortality.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are described as counts and percentages. We used R software, version 2.8.1 (Free Software Foundation, Inc., Boston, MA, USA) for the statistical analysis. Given the differences in baseline characteristics and risk of long-term dialysis or all-cause mortality between the incident COPD and non-COPD groups, we matched the COPD patients with non-COPD patients using a greedy matching algorithm with a caliper width of 0.2 SD of the log of the odds of the estimated propensity score at a 1:1 ratio. Crude HRs with 95% CIs for the outcomes of interest were derived from Cox proportional hazards models, with matched individuals without COPD constituting the reference group. Because of the high mortality rate in patients with COPD and advanced CKD, competing risk regression was also performed using the Fine and Gray model considering the sub-distribution hazard.18,19
Results
Patient characteristics
Among the 33,399 patients, 31,536 did not have COPD (non-COPD group) and 1,863 patients had COPD (COPD group). Significant differences were noted between the two groups in demographic characteristics and baseline comorbidities. In addition, we found significant differences between the two groups in medications for hypertension and other diseases. Thus, pairwise matching (1:1) of the non-COPD and COPD groups was used and resulted in two similar subgroups with 1,820 patients in each. No significant differences were noted regarding age, gender, monthly income, hospital level, Charlson scores, underlying disease, and antihypertension and cardiovascular agents between these two groups (Table 1).
Table 1.
Demographics and characteristics of COPD cohort and matched non-COPD cohort
| Variables | Non-COPD cohort (n=1,820) | COPD cohort (n=1,820) | Standardized difference |
|---|---|---|---|
| Age (years, mean ± SD) | 71.53±10.86 | 71.56±10.88 | −0.003 |
| Male gender | 1,145 (62.91%) | 1,142 (62.75%) | −0.003 |
| Monthly income (New Taiwan dollar), n (%) | |||
| <19,100 | 991 (54.45%) | 973 (53.46%) | 0.010 |
| 19,100–41,999 | 749 (41.15%) | 784 (43.08%) | −0.026 |
| ≥42,000 | 80 (4.40%) | 63 (3.46%) | 0.048 |
| Hospital level, n (%) | |||
| Urban | 685 (37.64%) | 697 (38.30%) | −0.008 |
| Suburban | 453 (24.89%) | 455 (25.00%) | −0.002 |
| Rural | 682 (37.47%) | 668 (36.70%) | 0.014 |
| Baseline comorbidities | |||
| Charlson score | 3.63±1.74 | 3.57±1.75 | 0.034 |
| Myocardial infarction | 36 (1.98%) | 45 (2.47%) | 0.034 |
| Congestive heart failure | 368 (20.22%) | 368 (20.22%) | 0.000 |
| Peripheral vascular disease | 31 (1.70%) | 24 (1.32%) | −0.032 |
| Cerebrovascular disease | 201 (11.04%) | 195 (10.71%) | −0.011 |
| Dementia | 42 (2.31%) | 52 (2.86%) | 0.035 |
| Rheumatologic disease | 10 (0.55%) | 19 (1.04%) | 0.056 |
| Peptic ulcer disease | 380 (20.88%) | 374 (20.55%) | −0.008 |
