Abstract
Purpose
Breast cancer treatments may lead to chronic pain. For some breast cancer survivors (BCS), this experience can develop into the perception of living with chronic pain. The majority of BCS are postmenopausal and have hormone receptor-positive (HR+) breast cancer requiring aromatase inhibitors (Als). Neither the prevalence nor risk factors associated with the perception of living with chronic pain among this population are well defined.
Methods
We conducted a cross-sectional survey among postmenopausal, HR+ BCS who previously took or were currently taking Als. The primary outcome was patients’ perception of living with chronic pain over the past 6 months. We measured pain and demographic and clinical variables. Multivariable logistic regression analysis was performed to evaluate risk factors associated with the perception of chronic pain.
Results
Among 1280 participants, 167 (13%) reported having the perception of living with chronic pain before their breast cancer diagnosis; 426 (34%) reported this perception after completion of non-hormonal cancer treatment. Seventy-eight percent of BCSs reported experiencing at least one type of treatment-related pain within the past 7 days, with 23% experiencing at least three types. The most common types of pain were Al-induced musculoskeletal pain (49%) and pain at the surgery or radiation site (31%). Younger age (< 56), BMI > 25, and the perception of living with chronic pain before diagnosis were risk factors associated with the perception of living with chronic pain.
Conclusions
One in three postmenopausal, HR+ BCS considered themselves to be living with chronic pain. Effective interventions to reduce chronic pain are needed.
Keywords: Breast cancer, Chronic pain, Aromatase inhibitors, Musculoskeletal pain
Introduction
In 2017, an estimated 252,710 new cases of invasive breast cancer and 63,410 new cases of non-invasive breast cancer will be diagnosed in the U.S. and, overall, approximately 40,610 women will die from this disease [1]. Although the majority of women diagnosed with breast cancer will become cancer survivors, many will experience late effects of their breast cancer treatment. Chronic pain is one of the most troublesome side effects of breast cancer treatment and has been associated with worse quality of life [2].
Different types of pain have been reported as a result of breast cancer treatments. Chronic post-surgical pain has been reported in 25–60% of breast cancer survivors, and is consistently correlated with younger age and axillary lymph node dissection [3–6]. Adjuvant hormonal treatments, including aromatase inhibitors (Als), have been associated with persistent musculoskeletal pain in up to 47% of patients [7, 8]. Up to 58% of breast cancer survivors have reported long-term chemotherapy-induced peripheral neuropathy, with symptoms such as tingling, numbness, and pain [9, 10]. Post-chemotherapy arthralgia has also been reported, which usually improves significantly within 3 months of chemotherapy completion [11–13]. In addition, pain associated with rotator cuff syndrome and lymphedema have also been reported [14, 15].
Previous work on breast cancer survivors’ experiences of chronic pain has focused on separate pain symptoms related to the various types described above, and these symptoms have not been correlated with patients’ overall experiences with the pain. Patients’ illness perceptions have been found to play an important role in a range of health experiences and outcomes [16, 17]. However, the association between breast cancer treatments and the types of chronic pain they experience, as well as if they feel that they are living with chronic pain (have the perception of living with chronic pain) is not well understood.
Considering the significant number of breast cancer survivors affected by chronic pain and the multiple possible coexisting pain syndromes that may exist in a given patient, there is a dearth of research examining the overall experience of this often-debilitating experience. Our current study aimed to fill this gap by describing the prevalence of the perception of living with chronic pain and identifying the variables associated with having this perception among a large cohort of breast cancer survivors who were taking or had taken an AI. Going forward, the information generated from this study will enable us to identify both patients at a higher risk of developing the perception of living with chronic pain, as well as potential treatment targets and corresponding strategies for pain management. As such, our findings may help direct interventions to improve quality of life in breast cancer survivors.
