Abstract
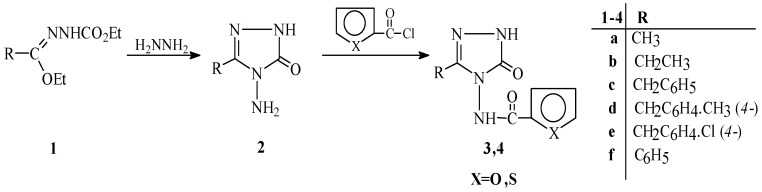
3-Alkyl(Aryl)-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones (2) reacted with 2-furoyl chloride and thiophene-2-carbonyl chloride to afford the corresponding 3-alkyl(aryl)-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (3) and 3-alkyl(aryl)-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (4), respectively. The new compounds synthesized were characterized by using IR, 1H-NMR, 13C-NMR and UV spectral data together with elemental analysis. In addition, to investigate the effects of solvents and molecular structure upon acidity, compounds 3 and 4 were titrated potentiometrically with tetrabutylammonium hydroxide in four non-aqueous solvents (isopropyl alcohol, tert-butyl alcohol, N,N-dimethylformamide and acetonitrile). The half-neutralization potential values and the corresponding pKa values were determined for all cases.
Keywords: 4,5-Dihydro-1H-1,2,4-triazol-5-ones; acylation; acidity; potentiometric titration; syntheses
Introduction
1,2,4-Triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives are reported to show a broad spectrum of biological activities such as antifungal, antimicrobial, hypoglycemic, antihypertensive, analgesic, antiparasitic, hypocholesteremic, antiviral, anti-inflammatory, antitumor and anti-HIV properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. These observations prompted us to synthesize some new 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives with potential biological activity. In addition, several articles, involving the acylation of 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives, have also been published up to date [11,12,15,16].
On the other hand, it is known that 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one rings have weak acidic properties, so some 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives were titrated potentiometrically with tetrabutylammonium hydroxide in non-aqueous solvents, and the pKa values of the compounds were determined [11,17,18,19,20]. We have previously described the synthesis and potentiometric titrations of some new 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives in different non-aqueous medium [21,22], where we determined the pKa values of these compounds for each non-aqueous solvent.
The aim of this work is to synthesize a series of 3-alkyl(aryl)-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (3) and 3-alkyl(aryl)-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (4) from the reactions of 3-alkyl(aryl)-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones (2) with 2-furoyl chloride and thiophene-2-carbonyl chloride, respectively (Scheme 1). Moreover, the synthesized compounds 3 and 4 were titrated potentiometrically with tetrabutylammonium hydroxide (TBAH) in four non-aqueous solvents, including isopropyl alcohol, tert-butyl alcohol, N,N-dimethylformamide and acetonitrile to determine their pKa values. For each new compound synthesized, the half-neutralization potential (HNP) and the corresponding pKa value were determined in the four mentioned non-aqueous solvents. The data obtained from the potentiometric titrations were interpreted, and the effect of the C-3 substituent and solvent effects were studied [17,18,19,20,21,22]. Determination of pKa values of active constituents of certain pharmaceutical preparations is important, because their distribution, transport behavior, bonding to receptors, and contributions to metabolic behavior depend on the ionization constant [23].
Scheme 1.
Results and Discussion
In this study, the structures of the newly synthesized 3-alkyl(aryl)-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (3) and 3-alkyl(aryl)-4-(2-thienyl-carbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones (4) were identified using elemental analysis and IR, 1H-NMR, 13C-NMR and UV spectral data, and these obtained spectral values were seen to be compatible with literature reports [24,25]. In addition, these newly synthesized compounds 3 and 4 were titrated potentiometrically with tetrabutyl-ammonium hydroxide (TBAH) in non-aqueous solvents such as isopropyl alcohol (ε=19.4), tert-butyl alcohol (ε=12), N,N-dimethylformamide (ε=37) and acetonitrile (ε=36).
The mV values were plotted versus TBAH volumes (mL) added, and thus potentiometric titration curve was formed for all the cases. From these curves, the HNP values were measured, and the corresponding pKa values were calculated.
