Abstract
With a growing list of new platforms, end-user acceptability is an evolving topic in point-of-care (POC) test development. While technical reports of new experimental POC tests are common, it is rare to find reports which evaluate the end-user acceptability of such innovations. This work illustrates an example of bridging that gap by evaluating the end-user acceptability of an experimental POC test platform with novel technical features. A prototype smartphone-based STI tests was evaluated by ED technicians, followed by a survey of acceptability factors. Our findings suggest that the end-user acceptability of some design features implemented in the prototype.
Keywords: End-user acceptability, point-of-care testing, sexually transmitted infections, chlamydia, episodic care, emergency departments
Introduction
Point-of-care (POC) tests hold much promise for use in sexually transmitted infections (STIs), where immediate diagnosis and treatment of patients can help facilitate new strategies to address the persistent STI public health burden1,2. Recent POC platforms have successfully incorporated nucleic acid amplification tests (NAAT) to achieve analytical performance on par with laboratory-based reference platforms3. Despite such improvement, barriers that limit the successful implementation of POC tests in clinical settings exist. Insufficient collaboration between developers and end-users can lead to complexity in the use of the device which translate poorly in the clinical setting, resulting in gaps or deficiencies in the usability of the POC device4,5. Furthermore, these fundamental issues reoccur with new emerging POC devices due to insufficient clinical needs assessments from end-users6.
MobiNAAT is a prototype NAAT platform developed for testing chlamydia in episodic care settings using vaginal swabs7. The mobiNAAT assay is a cartridge-based isothermal test, which completes automatically following a few minutes of hands-on sample preparation time in 1 hour. Several design features of this platform address portability, ease of use, and affordability. Namely, the cartridge utilizes a battery-operated, mug-sized (3.4′ × 4.6′ × 6′) instrument for process automation. The platform operates using a smartphone application that contains an on-board tutorial video to train first-time users without direct supervision. The cartridge utilizes droplet magnetofluidic technology to minimize reagent consumption and reduces assay cost to $2 per test. In this study, we sought feedback on these design features from the target end-users based on their primary exposure to the experimental prototype.
Materials and Methods
A cross-sectional survey was conducted in an urban academic Emergency Department (ED) serving a socioeconomically disadvantaged inner-city population. This study was approved by the Johns Hopkins Medicine Institutional Review Board, application number IRB 00088884, and all participants provided written informed consent prior to any study-related activities.
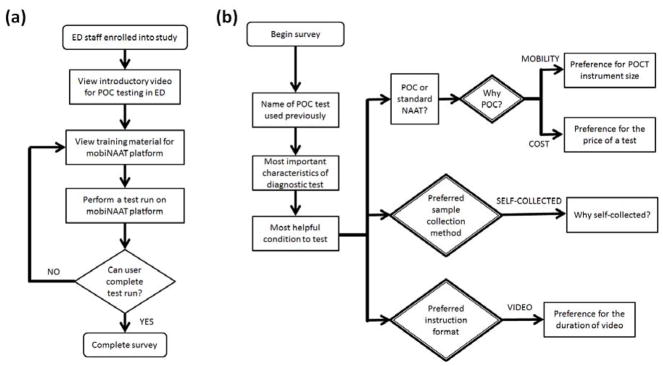
A dedicated study coordinator enrolled 30 ED technicians in August 2016 to assess their acceptability of a prototype microfluidic device for POC testing for genital chlamydial infection. ED technicians (e.g., certified nursing assistants, or patient care technicians) were identified as ideal end-users, as they commonly perform CLIA-waived POC testing such as urinalysis and blood glucose test in urgent care settings. The study workflow is presented in Figure 1a. Test setup included the mobiNAAT instrument, a microcentrifuge tube containing a previously prepared test sample, an assay cartridge, and a micropipettor for sample loading. The 6-minute video tutorial on the cell phone for the POC platform was prefaced with a 2-minute introductory section solely for the purpose of this study, outlining the purpose of POC STI testing in acute care settings. Assay cartridges were assembled and delivered to the ED site up to one week prior to use. Participants watched the video tutorial with instructions on how to perform testing, performed a test run using the microfluidics device, and lastly, completed a brief structured survey assessing acceptability (Figure 1b). The survey was designed to identify end user priorities and preferences for various design parameters such as the assay target, instrument size, instruction time, cost per test, and the format of the instruction materials. In order to gauge end user preference for the instrument size, the survey utilized familiar objects with relative differences in size and geometry (e.g. a watch, a mug or a tablet) as opposed to absolute dimensions. A typical session involved about 15 minutes of instruction and a hands-on setup of the test run by the participant, followed by the completion of the survey.
Figure 1.
Flowcharts describing study workflow and structured survey used in the study. (a) Study workflow. Each participant viewed introductory video tutorial, performed a test run on the experimental platform, and completed the acceptability survey. (b) Structured survey flowchart. The survey asked a series of general questions regarding POC testing, preference for POC test features, sample collection approach, and test instruction format. Results obtained from the decision blocks (double-lined diamond) are shown in Figure 2.
