Abstract
We present a case of a patient with Anton's syndrome due to decompression illness (DCI) after recreational scuba diving. Visual anosognosia, or denial of loss of vision, which is associated with lack of awareness regarding visual loss in the setting of cortical blindness, is known as Anton’s syndrome (also termed Anton-Babinski syndrome). Our patient presented with progressive neurological DCI treated with repeated recompression. The anosognosia resolved after 48 h. Subsequent echocardiography revealed a persistent (patent) foramen ovale.
Keywords: Decompression sickness, Central nervous system, Anosognosia, Case reports
Introduction
Decompression illness (DCI) is caused by bubble formation in blood or tissues after a reduction in ambient pressure following compressed gas diving. Clinically, DCI involving the central nervous system may present with a spectrum of neurological symptomatology, including rare and atypical syndrome presentations, making diagnosis difficult. We present one of these rare cases, with anosognosia presenting as Anton’s syndrome post recreational diving.
Case report
A 57-year-old male PADI-certified recreational Master Diver with 260 logged dives, with a background of essential hypertension, as well as a longstanding history of recurrent migraine with aura, was brought to the emergency department. He complained of a sudden onset of severe back pain, followed by lethargy, tingling sensation and inability to move both legs 45 minutes after surfacing from his second dive of the day. This was to a maximum depth of 29 metres' sea water (msw) for a total dive duration of 44 minutes on air. He had performed another dive on the same day to a maximum depth of 30.7 msw for a total dive duration of 47 minutes on air, with a 105-minute surface interval between the dives, wherein he ate a beef burger and drank a bottle of carbonated beverage. This was the last of seven separate dives over four days, and he admitted drinking five units of alcohol on the evening before the day of the incident.
On admission, the patient was obtunded and disorientated, and exhibited bilateral lower limb weakness of 2/5 on the MRC Muscle Strength Scale, with up-going left plantar reflex, together with brisk knee and ankle jerks bilaterally; neurological examination of his upper limbs was unremarkable. The patient was unable to perform Romberg's or Unterberger's tests in view of his inability to stand. On cranial nerve testing the most striking clinical feature on examination was severe impairment of visual acuity, with only light perception in both eyes. Despite an objective diminution of his vision, our patient exhibited anosognosia, with regards to his visual defect; when asked to grasp a simple object, he was quick to reach out to grasp it, but he was unable to visually locate it. He started confabulating answers at finger counting wherein no fingers were being displayed by the examining doctor. Pupillary reflexes were intact (suggesting an intact anterior visual pathway), with fundoscopy being unremarkable. His body-mass index was calculated at 39 kg·m⁻², and he was eupnoeic, afebrile, and hypertensive with a blood pressure of 190/100 mmHg, a blood glucose of 9.9 mmol·L⁻² and oxygen saturation of 96% on air. He was able to pass urine normally. He was administered one litre 0.9% sodium chloride intravenously prior to being transferred urgently to the hyperbaric unit for recompression therapy of spinal and cerebral DCI.
He was treated with a COMEX 30 table with 50/50 heliox, with the first 60 minutes at 30 msw (405 kPa), and a total therapeutic table time of 450 minutes excluding compression. The treatment was complicated by profuse vomiting of dark material after reaching 18 msw (284 kPa) during the decompression profile. He exhibited significant resolution of lower limb weakness within the first 60 minutes at 30 msw (405 kPa) and continued to pass urine normally whilst in the recompression chamber. He was admitted to the neurology inpatient service and managed with 4 mg dexamethasone intravenously, urinary catheterization, physiotherapy, intravenous rehydration with 0.9% saline, thromboembolic deterrent (TED) stockings and subcutaneous low molecular weight heparin. The cortical visual defect with associated anosognosia persisted for the first 48 hours, but resolved on follow-up treatment with five daily US Navy Treatment Table 5.
Magnetic resonance imaging (MRI) of the brain demonstrated evidence of widespread, bilateral multiple foci of gyral oedema throughout the cerebral hemispheres and also involving the cerebellum, with corresponding restricted diffusion noted in these regions, consistent with multiple acute emboli bilaterally. Magnetic resonance (MRI) of the spine showed no discernible pathology, despite the patient showing overt neurological signs of spinal DCI. Subsequent trans-thoracic echocardiography showed a persistent (patent) foramen ovale (PFO), with manifest right-to-left shunting through the PFO. All his symptomatology and signs resolved, except for residual bilateral lower limb weakness at 4/5 on the MRC Muscle Strength Scale.
