Abstract
A series of novel 11b-substituted 1,6,7,11b-tetrahydropyrimido[6,1-a]-isoquinoline-2,4-diones and 4-thioxo-1,3,4,6,7,11b-hexahydropyrimido[6,1-a]isoquinolin-2-ones were synthesized, utilizing two alternative strategies for ring closure of tetrahydroisoquinoline intermediates obtained from N-phenethyl enaminones.
Keywords: Tetrahydroisoquinolines; pyrimido[6,1-a]isoquinolines; enaminones
Introduction
A broad range of biological activities has been reported for compounds containing the pyrimido-[6,1-a]isoquinoline ring system and a number of these compounds are patented as potential therapeutic agents [1,2]. Amongst the reported ones are antihypertensive, bronchodilator and anti-allergic activities. The antihypertensive agent Trequinsin [3], for instance, is one of the most potent in vitro inhibitors of platelet phosphodiesterase and platelet aggregation known to date [4]. This makes the pyrimido[6,1-a]-isoquinoline skeleton an important synthetic target and a lot of research has been directed towards it. Compounds of this type are usually synthesized by ring closure of suitable tetrahydroisoquinolines [1,3,5,6,7,8,9,10,11], Bischler-Napieralsky cyclization of 1-(3,4-dimethoxyphenylethyl)barbituric acid [2] or other appropriate N-phenethyl amides [12,13]. Cyclizations of pyrimidine intermediates via N-acyliminium ions are also known [14,15]. Despite these numerous publications, however, there are very few examples of 11b-substituted pyrimido[6,1-a]isoquinolines [11,15], a fact attributable to the lack of suitable starting materials.
In a previous communication we reported on the synthesis of 1,1-disubstituted tetrahydro-isoquinolines via Pictet-Spengler reaction of N-phenetyl enaminones [16]. Some of these compounds are an excellent starting point for construction of the pyrimido[6,1-a]isoquinoline skeleton. To demonstrate this, now we have synthesized a series of novel 1,6,7,11b-tetrahydropyrimido[6,1-a]-isoquinoline-2,4-diones and 4-thioxo-1,3,4,6,7,11b-hexahydropyrimido[6,1-a]isoquinolin-2-ones (5) bearing methyl or phenyl substituents at the 11b position.
Results and Discussion
The synthesis of the targets 5 was accomplished by two different strategies (Scheme 1), starting from tetrahydroisoquinolin-1-yl acetamides (1, Path A) or ethyl esters of tetrahydroisoquinolin-1-yl acetic acids (2, Path B).
Scheme 1.
Since compounds of type 1 are generally available in higher yields than those of type 2 [16], our initial studies focused on Path A. The reactions of 1a-e with ethyl chlorocarbonate were carried out in the presence of Et3N as HCl scavenger and afforded the corresponding urethanes 3a-e in good yields (80 – 90%). The intermediates 3 obtained in this way were cyclized to 5 with the aid of strong bases like NaNH2 or t-BuOK. The yields of 5 are nearly quantitative after refluxing 3 and 1.2 equiv. of base in THF as the solvent for 1h. The rate of cyclization in general increases with the acidity of the amide group NH in 3. As an alternative, compounds 5 could be synthesized by cyclocondensation of 2 and isocyanates – Path B. To prove this, compounds 2d,e were reacted with phenyl isocyanate and the resulting ureas 4d,e were cyclized at r.t. in THF solution in the presence of t-BuOK or LDA. The products 5d and 5e obtained in this way were identical with the ones obtained via path A.
Path B also allows for the synthesis of the 4-thioxo derivatives 5f and 5g, when isothiocyanates are used instead of isocyanates. The limiting step in this case was the formation of the thioureas 4f,g. In contrast to the isocyanates, the isothiocyanates did not react with 2 at r.t., although the reaction proceeded smoothly at elevated temperatures (100 - 120 oC), leading directly to the final products 5f,g. The intermediate thioureas 4f,g are much more reactive than the corresponding oxo-analogues and cyclize immediately upon formation. For this reason they were not isolated and characterized. The reactions of 2 with isothiocyanates in refluxing solvents, like toluene or xylene, required prolonged heating and the yields of 5 did not exceed 65%. Better results were obtained under solvent-free conditions – thus heating of 2 with two equiv. of methyl or phenyl isothiocyanate for 10 min. at 120 oC afforded the expected products 5f, g in 87 and 81 % yields respectively.
Table 1.
