Abstract
Ten new azo Schiff bases 5a-h and 7a-b were prepared in excellent yields via the condensation of different aromatic amines and a new azoaldehyde, 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo)benzaldehyde (4) by two different methods. All new compounds were tested against five microorganisms: Staphylococcus aureus (Gram positive and methicillin resistant), Bacillus subtilis (Gram positive), Kelebsiella pneumonia, Pseudomonas aeruginosa and Escherichia coli (all Gram negative). Compounds 4, 5a, 5c, 5d and 5g were moderately active against Staphylococcus aureus and Bacillus subtilis. Compound 7b was highly active against Bacillus subtilis and moderately active against Staphylococcus aureus. Other compounds were inactive against these strains of bacteria. The antifungal activities of these compounds were also tested against eight different fungal species. None of them were active against the fungi species tested.
Keywords: Schiff bases, azo compounds, antibacterial and antifungal activity, o-vanillin
Introduction
Schiff bases are important intermediates for the synthesis of some bioactive compounds such as β‑lactams [1,2,3]. Furthermore, they are reported to show a variety of interesting biological actions, including antibacterial [4,5,6,7,8,9], antifungal [4,5,10], anti mouse hepatitis virus (MHV) [11], inhibition of herpes simplex virus type 1 (HSV-1) and adenovirus type 5 (Ad 5) [12], anticancer [13,14,15,16], anti mosquito larvae [17] and herbicidal activities [18]. It is also known that the presence of a chloro and an azo moiety in different types of compounds can lead them to exhibit pesticidal activity [18]. Some azo compounds synthesized by Jolly and coworkers have shown good antibacterial activity [19]. Both Schiff bases and azo compounds are important structures in the medicinal and pharmaceutical fields [20] and it has been suggested that the azomethine linkage might be responsible for the biological activities displayed by Schiff bases [16]. In light of the interesting variety of biological activities seen in compounds containing azo, methoxy groups and azomethine linkages, it was thought of interest to examine the effect of having all of above functionalities present simultaneously in one structure. Based on this notion we thus decided to synthesize ten new azo Schiff bases and to test them against Staphylococcus aureus, Bacillus subtilis, Kelebsiella pneumonia, Pseudomonas aeruginosa and Escherichia coli. These new azo Schiff bases were also tested against eight fungi including Candida albicans, Cryptococus neoformans, Tricophyton mentagrophytes, Microsporium canis, Epidermophyton floccosum, Aspergillus fumigatus, Aspergillus niger and Alternaria.
Results and Discussion
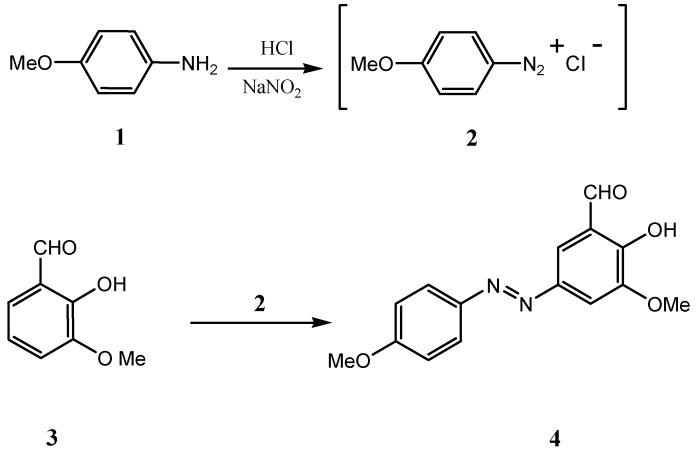
Diazonium salt 2 was prepared according to a reported method [21]. Treatment of compound 2 with 2-hydroxy-3-methoxybenzaldehyde (o-vanillin, 3) gave the azoaldehyde 2-hydroxy-3-methoxy-5 (4-methoxyphenylazo)benzaldehyde (4, Scheme 1) as a brown solid which was purified by column chromatography (eluent CH2Cl2/n-hexane) to give the pure material in 74% yield [22]. Azoaldehyde 4 could also be purified in 76.2 % yield by recrystallization from warm 95% ethanol.
Scheme 1.