Figure 1.

Occipital lobe involvement, showing right occipital enhancement on MRI (FLAIR)
Figure 2.
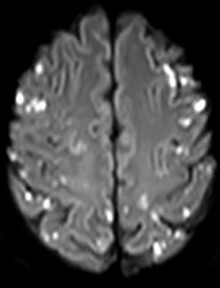
Parietal lobe involvement, showing enhancement subsequent to multiple emboli on MRI (FLAIR)
Discussion
The first documented description of a patient who appeared unaware of his own blindness was by the French writer Montaigne (1533–1592), as he described it in his second book of Les Essais.[ 1] Three centuries later, the neuropsychiatrist Gabriel Anton (1858–1933) described a cohort of patients with blindness and deafness who showed a distinctive lack of awareness of their deficits associated with brain pathology.[ 2] Joseph François Babinski (1857–1932) later coined the term "anosognosia" to describe this unusual symptomatology.
Anton's syndrome is the blatant denial of loss of vision (visual anosognosia) associated with confabulation in the setting of overt visual loss and cortical blindness. Frequently, patients with damage to the occipital lobes bilaterally also have damage to their visual association cortex, which may potentially explain their lack of awareness of the visual deficit.[ 4] Additionally, as suggested by Anton, damaged visual areas are effectively detached from functioning areas, such as speech-language areas; these often confabulate a response to the missing sensory visual information.[ 5] Cerebrovascular disease is by far the most commonly recognized cause of Anton's syndrome,[ 6] although the syndrome has also been reported in hypertensive encephalopathy with preeclampsia,[ 7] obstetric haemorrhage with hypo-perfusion[ 8]; and trauma.[ 9]
Our patient with embolic occipital infarcts causing cortical blindness and visual anosognosia appears to fulfil the classical description for Anton's syndrome. He maintained a striking belief in his visual aptitude despite an obvious deficit. The mechanism for cerebral injury in this case is shunting of nitrogen bubbles through a demonstrated PFO. The time of onset of symptoms, 45 minutes in our case, supports this aetiology, wherein in cases of cerebral arterial gas embolism secondary to pulmonary barotrauma, the onset of symptoms is usually close to or immediately post-surfacing from the dive in question.[ 10] We believe this is the first published case of Anton's syndrome in a recreational scuba diver as part of the clinical presentation of DCI.
We decided to proceed with a COMEX 30 table as opposed to a fully extended US Navy Treatment Table 6 (USN TT6) based on international guidelines, such as DMAC 23 rev.1,[ 11] and on the 30 years of experience at our hyperbaric centre. In cases of life-threatening DCI, USN TT6 may be insufficient, even if extended; the Comex 30 is a more radical tool for shrinking any remaining bubbles and flushing out nitrogen from tissues. Dexamethasone was prescribed by the admitting neurologist, despite being advised by the hyperbaric consultant that there is no evidence base for its use in DCI.[ 12] We postulate that the patient's episode of vomiting during the decompression phase was due to central nervous system oxygen toxicity whilst breathing 100% oxygen at a partial pressure of 284 kPa.[ 13] The ingestion of carbonated beverages and a heavy meal immediately prior to diving, with a gastric carbonated gas bubble prone to expansion in keeping with Boyle's law on ascent, could also have contributed to his episode of vomiting during recompression.
Our patient's diving history and profiles are considered to be provocative for DCI in terms of multi-day repetitive diving, obesity, dehydration and alcohol ingestion. His history of recurrent migraine with aura led us to suspect the possible presence of a right-to-left shunt at either pulmonary or cardiac level, and the presence of a PFO was confirmed. PFO is associated with an increased risk for the development of neurological DCI.[ 14]
MRI of the brain of divers suffering from DCI frequently shows evidence of cortical involvement, especially on FLAIR sequences, although imaging of the spinal cord sometimes fails to evidence any overtly discernible pathology.[ 15] Our patient had clinical evidence of spinal DCI despite the absence of discernible pathology on MRI.
