Abstract
A flavanone and a flavanone glycoside, together with vomifoliol, have been isolated for the first time from the aerial parts of the plant Echiochilon fruticosum and identified. Their structures were established on the basis of spectroscopic measurements, mainly 2D NMR using COSY, HMQC and HMBC experiments.
Keywords: Flavanone, heteroside, vomifoliol, Echiochilon fruticosum, NMR
Introduction
This research is a contribution to the chemical study of some plants growing in Tunisia [1], with the objective of discovering new natural products with potential biological activity. We have chosen for this purpose the plant Echiochilon fruticosum (Boraginaceae). Only some botanical and agronomical studies have been carried out in this plant, and, to the best of our knowledge, so far it has not been the subject of any phytochemical investigations. Echiochilon fruticosum may be considered one of four important herbs found in the northern desert of Egypt [2]. The ecological relationships of this plant in siliceous sand deposits north of Wadi El-Natrum (Egypt) were studied and it showed in particular a high correlation with soil salinity [3]. The nutritive value of Echiochilon fruticosum from the western Mediterranean desert of Egypt has been investigated and the possibility of its use as forage for domestic animals (mainly sheep and goats) was demonstrated [4]. We note that in Tunisia, this species is widespread in the region of Chott Meriem (Sousse, Tunisia) and in the desert, where it is used as grazing for animals [5,6]. We report here the isolation and the structure elucidation of a flavanone, naringenin 5-methyl ether (1), a flavanone glycoside, pinocembrin-7-glycoside (2) and vomifoliol (3) from the ethyl acetate aerial part extract of Echiochilon fruticosum. The structures were established on the basis of 2D NMR spectroscopic experiments and by comparison of the NMR data with literature values [7,8,9,10].
Results and Discussion
Compound 1
A molecular formula of C16H14O5 was obtained from the ESMS [MH+] at m/z 287 and from the 13C-NMR analysis. Furthermore, the IR spectrum revealed absorptions attributable to free hydroxyl (3366 cm-1) and ketone (1642 cm-1) groups. An examination of its NMR data (Table 1) and a comparison with the literature [7,8] suggested that compound 1 was a flavanone. Thus, its 1H-NMR spectrum revealed characteristic resonances of aromatic protons such as H2’,6’ (δ 7.35, d, J=8.4 Hz), H3’,5’ (δ 6.90, d, J=8.4 Hz), H8 (δ 6.02, s, 1H) and H6 (δ 6.09, s, 1H). A typical methoxyl signal at δ 3.82 ppm was also observed. The multiplicities and the weak coupling constants of H6 and H8 were in agreement with the existence of the hydroxyl group at C7 (δ 162.9). The presence in the 1H-NMR spectrum of signals at δ 5.38 (1H, dd, J=13, 2.9 Hz), δ3.12 (1H, dd, J=17.1, 2.9 Hz) and δ 2.85 (1H, dd, J=17.1, 13.1 Hz), in conjunction with the 13C-NMR signals at δ 78.6, and 129.9 ppm, inferred from HMQC and HMBC spectra (Table 1), pointed to the presence of an -O-CH-CH2-CO- system in the C-ring.
Table 1.
1H- and 13C-NMR spectral data for compounds 1, 2 and 3.