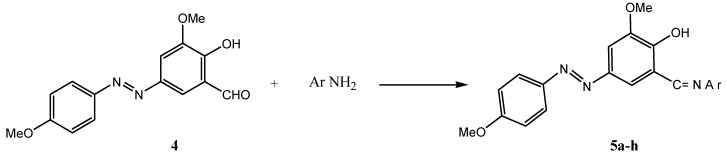
Treatment of azoaldehyde 4 with different aromatic amines, either in dry dichloromethane in the presence of anhydrous MgSO4 (method A) [23] or in refluxing absolute ethanol (method B) yielded the novel azo Schiff bases 5a-h in excellent yields (Scheme 2, Table 1).
Scheme 2.
Table 1.
Azo Schiff bases
| Compound | Ar | Reaction Method | Time(hrs) | Color | Yield % |
|---|---|---|---|---|---|
| 5 a | C6H5 | A | 8 | Dark oxblood | 99 |
| 5 b | C6H5CH2 | A | 8 | Dark orange | 98 |
| 5 c | m-HOC6H4 | A | 8 | Light red | 98 |
| 5 d | m-CH3C6H4 | B | 3 | Liver-coloured | 98 |
| 5 e | o-CH3 C6H4 | B | 3 | Light brick-red | 99 |
| 5 f | p-MeOC6H4 | B | 2 | Crimson | 99 |
| 5 g | m-MeOC6H4 | B | 3 | Red | 98 |
| 5 h | o-MeOC6H4 | B | 3 | Dark red | 99 |
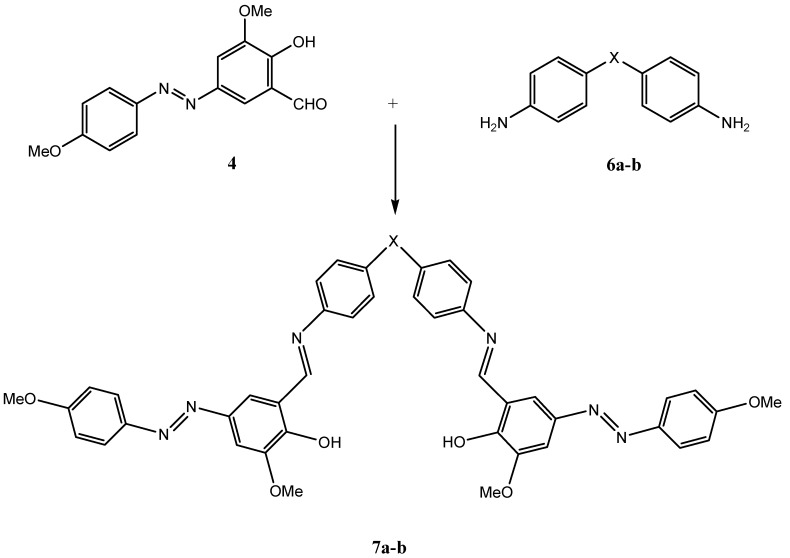
We next decided to prepare diimines 7a-b using 4,4ˊ-diaminodiphenyl ether (6a) and 4,4’-diamino diphenyl sulfone (6b) as the starting diamines. Treatment of two moles of azoaldehyde 4 with one mole of diamine in refluxing absolute ethanol (Method B) gave in excellent yields the novel compounds bis [5-(4-methoxyphenylazo)-2-hydroxy-3-methoxy benzaldehyde]-4,4ˊ-diimino phenyl ether (7a) and bis [5-(4-methoxyphenylazo)-2-hydroxy-3-methoxy benzaldehyde]-4,4ˊ-diimino phenyl sulfone (7b) as dark brick-red and oxblood colored crystals, respectively (Table 2, Scheme 3).
Table 2.
Bis azo Schiff bases
| Compound | X | Reaction Method | Time(hrs) | Colour | Yield % |
|---|---|---|---|---|---|
| 7a | O | B | 3 | Dark brick-red | 98 |
| 7b | SO2 | B | 3 | Oxblood | 98 |
Scheme 3.