Design and methods
Study population
All participants were patients enrolled in the baseline assessment of the Wellness after Breast Cancer (WABC) trial, a longitudinal prospective study focused on identifying biological determinants of symptom distress and disease outcomes in women with hormone receptor-positive breast cancer taking Als. Details of the study design have been published previously [18]. We initially recruited survey participants from breast cancer clinics in an academic tertiary care teaching hospital and a community hospital between November 2011 and June 2014. Eligible participants were postmenopausal women aged 18 or older with a history of stage I to stage III hormone receptor-positive breast cancer who had taken or were currently taking an AI. Trained research assistants approached potential study subjects in the waiting areas of oncology clinics and, after obtaining written informed consent, asked participants to complete a survey. The Institutional Review Board of the University of Pennsylvania approved the study protocol.
Study variables
The primary outcome, the patient’s perception of chronic pain, was assessed with the following yes or no question: “During the past 6 months, do you consider yourself to be a person who lives with chronic pain?” To understand the type of pain patients experienced, we asked them to list their painful symptoms and the medication that they had taken for pain within the past 7 days. In addition, we measured pain severity and pain interference with function on an 11-point numerical rating scale using the Brief Pain Inventory (BPI) [19]. Socio-demographic factors, including age, race, education, employment, and marital/relationship status, were also collected.
In addition, research assistants abstracted medical records to obtain clinical treatment characteristics, including most recent body mass index (BMI), time since diagnosis, treatment types [chemotherapy (yes or no), type of chemotherapy agent, type of surgery, reconstruction surgery (yes or no)], radiation (yes or no), and AI treatment-specific information. Since the existing literature indicates that previous pain experience is the most significant predictor of future pain [20], we measured each patient’s perception of living with chronic pain prior to breast cancer diagnosis with the following yes or no question: “Before your breast cancer diagnosis, did you consider yourself to be a person who lived with chronic pain?”
Statistical analyses
We performed statistical analyses using STATA 13.0 (StataCorp, College Station, TX). We summarized and reported demographic and clinical variables as well as study outcomes using standard descriptive statistics. Univariate logistic regressions were used to identify independent predictors of the perception of living with chronic pain. We built the multivariable logistic regression model with the perception of living with chronic pain versus no such perception as the dependent variable. Demographic and clinical variables associated with the perception of living with chronic pain at a p value of < 0.10 in the univariate logistic regression were included in the multivariable logistic regression model. Statistical tests were two-sided, and a p value of < 0.05 was considered statistically significant.
Results
Of 1518 consecutive patients screened, 1321 (87%) agreed to participate and provided consent. Among the 197 (13%) who declined (13%), the main reasons were lack of time to complete the survey (n = 62, 31%), did not want to participate in research (n = 85, 43%), and ineligible (n = 50, 25%). Additionally, 15 subjects withdrew from the study and 26 subjects did not return or adequately complete their surveys, resulting in the final sample size of 1280 (87% of eligible subjects).
Study population
Demographic and clinical characteristics of the study population are presented in Table 1. The majority (56.3%) aged 56–70 years, were white (82.3%), overweight (29.4%) or obese (31.2%), and within 5 years of their cancer diagnosis (65.6%). At the time of the survey, 901 (70.5%) of the participants were currently taking an AI and 778 (60.4%) had been on an AI for less than 3 years.
Table 1.