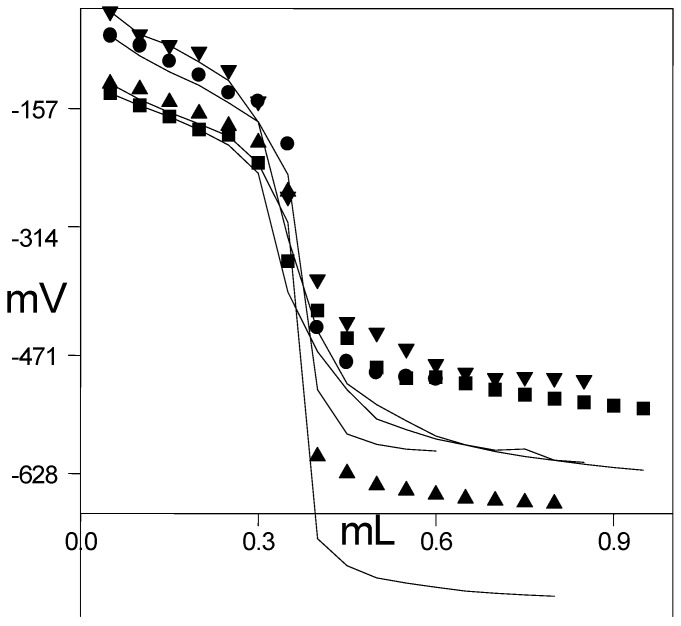
As an example, the potentiometric titration curves for 0.001 M 3-Benzyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3c) solutions titrated with 0.05 M TBAH in isopropyl alcohol, tert-butyl alcohol, N,N-dimethylformamide and acetonitrile are given in Figure 1. As it is clearly seen in Figure 1, a typical S-shaped titration curve was obtained.
Figure 1.
Potentiometric titration curves of 10-3 M 3-Benzyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3c) solutions titrated with 0.05 M TBAH in isopropyl alcohol (●), tert-butyl alcohol (▲), N,N-dimethylformamide (■) and acetonitrile (▼) at 25 °C.
The half-neutralization potentials (HNP) and the corresponding pKa values for compounds 3 and 4, obtained from the potentiometric titrations with 0.05 M TBAH in isopropyl alcohol, tert-butyl alcohol, N,N-dimethylformamide and acetonitrile, are given in Table 1.
Table 1.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3 and 4 in isopropyl alcohol, tert-butyl alcohol, N,N-dimethylformamide and acetonitrile.
| Compd. no | Isopropyl alcohol | Tert-butyl alcohol | N,N-Dimethyl formamide | Acetonitrile | ||||
|---|---|---|---|---|---|---|---|---|
| HNP (mV) | pKa | HNP (mV) | pKa | HNP (mV) | pKa | HNP (mV) | pKa | |
| 3a | -292 | 11.63 | -206 | 9.84 | -447 | 14.56 | -430 | 14.57 |
| 3b | -298 | 11.69 | -260 | 11.01 | -459 | 15.69 | -388 | 13.69 |
| 3c | -111 | 8.79 | -162 | 9.49 | -172 | 10.38 | -96 | 8.48 |
| 3e | -290 | 11.52 | -248 | 10.76 | -443 | 15.30 | -409 | 14.10 |
| 3f | -312 | 11.97 | -186 | 9.52 | -491 | 15.26 | -329 | 12.48 |
| 4a | -275 | 11.07 | -273 | 11.10 | -450 | 14.37 | -374 | 13.38 |
| 4b | -297 | 11.87 | -254 | 10.86 | -324 | 12.39 | -333 | 12.54 |
| 4c | -291 | 11.42 | -175 | 11.63 | -347 | 12.42 | -352 | 12.91 |
| 4d | -214 | 10.66 | -285 | 11.84 | -320 | 12.82 | -227 | 11.52 |
| 4e | -286 | 11.48 | -277 | 11.26 | -436 | 14.23 | -284 | 11.60 |
| 4f | -293 | 11.47 | -291 | 11.40 | -388 | 13.17 | -333 | 12.51 |
As it is well known, the acidity of a compound depends on some factors. The two most important factors are the solvent effect and molecular structure [18,19,20,21,22,26,27,28]. Table 1 shows that the HNP values and the corresponding pKa values obtained from potentiometric titrations depend on the non-aqueous solvents used. The results obtained illustrate that tert-butyl alcohol is the best solvent. As can be observed in Figure 1, for example, the potential jump of compound 3c in the end-point is very large for tert-butyl alcohol ranging from –266 mV to –599 mV. In addition, Table 1 shows that the molecular structure of titrated compounds affects the HNP and corresponding pKa values depending on the substituents at C-3 in the same solvent.