Antibacterial activity tests
To determine the antibacterial activity of these agents the disk-diffusion (Kirby-Bauer) method was carried out using Ampicillin (10 μg) and Streptomycin (10 μg) as the reference antibiotics. The prepared compounds were examined against one strain each of a gram positive and methicillin resistant (Staphylococcus aureus), two gram negative (Pseudomonas aeruginosa and Escherichia coli), one capsulated gram negative (Kelebsiella pneumonia) and a gram-positive spore forming bacteria (Bacillus subtilis). The test results presented in Table 3 suggest that compounds 4, 5a, 5c, 5d and 5g were moderately active against tested gram-positive bacteria, while compound 7b was highly active against B. subtilis and moderately active against S. aureus. None of the prepared compounds affected K. pneumonia, P. aeruginosa and E. coli.
Table 3.
Effect of new azo Schiff bases on the growth of tested bacteria
| Gram positive | Gram negative | |||||
|---|---|---|---|---|---|---|
| Compound | S. aureus | B. subtilis | K. pneumonia | P. aeruginosa | E. coli | |
| Ampicillin | - | - | - | - | - | |
| Streptomycin | + + | + + + | + + + | + + + | + + + | |
| 4 | + + | + + | - | - | - | |
| 5a | + + | + + | - | - | - | |
| 5b | - | - | - | - | - | |
| 5c | + + | + + | - | - | - | |
| 5d | + + | + + | - | - | - | |
| 5e | - | - | - | - | - | |
| 5f | - | - | - | - | - | |
| 5g | + + | + + | - | - | - | |
| 5h | - | - | - | - | - | |
| 7a | - | - | - | - | - | |
| 7b | + + | + + + | - | - | - | |
- Highly active = + + + (inhibition zone > 12 mm)
- Moderately active = + + (inhibition zone 9-12 mm)
- Slightly active = + (inhibition zone 6-9 mm)
- Inactive = - (inhibition zone < 6 mm)
Antifungal activity tests
The antifungal activities of ten new azo Schiff base were tested against eight different fungus by the disk-diffusion method. The species of fungi used included: yeasts (Candida albicans and Cryptococus neoformans), dermatophytes (Triscophyton mentagrophytes, Microsporium canis and Epidermophyton floccosum) and opportunistic filamentous fungi (Aspergillus fumigatus, Aspergillus niger and Alternaria).
Experimental
General:
All melting points were taken in open capillaries on a Büchi 530 apparatus and are uncorrected. FT-IR spectra were recorded on a Shimadzu 8000 instrument. 1H-NMR and 13C-NMR were run on a Brucker Avance DPX-250 (1H-NMR 250 MHz, 13C-NMR 62.9 MHz) using TMS as internal standard. Mass spectra were recorded on a Shimadzu GC MS–QP1000 EX at 70 ev. Column chromatography was carried out on Merck silica gel 60 (30 – 270 mesh).
Synthesis of 2-hydroxy-3-methoxy-5(4-methoxyphenylazo)benzaldehyde (4)
o-Vanillin 3 (1.24 g, 8.12 mmol) was dissolved in aqueous 2 M NaOH (10.00 mL, 0.80 g, 20.00 mmol) and the resulting solution was added slowly to a solution of diazonium chloride 2 (1.39 g, 8.12 mmol) in water at 0oC. The reaction mixture was stirred for one h at 0oC and then allowed to warm slowly to room temperature. The brown precipitate thus obtained was filtered off and washed with H2O (3 x 20 mL), dissolved in CH2Cl2 and dried (Na2SO4), filtered and solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (SiO2, 1:1 v/v CH2Cl2/n-hexane) to give 2-hydroxy-3-methoxy-5(4-methoxyphenylazo)benzaldehyde (4, 1.72 g, 74 %) or by recrystallization from warm 95% ethanol (1.77 g, 76.20 %); m.p. 169-171oC; IR (KBr) (cm-1): 1600 (C=C), 1670 (C=O), 3030-3580 (OH); 1H-NMR (CDCl3) δ (ppm): 4.01 (6H, s, 2 x OCH3), 7.07- 8.26 (6H, m, 2 x Ph), 10.06 (1H, s, CHO), 12.78 (1H, br, OH); 13C-NMR (CDCl3) δ (ppm): 56.82 (OCH3), 108.70-125.19 (aromatic carbons), 196.91(CHO); MS (m/z, %): 286 (M+, 65.5), 151 (o-vanillin, 19.8), 135 (MeOC6 H4N2, 40.3), 107 (MeOC6 H4, 100.0), 92 (C6 H4 O, 20.3).