Good recovery of visual function has been noted in hypertensive encephalopathy and cortical hypo-perfusion causing Anton’s syndrome.[ 07 , 08] Correction of the causative factor often leads to resolution of symptoms, and, in our case, prompt recompression therapy led to resolution of the visual deficit and anosognosia within 48 hours from presentation.
Conclusion
This case illustrates the need to maintain a high index of suspicion in assessing the possibility of DCI, in view of the deceptive presentation of DCI when the diver himself is unaware of his deficit and thus not in a position to forward any symptomatology. A thorough neurological examination is an essential part of the assessment of any diver presenting to medical attention post diving.
Footnotes
Conflict of interest: nil
Acknowledgement
We thank the patient for giving written consent after his recovery for publication of his case report and the accompanying images.
Contributor Information
CP Azzopardi, Hyperbaric Unit, Mater Dei Hospital, Msida, Malta.
L Matity, Hyperbaric Unit, Mater Dei Hospital, Msida, Malta.
S Muscat, Hyperbaric Unit, Mater Dei Hospital, Msida, Malta.
References
- Langelier A, editor. Les essais, book 2, de Montaigne M. Vol. 1595. Bordeaux: Simon Millanges; The underwater environment: cardiopulmonary, thermal, and energetic demands . (Fre). [Google Scholar]
- Anton G. Über die selbstwahrnehmung der herderkrankungen des gehirns durch den kranken bei rindenblindheit und rindentaubheit . Arch Psychiatrie Nervenkrankh. 1899;32:86–127. (Ger). [Google Scholar]
- Babinski J. Contribution a l'étude des troubles mentaux dans l'hémiplégie organique (anosognosie) . Revue Neurol. 1914;27:845–848. (Fre). [Google Scholar]
- Heilman KM. Anosognosia: possible neuropsychological mechanisms . Awareness of deficit after brain injury: clinical and theoretical issues. In: Prigatano FP, Schacter DL, editors. Oxford: Oxford University Press; 1991. pp. 53–62. [Google Scholar]
- Gazzaniga MS, editor. New York: Appleton-Century-Crofts; 1970. The bisected brain . [Google Scholar]
- Aldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: etiology, diagnosis, and prognosis . Ann Neurol. 1987;21:149–158. doi: 10.1002/ana.410210207. [DOI] [PubMed] [Google Scholar]
- Misra M, Rath S, Mohanty AB. Anton syndrome and cortical blindness due to bilateral occipital infarction . Indian J Ophthalmol. 1989;37:196. [PubMed] [Google Scholar]
- Argenta PA, Morgan MA. Cortical blindness and Anton syndrome in a patient with obstetric haemorrhage . Obstet Gynecol. 1998;91:810–812. doi: 10.1016/s0029-7844(97)00718-7. [DOI] [PubMed] [Google Scholar]
- McDaniel KD, McDaniel LD. Anton's syndrome in a patient with posttraumatic optic neuropathy and bifrontal contusions . Arch Neurol. 1991;48:101–105. doi: 10.1001/archneur.1991.00530130113028. [DOI] [PubMed] [Google Scholar]
- Francis TJR, Pearson RR, Robertson AG, Hodgson M, Dutka AJ, Flynn ET. Central nervous system decompression sickness: latency of 1070 human cases . Undersea Biomedical Research. 1988;15:403–417. [PubMed] [Google Scholar]
- Diving Medical Advisory Committee . The use of heliox in treating decompression illness . DMAC. 2014. Jun, [cited 2017 January]. Available at: http://www.dmacdiving. org/guidance/DMAC23.pdf.
- Bennett MH, Lehm SJ, Mitch SJ, Wasiak S. Recompression and adjunctive therapy for decompression illness . Cochrane Database Syst Rev. 2012 May;16(5):CD005277. doi: 10.1002/14651858.CD005277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman N. CNS oxygen toxicity . Undersea Hyperb Med. 2004;31:63–72. [PubMed] [Google Scholar]
- Wilmshurst P, Bryson P. Relationship between the clinical features of neurological decompression illness and its causes . Clin Sci. 2000;99:65–75. [PubMed] [Google Scholar]
- Gorman D, Sames C, Drewry A, Bodicoat S. A case of type 3 DCS with a radiologically normal spinal cord . Internal Medicine Journal. 2006;36:193–196. doi: 10.1111/j.1445-5994.2006.01026.x. [DOI] [PubMed] [Google Scholar]