Experimental
General
Melting points were taken on a Electrothermal digital melting point apparatus and are uncorrected. IR spectra were registered using KBr disks on a Perkin-Elmer 1600 FTIR spectrometer. 1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 with TMS as internal standard on a Varian Mercury spectrometer at 200 MHz and 50 MHz, respectively. UV absorption spectra were measured for ethanol solutions in 10 mm quartz cells between 200 and 400 nm using a Shimadzu UV-1201 spectrophotometer. For potentiometric titrations, a Jenway 3040 ion analyser pH meter (calibrated according to the instructions of the manufacturer) equipped with an Ingold pH electrode were used. During the titrations, the titrant was added in increments of 0.05 mL after each stable reading, and the corresponding mV values were recorded. Chemicals were supplied from Fluka and Merck. After purification, isopropyl alcohol was used to prepare 0.05 M tetrabutylammonium hydroxide (TBAH). For all potentiometric titrations, 0.05 M TBAH in isopropyl alcohol was used. The starting compounds 2a-e were prepared from the reactions of the corresponding ester ethoxycarbonylhydrazones (1a-e) with hydrazine hydrate according to literature [16,29].
General Method for the Preparation of 3-alkyl(aryl)-4-[2-furoylamino]-4,5-dihydro-1H-1,2,4-triazol-5-ones (3) or 3-alkyl(aryl)-4-[2-thienylcarbonyamino]-4,5-dihydro-1H-1,2,4-triazol-5-ones (4):
3-Alkyl(aryl)-4-amino-4,5-dihydro-1H-1,2,4-triazol-5-one (2) (0.01 mol) was refluxed with a solution of the appropriate heteroaroyl chloride (2-furoyl chloride or 2-thiophenecarbonyl chloride) (0.01 mol) in n-butyl acetate (40 mL) for 6 hours and then allowed to cool. The product was recrystallized from an appropriate solvent to give 3 or 4. The following compounds were prepared applying this procedure:
3-Methyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3a). Yield 90%; m.p. 83 °C (H2O); Calculated for C8H8N4O3 (208.18): 46.16% C, 3.87% H, 26.91% N; found: 46.70% C, 4.18% H, 26.79% N. 1H-NMR: δ 2.06 (s, 3H, CH3), 6.76 (s, 1H, Ar-H), 7.40 (s, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 11.38 (s, 1H, NH); 11.73 (s, 1H, NH); 13C-NMR: δ 10.72 (aliphatic carbon), 112.65, 116.87, 145.05, 147.07 (aromatic carbons), 145.36 (triazole C3), 152.89 (triazole C5), 157.24 (C=O); IR: 3500, 3170 (NH), 1715, 1680 (C=O), 1596 (C=N) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 253 (26350), 212 (16510) nm.
3-Ethyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3b). Yield 87%; m.p. 111-112 °C (EtOH-toluene, 1:3); Calculated for C9H10N4O3 (222.20): 48.65% C, 4.54% H, 25.21% N; found: 48.35% C, 4.74% H, 24.93% N. 1H-NMR: δ 1.11 (t, 3H, CH3), 2.33 (q, 2H, CH2), 6.70 (s, 1H, Ar-H), 7.33 (s, 1H, Ar-H), 7.95 (s, 1H, Ar-H), 11.33 (s, 1H, NH); 11.70 (s, 1H, NH); 13C-NMR: δ 9.88, 18.17 (aliphatic carbons), 112.57, 116.74, 145.00, 149.10 (aromatic carbons), 147.00 (triazole C3), 152.96 (triazole C5), 157.14 (C=O); IR: 3450, 3260 (NH), 1715, 1695 (C=O), 1595 (C=N) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 211 (11390) nm.