Characteristics associated with patient perception of living with chronic pain
| n = 1280 | Overall |
Perception of currently living with chronic pain |
|||
|---|---|---|---|---|---|
| n | Row (%) | n | Row (%) | p value | |
| Overall | 1280 | – | 426 | 33.6 | |
| Socio-demographic | |||||
| Age | 0.002 | ||||
| < 56 | 255 | 19.9 | 108 | 42.9 | |
| 56–70 | 721 | 56.3 | 221 | 31.0 | |
| > 70 | 304 | 23.8 | 97 | 32.0 | |
| Race | 0.80 | ||||
| White | 1053 | 82.3 | 352 | 33.8 | |
| Non-white | 227 | 17.7 | 74 | 32.9 | |
| Education | 0.54 | ||||
| High school or less | 263 | 20.6 | 87 | 33.5 | |
| Some college or trade school | 253 | 19.9 | 93 | 37.0 | |
| 4-year college degree | 315 | 24.7 | 98 | 31.2 | |
| Graduate or professional degree | 443 | 34.8 | 147 | 33.4 | |
| Employment | 0.59 | ||||
| Employed | 623 | 48.7 | 211 | 34.4 | |
| Not currently employed | 656 | 51.3 | 215 | 32.9 | |
| Marital/relationship status | 0.74 | ||||
| Living alone | 500 | 39.1 | 164 | 33.1 | |
| Married/living with partner | 779 | 60.9 | 262 | 34.0 | |
| Clinical | |||||
| BMI | < 0.001 | ||||
| Underweight and normal (< 25) | 504 | 39.4 | 127 | 25.5 | |
| Overweight (25–30) | 376 | 29.4 | 129 | 34.6 | |
| Obese (> 30) | 400 | 31.2 | 170 | 42.9 | |
| Time since diagnosis (years) | 0.040 | ||||
| < 5 | 840 | 65.6 | 298 | 35.9 | |
| 5–10 | 296 | 23.1 | 91 | 30.9 | |
| > 10 | 144 | 11.3 | 37 | 26.1 | |
| Surgery | 0.060 | ||||
| Lumpectomy | 737 | 57.6 | 229 | 31.5 | |
| Mastectomy | 542 | 42.4 | 197 | 36.6 | |
| Axillary surgery | 0.60 | ||||
| Sentinel | 794 | 62.8 | 255 | 32.5 | |
| ALND | 423 | 33.5 | 147 | 35.0 | |
| None | 47 | 3.7 | 17 | 37.0 | |
| Reconstruction surgery | 0.077 | ||||
| No | 876 | 68.6 | 278 | 32.0 | |
| Yes | 401 | 31.4 | 148 | 37.1 | |
| Chemotherapy | 0.37 | ||||
| No chemo | 621 | 48.5 | 194 | 31.7 | |
| Yes, did not included taxane | 128 | 10.0 | 46 | 35.9 | |
| Yes, included taxane | 531 | 41.5 | 186 | 35.3 | |
| Radiation therapy | 0.26 | ||||
| No | 399 | 31.2 | 142 | 35.9 | |
| Yes | 881 | 68.8 | 284 | 32.6 | |
| Currently taking an aromatase inhibitor | 0.41 | ||||
| No | 377 | 29.5 | 119 | 31.8 | |
| Yes | 901 | 70.5 | 305 | 34.2 | |
| Aromatase inhibitor | 0.16 | ||||
| Anastrozole (arimidex) | 1030 | 80.5 | 356 | 34.9 | |
| Exemestane (aromasin) | 70 | 5.5 | 19 | 27.1 | |
| Letrozole (femara) | 180 | 14.0 | 51 | 29.0 | |
| Duration of AI therapy (years) | 0.002 | ||||
| < 1 | 238 | 18.6 | 88 | 37.8 | |
| 1–3 | 535 | 41.8 | 197 | 37.2 | |
| > 3 | 506 | 39.6 | 140 | 27.8 | |
| Chronic pain before cancer | < 0.001 | ||||
| No | 1102 | 86.8 | 275 | 25.0 | |
| Yes | 167 | 13.2 | 150 | 90.4 | |
The bold italic value means <0.05 (statistically significant)
Perception of living with chronic pain and characteristics of pain
Among the 1280 participants, 167 (13%) patients reported having the perception of living with chronic pain prior to their cancer diagnosis. At the time of the survey, after breast cancer diagnosis and completion of non-hormonal cancer treatment, 426 (34%) patients reported having the perception of living with chronic pain over the past 6 months. Among the 167 patients who reported having the perception of living with chronic pain prior to cancer diagnosis, 150 (90%) also reported having this perception after completion of non-hormonal cancer treatments. The most common chronic pain conditions prior to breast cancer diagnosis were lower back pain (88, 53%), osteoarthritis (85, 51%), and neck pain (41, 25%). In this cohort of patients, having undergone lumpectomy versus mastectomy did not affect whether or not they had the perception of living with chronic pain after their non-hormonal treatment (87.2% vs. 94.6%). Among the 1113 patients without chronic pain perception before cancer diagnosis, 275 (25%) reported de novo perception of living with chronic pain after completion of non-hormonal cancer treatment.