Yields of intermediates (3, 4) and end products 5 obtained according to Scheme 1
| Entry | R1 | R2 | X | Path | Yield (%)a | ||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | |||||
| a | C6H5 | CH3 | O | A | 87 | – | 93 |
| b | CH3 | H | O | A | 85 | – | 91 |
| c | CH3 | CH3 | O | A | 89 | – | 95 |
| d | CH3 | C6H5 | O | A | 90 | – | 93 |
| B | – | 95 | 94 | ||||
| e | C6H5 | C6H5 | O | A | 84 | – | 86 |
| B | – | 93 | 88 | ||||
| f | CH3 | CH3 | S | B | – | – | 87 |
| g | CH3 | C6H5 | S | B | – | – | 81 |
aExcept for entries f and g, yields of 5 are based on the corresponding intermediate 3 or 4.
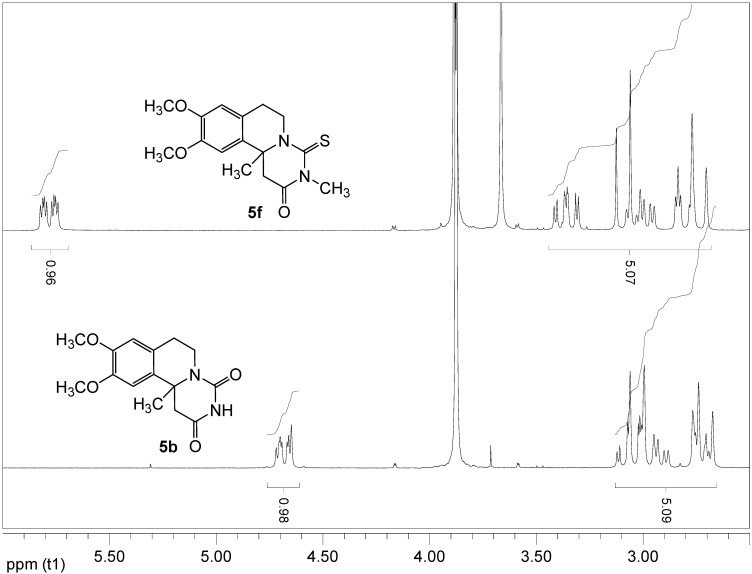
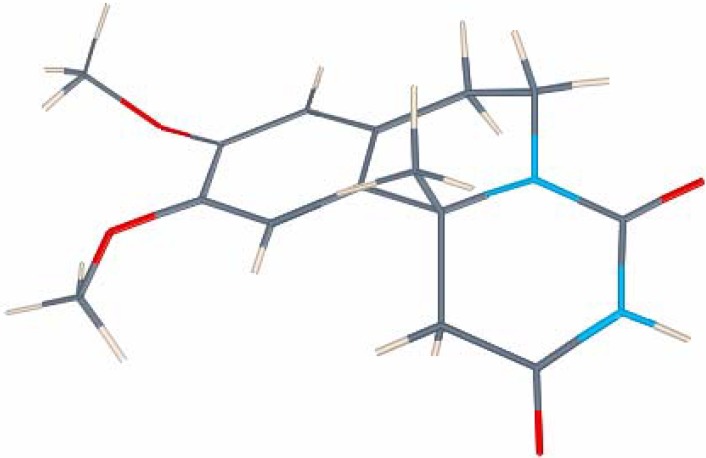
Apart from the standard NMR measurements, DEPT and HMQC experiments were used to confirm the structures of 5. A peculiarity common to all of the final products 5 is the very strongly pronounced nonequivalency of the diastereotopic protons in the C-6 methylene group – the signals for these protons appear as multiplets separated by average 1.5 ppm apart from each other (Figure 1, 5b). This could be explained as a combined effect of the conformational constraint of the ring system and the magnetic anisotropy of the nearby C-4 carbonyl group. Thus, the equatorial hydrogen at C-6 is fixed in closer proximity to the carbonyl group and falls within its deshielding zone. The AM1-optimized geometry [17] of 5b clearly illustrates the different orientations of the hydrogens at C-6 (Figure 2). Because of the larger deshielding zone of the thiocarbonyl group the downfield shift of the equatorial hydrogen is greater in the thioxo compounds 5f,g. In these two compounds the distance between the two signals for CH2N reaches 2.5 ppm (Figure 1, 5f).
Figure 1.
Representative 1H-NMR cuts (2.5 – 6.0 ppm region) showing the methylene signals and the downfield shift of the equatorial hydrogen at C-6.
Figure 2.