General procedures for the synthesis of Schiff bases.
Method A. Aniline (0.93 g, 10.00 mmol) and a large excess of anhydrous MgSO4 were added in succession to a stirred solution of aldehyde 4 (2.86 g, 10.00 mmol) in dry CH2Cl2 (40 mL) at 0 oC. The resulting mixture was stirred for 8 h at room temperature, then filtered and the solvent was evaporated under reduced pressure to give the azo Schiff base 2-methoxy-4-(4-methoxyphenylazo)-6-phenyliminomethylphenol (5a, 3.57 g, 99%) as a dark oxblood colored solid which was recrystallized from ethanol; m.p. 120-122 oC; IR (KBr, cm-1): 1623.9 (C=N), 3154.4-3659.7 (OH); 1H-NMR (CDCl3) δ (ppm): 3.88 (6H, s, 2 x OMe), 6.91-7.85 (11H, m, 3 x Ph), 8.65 (1H, s, HC=N), 14.49 (1H, br, OH); 13C‑NMR (CDCl3) δ (ppm): 55.97 (OMe), 105.24-161.64 (aromatic carbons), 162.10 (HC=N); MS (m/z, %): 361 (M+, 52.4), 226 (MeOC6H2OHC=NC6H5, 13.9), 195 (HOC6H2C=NC6H5, 1.5), 135 (MeOC6H4 N=N, 36), 107 (MeOC6H4, 100.0), 77 (C6H5, 46.5).
Method B. A mixture of 4,4ˊ-diaminodiphenyl ether (6a, 2.00 g, 10.00 mmol) and 2-hydroxy-3-methoxy-5(4-methoxyphenylazo)benzaldehyde (4, 5.73 g, 20.00 mmol) in absolute ethanol (50.00 mL) was refluxed for 2 h to give the crude azo Schiff base bis[5-(4-methoxyphenylazo)-2-hydroxy-3-methoxybenzaldehyde]-4,4ˊ-diiminophenyl ether (7a) as a solid. The precipitate was filtered off and washed with ethanol to give pure compound 7a (7.21 g, 98%); m.p. 238-240 oC; IR (KBr, cm-1): 1620.1 (HC=N), 3261.4-3668.9 (OH); 1H-NMR (CDCl3) δ (ppm): 3.79 (4 x OMe, s, 12 H), 6.91-7.82 (6 x Ph, m, 20H), 8.61 (2 x HC=N, s, 2H), 14.36 (2 x OH, br, 2H); 13C-NMR (CDCl3) δ (ppm): 56.94 (OMe), 105.33- 161.20 (aromatic carbons), 162.10 (HC=N). MS (m/z, %): 494 (MeOHOC6H2C=NC6H4N=C C6H2OHOMeN=N, 3.0), 376 (OC6H4 N=CC6H2OHOMe, 1.5), 257 (MeOC6H4N=NC6H2OHOMe, 1.9), 241 (OC6H4N=C C6H2OHOMe, 4.3), 226 (C6H4N=NC6H4OHO Me, 22.2), 135 (MeOC6H4N=N, 12.7), 108 (C6H5OMe, 100.0), 107 (C6H4 OMe, 44.3), 92 (C6H4O, 13.6).
Using these methods the following compounds were similarly prepared:
2-(Benzyliminomethyl)-6-methoxy-4-(4-methoxyphenylazo)phenol (5b): m.p. 149-151 oC; IR (KBr, cm‑1): 1635.5 (C=N), 3319.3-3600.9 (OH); 1H-NMR (CDCl3) δ (ppm): 3.8 (2 x OCH3, s, 6H), 4.76 (CH2, s, 2H), 6.90-7.84 (3 x Ph, m, 11H), 8.28 (HC=N, s, 1H), 14.49 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 56.38 (OCH3), 60.51 (CH2), 104.25-160.75 (aromatic carbons), 165.35 (C=N); MS (m/z, %): 375 (M+, 100.0), 284 (MeOC6H4N=NC6H2OMeOHC=N, 15.0), 240 (MeOC6H4 N=NC6H2OMe, 10.8), 135 (MeOC6H4N=N, 20.2), 107 (MeOC6H4, 51.7), 91 (C6H5CH2, 95.4), 77 (C6H5, 23.4).