Overall, 1002 (78%) participants reported experiencing at least one out of six types of breast cancer treatment-related pain (listed below) over the past 7 days; 596 (47%) reported experiencing at least two types of pain, and 292 (23%) reported at least three types of pain over the past 7 days. Among these participants, AI-induced musculoskeletal pain, pain at the site of surgery or radiation, and neuropathy symptoms (tingling, numbness, or pain) were the most common types of pain reported (Fig. 1). Patients with the perception of living with chronic pain reported a higher proportion of all six types of pain than those without such a perception (Fig. 1).
Fig. 1.
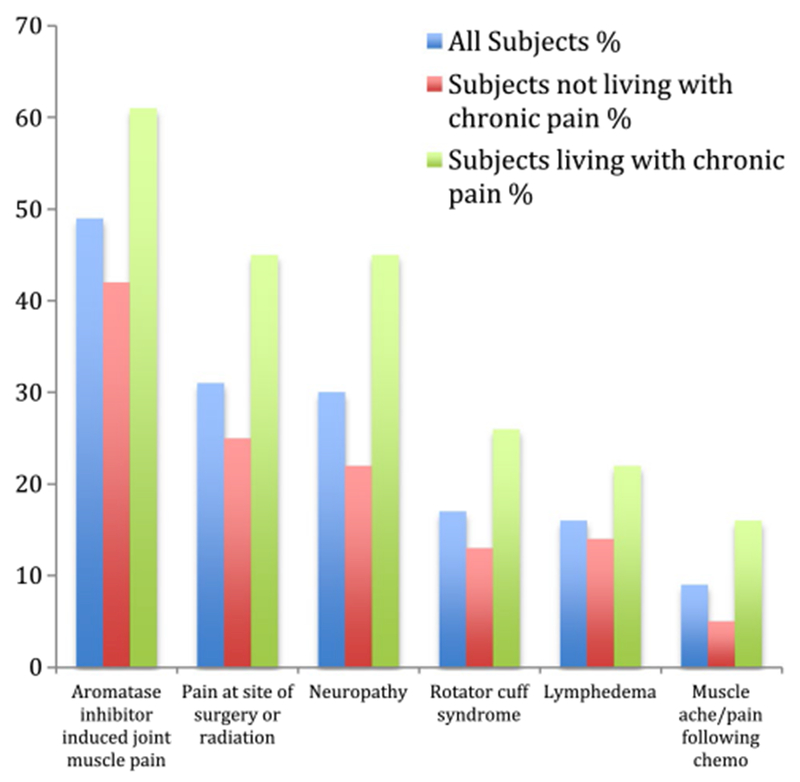
Different pain experienced among subjects over the past 7 days
Risk factors associated with the perception of chronic pain
Chi-square (χ2) analyses (Table 1) showed that age younger than 56 years, BMI greater than 25, < 5 years since diagnosis, less than 3 years of duration on an AI, and having the perception of living with chronic pain before cancer diagnosis were all statistically significantly associated with the perception of living with chronic pain after cancer diagnosis and initial treatment (all p < 0.05). Importantly, race, education level, employment status, marital status, axillary lymph node surgery type, chemotherapy or radiation therapy, currently taking an AI or not, and type of AI were not associated with the perception of living with chronic pain. Having had a mastectomy showed a trend towards an association with a higher perception of living with chronic pain compared to having had a lumpectomy, but did not reach statistical significance. Having had reconstruction surgery showed a trend towards an association with a higher perception of living with chronic pain compared to not having it done, but did not reach statistical significance either.