| Hemiplegia or paraplegia | 9 (0.49%) | 10 (0.55%) | 0.008 |
| Diabetes | 829 (45.55%) | 794 (43.63%) | −0.039 |
| Moderate or severe liver disease | 106 (5.82%) | 82 (4.51%) | −0.060 |
| Asthma | 187 (10.27%) | 190 (10.44%) | 0.005 |
| Medication for hypertension | |||
| Alpha-blocker | 399 (21.92%) | 420 (23.08%) | 0.028 |
| Beta-blocker | 818 (44.95%) | 814 (44.73%) | −0.004 |
| Calcium channel blocker | 1,358 (74.62%) | 1,360 (74.73%) | 0.003 |
| Diuretic | 1,255 (68.96%) | 1,258 (69.12%) | 0.004 |
| ACEI or ARB | 1,051 (57.75%) | 1,044 (57.36%) | −0.008 |
| Other medications | |||
| Aspirin | 193 (10.60%) | 197 (10.82%) | 0.007 |
| Clopidogrel | 142 (7.80%) | 155 (8.52%) | 0.026 |
| Ticlopidine | 49 (2.69%) | 57 (3.13%) | 0.026 |
| Dipyridamole | 656 (36.04%) | 666 (36.59%) | 0.011 |
| Nitrate | 30 (1.65%) | 36 (1.98%) | 0.025 |
| Statin | 398 (21.87%) | 407 (22.36%) | 0.012 |
| Proton pump inhibitor | 264 (14.51%) | 280 (15.38%) | 0.025 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
The incidence of ESRD and mortality
Table 2 summarizes the event rates of ESRD and mortality in the matched COPD and non-COPD cohorts. The incidence of ESRD was 744.2 per 1,000 person-years in the COPD group and 724.6 per 1,000 person-years in the non-COPD group. However, this difference did not reach statistical significance (aHR 1.04; 95% CI 0.96–1.12; Table 2). A similar trend was noted in competing analysis in which we treated mortality as a competing risk. The mortality rate was 151.7 per 1,000 person-years in the COPD group and 125.5 per 1,000 person-years in the non-COPD group. Overall, the patients with COPD had a higher risk of mortality (aHR 1.22; 95% CI 1.11–1.33) than those without COPD (Table 2). The cumulative incidence rates of ESRD were similar between the COPD and non-COPD groups (log-rank test, P=0.356; Figure 1). In contrast, the mortality rate was higher in the COPD group than in the non-COPD group (log-rank test, P<0.001; Figure 2).
Table 2.
Cumulative incidence rates and HRs of ESRD and mortality in COPD and non-COPD patients
| COPD
|
Non-COPD
|
Crude HR (95% CI) |
aHRa (95% CI) | Competing risk
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Person-year | IRb | Event | Person-year | IRb | aHRa (95% CI) | |||
| ESRD | 1,443 | 1,939.08 | 744.2 | 1,429 | 1,972.08 | 724.6 | 1.03 (0.96, 1.11) | 1.04 (0.96, 1.12) | 1.04 (0.96, 1.11) |
| Mortality | 994 | 6,553.03 | 151.7 | 838 | 6,679.34 | 125.5 | 1.21 (1.10, 1.33) | 1.22 (1.11, 1.33) | |
Notes:
Adjusted for propensity score.
IR per 1,000 person-years.
Abbreviations: aHR, adjusted HR; ESRD, end-stage renal disease; IR, incidence rate.
Figure 1.
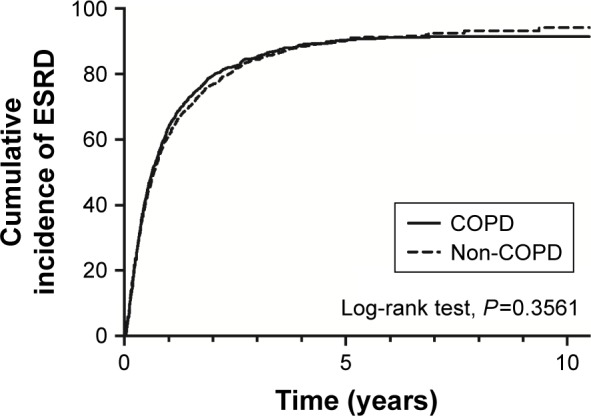
Cumulative incidence of ESRD in patients with or without COPD.
Abbreviation: ESRD, end-stage renal disease.
Figure 2.
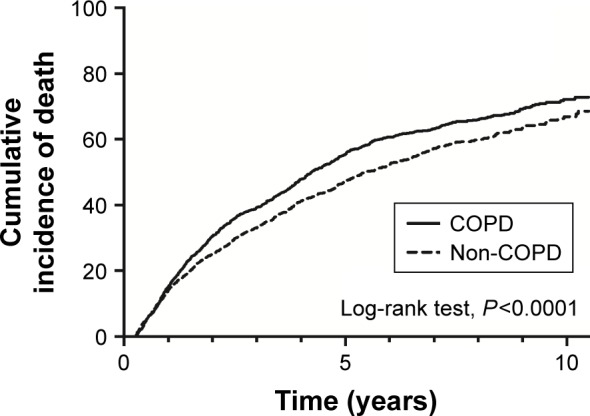
Cumulative incidence of death in patients with or without COPD.