Results
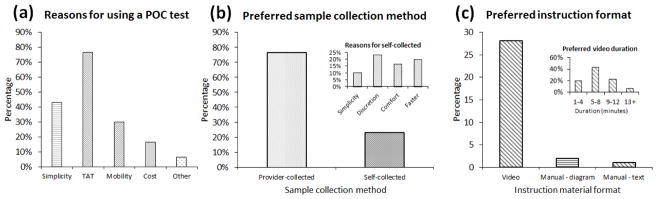
Of the 30 technicians enrolled, 26 (86.7%) had previous experience with a POC test, and POC tests were strongly preferred (83.3%) over standard laboratory-based NAAT (16.7%). Results of the survey indicated that turnaround time (70.0 %), sensitivity (36.7 %), and ease of use (23.3 %) were the parameters perceived to be of the highest importance in an STI diagnostic test. Chlamydia (96.7 %) and gonorrhea (96.7 %) were identified as targets that would be the most helpful to test for among a list of the most prevalent STIs, duplicating this preference among clinician end-users5. As shown in Figure 2a, participants favored the use of POC tests over laboratory-based tests due to their fast turnaround time (76.7 %), simplicity (43.3 %), and mobility (30.0 %). This finding suggests that the primary value of a POC STI test to end-users in an acute care setting is the potential to save time, expedite patient care decisions, and reduce length of stay. Including the time spent viewing instruction videos, a typical assay took 15 minutes to set up by a first-time user and was completed in 1 hour. Notably, cost appears to be a secondary consideration to the end-users, reflecting some difference in perspective from the clinicians who prioritize cost over time8. A tablet-sized device was overwhelmingly preferred (30.0 %) over a mug or a watch, from which we could infer that the technicians preferred the familiarity of handheld devices to miniaturization. As for the cost, most participants preferred low-cost devices ranging from $1 to $15 (38.1%), followed by $16 to $30 (28.6%), and $31 to $50 (23.8%).
Figure 2.
Survey results. (a) Breakdown of reasons for using a POC test. Multiple responses were permitted. (b) Preferred sample collection method. Inset shows the breakdown of reasons for respondents who chose self-collected as the preferred method. (c) Preferred instruction format. Inset shows breakdown for the preferred duration of instructional video material.
Interestingly, most participants preferred provider-collected samples (73.3 %) as shown in Figure 2b. This observation is in contrast to patients and providers, where the majority of respondents preferred self-collected methods9. Of note, participants strongly preferred a short video tutorial (90.0 %) with runtime of 5–12 minutes to the conventional text and graphic instructions (6.7 %). Furthermore, most participants favored the use of POC tests in the ED (83.3 %).
Discussion
Our study was primarily designed to evaluate the end-user feedback on prototype POC test platform. While other studies to date focus on clinical parameters such as turnaround time and diagnostic performance, our study included issues regarding technical and design parameters that the platform developers found to be important. Survey results show that strong end-user preferences can be identified in these categories, highlighting the importance of directly engaging end-users for their feedback throughout platform development.
A novel feature of this study was the use of an experimental device as a way to illustrate key design features directly to the participants prior to administering the survey. Implications of new design features such as the format and length of video instruction material would be difficult to convey without incorporating an interactive demonstration as performed in the present study. In that regard, the format of this study is ideally suited for vetting previously untested design features in a POC device directly with potential end users.
In summary, our findings suggest a high level of acceptability for POC tests among ED technicians. In particular, our study encourages further investigation into the format of instructional materials and device dimensions as factors that may affect end user preferences.
Acknowledgments
We would like to thank our funding sources the National Institutes of Health (U54EB007958, R01AI117032) and the National Science Foundation (1159771, 1033744) for supporting this work.
References
- 1.Gaydos CA, Hardick J. Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Rev Anti Infect Ther. 2014 Jun;12(6):657–672. doi: 10.1586/14787210.2014.880651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect. 2017 Sep;93(6):424–429. doi: 10.1136/sextrans-2016-052988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaydos CA, Van der Pol B, Jett-Goheen M, et al. Performance of the Cepheid CT/NG Xpert Rapid PCR Test for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013 Jun;51(6):1666–1672. doi: 10.1128/JCM.03461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh YH, Hogan MT, Barnes M, et al. Perceptions of an ideal point-of-care test for sexually transmitted infections–a qualitative study of focus group discussions with medical providers. PLoS One. 2010 Nov 30;5(11):e14144. doi: 10.1371/journal.pone.0014144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh YH, Gaydos CA, Hogan MT, et al. What qualities are most important to making a point of care test desirable for clinicians and others offering sexually transmitted infection testing? PLoS One. 2011 Apr 29;6(4):e19263. doi: 10.1371/journal.pone.0019263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weigl BH, Gaydos CA, Kost G, et al. The value of clinical needs assessments for point-of-care diagnostics. Point of care. 2012 Jun;11(2):108. doi: 10.1097/POC.0b013e31825a241e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DJ, Athamanolap P, Chen L, et al. Mobile nucleic acid amplification testing (mobiNAAT) for Chlamydia trachomatis screening in hospital emergency department settings. Scientific Reports. 2017 Jul 3;7:e4495. doi: 10.1038/s41598-017-04781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh YH, Gaydos CA, Hogan MT, et al. Perceptions on point-of-care tests for sexually transmitted infections: comparison between frontline clinicians and professionals in industry. Point of Care. 2012 Jun 1;11( 2):126–129. doi: 10.1097/POC.0b013e31825a25e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh J, Howard M, Lytwyn A. Self-collection for vaginal human papillomavirus testing: systematic review of studies asking women their perceptions. Journal of lower genital tract disease. 2010 Oct 1;14(4):356–62. doi: 10.1097/LGT.0b013e3181dc115b. [DOI] [PubMed] [Google Scholar]