After adjusting for socio-demographic and clinical covariates (Table 2, multivariable logistic model), younger age [< 56: adjusted odds ratio (AOR) 1.69, 95% confidence interval (CI) 1.10, 2.60; p = 0.016], higher BMI (between 25 and 30: AOR 1.57, 95% CI 1.13–2.19, p = 0.007; BMI > 30: AOR 1.86, 95% CI 1.34-2.58; p < 0.001), and chronic pain prior to cancer diagnosis (AOR 27.9; 95% CI 16.23–47.96; p < 0.001) were all associated with increased odds of having the perception of living with chronic pain.
Table 2.
Multivariate model of patient perception of living with chronic pain
| Factors | Univariate | Model 1 |
||||
|---|---|---|---|---|---|---|
| Multivariate logistic model | ||||||
| OR | 95% CI | p value | AOR | 95% CI | p value | |
| Age | ||||||
| > 70 (reference) | 1 | – | – | 1 | – | – |
| 56–70 | 0.95 | 0.72–1.28 | 0.76 | 0.96 | 0.68–1.35 | 0.80 |
| ≤ 55 | 1.59 | 1.12–2.25 | 0.009 | 1.69 | 1.10–2.60 | 0.016 |
| BMI | ||||||
| Underweight and normal (< 25) (reference) | 1 | – | – | 1 | – | – |
| Overweight (25–30) | 1.54 | 0.15–2.07 | 0.004 | 1.57 | 1.13–2.19 | 0.007 |
| Obese (> 30) | 2.20 | 1.65–2.92 | < 0.001 | 1.86 | 1.34–2.58 | < 0.001 |
| Time since diagnosis (years) | ||||||
| < 5 | 1 | – | – | 1 | – | – |
| 5–10 | 0.80 | 0.60–1.07 | 0.13 | 1.18 | 0.83–1.67 | 0.36 |
| > 10 | 0.63 | 0.42–0.94 | 0.024 | 0.99 | 0.62–1.58 | 0.98 |
| Surgery | ||||||
| Lumpectomy (reference) | 1 | – | – | 1 | – | – |
| Mastectomy | 1.25 | 0.99–1.58 | 0.060 | 1.04 | 0.74–1.48 | 0.82 |
| Reconstructive surgery | ||||||
| No (reference) | 1 | – | – | 1 | – | – |
| Yes | 1.25 | 0.98–1.60 | 0.077 | 1.19 | 0.81–1.74 | 0.37 |
| Duration of AI therapy (years) | ||||||
| < 1 (reference) | 1 | – | – | 1 | – | – |
| 1–3 | 0.98 | 0.71–1.34 | 0.89 | 1.09 | 0.75–1.58 | 0.66 |
| > 3 | 0.63 | 0.46–0.88 | 0.007 | 0.7 | 0.47–1.06 | 0.096 |
| Lived with chronic pain before cancer | ||||||
| No (reference) | 1 | – | – | 1 | – | – |
| Yes | 28.10 | 16.5–47.9 | < 0.001 | 27.90 | 16.23–47.96 | < 0.001 |
The bold italic value means <0.05 (statistically significant)
BPI and medication usage associated with the perception of living with chronic pain
Compared with patients who did not perceive themselves to be living with chronic pain, patients with the perception of living with chronic pain had significantly higher pain severity (mean ± standard deviation: 4.0 ± 2.1 vs. 1.7 ± 1.9) and pain-related interference (3.4 ± 2.4 vs. 1.0 ± 1.6) as measured by the BPI on a 0–10 point scale (Fig. 2). At the time of survey, patients with the perception of living with chronic pain had higher pain medication usage compared to those without this perception (Fig. 3). Importantly, 13.8% of patients with the perception of living with chronic pain versus 2.3% of patients without such perception reported having used narcotics/opioids within the past 7 days (Fig. 3).
Fig. 2.