Subgroup analysis
Although we performed further subgroup analysis, we did not find significant differences in terms of ESRD among the age and gender groups. However, a trend of a higher risk of ESRD with age was observed (Table 3). For the CKD patients aged 55–69 and ≥70 years, those with COPD had a significantly higher risk of mortality than those without COPD. In contrast, no significant difference was found in the CKD patients aged 40–54 years. A similar trend was observed in the male patients. For the female patients, those with COPD had a higher risk of mortality than those without COPD; however, none of the differences reached statistical significance (Table 4).
Table 3.
Rates and risk ratio of cumulative incidence of ESRD in COPD and non-COPD patients by age and gender
| Cumulative incidence by age
|
||||
|---|---|---|---|---|
| All age | 40–54 years | 55–69 years | >70 years | |
| COPD patients | ||||
| ESRD (n) | 1,443 | 140 | 459 | 844 |
| COPD patients (n) | 1,820 | 153 | 525 | 1,142 |
| Rate in COPD patients | 0.79 | 0.92 | 0.87 | 0.74 |
| Non-COPD patients | ||||
| ESRD (n) | 1,429 | 146 | 474 | 809 |
| Non-COPD patients (n) | 1,820 | 153 | 531 | 1,136 |
| Rate in non-COPD patients | 0.79 | 0.95 | 0.89 | 0.71 |
| Risk ratio (95% CI) | 1.01 (0.98, 1.04) | 0.959 (0.90, 1.02) | 0.98 (0.94, 1.02) | 1.04 (0.99, 1.09) |
| COPD male | ||||
| ESRD (n) | 889 | 80 | 276 | 533 |
| COPD male (n) | 1,142 | 89 | 322 | 731 |
| Rate in COPD male | 0.78 | 0.90 | 0.86 | 0.73 |
| Non-COPD male | ||||
| ESRD (n) | 889 | 91 | 296 | 502 |
| Non-COPD male (n) | 1,145 | 95 | 330 | 720 |
| Rate in non-COPD male | 0.78 | 0.96 | 0.90 | 0.70 |
| Risk ratio | 1.00 (0.96, 1.05) | 0.94 (0.87, 1.02) | 0.96 (0.90, 1.01) | 1.05 (0.98, 1.12) |
| COPD female | ||||
| ESRD (n) | 554 | 60 | 183 | 311 |
| COPD female (n) | 678 | 64 | 203 | 411 |
| Rate in COPD female | 0.82 | 0.94 | 0.90 | 0.76 |
| Non-COPD female | ||||
| ESRD (n) | 540 | 55 | 178 | 307 |
| Non-COPD female (n) | 675 | 58 | 201 | 416 |
| Rate in non-COPD female | 0.80 | 0.95 | 0.89 | 0.74 |
| Risk ratio | 1.02 (0.97, 1.08) | 0.99 (0.91, 1.08) | 1.02 (0.95, 1.09) | 1.03 (0.95, 1.11) |
Abbreviation: ESRD, end-stage renal disease.
Table 4.
Rates and risk ratio of cumulative incidence of death in COPD and non-COPD patients by age and gender
| Cumulative incidence by age
|
||||
|---|---|---|---|---|
| All age | 40–54 years | 55–69 years | >70 years | |
| COPD patients | ||||
| Death (n) | 994 | 35 | 258 | 701 |
| COPD patients (n) | 1,820 | 153 | 525 | 1,142 |
| Rate in COPD patients | 0.55 | 0.23 | 0.49 | 0.61 |
| Non-COPD patients | ||||
| Death (n) | 838 | 32 | 206 | 600 |
| Non-COPD patients (n) | 1,820 | 153 | 531 | 1,136 |
| Rate in non-COPD patients | 0.46 | 0.21 | 0.39 | 0.53 |
| Risk ratio (95% CI) | 1.19 (1.11, 1.27) | 1.09 (0.72, 1.67) | 1.27 (1.10, 1.45) | 1.16 (1.08, 1.25) |
| COPD male | ||||
| Death (n) | 652 | 22 | 176 | 454 |
| COPD male (n) | 1,142 | 89 | 322 | 731 |
| Rate in COPD male | 0.57 | 0.25 | 0.55 | 0.62 |
| Non-COPD male | ||||
| Death (n) | 523 | 24 | 132 | 367 |
| Non-COPD male (n) | 1,145 | 95 | 330 | 720 |
| Rate in non-COPD male | 0.46 | 0.25 | 0.40 | 0.51 |
| Risk ratio | 1.25 (1.15, 1.36) | 0.98 (0.59, 1.62) | 1.37 (1.16, 1.61) | 1.22 (1.11, 1.34) |
| COPD female | ||||
| Death (n) | 342 | 13 | 82 | 247 |
| COPD female (n) | 678 | 64 | 203 | 411 |
| Rate in COPD female | 0.50 | 0.20 | 0.40 | 0.60 |
| Non-COPD female | ||||
| Death (n) | 315 | 8 | 74 | 233 |
| Non-COPD female (n) | 675 | 58 | 201 | 416 |
| Rate in non-COPD female | 0.47 | 0.14 | 0.37 | 0.56 |
| Risk ratio | 1.08 (0.97, 1.21) | 1.47 (0.66, 3.30) | 1.10 (0.86, 1.40) | 1.07 (0.96, 1.21) |
Discussion
