Abstract
Current progress on the mechanism and substrate recognition by sterol methyl transferase (SMT), the role of mechanism-based inactivators, other inhibitors of SMT action to probe catalysis and phytosterol synthesis is reported. SMT is a membrane-bound enzyme which catalyzes the coupled C-methylation-deprotonation reaction of sterol acceptor molecules generating the 24-alkyl sterol side chains of fungal ergosterol and plant sitosterol. This C-methylation step can be rate-limiting in the post-lanosterol (fungal) or post-cycloartenol (plant) pathways. A series of sterol analogs designed to impair SMT activity irreversibly have provided deep insight into the C-methylation reaction and topography of the SMT active site and as reviewed provide leads for the development of antifungal agents.
Keywords: Ergosterol, sitosterol, stereochemistry, sterol biosynthesis inhibitors
Introduction
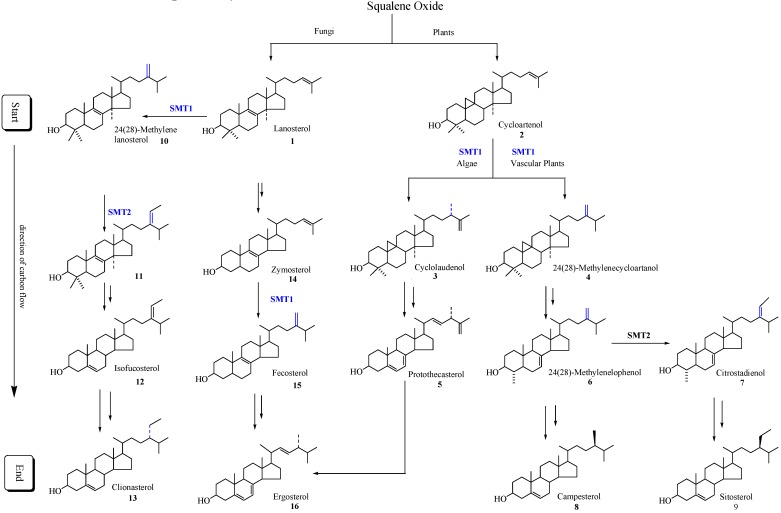
Sterol synthesis, particularly C-methylation of the sterol side chain at C-24, is an important area of biochemical difference between animals and fungi, as well as plants, that can be exploited in the development of new antifungal agents and engineered to generate value-added traits in crops. Whereas the major sterol of animals is the Δ5-sterol cholesterol, fungi and plants produce Δ5-sterols in which the cholesterol skeleton has been modified by the addition of one or two supernumerary carbon atoms at C-24 with either α or β chirality [1,2]. The 24-alkyl group is characteristic of all phytosterols and is preserved during subsequent steroid metabolism in both fungi and plants to give hormones that regulate growth and reproduction in a manner similar to animals [1,2]. The isopentenoid pathway to squalene oxide can bifurcate to generate the start of the sterol pathway which in fungi begins with the formation of lanosterol and in plants begins with the formation of cycloartenol. The direction of carbon flux proceeds forward to produce Δ5-sterols (Scheme 1).
Scheme 1.
Typical phytosterol pathways in plants and fungi. Only the major intermediates and end products are illustrated. SMT1 and SMT2 refer to sterol methyltransferase catalyzing the first and second C1-transfer activity, respectively.