2-[(3-Hydroxyphenylimino)methyl]-6-methoxy-4-(4-methoxyphenylazo)phenol (5c): m.p. 229-231 oC. IR (KBr, cm-1): 1604.7 (C=N), 3300.0-3610.5 (OH); 1H-NMR (DMSO) δ (ppm): 3.86 (2 x OMe, s, 6H), 6.77-7.87 (3 x Ph, m, 10H), 8.84 (HC=N, s, 1H), 9.78 (OH, br, 1H), 14.39 (OH, br, 1H); 13C‑NMR (DMSO) δ (ppm): 56.08 (OMe), 104.92-161.68 (aromatic carbons), 162.34 (C=N); MS (m/z, %): 377 (M+, 21.6), 257 (MeOC6H4N=NC6H2OMeOH, 3.2), 242 (MeOC6H2OHC=N C6H4OH, 5.0), 149 (MeO C6H2OHC=N, 6.0), 135 (MeOOC6H4N=N, 51.5), 107 (MeOC6H4, 100.0).
2-Methoxy-4-(4-methoxyphenylazo)-6-(m-tolyliminomethyl)phenol (5d): m.p. 124-126 oC; IR (KBr, cm-1): 1604.7 (C=N), 3222.8-3640.4 (OH); 1H-NMR (CDCl3) δ (ppm): 2.27 (Me, s, 3H), 3.84 (2 x OMe, s, 6H), 6.88-7.87 (3 x Ph, m, 10H), 8.47 (HC=N, s, 1H), 14.64 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 21.83 (Me), 55.99 (OMe), 105.11-129.98 (aromatic carbons), 161.10 (HC=N); MS (m/z, %): 375 (M+, 37.2), 240 (MeC6H4N=CC6H2OHOMe, 10.9), 225 (C6H4N=CC6H2OHOMe, 6.8), 135 (MeOC6H4N=N, 25.9), 118 (MeC6H4N=CH, 2.2), 107 (MeO C6H4, 100.0), 91 (MeC6H4, 9.3).
2-Methoxy-4-(4-methoxyphenylazo)-6-(o-tolyliminomethyl)phenol (5e): m.p. 121-123 oC; IR (KBr, cm‑1): 1609.5 (C=N), 3184.2-3659.7 (OH). 1H-NMR (CDCl3) δ (ppm): 2.47 (Me, s, 3H), 3.88 (2 x OMe, s, 6H), 6.98-7.91 (3 x Ph, m, 10H), 8.51 (HC=N, s, 1H), 14.99 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 20.01 (Me), 57.38 (OMe), 106.39-152.06 (aromatic carbons), 163.43 (HC=N); MS (m/z, %): 375 (M+, 100.0), 240 (MeC6H4N=CC6H2OMeOH, 22), 225 (C6H4N=CC6H2 OMeOH, 16.3), 135 (MeO-C6H4N=N, 13.4), 107 (MeOC6H4 , 71), 91 (C6H4CH3, 11).
2-Methoxy-4-(4-methoxyphenylazo)-6-[(4-methoxyphenylimino)methyl]phenol (5f): m.p. 189 –191 oC; IR (KBr, cm-1): 1620.1 (HC=N), 3309.6-3600.9 (OH).; 1H-NMR (CDCl3) δ (ppm): 3.89 (3 x OMe, s, 9H), 6.88-7.84 (3 x Ph, m, 10H), 8.62 (HC=N, s, 1H), 14.68 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 56.59 (OMe), 105.02-150.15 (aromatic carbons), 159.70 (HC=N); MS (m/z, %): 391 (M+, 96.7), 256 (MeOC6H2OHC=NC6H4OMe, 18.8), 135 (MeOC6H4N=N, 14.6), 107 (MeOC6H4, 51.6), 43 (C-OCH3, 100.0).
2-Methoxy-4-(4-methoxyphenylazo)-6-[(3-methoxyphenylimino)methyl]phenol (5g): m.p. 136-138 oC; IR (KBr, cm-1): 1633.60 (HC=N), 3174.6-3629.8 (OH); 1H-NMR (CDCl3) δ (ppm): 3.80 (3 x OMe, s, 9H), 6.77-7.83 (3 x Ph, m, 10H), 8.66 (HC=N, s, 1H), 14.42 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 55.96 (OMe), 105.26-161.02 (aromatic carbons), 162.06 (HC=N); MS (m/z, %): 391 (M+, 40.9), 256 (MeOC6H2OHC=NC6H4OMe, 7.3), 226 (MeOC6H2OH N=NC6H4, 2.6), 135 (MeOC6H4N=N, 29.6), 107 (MeOC6H4, 100.0).