| Compound 1 | Compound 2 | Compound 3 | ||||
| Position | 13C | 1H | 13C | 1H | 13C | 1H |
| 1 | 78.9 | |||||
| 2 | 78.6 | 5.38; dd ; 13.1 ; 2.9 | 79.3 | 5.48; dd ; 2.9 ; 13.1 | 41.0 | |
| 3a | 52.9 | 3.12; dd; 2.9; 17.1 | 43.2 | 2.86; dd; 17.1; 2.9 | 49.6 | 2.25 (a); d; 16.9 |
| 3b | 2.85; dd; 17.1; 13.1 | 3.12; dd; 17.1; 13.1 | 2.45 (b); d; 16.9 | |||
| 4 | 196.4 | 196.5 | 195.5 | |||
| 5 | 168.0 | 162.9 | 127.9 | 5.90; m | ||
| 6 | 93.8 | 6.09; d; 2.17 | 97.0 | 6.21; d; 2.1 | 162.2 | |
| 7 | 162.9 | 165.6 | 129.0 | 5.81; m | ||
| 8 | 96.3 | 6.02; d; 2.3 | 95.8 | 6.23; d; 2.1 | 135.7 | 5.84; m |
| 9 | 164.0 | 162.8 | 68.1 | 4.41; m | ||
| 10 | 102.0 | 105.0 | 23.7 | 1.29; d; 6.4 | ||
| 11 | 24.0 | 1.01; s | ||||
| 12 | 23.0 | 1.08; s | ||||
| 13 | 18.8 | 1.89; s | ||||
| 1’ | 129.9 | 138.3 | ||||
| 2’ | 127.7 | 7.35; d; 8.4 | 126.0 | 7.48; d; 7.5 | ||
| 3’ | 115.4 | 6.90; d; 2.3 | 129.6 | 7.41; m | ||
| 4’ | 156.4 | 128.6 | 7.39; m | |||
| 5’ | 115.4 | 6.90; d; 8.4 | 129.6 | 7.41; m | ||
| 6’ | 127.7 | 5.35; d; 8.4 | 126.0 | 7.48; d; 7.5 | ||
| 1’’ | 101.0 | 4.97; d; 7.1 | ||||
| 2’’ | ||||||
| 3’’ | ||||||
| 4’’ | ||||||
| 5’’ | ||||||
| 6”a | 61.1 | 3.74; dd | ||||
| 6”b | 61.1 | 3.87; dd |
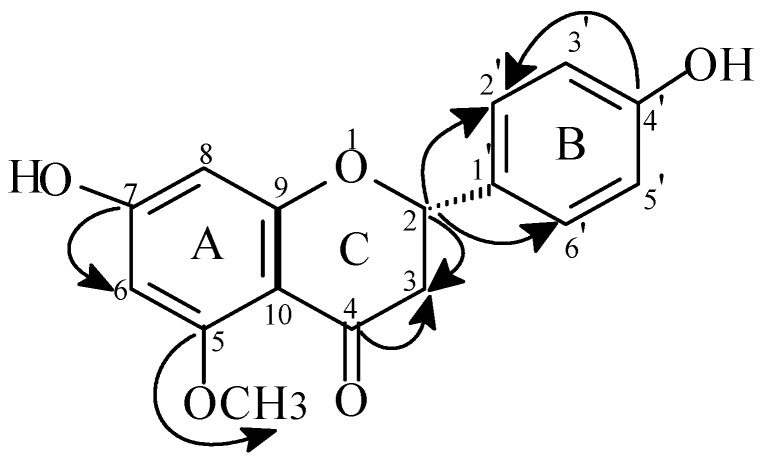
This conclusion was reinforced by the peak correlating signals at δ 3.12 and 5.38 ppm observed in the 1H-1H COSY spectrum. Other heteronuclear correlations deduced from the HMBC spectrum were also in agreement with the proposed structure (Figure 1). Elucidation of the absolute configuration at C2 was based on the values of the coupling constants with the methylenic protons H3α,β. (Jax-ax=13.1 and Jax-eq= 2.9 Hz). The close similarity of the H2 chemical shifts (Table I) with those of the literature [7,8] thus confirmed the S- configuration of C2.
Figure 1.
Heteronuclear multiple-bond correlations for 1. Arrows point from carbon to proton.
Compound 2
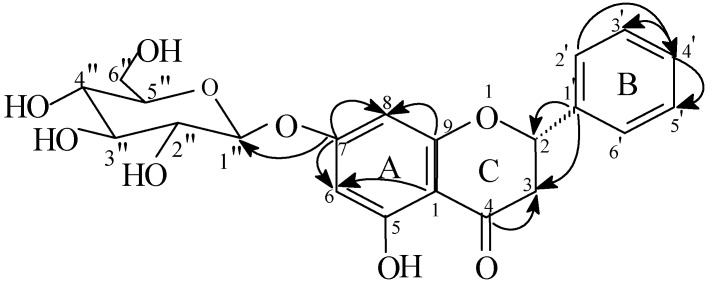
A molecular formula of C21H22O9 was deduced from the ES MS [MNa+] at m/z 441. The IR spectrum exhibited absorptions corresponding to hydroxyl (3321 cm-1) and ketone (1652 cm-1) groups. The comparison of the 1H-NMR spectrum with that of 1 (Table I) permitted us to note on the one hand, the presence of all proton signals of 1 with the exception of that of the methoxyl group at 3.82 ppm, and on the other, one additional signal at δ 7.39 (m, H4’). This observation suggests that the aglycone is pinocembrin. This hypothesis was confirmed on the basis of the correlation observed between the H4’ proton and C2’(δ 127.7) in the HMBC spectrum (Figure 2). The identification of the sugar as β-D-glycopyranoside was easily deduced from the analysis of the 1H-1H COSY spectrum using the anomeric proton as a starting point. The attachment of a glucose unit at C7 (δ 165.6 ppm) was apparent from the heteronuclear H1’’-C7 HMBC connectivity (Figure 2). As in compound 1, the absolute configuration of C2 was deduced from the comparison of H2 chemical shifts of 2 with the reported literature values [7,8].
Figure 2.