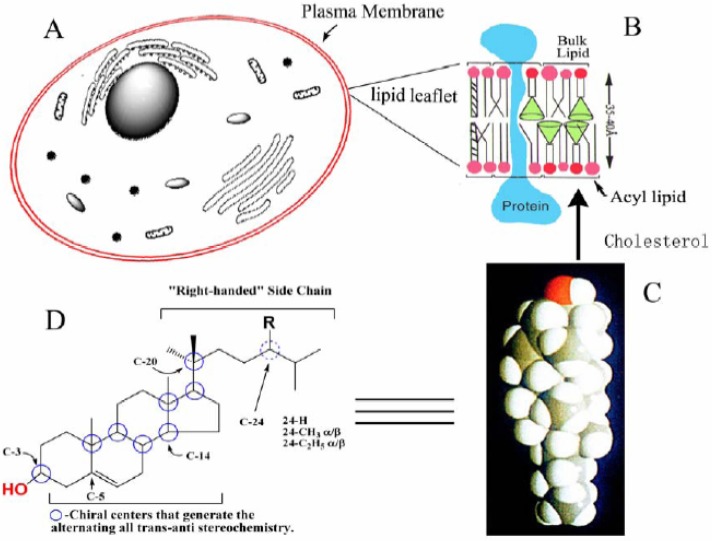
A striking feature of phytosterols is their amphipathic properties, established by a polar C-3 hydroxyl group and hydrophobic nucleus and side chain, flat shape, established by the alternating all-trans-anti stereochemistry of the ring system and “right-handed” side chain conformation, established by the 20R stereochemistry (Figure 1). The Δ5 -phytosterols often constitute < 80% of the total sterol fraction of cells and accumulate in the plasma membrane [2,3,4,5]. The addition of a 24-alkyl group to the C8 side chain of cholesterol can allow the side chain to sweep out a larger cone in the bulk lipids of the membrane than the cone swept out by a C8 isooctyl side chain thereby providing greater potential of phytosterols to disrupt lipid-lipid interactions which in turn can affect fluidity of the lipid leaflet.
Figure 1.
Structure of sterol and its association in the lipid leaflet of the plasma membrane. A, Generic picture of the cell illustrating subcellular structures; B, The lipid leaflet of the plasma membrane; C, A space-filling model of cholesterol; D, Sterol features involved with sterol-lipid and sterol-protein interactions.
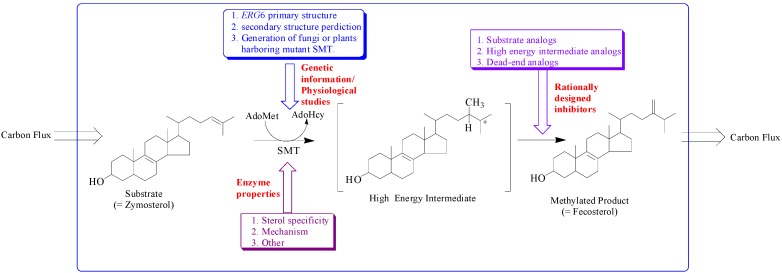
The sterol features engineered by enzyme catalysis to promote sterol homeostasis are intimately associated with the structure of the active site topology of the sterol methyltransferase (SMT). Different approaches have been utilized to understanding how SMT manages carbon flux relates to the characterization of genetic information, morphology and growth response of cells/plants harboring mutant SMT cDNA, enzyme properties and testing of rationally designed inhibitors of the SMT (Figure 2) [6,7,8,9,10,11,12,13].
Figure 2.
Hypothetical model illustrating approaches to study sterol methyltransferase positioned to control carbon flux to Δ5 -sterol end products.
The focus of this review is the enzymology and inhibition of SMT and its several isoforms, particularly recent mechanistic findings and the results of a new class of mechanism-based inactivators designed to impair SMT action.
SMT Properties
A full understanding of SMT action and its control genetically or by modulators requires elucidation of the properties of the enzyme. SMT is a membrane-bound protein typically studied as a crude microsomal/detergent solubilized preparation [6]. The ability for a range of Δ24-sterols to serve as substrates suggested that a single SMT might be responsible for the various catalytic activities in a given fungus or plant. However, recent cloning and functional expression of SMT cDNA in a yeast system erg6 defective in SMT synthesis revealed the existence of several SMT isoforms classified as SMT1 for the first C1-transfer activity and SMT2 for the second C1-transfer activity and each of these isoforms are considered to possess unique substrate specificities [14]. Using molecular biology and modern chromatographic techniques, we successfully transferred the cloned cDNA of SMT from yeast and vascular plants to Escherichia coli and purified the resulting recombinant enzyme to homogeneity [15,16,17]. From 1 liter of bacteria culture about 5 to 10 mg of pure protein can be generated against a sterol-free background. By exploiting these efforts, the estimation of actual enzyme concentration and thereby, the comparison of exacting kinetic parameters, as well as, the determination of accurate product distributions and reaction pathways have been determined for the first time. As predicted, the different SMT isoforms were found to possess unique sterol specificities.