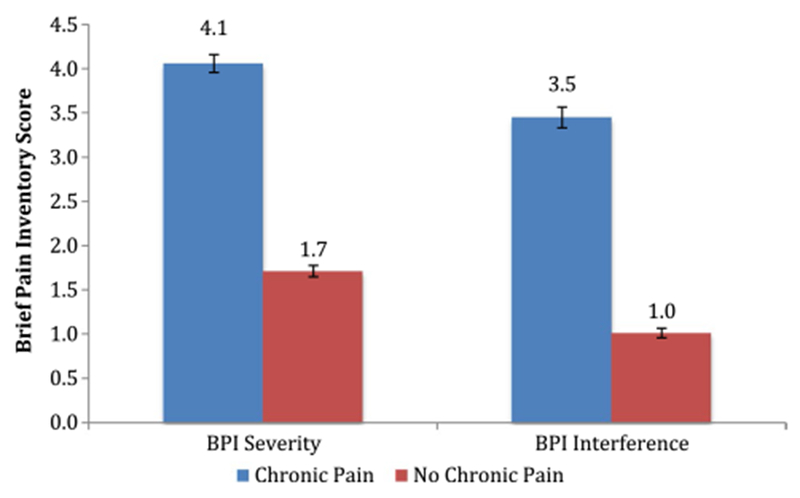
Pain severity and interference scores: chronic pain versus no chronic pain
Fig. 3.
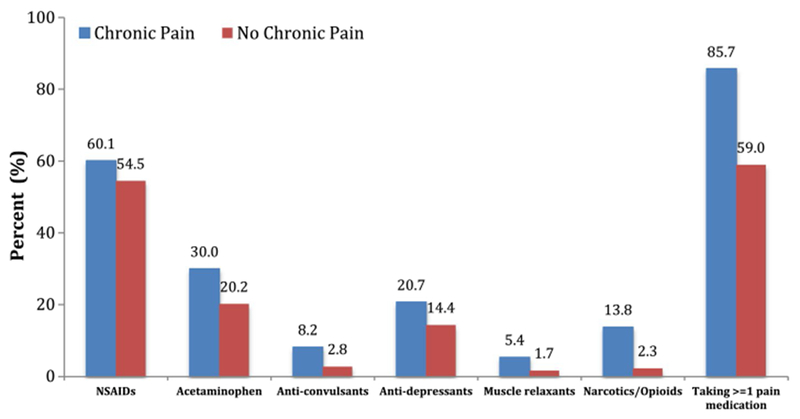
Percentage of subjects using medication for pain management
Discussion
To the best of our knowledge, this is the first study with a large sample size to assess patients’ perception of living with chronic pain within the prior 6 months. Our study demonstrates that among breast cancer survivors treated with an AI, patients’ perception of living with chronic pain almost tripled after non-hormonal cancer treatment. Importantly, 25% of such patients developed de novo chronic pain perception, suggesting that their chronic pain resulted from breast cancer treatments.
Such a sharp increase in having the perception of living with chronic pain is likely due to the different types of pain that resulted from cancer treatments. By providing specific information about the types of conditions and impact of chronic pain perception, our results can help clinicians to better identify and manage chronic pain and factors associated with the perception of chronic pain among their patients. Echoing the recent ASCO clinical practice guideline, “Management of Chronic Pain in Survivors of Adult Cancer,” our results emphasize the need for clinicians to screen for pain at each cancer survivor encounter and provide symptom-targeted pain management [21].
Existing literature focused on post-operative pain has identified a number of potential risk factors for having this type of pain, including younger age, preoperative pain, acute post-operative pain, mastectomy, axillary lymph node dissection, chemotherapy, and radiation [3, 5, 22–25]. Our study showed that the perception of living with chronic pain was not associated with the type of surgery (mastectomy), axillary lymph node dissection, chemotherapy, radiation, or reconstruction surgery. Importantly, current AI usage was not significantly associated with the perception of living with chronic pain among our cohort of patients. This highlights the complex, multifaceted nature of chronic pain among breast cancer survivors on AIs and suggests heterogeneity of sources of chronic pain in this population, including both AI and non-AI causes.