3D Model of 5b, showing the different orientations of the hydrogens at C-6. Calculations were performed using the PC GAMESS version [18] of the GAMESS (US) QC package [19].
Experimental
General
Melting points were determined on a Boëtius hotstage apparatus and are uncorrected. NMR spectra were recorded on a Bruker DRX-250 device using CDCl3 as solvent. Chemical shifts (δ, ppm) are downfield from TMS as internal standard and coupling constants are in Hz. IR spectra were recorded on a Perkin-Elmer 1750 FTIR device in KBr pellets and absorption is given in cm-1. All new compounds had elemental analyses within 0.4% of the theoretical values as well as correct molecular ion peaks by mass spectrometry.
Synthesis of tetrahydroisoquinolines 1 and 2
Compounds 1b,d and 2d-g are described in reference [16]. Compounds 1a,c,e are novel and were synthesized as follows: the corresponding β-ketoamide [20] (2 mmol) and Na2SO4 (0.6 g) were added to a solution of homoveratrylamine (2 mmol) in CH2Cl2 (10 mL). The mixtures were stirred at r.t. for 14 days (entries a,e) or 2 days (entry c). Then, the sulfate was filtered off and the solvent distilled. To the enaminones obtained in this way methanesulfonic acid (7 – 8 mL) was added and the mixtures were stirred at r.t. for 4 days (entries a,e) or 2 days (entry c). After that, water (200 mL) was added, the pH brought to 9-10 with 25% aq. NH3 and the tetrahydroisoquinolines 1 were extracted with CH2Cl2 (3 × 50 mL) After distillation of the solvent the products crystallize upon trituration with ether (or petroleum ether for 1c). The mother liquors could be further purified by column chromatography on neutral alumina, using ether as eluent.
2-(6,7-Dimethoxy-1-phenyl-1,2,3,4-tetrahydroisoquinolin-1-yl)-N-methyl-acetamide (1a): Yield 83 %; mp 107 – 108 oC; IR: νNH 3481, 3269; νC=O 1651; 1H-NMR: 2.56 (d, 3J = 4.8, 3H, CONHCH3), 2.68 – 3.10 (m, 5H, CH2CH2NH), 3.15 – 3.34 (AB quartet, 2J = 15.9, 2H, CH2CO), 3.74 (s, 3H, CH3O), 3.87 (s, 3H, CH3O), 6.50 (s, 1H, Ar-H), 6.60 (s, 1H, Ar-H), 7.14 – 7.32 (m, 5H, C6H5), 8.63 (br, 1H, CONHCH3); 13C-NMR: 25.17, 30.10, 39.87, 48.73, 55.54, 55.77, 60.23, 110.15, 111.22, 120.89, 125.53, 128.34, 129.91, 135.65, 138.30, 146.36, 147.73, 171.35.
2-(6,7-Dimethoxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl)-N-methyl-acetamide (1c): Yield 90 %; mp 68 – 70 oC; IR: νNH 3416, 3268; νC=O 1657; 1H-NMR: 1.47 (s, 3H, CH3), 1.78 (br s, 1H, NH), 2.45 (d, 2J = 15.6, 1H, 0.5 × CH2CO), 2.69 – 3.20 (m, 4H, CH2CH2NH), 2.72 (d, 3J = 4.8, 3H, CONHCH3), 2.79 (d, 2J = 15.6, 1H, 0.5 × CH2CO), 3.85 (s, 6H, 2 × CH3O), 6.52 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 8.45 (br, 1H, CONHCH3); 13C-NMR: 25.11, 28.54, 30.24, 39.66, 46.47, 55.78, 55.79, 60.19, 109.05, 111.13, 129.07, 134.51, 146.06, 148.12, 172.67.
2-(6,7-Dimethoxy-1-phenyl-1,2,3,4-tetrahydroisoquinolin-1-yl)-N-phenyl-acetamide (1e): Yield 76 %; mp 202 – 204 oC; IR: νNH 3317, 3240; νC=O 1684; 1H-NMR: 2.69 – 3.05 (m, 5H, CH2CH2NH), 3.13 (d, 2J = 15.8, 1H, 0.5 × CH2CO), 3.36 (d, 2J = 15.8, 1H, 0.5 × CH2CO), 3.73 (s, 3H, CH3O), 3.82 (s, 3H, CH3O), 6.54 (s, 1H, Ar-H), 6.57 (s, 1H, Ar-H), 6.96 – 7.37 (m, 10H, 2 × C6H5), 11.12 (s, 1H, CONHC6H5); 13C-NMR: 29.12, 38.40, 47.94, 55.58, 55.90, 61.02, 110.11, 111.16, 119.78, 123.34, 126.47, 127.18, 127.31, 128.28, 128.59, 128.69, 138.30, 146.66, 147.39, 147.80, 168.84.