3-Benzyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3c). Yield 82%; m.p. 160-162 °C (EtOH); Calculated for C14H12N4O3 (284.27): 59.15% C, 4.25% H, 19.71% N; found: 59.48% C, 4.18% H, 18.79% N. 1H-NMR: δ 3.72 (s, 2H, CH2), 6.74 (s, 1H, Ar-H), 7.20-7.36 (m, 7H, Ar-H), 11.71 (s, 1H, NH); 11.90 (s, 1H, NH); 13C NMR: δ 30.40 (aliphatic carbon), 112.80, 117.10, 126.89, 128.47 (3C), 128.63 (3C), 134.60 (aromatic carbons), 146.20 (triazole C3), 152.00 (triazole C5), 163.30 (C=O); IR: 3450, 3230 (NH), 1746, 1715 (C=O), 1590 (C=N), 770, 705 (monosubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 246 (28720), 221 (23950) nm.
3-(4-Chlorobenzyl)-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3e). Yield 90%; m.p. 177-178 °C (EtOH-H2O, 1:3); Calculated for C14H11N4O3Cl (318.72): 52.76% C, 3.48% H, 17.58% N; found: 52.45% C, 3.87% H, 17.56% N. 1H-NMR: δ 3.82 (s, 2H, CH2), 6.75 (s, 1H, Ar-H), 7.25-7.38 (m, 5H, Ar-H), 8.02 (s, 1H, Ar-H), 11.36 (s, 1H, NH); 11.92 (s, 1H, NH); 13C-NMR: δ 30.30 (aliphatic carbon), 112.40, 117.10, 128.58 (3C), 130.96 (2C), 131.90, 134.10, 147.03 (aromatic carbons), 145.00 (triazole C3), 153.00 (triazole C5), 157.30 (C=O); IR: 3400, 3210 (NH), 1725, 1688 (C=O), 1595 (C=N), 810 (1,4-disubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 256 (14340), 221 (16830) nm.
3-Phenyl-4-(2-furoylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3f). Yield 79%; m.p. 278-239 °C (H2O); Calculated for C13H16N4O3 (270.25): 57.78% C, 3.73% H, 20.73% N; found: 58.02% C, 3.50% H, 20.43% N. 1H-NMR: δ 6.75 (s, 1H, Ar-H), 7.47-7.52 (m, 4H, Ar-H), 7.85-7.86 (m, 2H, Ar-H), 8.03 (s, 1H, Ar-H), 11.82 (s, 1H, NH); 12.49 (s, 1H, NH); 13C-NMR: δ 112.80, 117.20, 126.25, 127.14 (2C), 129.30 (2C), 130.91, 146.53, 147.17 (aromatic carbons), 145.21 (triazole C3), 153.62 (triazole C5), 157.45 (C=O); IR: 3450, 3150 (NH), 1713, 1670 (C=O), 1590 (C=N), 760, 695 (monosubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 246 (29600), 216 (22900) nm.
3-Methyl-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4a). Yield 83%; m.p. 255-256 °C (EtOH); Calculated for C8H8N4O2S (224.24): 42.85% C, 3.60% H, 24.99% N; found: 43.26% C, 3.45% H, 24.98% N. 1H-NMR: δ 1.99 (s, 3H, CH3), 7.06-7.29 (m, 1H, Ar-H), 7.45-8.22 (m, 2H, Ar-H), 11.46 (s, 1H, NH); 11.70 (s, 1H, NH); 13C-NMR: δ 11.87 (aliphatic carbon), 129.30, 131.56, 134.00, 135.84 (aromatic carbons), 146.21 (triazole C3), 153.82 (triazole C5), 161.84 (C=O); IR: 3300, 3150 (NH), 1720, 1660 (C=O), 1610 (C=N) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 248 (29140), 212 (19790) nm.