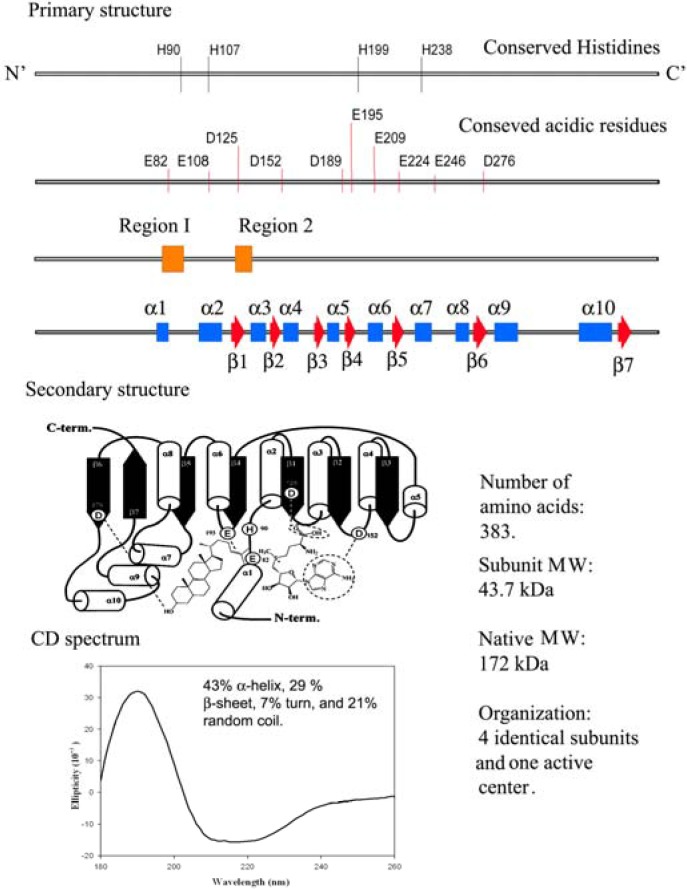
The addition of one or two carbon atoms at C-24 involves either a single or double methylation, and distinct mechanisms operate variably in nature. These SMTs have catalytic activities that can create diversity at the level of side chain stereochemistry and function. The SMT catalyzes the bi-substrate C-methyl transfer reaction whereby a methyl group of (S)-adenosyl-L-methionine (AdoMet) is transferred to C-24 of a sterol acceptor molecule, a high energy intermediate (HEI) is formed during the reaction progress and two compounds are produced, AdoHcy and a methylated sterol (Figure 2). In the case of yeast, the cDNA encoding SMT is the ERG6 gene and genetic studies indicate the importance of ERG6 for normal membrane functions and resistance to antifungal drugs [10,11,12]. Functional analysis of highly conserved amino acids in the primary structure of ERG6 by site-directed mutagenesis have led to a secondary structure prediction of the enzyme as shown in Figure 3 [18,19,20].
Figure 3.
Comparison of the genetic information with physical measurements of the yeast sterol methyl transferase.
Different SMT isoforms are now recognized to be synthesized in the cycloartenol-sitosterol pathway and lanosterol/cycloartenol-ergosterol pathway (Scheme 1) [6,14,21]. Substrate specificity, the basis for the reclassification of the enzyme commission nomenclature of SMTs, differs between the SMT1 isoforms of fungal and plant origin and between the SMT1 and SMT2 isoforms synthesized by the same plant. For example, zymosterol is the preferred substrate of fungal SMT1 (E.C. 2.1.1.41), cycloartenol is the preferred substrate plant of SMT1 (E.C. 2.1.1.142) and 24(28)-methylenelophenol is the preferred substrate of the plant SMT2 (E.C. 2.1.1.143) [6,14,18,21,22,23].
In all plants the critical slow step in phytosterol synthesis is associated with the C-methylation of cycloartenol [24,25,26]. In vascular plants and many fungi such as Pneumocystis carinii, the product of the SMT1 catalyzed reaction can be processed to be the substrate of SMT2 that controls the ratio of 24-methyl to 24-ethyl Δ5-sterols. Down-regulation of vascular plant SMT by sitosterol, but not by ergosterol or cholesterol and in the case of yeast SMT by ergosterol but not by sitosterol or cholesterol, suggest plant/fungal-specific membrane inserts can feed-back and modulate the post-lanosterol/cycloartenol synthetic pathway (Table 1) [6]. Coordinated sterol synthesis with membrane function and cell growth is also indicated by the slow turnover rate of the fungal SMT and fungal 14α-demethylase enzymes at ca. 0.01 s-1 [17,27,28].