Synthesis of urethanes 3 – general procedure:
The solvents used in these preparations were 1,2-dichloroethane (3c,d), toluene (3a,e) and acetonitrile (3b). Ethyl chlorocarbonate (2 mmol) was added to a stirred solution of the corresponding tetrahydroisoquinoline 1 (1 mmol) and Et3N (1 mmol) in the specified solvent (15 mL) and the mixture was heated at reflux temperature for 1 h. The solvent was then evaporated in vacuo, the solid residue taken with CH2Cl2 (100 mL) and washed with 5% aq. hydrochloric acid (150 mL). The organic phase was dried, the solvent distilled off and the residue triturated with Et2O or petroleum ether to crystallize.
6,7-Dimethoxy-1-methylcarbamoylmethyl-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (3a): mp 127 – 128 oC; IR: νNH 3349; νC=O 1701, 1650; 1H-NMR: 1.21 (t, 3J = 7, 3H, CO2CH2CH3), 2.64 (d, 3J = 4.8, 3H, CONHCH3), 2.84 – 2.89 (m, 2H, CH2CH2N), 3.10 (d, 2J = 14.1, 1H, 0.5 × CH2CO), 3.47 (q, 3J = 7, 2H, CO2CH2CH3), 3.60 (s, 3H, CH3O), 3.85 (s, 3H, CH3O), 3.90 – 4.20 (m, 3H, CH2CH2N + 0.5 × CH2CO), 5.18 (br s, 1H, CONHCH3), 6.26 (s, 1H, Ar-H), 6.58 (s, 1H, Ar-H), 7.12 – 7.31 (m, 5H, C6H5).
1-Carbamoylmethyl-6,7-dimethoxy-1-methyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (3b): mp 154 – 155 oC; IR: νNH 3416, 3347, 3227; νC=O 1685, 1658; 1H-NMR: 1.31 (t, 3J = 7, 3H, CO2CH2CH3), 1.75 (s, 3H, CH3), 2.65 – 2.91 (m, 2H, CH2CH2N), 2.75 (d, 2J = 13.7, 1H, 0.5 × CH2CO), 3.44 – 3.51 (m, 1H, 0.5 × CH2CH2N), 3.86 (s, 3H, CH3O), 3.88 (s, 3H, CH3O), 3.92 (d, 2J = 13.7, 1H, 0.5 × CH2CO), 3.94 – 4.04 (m, 1H, 0.5 × CH2CH2N), 4.18 (q, 3J = 7, 2H, CO2CH2CH3), 5.42 (br s, 1H, 0.5 × CONH2), 5.69 (br s, 1H, 0.5 × CONH2), 6.55 (s, 1H, Ar-H), 6.83 (s, 1H, Ar-H).
6,7-Dimethoxy-1-methyl-1-methylcarbamoylmethyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (3c): mp 144 – 145 oC; IR: νNH 3360; νC=O 1692, 1640; 1H-NMR: 1.33 (t, 3J = 7, 3H, CO2CH2CH3), 1.71 (s, 3H, CH3), 2.61 – 2.90 (m, 2H, CH2CH2N), 2.68 (d, 3J = 4.8, 3H, CONHCH3), 2.70 (d, 2J = 13.5, 1H, 0.5 × CH2CO), 3.41 – 3.50 (m, 1H, 0.5 × CH2CH2N), 3.86 (s, 3H, CH3O), 3.87 (s, 3H, CH3O), 3.93 (d, 2J = 13.5, 1H, 0.5 × CH2CO), 3.93 – 4.05 (m, 1H, 0.5 × CH2CH2N), 4.16 (q, 3J = 7, 2H, CO2CH2CH3), 5.23 (br s, 1H, CONHCH3), 6.57 (s, 1H, Ar-H), 6.81 (s, 1H, Ar-H).