3-Ethyl-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4b). Yield 82%; m.p. 202-203 °C (EtOH-H2O, 1:3); Calculated for C9H10N4O2S (238.26): 45.37% C, 4.23% H, 23.51% N; found: 45.03% C, 4.18% H, 23.57% N. 1H-NMR: δ 1.14 (t, 3H, CH3), 2.41 (q, 2H, CH2), 7.27-7.29 (m, 1H, Ar-H), 7.97-7.99 (m, 2H, Ar-H), 11.47 (s, 1H, NH); 11.79 (s, 1H, NH); 13C-NMR: δ 10.11, 18.34 (aliphatic carbons), 128.79, 130.76, 133.47, 135.40 (aromatic carbons), 149.26 (triazole C3), 153.19 (triazole C5), 161.05 (C=O); IR: 3500, 3175 (NH), 1720, 1675 (C=O), 1600 (C=N) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 250 (22220), 207 (16500) nm.
3-Benzyl-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4c). Yield 86%; m.p. 220-222 °C (EtOH-H2O, 1:3); Calculated for C14H12N4O2S (300.33): 55.99% C, 4.03% H, 18.65% N; found: 55.73% C, 3.73% H, 18.38% N. 1H-NMR: δ 3.82 (s, 2H, CH2), 7.26 (s, 6H, Ar-H), 7.95 (s, 2H, Ar-H), 11.46 (s, 1H, NH); 11.94 (s, 1H, NH); 13C-NMR: δ 31.10 (aliphatic carbon), 127.18, 128.76 (3C), 129.08 (2C), 131.10, 133.90, 135.40, 135.90 (aromatic carbons), 147.90 (triazole C3), 153.40 (triazole C5), 161.20 (C=O); IR: 3250, 3100 (NH), 1725, 1660 (C=O), 1600 (C=N), 770, 705 (monosubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 251 (22220), 210 (30440) nm.
3-(4-Methylbenzyl)-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4d). Yield 77%; m.p. 167-168 °C (EtOH-H2O, 1:3); Calculated for C15H14N4O2S (314.36): 57.31% C, 4.48% H, 17.82% N; found: 57.10% C, 4.27% H, 17.93% N. 1H-NMR: δ 2.27 (s, 3H, CH3), 3.95 (s, 2H, CH2), 7.11-7.27 (m, 5H, Ar-H), 7.95-8.00 (m, 2H, Ar-H), 11.43 (s, 1H, NH); 11.89 (s, 1H, NH); IR: 3250, 3120 (NH), 1760, 1670 (C=O), 1615 (C=N), 790 (1,4-disubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 269 (23580), 211 (24660) nm.
3-(4-Chlorobenzyl)-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4e). Yield 90%; m.p. 190-191 °C (EtOH-H2O, 1:3); Calculated for C14H11N4O2SCl (334.78): 50.23% C, 3.31% H, 16.74% N; found: 50.55% C, 2.99% H, 16.50% N. 1H-NMR: δ 3.82 (s, 2H, CH2), 7.24-7.37 (m, 5H, Ar-H), 7.93-7.97 (m, 2H, Ar-H), 11.41 (s, 1H, NH); 11.93 (s, 1H, NH); 13C-NMR: δ 29.00 (aliphatic carbon), 127.25 (3C), 129.39, 129.57 (2C), 130.90, 131.99, 132.63, 134.30 (aromatic carbons), 145.90 (triazole C3), 152.90 (triazole C5), 159.80 (C=O); IR: 3220, 3100 (NH), 1730, 1665 (C=O), 1595 (C=N), 805 (1,4-disubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 251 (15100), 222 (24400) nm.
3-Phenyl-4-(2-thienylcarbonylamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4f). Yield 85%; m.p. 160-161 °C (EtOH-H2O, 1:3); Calculated for C13H10N4O2S (286.31): 55.54% C, 3.52% H, 19.57% N; found: 55.16% C, 3.52% H, 19.43% N. 1H-NMR: δ 7.29-8.05 (m, 8H, Ar-H), 11.85 (s, 1H, NH); 12.46 (s, 1H, NH); 13C-NMR: δ 126.10, 126.98 (2C), 127.10, 129.25 (2C), 130.90 (2C), 133.80, 135.50 (aromatic carbons), 146.20 (triazole C3), 153.70 (triazole C5), 161.10 (C=O); IR: 3250 (NH), 1730, 1675 (C=O), 1610 (C=N), 770, 700 (monosubstituted benzenoid ring) cm-1; UV λmax nm, (ε, L·mol-1·cm-1): 253 (25710), 210 (16990) nm.
Footnotes
Sample availability: Contact the authors.