Table 1.
Comparison of the molecular and kinetics parameters for recombinant SMT1 and SMT2 from fungi (Saccharomyces cerevisiae) and plants (Glycine max and Arabidopsis thaliana).
| Source | Preferred substrate (Km) | kcat | Enzyme generated product | Reaction mechanism | Kinetics mechanism | Down-Regulation of SMT activity | Rf |
|---|---|---|---|---|---|---|---|
|
S .cerevisiae (yeast) SMT1 |
Zymosterol (30µM) | 0.01s-1 | First C1- transfer is fecosterol | SN2 -type and non-stop | Ternary complex mechanism; Random pathway | Ergosterol inhibits SMT with Ki= 65 µM neither sitosterol or cholesterol inhibits activity | [21], [28] |
| No second C1- transfer activity | Not applicable | ||||||
|
G. max (soybean) SMT1 |
Cycloartenol (30µM) | 0.01s-1 | First C1- transfer is 24(28) methylenecycloartanol | SN2 -type and non- stop | Ternary complex mechanism; Ordered pathway with AdoMet binding first | Sitosterol inhibits SMT Ki=100 µM; neither ergosterol or cholesterol inhibits activity | [15], [29] |
| Second C1- transfer is a mixture of 24-ethyl olefins | SN2 -type and step-wise | ||||||
|
A. thaliana
SMT2 |
24(28)-Methylene lophenol (28µM) | 0.01s-1 | First C1- transfer is 24(28) methylenecycloartanol | SN2 -type and non- stop | Unknown | Sitosterol inhibits first C1-transfer at 200µM and second C1-transfer at 297 µM | [16] |
| Second C1- transfer is a mixture of 24-ethyl olefins | SN2 -type and step-wise | Unknown |
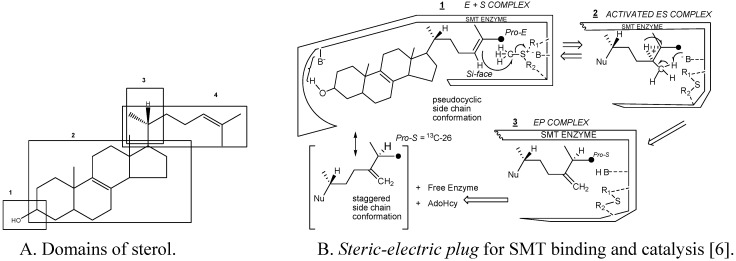
Substrate studies using a series of sterol analogs of the putative preferred substrate for the plant and fungal SMTs suggest the size, shape and electronics of the sterol molecule are involved in binding and can give rise to distinct enzymatic mechanisms and these features are characterized in at least 4 molecular domains (Fig. 4) [6,15,16,17,28,29].
Figure 4.
Sterol features that contribute to catalysis.
For example, hydrogen bonding interactions between the sterol and SMT involve the methyl groups at C-4 and C-14 and the olefin Δ24(25) or Δ24(28 ) structures as embodied in the steric-electric plug model (Figure 4) [6]. The configuration at C-20 influences the conformation of the sterol side chain whereas the number and position of double bonds in the nucleus influence molecular shape. Variation in the conformation properties of the nucleus can affect the tilt of C-3 hydroxyl group and tilt of the 17(20)-bond sterol side chain. The possibility of cycloartenol to be “bent” is no longer credible, rather the sterol is thought to assume a planar structure with the side chain to the right when the substrate is bound to SMT or functioning as a membrane insert [3,30,31]. The global substrate features recognized by the SMT and turnover numbers developed from the Michaelis complex and related properties, including subunit architecture (4 identical subunits with native molecular weights between 161 kDa to 172 kDa), pH dependence (centered at pH 7.5) and activation energies (ca. 45 kJ/mol) are similar for SMT1 and SMT2 type enzymes (Table 1), suggesting the active site topography is similar amongst SMTs.