6,7-Dimethoxy-1-methyl-1-phenylcarbamoylmethyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (3d): mp 169 – 170 oC; IR: νNH 3300; νC=O 1689, 1668; 1H-NMR: 1.35 (t, 3J = 7, 3H, CO2CH2CH3), 1.69 (s, 3H, CH3), 2.58 – 2.88 (m, 2H, CH2CH2N), 2.83 (d, 2J = 14.0, 1H, 0.5 × CH2CO), 3.38 – 3.49 (m, 1H, 0.5 × CH2CH2N), 3.87 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 3.90 – 4.04 (m, 1H, 0.5 × CH2CH2N), 4.01 (d, 2J = 14.0, 1H, 0.5 × CH2CO), 4.20 (q, 3J = 7, 2H, CO2CH2CH3), 6.56 (s, 1H, Ar-H), 6.79 (s, 1H, Ar-H), 7.02 – 7.61 (m, 6H, CONHC6H5).
6,7-Dimethoxy-1-phenyl-1-phenylcarbamoylmethyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (3e): mp 172 – 173 oC; IR: νNH 3355; νC=O 1699, 1658; 1H-NMR: 1.25 (t, 3J = 7, 3H, CO2CH2CH3), 2.80 – 2.86 (m, 2H, CH2CH2N), 3.12 (d, 2J = 14.1, 1H, 0.5 × CH2CO), 3.53 (q, 3J = 7, 2H, CO2CH2CH3), 3.68 (s, 3H, CH3O), 3.84 (s, 3H, CH3O), 3.87 – 4.19 (m, 2H, CH2CH2N), 4.03 (d, 2J = 14.1, 1H, 0.5 × CH2CO), 6.30 (s, 1H, Ar-H), 6.57 (s, 1H, Ar-H), 6.72 (s, 1H, CONHC6H5), 6.95 – 7.33 (m, 10H, 2 × C6H5).
Synthesis of ureas 4 – general procedure:
Phenyl isocyanate (1.1 mmol) was added to a solution of 2 (1 mmol) in ether (10 - 20 mL) and the mixture was stirred overnight at r.t. The precipitated crystals were filtered off, washed with petroleum ether and dried.
(6,7-Dimethoxy-1-methyl-2-phenylcarbamoyl-1,2,3,4-tetrahydroisoquinolin-1-yl)-acetic acid ethyl ester (4d): mp 154 – 155 oC; IR: νNH 3375; νC=O 1709, 1656; 1H-NMR: 1.05 (t, 3J = 7.1, 3H, CO2CH2CH3), 1.84 (s, 3H, CH3), 2.84 – 2.89 (m, 2H, CH2CH2N), 2.97 (d, 2J = 16.0, 1H, 0.5 × CH2CO), 3.67 – 3.85 (m, 2H, CH2CH2N), 3.85 (s, 3H, CH3O), 3.87 (s, 3H, CH3O), 3.91 (q, 3J = 7.1, 2H, CO2CH2CH3), 4.23 (d, 2J = 16.0, 1H, 0.5 × CH2CO), 6.58 (s, 1H, Ar-H), 6.72 (s, 1H, CONHC6H5), 6.77 (s, 1H, Ar-H), 6.98 – 7.31 (m, 5H, C6H5).
(6,7-Dimethoxy-1-phenyl-2-phenylcarbamoyl-1,2,3,4-tetrahydroisoquinolin-1-yl)-acetic acid ethyl ester (4e): mp 159 – 160 oC; IR: νNH 3281; νC=O 1732, 1636; 1H-NMR: 1.14 (t, 3J = 7.1, 3H, CO2CH2CH3), 2.85 – 3.17 (m, 2H, CH2CH2N), 3.54 (d, 2J = 13.8, 1H, 0.5 × CH2CO), 3.55 – 3.66 (m, 1H, 0.5 × CH2CH2N), 3.65 (s, 3H, CH3O), 3.84 (s, 3H, CH3O), 3.96 (d, 2J = 13.8, 1H, 0.5 × CH2CO), 3.99 (q, 3J = 7.1, 2H, CO2CH2CH3), 4.39 – 4.51 (m, 1H, 0.5 × CH2CH2N), 6.33 (s, 1H, Ar-H), 6.40 (s, 1H, CONHC6H5), 6.59 (s, 1H, Ar-H), 6.92 – 7.54 (m, 10H, 2 × C6H5).
Tetrahydropyrimido[6,1-a]isoquinoline-2,4-diones 5a-e – General Procedure Path A:
A stirred suspension of the corresponding urethane 3 (1 mmol) and powdered sodium amide (1.2 equiv) in THF (10 – 15 mL) was heated at reflux temperature for 1h. The solvent was then evaporated, the solid residue was taken up with small amount of water and extracted with CH2Cl2 (75 – 100 mL). The organic phase was washed with water, dried and the solvent distilled off. All products crystallize upon trituration with Et2O.