Heteronuclear multiple-bond correlations for 2. Arrows point from carbon to proton
Compound 3
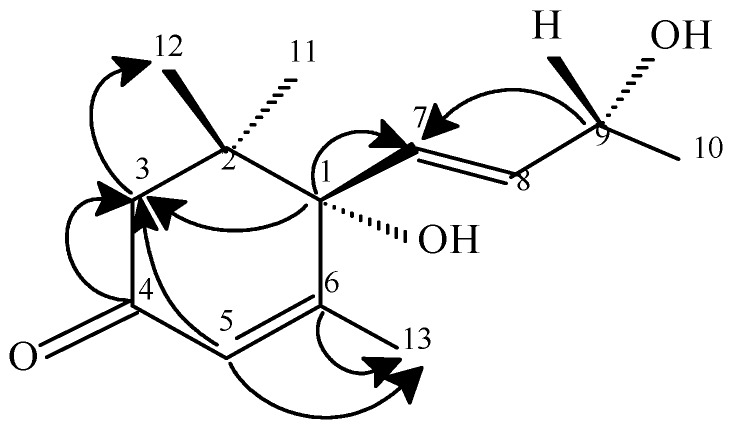
The ESMS of this compound showed a molecular ion [MNa+] at m/z 247, suggesting a molecular formula of C13H20O3. The comparison of the corresponding NMR data (Table 1) with those reported in literature [9,10] led to its identification as vomifoliol. The use of some 2D NMR experiments has permitted the confirmation of this structure. The cyclohexenone structure was confirmed by the observation in the HMBC spectrum of correlations between the AB system H3ab (δ 2.25, δ 2.45, J1=J2=16.9Hz) and C4 (δ 195.5), C1 (δ 78.9) and C5 (δ 127.9). The location of the two high field methyl groups CH3-11 and CH3-12 at C2 was ascertained by the detection of the H3a,b-C11 and H3a,b-C12 correlations. The position of the side chain on C1 of the cyclohexenone ring is confirmed by the observation in the HMBC spectrum of the H7-C1 correlation. The COSY spectrum revealing correlations between the olefinic signal H7 (δ 5.81) with the other olefinic proton H8 (δ 5.84) which in turn had a cross peak with H9 (δ 4.41) permitted us to confirm the structure of this side chain. All these NMR data together with the mass spectrum suggested the structure of vomifoliol reported for compound 3.
Figure 3.
Heteronuclear multiple-bond correlations for 3. Arrows point from carbon to proton.
Conclusions
Echiochilon fruticosum, which has not been previously chemically investigated, was found to contain three known compounds, namely a flavanone: naringenin 5-methyl ether (1), a flavanone glycoside: pinocembrin glycoside (2) and a cyclohexenone derivative: vomifoliol (3). Their structures were established on the basis of 2D NMR experiments. With regards to possible biologically interesting propeties of these compounds, it is well known that some flavanones have distinct taste properties, being bitter, sweet or bittersweet. Furthermore, some of them, such as naringenin, are reported to have inhibitory effects on microorganisms, whereas, the corresponding glycosides appeared to be inactive [11]. Vomifoliol has been reported to play an important role as an endogenous regulator of the stomatal aperture [10].
Experimental
General
Mass spectra were obtained with a Micromass spectrometer (Q-ToF Micro) linked to an ESI source. 1H-, 13C- and 2D-NMR spectra of 1 were recorded in CDCl3 with a Bruker NMR-300 spectrometer, operating at 300 and 75 MHz, respectively. The 1H-, 13C- and 2D-NMR spectra of 2 and 3 were obtained in a mixture of CDCl3 and CD3OD (compound 2) and CDCl3 (compound 3) on a Bruker Avance AM-400 spectrometer. Chemical shifts (δ) are given in ppm and coupling constants (J) in Hz.
Plant material
The aerial parts of Echiochilon fruticosum were harvested in March 2000 in Chott Meriem, Sousse (Tunisia). A herbarium specimen of this plant was deposited in the herbarium of the Laboratoire de Biologie Végétale et Botanique, Ecole Supérieure d’Horticulture et d’Elevage de Chott Mériem, Université du Centre, Sousse, Tunisia.
Extraction
Dried and finely powdered Echiochilon fruticosum aerial parts (1200 g) were extracted with methanol in a Soxhlet apparatus during 7 days. The crude residue (150 g) obtained after filtration and evaporation of the solvent was dissolved in water then extracted successively with petroleum ether, EtOAc and BuOH, yielding 86 g, 10 g and 24 g fractions, respectively.
Isolation of pure compounds 1-3
The ethyl acetate extract (10g) was resolved by silica gel column chromatography (SDS, ref. 2100027, petroleum ether, AcOEt, methanol gradients) so that ten main fractions were collected. Fraction 1 was in turn chromatographed over silica gel with CHCl3 to provide 10 subfractions. Subfraction 6 (120 mg) was rechromatographed on silica gel into 65 fractions using as eluent a 9.8:0.2 CHCl3/MeOH mixture. The combined fractions 31 to 47 (55 mg) were further purified on a silica gel column to give compound 1 as an oily substance (26 mg); [α]D 22 –14 (C = 0.17; CHCl3), Rf = 0.5 (CHCl3/MeOH 9.5:0.5); IR (KBr) νmax cm-1 3366 (br OH), 1642 (C=O); for 1H- and 13C-NMR data see Table I.