Mechanisms of catalysis
The stereochemical restrictions of the active site have been determined using 2H- and 13C-isotopically labeled substrates assayed with SMT and characterization of the enzyme-generated products by NMR. In his seminal report, Arigoni defined the stereochemistry at C-25 in fungal ergosterol to be 25S [32]. We confirmed that ergosterol and sitosterol are the methylene products of SMT1 and that they possessed the 25S stereochemistry [33,34,35] which is distinct from that of animal cholesterol which assumes the 25R stereochemistry following reduction of the 24, 25-double bond [36]. The stereochemistry at C-25 is retained during the second alkylation step [37].
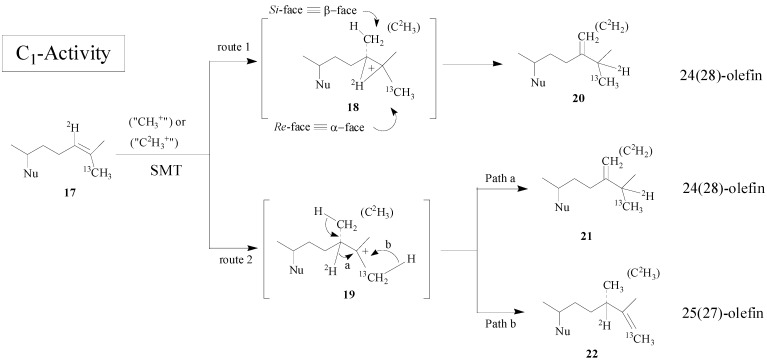
Recent studies indicate that the overall mechanism can be different in the first and second C1-transfer activities, concerted or step-wise, respectively depending on the source of the SMT isoform [15]. In the case of wild-type yeast SMT1 a single catalytic pathway is followed but in the case of select mutants catalysis to generate alternate products can be induced through site-directed mutagenesis of amino acids in the active center [17,18,19]. The overall methyl transfer reaction catalyzed by SMT is proposed to proceed through a nucleophilic attack by the π-electrons of the Δ24-bond on the S- methyl group of AdoMet. The reaction can lead to a high energy intermediate (HEI) possessing a 24β- (si-face attack) methyl or ethyl at C-24 and a bridged carbenium ion across the 24, 25-bond or a transient C-25 carbocation. After a 1, 2-hydride transfer of H-24 to C-25 (first C1-transfer), an elimination of a proton at C-28 occurs, giving rise to a 24(28)-methylene structure (Scheme 2).
Scheme 2.
Alternate C-methylation pathways catalyzed by the wild-type (route 1; nonstop) and mutant D79L (route 2; step-wise) SMT from S. cerevisiae.
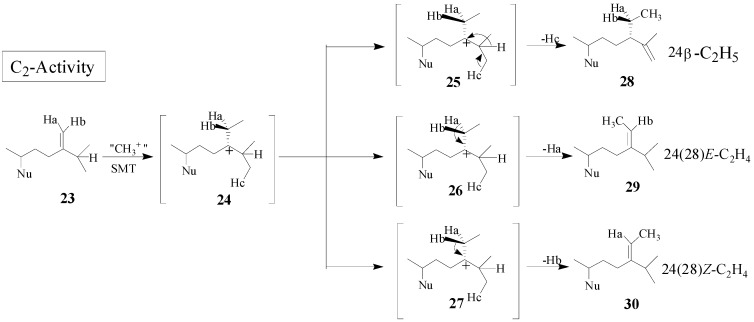
Although the catalysis involves a conformationally flexible sterol substrate and very reactive ionic intermediates, the reaction progress is efficiently channeled through a coupled methylation-deprotonation step to produce single or sets of products with complete structural and stereochemical control (Scheme 3).
Scheme 3.
C-methylation pathways catalyzed by SMT to generate multiple olefin products.
The kinetics of the SMT catalyzed reaction involving two substrates have been hypothesized to proceed by one of two types of limiting mechanisms in which either a ternary or binary (ping pong) complex is formed [28,38]. These two mechanisms can be readily distinguished by steady-state kinetic velocities since they produce different kinetic patterns. The ternary complex can proceed by one of two possible routes, random or ordered, and can be distinguished by measuring the effect of product inhibition on the initial rate. In the case of the yeast SMT1, recent experimental evidence reveals a sequential reaction operates randomly and in the case of soybean SMT1 the mechanism operates in ordered fashion with AdoMet binding before sterol and methylated sterol product released before AdoHcy [15,28].