Tetrahydropyrimido[6,1-a]isoquinoline-2,4-diones 5d, e – General Procedure Path B:
To a solution of the corresponding urea 4 (1 mmol) in THF (10 – 15 mL) was added LDA (1.2 equiv) and the mixture was stirred for 30 min. at r.t. After that, the solvent was evaporated in vacuo, and the solid residue was worked up as described above.
9,10-Dimethoxy-3-methyl-11b-phenyl-1,6,7,11b-tetrahydropyrimido[6,1-a]isoquinoline-2,4-dione (5a): mp 205 – 206 oC; IR: νC=O 1707, 1663; 1H-NMR: 2.70 – 3.02 (m, 2H, CH2CH2N), 3.08 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 3.17 (s, 3H, NCH3), 3.59 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 3.60 – 3.69 (m, 1H, 0.5 × CH2CH2N), 3.77 (s, 3H, CH3O), 3.88 (s, 3H, CH3O), 4.34 – 4.43 (m, 1H, 0.5 × CH2CH2N), 6.53 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H), 7.19 – 7.30 (m, 5H, C6H5); 13C-NMR: 27.67, 28.16, 40.21, 45.49, 55.82, 56.04, 59.62, 108.99, 111.12, 126.04, 126.51, 127.90, 128.69, 130.44, 142.84, 147.94, 148.34, 153.22, 168.15.
9,10-Dimethoxy-11b-methyl-1,6,7,11b-tetrahydropyrimido[6,1-a]isoquinoline-2,4-dione (5b): mp 257 – 258 oC; IR: νNH 3180; νC=O 1722, 1668; 1H-NMR: 1.63 (s, 3H, CH3), 2.70 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 2.70 – 3.12 (m, 3H, CH2CH2N + 0.5 × CH2CH2N), 3.05 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 3.87 (s, 3H, CH3O), 3.88 (s, 3H, CH3O), 4.65 – 4.70 (m, 1H, 0.5 × CH2CH2N), 6.58 (s, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 8.84 (s, 1H, NH); 13C-NMR: 25.15, 28.80, 36.67, 45.76, 55.82, 55.87, 56.02, 107.75, 111.29, 125.29, 130.81, 148.08, 151.94, 168.58.
9,10-Dimethoxy-3,11b-dimethyl-1,6,7,11b-tetrahydropyrimido[6,1-a]isoquinoline-2,4-dione (5c): mp 185 – 186 oC; IR: νC=O 1707, 1657; 1H-NMR: 1.57 (s, 3H, CH3), 2.71 (d, 2J = 16.2, 1H, 0.5 × CH2CO), 2.71 – 3.13 (m, 3H, CH2CH2N + 0.5 × CH2CH2N), 3.07 (d, 2J = 16.2, 1H, 0.5 × CH2CO), 3.27 (s, 3H, NCH3), 3.87 (s, 3H, CH3O), 3.88 (s, 3H, CH3O), 4.65 – 4.72 (m, 1H, 0.5 × CH2CH2N), 6.58 (s, 1H, Ar-H), 6.63 (s, 1H, Ar-H); 13C-NMR: 24.88, 27.68, 28.85, 37.44, 46.13, 54.38, 55.77, 55.96, 107.60, 111.21, 125.33, 130.99, 148.00, 152.76, 168.24.
9,10-Dimethoxy-11b-methyl-3-phenyl-1,6,7,11b-tetrahydropyrimido[6,1-a]isoquinoline-2,4-dione (5d): mp 263 – 264 oC; IR: νC=O 1718, 1666; 1H-NMR: 1.75 (s, 3H, CH3), 2.73 – 3.19 (m, 3H, CH2CH2N + 0.5 × CH2CH2N), 2.92 (d, 2J = 16.2, 1H, 0.5 × CH2CO), 3.23 (d, 2J = 16.2, 1H, 0.5 × CH2CO), 3.87 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 4.65 – 4.72 (m, 1H, 0.5 × CH2CH2N), 6.62 (s, 1H, Ar-H), 6.65 (s, 1H, Ar-H), 7.19 – 7.48 (m, 5H, C6H5); 13C-NMR: 25.53, 27.82, 36.38, 45.89, 55.41, 55.68, 56.73, 107.98, 111.09, 125.94, 127.13, 128.52, 131.07, 140.34, 145.34, 148.11, 153.05, 168.12.