References
- 1.Bhat A. R., Bhat G. V., Shenoy G. G. Synthesis and in-vitro antimicrobial activity of new 1,2,4-triazoles. J. Pharm. Pharmacol. 2001;53:267–272. doi: 10.1211/0022357011775307. [DOI] [PubMed] [Google Scholar]
- 2.Modzelewska-Banachiewicz B., Jagiello-Wojtowicz E., Tokarzewska-Wielosz E. Synthesis and biologically activity of BIS-1,2,4-triazole and BIS-1,3,4-thiadiazole derivatives. Acta Pol. Pharm.-Drug Res. 2000;57:199–204. [PubMed] [Google Scholar]
- 3.Varvaresou A., Tsantili-Kakoulidou A., Siatra-Papastaikoudi T., Tiligada E. Synthesis and biological evaluation of indole containing derivatives of thiosemicarbazide and their cyclic 1,2,4-triazole and 1,3,4-thiadiazole analogs. Arzneim.-Forsch./Drug Res. 2000;50:48–54. doi: 10.1055/s-0031-1300163. [DOI] [PubMed] [Google Scholar]
- 4.Ulusoy N., Gursoy A., Otuk G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Pharmaco. 2001;56:947–952. doi: 10.1016/s0014-827x(01)01128-4. [DOI] [PubMed] [Google Scholar]
- 5.Witkowski J. T., Robins R. K., Khare G. P., Sidwell R. W. Synthesis and antiviral activity of 1,2,4-triazole-3-thiocarboxamide and 1,2,4-triazole-3-thiocarboxamidine ribonucleosides. J. Med. Chem. 1973;16:935–937. doi: 10.1021/jm00266a014. [DOI] [PubMed] [Google Scholar]
- 6.Burzozowski Z. Synthesis and anti-HIV activity of some new 2-mercapto-N-(1,2,4-triazol-3-yl)benzenesulfonamide derivatives containing the 1,2,4-triazole moiety fused with variety of heteroaromatic rings. Acta Pol. Pharm.-Drug Res. 1998;55:473–480. [PubMed] [Google Scholar]
- 7.Hui X.-P., Zhang L.-M., Zhang Z.-Y., Wang Q., Wang F. Synthesis and antibacterial activity of s-triazoles, s-triazolo[3,4-b]-1,3,4-thiadiazines and s-triazolo[3,4-b]-1,3,4-thiadiazoles of 5-methylisoxazole. J. Chin. Chem. Soc. 2000;47:535–539. [Google Scholar]
- 8.Katica C.-R., Vesna D., Vlado K., Dora G. M., Aleksandra B. Synthesis, antibacterial and antifungal activity of 4-substituted-5-aryl-1,2,4-triazoles. Molecules. 2001;6:815–824. doi: 10.3390/61000815. [DOI] [Google Scholar]
- 9.Wang Z., You T., Xu Y. Synthesis and biological activities of 2-substituted-5-(β-pyridyl)-2,3-dihydro-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Molecules. 1996;1:68–71. doi: 10.1007/s007830050011. [DOI] [Google Scholar]
- 10.Ikizler A. A., Ikizler A., Yüksek H., Serdar M. Antitumor activities of some 4,5-dihydro-1H-1,2,4-triazol-5-ones. Modelling, Measurement & Control C, AMSE Press. 1998;1:25–33. [Google Scholar]
- 11.Yüksek H., Demibaş A., Ikizler A., Johansson C. B., Çelik C., Ikizler A. A. Synthesis and antibacterial activities of some 4,5-dihydro-1H-1,2,4-triazol-5-ones. Arzneim.-Forsch./Drug Res. 1997;47:405–409. [PubMed] [Google Scholar]
- 12.Ikizler A. A., Demibaş A., Johansson C. B., Çelik C., Serdar M., Yüksek H. Synthesis and biological activities of some 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Acta Pol. Pharm.-Drug Res. 1998;55:117–123. [PubMed] [Google Scholar]
- 13.Ikizler A. A., Uçar F., Yüksek H., Aytin A., Yasa I., Gezer T. Synthesis and antifungal activity of some new arylidenamino compounds. Acta Pol. Pharm.-Drug Res. 1997;54:135–140. [PubMed] [Google Scholar]