The physical properties based on the secondary structure prediction from Circular Dichroism measurements of yeast and soybean SMT1 indicate different populations of α-helices, β-sheets and random coils (Figure 3) but the common core that contains the active center appears to be conserved based on sequence alignment of 16 SMTs and homology building experiments [19,20]. At the current time, a single base, histidine90, is thought to function as the C-28 deprotonating agent and a second, as yet, unknown polar residue can function as the base in C-27 deprotonation. Glutamic acid276 is considered to hydrogen bond to the C-3 hydroxyl group of the sterol [20]. The systematic perturbation of the enzymatic mechanism involving isotopically branching experiments and testing substrate analogs are in agreement with the suggestion [6] that distinct polar amino acids in the proximal and distal regions of the active center exist to recognize the acceptor nucleophilicity and channel the reaction pathway to Δ24(28) or Δ25(27) - olefin formation [19,20].
Rational Design of Potent Inhibitors of SMT
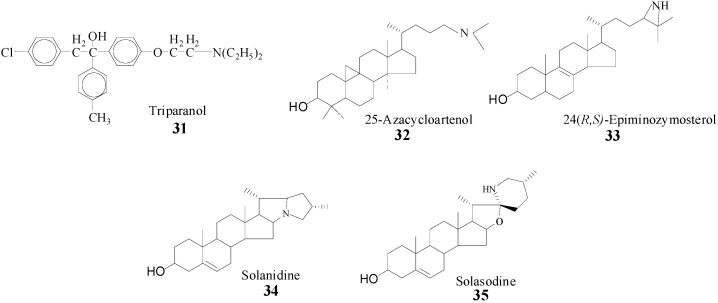
The treatment of fungal disease is usually by application of a non-steroidal, azole-type reversible inhibitor of the 14α-demethylase enzyme (14DM) or by subscription to a polyene antibiotic such as amphotericin B which complexes to ergosterol in the membrane [10]. However, these compounds suffer the disadvantage of being general cytochrome P450 inhibitors, their efficacy depending on a greater affinity for fungal P45014DM versus mammalian P45014DM or to be ineffective to invasive fungal infections when the fungus fails to synthesize ergosterol, respectively. Alternatively, the SMTs are appealing targets for the design of enzyme inhibitors, since they lie on a pathway that is key for the synthesis of membrane inserts in microbes and plants and the unique aspects of SMT structure and function make for the design of species-specific inhibitors that will not be harmful to mammalian physiology [6]. Evidence that SMT action can be impaired by sterol analogs with an ammonium function in the side chain prepared synthetically or from nature (Scheme 4) [22,23,28,39] prompted us to take a detailed analysis of SMT inhibitors and to prepare and test a range of diverse structures. It is assumed that the inhibitory power of the compounds in Scheme 4 is related to the tertiary amine function of these molecules which will be protonated at physiological pH and that therefore can mimic the high energy intermediate in the C-methylation reaction progress [22].
Scheme 4.
Synthetic (31, 32, 33) and natural products (34, 35) prepared to inhibit SMT.