In this study, we used a national database to assess the impact of COPD on the renal outcomes and mortality of advanced CKD patients. We found that the advanced CKD patients with COPD had a significantly higher risk of mortality than those without COPD (aHR 1.22; 95% CI 1.11–1.33). A similar finding was reported in the study based on CKD registry records at the Cleveland Clinic, in which COPD was associated with a 41% increased risk (95% CI 1.31–1.52) of mortality among patients with stage 3 and 4 CKD.9 Another survey20 of 3,358 patients who underwent vascular surgery revealed that both moderate and severe COPD were associated with increased mortality in CKD patients compared to patients without COPD (HR 1.27; 95% CI 1.03–1.56 and HR 1.61; 95% CI 1.10–2.35, respectively). Overall, our findings are in line with previous studies,9,20 suggesting that COPD can negatively impact mortality rates in patients with advanced CKD.
Navaneethan et al9 reported that significant associations between mortality and COPD were more pronounced in younger CKD patients and female patients with CKD. In contrast, the negative impact of COPD on the mortality rate of the advanced CKD patients in this study was noted only in patients aged older than 55 years and in male patients, but not in the female patients. Further studies are warranted to investigate this issue among specific groups.
Although the risk of ESRD was higher in the COPD group than in the non-COPD group in this study, the difference did not reach statistical significance (aHR 1.04; 95% CI 0.96–1.12). In the subgroup analysis according to age and gender, we still did not find any significant differences regarding the risk of ESRD between the COPD and non-COPD groups. Thus, our findings suggest that COPD does not affect the risk of ESRD among advanced CKD patients and also that COPD itself may not impact the renal outcomes of COPD patients.
In addition, the prevalence of COPD among the advanced CKD patients was about 5.6% (1,863/33,399) in this study. This result is consistent with the findings of one recent investigation in a large health care system among 5,960 patients with stage 3 and 4 CKD, of whom 4.7% (n=2,667) had underlying COPD.9 Another study of CKD and COPD among patients who had undergone vascular surgery reported that 4.9% (n=45) of 918 patients with a glomerular filtration rate of <60 mL/min/1.73 m2 had COPD and that the prevalence of COPD was inversely associated with renal function.20 Overall, these findings suggest that a significant proportion of CKD patients have underlying COPD and that clinicians should be alert to the presence of COPD among patients with CKD.
Because this study aimed to investigate the effect of COPD on the long-term risk of dialysis and mortality among patients with advanced CKD, we excluded patients who commenced dialysis before the index date and died within 90 days after the index date. However, CKD itself can also affect the long-term outcome of COPD patients,21 and even acute kidney injury was associated with inhospital mortality among patients with COPD exacerbation.22 Thus, the interaction between CKD and COPD could be bidirectional, and further study should be warranted to clarify their interaction.
This study had one major strength. We enrolled a large cohort of CKD patients with a long follow-up period. Therefore, we can include many known confounding factors to minimize their possible effects. However, this study had several limitations. Some confounding factors, such as lung function test and clinical symptoms/signs were not available from the NHIRD. Therefore, we cannot assess the severity of COPD. In addition, we can obtain only the data on all-cause mortality from NHIRD, so we cannot investigate specific causes of mortality. Moreover, data based on ICD-9-CM codes could not give information on pathology severity, functional status, or intensity of care given, including aggressive therapy and devices used. Diagnosis based on ICD-9-CM codes could be influenced from individual ability and codifying hospital procedures. Finally, we could not ascribe worsening renal function to any specific cause based on this database.