- 14.Demirbaş A., Johansson C. B., Duman N., Ikizler A. A. Synthesis and biological activities of some new 4,5-dihydro-1H-1,2,4-triazol-5-ones. Acta Pol. Pharm.-Drug Res. 1996;53:117–121. [PubMed] [Google Scholar]
- 15.Ikizler A., Doğan N., Ikizler A. A. The acylation of 4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones. Rev. Roum. Chim. 1998;43:741–746. [Google Scholar]
- 16.Ikizler A. A., Yüksek H. Acylation of 4-amino-4,5-dihydro-1H-1,2,4-triazol-5-ones. Org. Prep. Proc. Int. 1993;25:99–105. doi: 10.1080/00304949309457935. [DOI] [Google Scholar]
- 17.Ikizler A. A., Ikizler A., Şentürk H. B., Serdar M. The pKa values of some 1,2,4-triazole and 1,2,4-triazolin-5-one derivatives in nonaqueous media. Doğa-Tr. Kimya D. 1988;12:57–66. [Chem. Abstr.1988, 109, 238277q] [Google Scholar]
- 18.Ikizler A. A., Erdoğan Y. Determination of pKa values of some benzylideneamino compounds in nonaqueous media. Doğa-Tr. J. of Chem. 1991;15:337–344. [Chem. Abstr.1992, 116, 193614y] [Google Scholar]
- 19.Ikizler A. A., Şentürk H. B., Ikizler A. pK’a values of some 1,2,4-triazole derivatives in nonaqueous media. Doğa-Tr. J. of Chem. 1991;15:345–354. [Chem. Abstr.1992, 116, 173458x] [Google Scholar]
- 20.Erdoğan Y., Aslan A., Demirbaş A., Yaylı N. Potentiometric titration of two carboxylic acids and two triazole derivatives in non aqueous media. Modelling, Measurement & Control C, AMSE Press. 1994;46:49–54. [Google Scholar]
- 21.Bahçeci Ş., Yüksek H., Ocak Z., Azaklı A., Alkan M., Ozdemir M. Synthesis and potentiometric titrations of some new 4-(benzylideneamino)-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives in non-aqueous media. Collect. Czech. Chem. Commun. 2002;67:1215–1222. [Google Scholar]
- 22.Bahçeci Ş., Yüksek H., Ocak Z., Köksal C., Ozdemir M. Synthesis and non-aqueous medium titrations of some new 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Acta Chim. Slov. 2002;49:783–794. [Google Scholar]
- 23.Demirbaş A., Kula I., Erdoğan Y., Aslan A., Yaylı N., Karslıoğlu S. Non-aqueous medium titrations of some acidic compounds. Energy, Educ., Sci. Technol. 1998;1:1–6. [Google Scholar]
- 24.Ikizler A. A., Ikizler A., Yüksek H. 1H-NMR spectra of some 4,5-dihydro-1,2,4-triazol-5-ones. Magn. Reson. Chem. 1993;31:1088–1094. doi: 10.1002/mrc.1260311211. [DOI] [Google Scholar]
- 25.Ikizler A. A., Yüksek H. A study on 4,5-dihydro-1H-1,2,4-triazol-5-ones. Rev. Roum Chem. 1996;41:585–590. [Google Scholar]
- 26.Aslan A., Erdoğan Y., Demirbaş A., Karslıoğlu S. Potentiometric titration of some dicarboxylic acids in non-aqueous media. Pharmazie. 1997;52:309–310. [Google Scholar]
- 27.Aktaş A. H., Yaşar G., Alsancak G. Ö. Conductimetric and potentiometric titration of some hydroxylated cinnamic acids with tetrabutylammonium hydroxide in non-aqueous media. Turk. J. Chem. 2001;25:501–507. [Google Scholar]
- 28.Gündüz T. Susuz Ortam Reaksiyonları. Gazi Büro Kitabevi Tic. Ltd. Şti; Ankara: 1998. [Google Scholar]
- 29.Ikizler A. A., Un R. Reactions of ester ethoxycarbonylhydrazones with some amine type compounds. Chim. Acta Turc. 1979;7:269–290. [Chem. Abstr.1991, 94, 15645d] [Google Scholar]