2-Methoxy-4-(4-methoxyphenylazo)-6-[(2-methoxyphenylimino)methyl]phenol (5h): m.p. 180-182 oC; IR (KBr, cm-1): 1618.2 (HC=N), 3232.5-3668.5 (OH); 1H-NMR (CDCl3) δ (ppm): 3.85 (3 x OMe, s, 9H), 6.86-7.83 (3 x Ph, m, 10H ), 8.68 (HC=N, s, 1H), 15.23 (OH, br, 1H); 13C-NMR (CDCl3) δ (ppm): 56.35 (OMe), 104.05-158.24 (aromatic carbons), 161.71 (HC=N). MS (m/z, %): 391 (M+, 62.1), 256 (MeOC6H2OHC=NC6H4OMe, 12.2), 226 (MeOC6H2OHN=NC6H4, 3.3), 177 (N=CC6H2OHOMeN=N, 3.7), 149 (C=NC6H2OHOMe, 14.0), 135 (MeOC6H4 N=N, 27.5), 107 (C6H4OMe, 100.0).
Bis[5-(4-methoxyphenylazo)-2-hydroxy-3-methoxybenzaldehyde]-4,4ˊ-diiminophenyl sulfone (7b): m.p. 252-254 oC; IR (KBr, cm-1): 1620.10 (HC=N), 3271.0-3649.1 (OH); 1H-NMR (CDCl3) δ (ppm): 3.88 (4 x OMe, s, 12 H), 6.99-7.90 (6 x Ph, m, 20H), 8.70 (2 x HC=N, s, 2H), 14.45 (2 x OH, br, 2H); 13C‑NMR (CDCl3) δ (ppm): 55.94 (OMe), 105.37-161.26 (aromatic carbons), 162.06 (HC=N); MS (m/z, %): 497 (MeOHOC6H2C=NC6H4SO2N=CC6H2OMe, 1.2), 469 (C6H4N=NC6H2OHOMeC=N C6H4SO2C6H4, 1.9), 393 (N=NC6H2OMeOHC=NC6H4SO2C6H4, 2.5), 391 (MeOHOC6H2C=NC6H4 SO2N=C, 14.5), 257 (HC=NC6H4SO2C6H4N, 1.5), 135 (MeOC6H4N=N, 36.6), 107 (MeOC6H4, 100.0), 92 (C6H4O, 26.2).
Antibacterial activity tests
Sterile disks (Whatman No.3 filter paper, 5 mm diameter) each containing 2000 µg of the different agents were placed on the surface of a streaked nutrient agar plate inoculated with each bacterium. The plates were incubated at 37 ºC for 24 hours and inhibition zones of bacterial growth produced by the various agents were then measured (mm) [24].
Antifungal activity tests
Before testing the test species were cultured on potato dextrose agar. Mature colonies were covered with sterile water (approximately 2 mL). The agar plates (saboured glucose agar 2%) were inoculated by dipping a sterile cotton swab into the inoculum and evenly streaking the swab in three directions over the entire surface of the plates, which were then allowed to dry. The disks with compounds (200 μg/disk) were applied onto each inoculated plate and the plates were incubated at 37 oC for yeasts and 25 oC for filamentous fungi, with readings taken after 48 to 72 hours and 5 to 14 days respectively [25]. Inhibitory zone diameters for disks were measured and compared with Amphotericin B disks (15 μg/disk) used as controls. No inhibitory zone areas were observed at the end of incubation period and these compounds therefore seem to have no antifungal effects for fungi at 200 μg.
Acknowledgments
The authors thank the Shiraz University Research Council for financial support (Grant No. 83-GR-SC-31 ).