Conclusion
COPD increased the risk of mortality among the patients with advanced CKD in this study, especially the elderly and male patients. In contrast, COPD did not affect the risk of ESRD among the patients with advanced CKD.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Landis SH, Muellerova H, Mannino DM, et al. Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi: 10.2147/COPD.S61854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–494. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 4.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi: 10.1016/j.rmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 6.Gaddam S, Gunukula SK, Lohr JW, Arora P. Prevalence of chronic kidney disease in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med. 2016;16(1):158. doi: 10.1186/s12890-016-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CY, Liao KM. Chronic Obstructive Pulmonary Disease is associated with risk of Chronic Kidney Disease: A Nationwide Case-Cohort Study. Sci Rep. 2016;6:25855. doi: 10.1038/srep25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MY, Boo S, Yoo M, Lee J, Kang NR. Impact of chronic kidney disease among Korean adults with chronic obstructive pulmonary disease. Int Urol Nephrol. 2017;49(7):1225–1232. doi: 10.1007/s11255-017-1572-4. [DOI] [PubMed] [Google Scholar]
- 9.Navaneethan SD, Schold JD, Huang H, et al. Mortality Outcomes of Patients with Chronic Kidney Disease and Chronic Obstructive Pulmonary Disease. Am J Nephrol. 2016;43(1):39–46. doi: 10.1159/000444422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu TW, Liu JS, Hung SC, et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174(3):347–354. doi: 10.1001/jamainternmed.2013.12700. [DOI] [PubMed] [Google Scholar]
- 11.Li CH, Chen WC, Liao WC, et al. The association between chronic obstructive pulmonary disease and Parkinson’s disease: a nationwide population-based retrospective cohort study. QJM. 2015;108(1):39–45. doi: 10.1093/qjmed/hcu136. [DOI] [PubMed] [Google Scholar]
- 12.Huang JY, Jian ZH, Nfor ON, et al. The effects of pulmonary diseases on histologic types of lung cancer in both sexes: a population-based study in Taiwan. BMC Cancer. 2015;15:834. doi: 10.1186/s12885-015-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao KM, Chen CY. Incidence and risk factors of atrial fibrillation in Asian COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2523–2530. doi: 10.2147/COPD.S143691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu VC, Hu YH, Wu CH, et al. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014;67(10):1139–1149. doi: 10.1016/j.jclinepi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Ou SM, Chen YT, Chao PW, et al. Nonsteroidal anti-inflammatory drug use is associated with cancer risk reduction in chronic dialysis patients. Kidney Int. 2013;84(1):198–205. doi: 10.1038/ki.2013.79. [DOI] [PubMed] [Google Scholar]
- 18.Wu VC, Chueh SJ, Chen L, et al. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–1708. doi: 10.1097/HJH.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 19.Wu VC, Chang CH, Wang CY, et al. Risk of Fracture in Primary Aldosteronism: A Population-Based Cohort Study. J Bone Miner Res. 2017;32(4):743–752. doi: 10.1002/jbmr.3033. [DOI] [PubMed] [Google Scholar]
- 20.van Gestel YR, Chonchol M, Hoeks SE, et al. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrol Dial Transplant. 2009;24(9):2763–2767. doi: 10.1093/ndt/gfp171. [DOI] [PubMed] [Google Scholar]
- 21.Fedeli U, de Giorgi A, Gennaro N, et al. Lung and kidney: a dangerous liaison? A population-based cohort study in COPD patients in Italy. Int J Chron Obstruct Pulmon Dis. 2017;12:443–450. doi: 10.2147/COPD.S119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbian F, de Giorgi A, Manfredini F, et al. Impact of renal dysfunction on in-hospital mortality of patients with severe chronic obstructive pulmonary disease: a single-center Italian study. Int Urol Nephrol. 2016;48(7):1121–1127. doi: 10.1007/s11255-016-1272-5. [DOI] [PubMed] [Google Scholar]