From the ten main fractions indicated above, fraction 5 was divided into 5 subfractions on a silica gel column using CHCl3 as eluant. The most polar one was further purified on a silica gel column using CHCl3/MeOH gradients to provide compound 2 as a white solid (1.5 mg); [α]D 22 –35 (C = 0.01; MeOH), Rf = 0.5 (CHCl3/MeOH 8.5:1.5); IR (KBr) νmax cm-1 3321 (br OH), 1652 (C=O); for 1H- and 13C-NMR data see Table I.
Fraction 3 (1g) was passed through an RP18 reverse phase column using water/methanol to provide ten subfractions. The subfraction with medium polarity (15 mg) was further resolved on a silica gel column using a gradient of CHCl3/MeOH as eluent to afford 4 subfractions; the third one (3 mg) was finally purified on a preparative plate eluted with a 9:1 CHCl3/MeOH mixture to give compound 3 as a white solid (1 mg); [α]D 22 +41 (C = 0.01; CHCl3), Rf = 0.5 (CHCl3/MeOH 9:1); IR (KBr) νmax cm-1 3430 (br OH), 1657 (C=O); for 1H- and 13C-NMR data see Table I.
Acknowledgements
The authors wish to thank Dr F. Harzallah-Skhiri, Laboratoire de Biologie Végétale et Botanique, Ecole Supérieure d’Horticulture et d’Elevage Université du Centre Chott Mériem, Sousse, Tunisia, for botanical classification of the plant material.
Footnotes
Sample availability: Samples of compounds 1 (6 mg), 2 (1 mg) and 3 (0.8 mg) are available from the authors.
References
- 1.Hichri F., Ben Jannet H., Cheriaa J., Jegham S., Mighri Z. Antibacterial activities of a new prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chimie. 2003;6:473–483. doi: 10.1016/S1631-0748(03)00066-3. and references cited therein. [DOI] [Google Scholar]
- 2.Moursi M. A., Abdel Gawad A. A., Ibrahim K. M., Osman R. Pasture productivity in north west coastal region in Egypt. 1. Effect of location on chemical composition of some forage plants at Sidi-Barrani. Egypt. J. Agron. 1979;2:129–139. [Google Scholar]
- 3.El-Ghonemy A. A. Socio-ecological studies of the natural plant communities along a desert transect 200 km long between Alexandria and Cairo. III. Ecological relation of vegetation on siliceous deposit north of Wadi El-Natrun. Egypt. J. Bot. 1976;19:43–62. [Google Scholar]
- 4.El-Din A. S., El-Kady H. F. Nutritive value of the range plants in the western Mediterranean Desert of Egypt. Arab Gulf J. Sci. Res. 2001;19:19–27. [Google Scholar]
- 5.Alapetite G. P. Flore de la Tunisie. Publications Scientifiques Tunisiennes, Imprimerie officielle de la République Tunisienne; Tunis (Tunisia): 1981. [Google Scholar]
- 6.Issaoui A., Kallala A., Neffati M., Akrimi N. Plantes Naturelles du Sud Tunisien. Ministère de l’environnement et de l’amenagement du territoire; Tunis (Tunisia): 1996. pp. 68–69. [Google Scholar]
- 7.Singh V.P., Bineeta Y., Pandey V.B. Flavanone glycosides from Alhagi pseudalhagi. Phytochemistry. 1999;51:587–590. doi: 10.1016/S0031-9422(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 8.Kang T. H., Jeong S. J., Ko W. G., Kim Na. Y., Lee B. H., Inagaki M., Miyamoto T., Higuchi R., Chul Kim Y. Cytotoxic lavandulyl flavanones from Sophora flavescens. J. Nat. Prod. 2000;63:680–681. doi: 10.1021/np990567x. [DOI] [PubMed] [Google Scholar]
- 9.Demole E., Enggist P. Novel synthesis of 3,5,5-trimethyl-4-(2-butenylidene)-cyclohex-2-en-1- one, a major constituent of Burley Tobacco flavour. Helv. Chim. Acta. 1974;7:2087–2091. doi: 10.1002/hlca.19740570722. [DOI] [Google Scholar]
- 10.Andersson R., Lundgren R. Monoaryl and cyclohexenone glycosides from needles of Pinus sylverstris. Phytochemistry. 1988;27:559–562. doi: 10.1016/0031-9422(88)83141-8. [DOI] [Google Scholar]
- 11.Grayer R. J. Method. Plant Biochem. 1989;1:287–288. [Google Scholar]