Consistent with prior literature [5], our study showed that younger age is associated with the perception of living with chronic pain when adjusting for clinical and demographic factors only. This could be due to the fact that a higher proportion of younger breast patients have more locally advanced disease and tend to undergo mastectomy versus lumpectomy, receive chemotherapy, and have higher psychosocial stress. An additional study investigating the correlation between psychosocial factors and the perception of living with chronic pain should be conducted.
Also consistent with some prior research [26, 27], our study showed a direct correlation between higher BMI and increased perception of pain. Chronic pain has been associated with pro-inflammatory states such as up-regulation of pro-inflammatory cytokines [28–30]. Obesity has also been associated with elevated pro-inflammatory cytokines [31]. The elevated pro-inflammatory state may serve as a shared mechanism underlying the perception of chronic pain and obesity. Moreover, additional weight stresses joints more and may cause more pain. Lastly, patients with an increased perception of chronic pain are less likely to exercise and therefore, may be more obese. Additional studies to determine if weight loss interventions may help reduce chronic pain perception are warranted.
In our study, the most influential factor for chronic pain after treatment was a patient’s perception of living with chronic pain before cancer, which parallels the results of a longitudinal study conducted among breast cancer survivors in general [20]. While potentially impacted by recall bias, the effect was reinforced by a patient-reported chronic pain condition prior to diagnosis. Unlike most other risk factors, such as age and obesity, chronic pain prior to treatment can be addressed through an effective pain management plan. However, the current system tends to undermanage and undertreat these patients [32]. Our results further support the need for early integration of pain management into cancer care, especially in patients with chronic pain, prior to their cancer diagnosis.
Our study showed that the perception of living with chronic pain is significantly associated with an over twofold increase in pain intensity and three times worsened interference with function (Fig. 2). In addition, having the perception of living with chronic pain is associated with higher pain medication usage, with a sixfold increase in opioid usage (from 2.3 to 13.8%).
Our study’s main limitation is that it was an observational cross-section study and, accordingly, we can only provide an association between groups and cannot conclude if any of the associations are causal. The selection bias inherent in a survey study was minimized by the high participation rate. The recall bias about pre-cancer pain is another potential bias and may over-exaggerate the association between pre-cancer pain and post-breast cancer diagnosis pain. Another limitation was that our survey assessing the perception of living with chronic pain may be subjective and may not accurately capture pain. However, some literature suggests that the perception of an illness is a more important predictor of patient outcomes [16, 17]. Finally, the study is limited in its generalizability as it was conducted among breast cancer patients treated with an AI at a large, urban academic medical center.
Despite these limitations, our study is the first of its kind to analyze the perception of living with chronic pain among a large cohort of breast cancer survivors treated with an AI. We found that one in three women with breast cancer perceive that they are living with chronic pain. Younger age, higher BMI, and chronic pain prior to breast cancer were risk factors. In addition, having the perception of living with chronic pain was strongly associated with multiple types of cancer treatment-related pain, worsening pain, function, and pain medication use. These findings may help lead to the development of risk assessment models to manage pain among breast cancer patients being treated with AIs. As patients with chronic pain perception experience multiple types of pain for which they try multiple medications, our findings suggest that multi-model and team-based interventions may more effectively manage symptoms, and improve functions and quality of life for women with breast cancer.