9,10-Dimethoxy-3,11b-diphenyl-1,6,7,11b-tetrahydropyrimido[6,1-a]isoquinoline-2,4-dione (5e): mp 277 – 278 oC; IR: νC=O 1713, 1673; 1H-NMR: 2.78 – 3.10 (m, 2H, CH2CH2N), 3.27 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 3.69 – 3.78 (m, 1H, 0.5 × CH2CH2N), 3.75 (d, 2J = 16.4, 1H, 0.5 × CH2CO), 3.78 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 4.38 – 4.50 (m, 1H, 0.5 × CH2CH2N), 6.58 (s, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.96 – 7.39 (m, 10H, 2 × C6H5); 13C-NMR: 27.70, 41.02, 45.78, 56.12, 57.05, 58.97, 110.06, 111.31, 120.54, 123.13, 125.07, 127.30, 127.76, 128.45, 128.62, 128.69, 138.39, 147.06, 147.41, 148.09, 154.03, 168.36.
4-Thioxo-1,3,4,6,7,11b-hexahydropyrimido[6,1-a]isoquinolin-2-ones 5f,g – General Procedure:
A mixture of the corresponding tetrahydroisoquinoline 2 (1 mmol) and methyl or phenyl isothiocyanate (2 mmol) was heated in an open reaction vessel at 120 oC for 10 min. The mixture was then allowed to cool to r.t. and the obtained crystalline product was washed with ether.
9,10-Dimethoxy-3,11b-dimethyl-4-thioxo-1,3,4,6,7,11b-hexahydropyrimido[6,1-a]isoquinolin-2-one (5f): mp 188 – 190 oC; IR: νC=O 1702; 1H-NMR: 1.61 (s, 3H, CH3), 2.73 (d, 2J = 16.5, 1H, 0.5 × CH2CO), 2.78 – 3.42 (m, 3H, CH2CH2N + 0.5 × CH2CH2N), 3.09 (d, 2J = 16.5, 1H, 0.5 × CH2CO), 3.67 (s, 3H, NCH3), 3.87 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 5.74 – 5.82 (m, 1H, 0.5 × CH2CH2N), 6.57 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H); 13C-NMR: 23.89, 28.75, 34.69, 45.90, 46.08, 55.87, 56.07, 58.20, 107.54, 111.06, 125.62, 130.68, 148.21, 165.71, 180.43.
9,10-Dimethoxy-11b-methyl-3-phenyl-4-thioxo-1,3,4,6,7,11b-hexahydropyrimido[6,1-a]isoquinolin-2-one (5g): mp 280 oC (dec.); IR: νC=O 1703; 1H-NMR: 1.81 (s, 3H, CH3), 2.79 – 3.50 (m, 3H, CH2CH2N + 0.5 × CH2CH2N), 2.97 (d, 2J = 16.3, 1H, 0.5 × CH2CO), 3.23 (d, 2J = 16.3, 1H, 0.5 × CH2CO), 3.87 (s, 3H, CH3O), 3.89 (s, 3H, CH3O), 5.73 – 5.81 (m, 1H, 0.5 × CH2CH2N), 6.61 (s, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 7.19 – 7.51 (m, 5H, C6H5); 13C-NMR: 26.17, 27.62, 46.03, 46.29, 55.57, 55.73, 57.95, 108.07, 111.18, 126.13, 127.87, 128.02, 131.35, 140.97, 145.52, 148.01, 166.02, 180.32.
Acknowledgements
This project is supported in part by the Fund for Scientific Research of Plovdiv University - Grant NPD2004MU8. We also thank to the staff of the NMR laboratory at the Institute of Organic Chemistry - Sofia, for their kind assistance with the NMR analyses.
Footnotes
Sample Availability: Available from the authors.