Footnotes
Sample availability: Available from the authors
References
- 1.Venturini A., Gonzalez J.J. J. Org. Chem. 2002;67:9089–9092. doi: 10.1021/jo026188h. [DOI] [PubMed] [Google Scholar]
- 2.Taggi A.E., Hafez A.M., Wack H., Young B., Ferraris D., Lectka T.J. J. Am. Chem. Soc. 2002;124:6626–6635. doi: 10.1021/ja0258226. [DOI] [PubMed] [Google Scholar]
- 3.Delpiccolo C.M.L., Mata E.G. Tetrahedron: Asymmetry. 2002;13:905–910. [Google Scholar]
- 4.More P.G., Bhalvankar R.B., Pattar S.C. Indian Chem. J. Indian Chem. Soc. 2001;78:474–475. [Google Scholar]
- 5.Pandeya S.N., Sriram D., Nath G., De Clercq E. Il Farmaco. 1999;54:624–628. doi: 10.1016/s0014-827x(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 6.Baseer M.A., Jadhav V.D., Phule R.M., Archana Y.V., Vibhute Y.B. Orient. J. Chem. 2000;16:553–556. [Google Scholar]
- 7.El-Masry A.H., Fahmy H.H., Abdelwahed S.H.A. Molecules. 2000;5:1429–1438. [Google Scholar]
- 8.Karia F.D., Parsania P.H. Asian J. Chem. 1999;11:991–995. [Google Scholar]
- 9.Kabeer A.S., Baseer M.A., Mote N.A. Asian J. Chem. 2001;13:496–500. [Google Scholar]
- 10.Singh W.M., Dash B.C. Pesticides. 1988;22:33–37. [Google Scholar]
- 11.Wang P-H., Keck J.G., Lien E.J., Lai M.M.C.J. J. Med. Chem. 1990;33:608–614. doi: 10.1021/jm00164a023. [DOI] [PubMed] [Google Scholar]
- 12.Das A., Trousdale M.D., Ren S., Lien E.J. Antiviral Res. 1999;44:201–208. doi: 10.1016/S0166-3542(99)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai S.B., Desai P.B., Desai K.R. Hetrocycl. Commun. 2001;7:83–90. [Google Scholar]
- 14.Pathak P., Jolly V.S., Sharma K.P. Orient. J. Chem. 2000;16:161–162. [Google Scholar]
- 15.Kuz’min V.E., Lozitsky V.P., Kamalov G.L., Lozitskaya R.N., Zheltvay A.I., Fedtchouk A.S., Kryzhanovsky D.N. Acta Biochim. Polon. 2000;47:867–876. [PubMed] [Google Scholar]
- 16.Phatak P., Jolly V.S., Sharma K.P. Orient. J. Chem. 2000;16:493–494. [Google Scholar]
- 17.Das B.P., Choudhury R.T., Das K.G., Choudhury D.N., Choudhury B. Chem. Environ. Res. 1994;3:19–23. [Google Scholar]
- 18.Samadhiya S., Halve A. Orient. J. Chem. 2001;17:119–122. [Google Scholar]
- 19.Jolly V.S., Pathak P., Jain R. J. Indian Chem. Soc. 1993;70:505–507. [Google Scholar]
- 20.Halve A., Goyal A. Orient. J. Chem. 1996;12:87–88. [Google Scholar]
- 21.Furniss B.S., Hannaferd A.J., Rogers V., Smith P.W.G., Tatchell A.R. Vogel’sTextbook of Practical Organic Chemistry. 4th ed. Longman, Inc.; New York: 1981. p. 716. [Google Scholar]
- 22.Jarrahpour A.A., Esmaeilbeig A.R., Zarei M. Molbank. 2004:M371. [Google Scholar]
- 23.Matsui S., Hashimoto Y., Saigo K. Synthesis. 1998:1161–1166. [Google Scholar]
- 24.Forbes B.A., Sham D.F., Weissfeld A.S. Bailey and Scott‘s Diagnostic Microbiology. 11th ed. Mosby, Inc.; St. Louis (USA): 2002. pp. 236–240. [Google Scholar]
- 25.Rex J.H., Pfaller M.I., Walsh T.J., Chaturvdei V., Espinel-Ingroff A., Ghannoum M.A., Gosey L.L., Odds F.C., Rinaldi M.G., Sheehan D.G., Warnock D.W. Clin. Microbiol. Rev. 2001;14:643–658. doi: 10.1128/CMR.14.4.643-658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]