Acknowledgements
This study was funded in part by a National Cancer Institute R01CA158243 (Mao) and R21CA173263 (Bao), a Memorial Sloan Kettering Cancer Center P30 grant (P30-CA008748), and the Byrne Fund at Memorial Sloan Kettering Cancer Center. The funding sources had no involvement in the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Cancer Facts and Statistics 2017, American Cancer Society, 2017 [Google Scholar]
- 2.Peuckmann V, Ekholm O, Rasmussen NK et al. (2009) Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain 13:478–485 [DOI] [PubMed] [Google Scholar]
- 3.De Oliveira GS Jr, Chang R, Khan SA et al. (2014) Factors associated with the development of chronic pain after surgery for breast cancer: a prospective cohort from a tertiary center in the United States. Breast J 20:9–14 [DOI] [PubMed] [Google Scholar]
- 4.Schou Bredal I, Smeby NA, Ottesen S et al. (2014) Chronic pain in breast cancer survivors: comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manag 48:852–862 [DOI] [PubMed] [Google Scholar]
- 5.Gartner R, Jensen MB, Nielsen J et al. (2009) Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 302:1985–1992 [DOI] [PubMed] [Google Scholar]
- 6.Bruce J, Thornton AJ, Powell R et al. (2014) Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain 155:232–243 [DOI] [PubMed] [Google Scholar]
- 7.Crew KD, Greenlee H, Capodice J et al. (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883 [DOI] [PubMed] [Google Scholar]
- 8.Mao JJ, Strieker C, Bruner D et al. (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115:3631–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao T, Basal C, Seluzicki C et al. (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershman DL, Till C, Wright JD et al. (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J Clin Oncol 34:3014–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amiri AH, Rafiei A (2010) Analysis of patients with post-chemotherapy arthralgia and arthritis in breast cancer. Indian J Med Sci 64:197–203 [PubMed] [Google Scholar]
- 12.Almoallim H, Abdulaziz S, Fallatah E et al. (2017) Clinical characteristics and outcomes of cancer patients with post-chemotherapy arthritis: a retrospective case series report. Open Access Rheumatol 9:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loprinzi CL, Duffy J, Ingle JN (1993) Postchemotherapy rheumatism. J Clin Oncol 11:768–770 [DOI] [PubMed] [Google Scholar]
- 14.Ebaugh D, Spinelli B, Schmitz KH (2011) Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses 77:481–487 [DOI] [PubMed] [Google Scholar]
- 15.DiSipio T, Rye S, Newman B et al. (2013) Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 14:500–515 [DOI] [PubMed] [Google Scholar]
- 16.Kaptein AA, Schoones JW, Fischer MJ et al. (2015) Illness perceptions in women with breast cancer—a systematic literature review. Curr Breast Cancer Rep 7:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mickeviciene A, Vanagas G, Jievaltas M et al. (2013) Does illness perception explain quality of life of patients with prostate cancer? Medicina (Kaunas) 49:235–241 [PubMed] [Google Scholar]
- 18.Mao JJ, Su HI, Feng R et al. (2011) Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res 13:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 23:129–138 [PubMed] [Google Scholar]
- 20.Rief W, Bardwell WA, Dimsdale JE et al. (2011) Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast Cancer Res Treat 130:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paice JA, Portenoy R, Lacchetti C et al. (2016) Management of chronic pain in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 34:3325–3345 [DOI] [PubMed] [Google Scholar]
- 22.Tasmuth T, Kataja M, Blomqvist C et al. (1997) Treatment-related factors predisposing to chronic pain in patients with breast cancer—a multivariate approach. Acta Oncol 36:625–630 [DOI] [PubMed] [Google Scholar]
- 23.Sipila R, Estlander AM, Tasmuth T et al. (2012) Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Br J Cancer 107:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belfer I, Schreiber KL, Shaffer JR et al. (2013) Persistent post-mastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain 14:1185–1195 [DOI] [PubMed] [Google Scholar]
- 25.Poleshuck EL, Katz J, Andrus CH et al. (2006) Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 7:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright LJ, Schur E, Noonan C et al. (2010) Chronic pain, overweight, and obesity: findings from a community-based twin registry. J Pain 11:628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okifuji A, Hare BD (2015) The association between chronic pain and obesity. J Pain Res 8:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windebank AJ, Grisold W (2008) Chemotherapy-induced neuropathy. J Peripher Nerv Syst 13:27–46 [DOI] [PubMed] [Google Scholar]
- 29.Wang XM, Hamza M, Wu TX et al. (2009) Upregulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: correlation to clinical pain. Pain 142:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paley CA, Johnson MI (2016) Physical activity to reduce systemic inflammation associated with chronic pain and obesity: a narrative review. Clin J Pain 32:365–370 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt FM, Weschenfelder J, Sander C et al. (2015) Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 10:e0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisch MJ, Lee JW, Weiss M et al. (2012) Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 30:1980–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]