References and Notes
- 1.Lombardino J. G., McLamore W. M., Lavbach G. H. Azabenzopyridocolines. No. 3,021,331. US Patent. 1962
- 2.Lal B., D’Sa A., Dornauer H., de Sousa N. J. Processes for the Manufacture of Pyrimido[6,1-a]-isoquinolinones. No. 4,400,506. US Patent. 1983
- 3.Lal B., Dohadwalla A. N., Dadkar N. K., D’Sa A., de Sousa N. J. Trequinsin, a Potent New Antihypertensive Vasodilator in the Series of 2-(Arylimino)-3-alkyl-9,10-dimethoxy-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-ones. J. Med. Chem. 1984;27:1470. doi: 10.1021/jm00377a016. [DOI] [PubMed] [Google Scholar]
- 4.Ruppert D., Weithmann K. U. HL 725, an extremely potent inhibitor of platelet phosphodiesterase and induced platelet aggregation in vitro. Life Sci. 1982;31:2037. doi: 10.1016/0024-3205(82)90095-9. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti S., Srivastava M. C., Ila H., Junjappa H. aza-Annulation of Tetrahydroisoquinoline Derived Enamines: Efficient andConvergent Routes to Novel Functionalized Benzo[a]quinolizin-4-ones andPyrimido[6,1-a]isoquinoline Derivatives. Synlett. 2003:2369. [Google Scholar]
- 6.Fulop F., Semega E., Bernath G., Sohar P. Saturated Heterocycles. 162. Synthesis and Steric Structures of 3,4-Disubstituted 1,6,7,11b-Tetrahydropyrimido[6,1-a]isoquinolin-2-ones. J. Heterocyclic Chem. 1990;27:957. [Google Scholar]
- 7.Kobor J., Fulop F., El-Gharib M. S., Bernath G. Saturated Heterocycles. 57. Synthesis of 4-Substituted-9,10-dialkoxy-1,6,7,11b-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-2-ones. J. Hetero-cyclic Chem. 1984;21:149. [Google Scholar]
- 8.Granik V. G., Knyazeva V. F., Persianova I. V., Solov'eva N. P., Glushkov R. G. Acetals of Lactams and Acid Amides. 36. Some Reactions of Enamines of the Isoquinoline Series and Synthesis of Pyrimido(4,3-a)isoquinolines. Khim. Geterotsikl. Soedin. 1982;18:1095. [Google Scholar]
- 9.Kiss P., Holly S. Herstellung von Pyrimido[6,1-a]isochinolinen mit Formaldehyd. Chem. Ber. 1981;114:61. [Google Scholar]
- 10.Nair M. D., Mehta S. R. Reaction of 1-Methyl-3,4-dihydroisoquinolines with Phenyl Isocyanate & Isothiocyanate. Indian J. Chem. 1969;7:684. [Google Scholar]
- 11.Nair M. D., Desai J. A. Acetylenic Ester Adducts as Pictet-Spengler Intermediates in Isoquinoline Synthesis. Indian J. Chem. 1979;17B:277. [Google Scholar]
- 12.Yamazaki T. Yakugaku Zasshi. 1959;79:1008. [Chem Abstr.1961, 54, 5679] [Google Scholar]
- 13.Kametani T., Iida H., Kano S. Yakugaku Kenkyu. 1961;33:223. [Chem Abstr.1961, 55, 19933] [Google Scholar]
- 14.Kano S., Yuasa Y. A Diastereoselective Synthesis of 7-Arylpyrimido[6,1-a]isoquinolines Through N-Acyliminium Ion Cyclization. Heterocycles. 1985;23:2907. [Google Scholar]
- 15.Fisyuk A. S., Mukanov A. Yu. New Method for the Synthesis of 1,2,3,6,7,11b-Hexahydro-4H-pyrimido[6,1-a]isoquinoline-4-thiones and 2,3,6,7,12,12b-Hexahydropyrimido[6,1-a]-β-carboline-4(1H)-thiones. Khim. Geterotsikl. Soedin. 2003;39:307. [Google Scholar]
- 16.Venkov A. P., Angelov P. A. 1,1-Disubstituted Tetrahydroisoquinolines from Enaminones via Pictet-Spengler Reaction. Synth. Commun. 2003;33:3025. [Google Scholar]
- 17.Dewar M. J. S., Zoebisch E. G., Healy E. F., Stewart J. J. P. AM1: A New General Purpose Quantum Mechanical Molecular Model. J. Am. Chem. Soc. 1985;107:3902. [Google Scholar]
- 18.Granovsky A. A. http://classic.chem.msu.su/gran/gamess/index.html.
- 19.Schmidt M. W., Baldridge K. K., Boatz J. A., Elbert S. T., Gordon M. S., Jensen J. J., Koseki S., Matsunaga N., Nguyen K. A., Su S., Windus T. L., Dupuis M., Montgomery J. A. General Atomic and Molecular Electronic Structure System. J.Comput.Chem. 1993;14:1347. [Google Scholar]
- 20.N-Methyl-3-oxo-3-phenyl-propionamide was obtained according to a recently published procedure: Stefane, B.; Polanc, S. A New Regio- and Chemoselective Approach to β-Keto Amides and β-Enamino Carboxamides via 1,3,2-Dioxaborinanes. Synlett. 2004:698. [Google Scholar]