Inhibitors designed to impair phytosterol synthesis will bind reversibly or irreversibly with the enzyme. Reversible inhibitors can be classified based on whether they are (i) high energy intermediate analogs or (ii) dead-end analogs, and the irreversible inhibitors can be classified as (iii) mechanism-based inactivators. The compounds shown in Scheme 4 have been tested and found to be non-competitive inhibitors against the native substrate of the SMT [22,23,28,39]. In spite of numerous side chain modifications [38,40,41,42,43,44,45,46,47], only a limited number of structural variants exhibit binding affinities to the enzyme active site that are significantly greater than those of the native substrate. These binding affinities are measured as the equilibrium constants for the reversible binding complex of inhibitor and expressed as apparent Ki values. These Ki values can be compared to the analogous Michaelis-Menten Km values for substrate and SMT binding affinity. The in vitro potency of SMT inhibitors can be compared via the ratios of their Km/Ki values, thus the larger the ratio the greater the inhibition. The ammonium and sulfonium analogs were prepared with the expectation that the SMT in the ground state would recognize the “high energy intermediate” mimic, the assumption being that the active site binds the C-24 or C-25 heteroatom (N or S) substituted sterols to the same active center as the substrates [42] and the enzyme will undergo a conformational change during catalysis to recognize the inhibitory group of the substrate analog [6]. In work with the yeast SMT, 25-azalanosterol was discovered to bind to the active center with a Kd value of 4 μM similar to either the Kd values of zymosterol or AdoMet at 4 μM [20]. The expectation for tight binding was borne out by the results of the 24- and 25-heteroatom substituted sterols assayed with SMT1 and SMT2, the carbocation mimic was bound to the Michaelis complex many orders more tightly than the sterol (or AdoMet) substrate generating a Km/Ki ratio of approximately 1000 [16,23,38,40,41,42,43,44,45,46]. The efficacy of 25‑azacycloartenol, a substrate mimic, and solasodine have been compared in vitro and in vivo with the pathogenic yeast-like microbe P. wickerhamii which synthesizes ergosterol by the cycloartenol-cyclolaudenol route shown in Scheme 1. A correlation of growth impairment and inhibition SMT activity was observed with the two compounds; 25-azacycloartenol, IC50 = 35 nM and Ki = 3 nM and solasodine, IC50 = 2 μM and Ki = 75 μM [23]. In the case of 25-azacycloartenol, the Km(cycloartenol)/Ki(25-azacycloartenol) ratio = 30 μM/ 35 nM was approximate 1000. At the IC50 of inhibitor, the sterol composition of the treated cells was found to be dramatically modified with the bulk of the ergosterol replaced by cycloartenol, the native substrate of the SMT1. In the case of solasodine, the Km/Ki ratio was 0.5, suggesting the inhibitor to be much less potent than 25-azacycloartenol. Indeed, microbial growth was affected less by solasodine treatment. However, while the azasteroids can be fungicidal and powerful inhibitors of SMT, as noted by Ator et al. [41] clinical experience with triparanol indicate a range of side effects of sufficient severity to require the withdrawal of the drug from the market. Consequently, the clinical importance of these types of compounds has not been examined further and their study has been restricted to deciphering the substrate requirements for SMT catalysis.
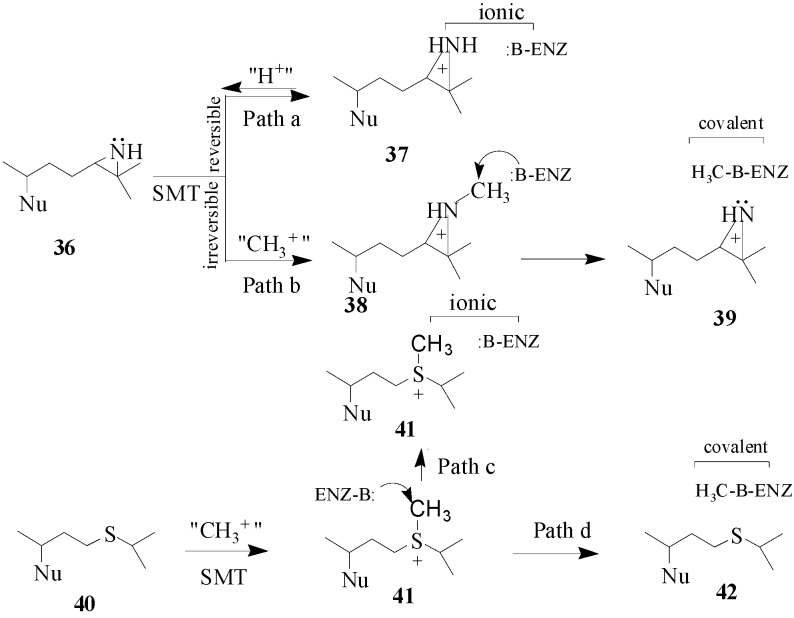
By the late 1980’s, a class of enzyme inhibitors known as mechanism-based or suicide substrates were evolving as alternative compounds to inhibit SMT and to serve as potential antifungal agents [46,47]. These compounds are expected to bear a strong resemblance to the relevant substrates and are not required to possess stereoelectronic properties similar to the presumptive cationic intermediate in the SMT reaction as will be the case for the high energy intermediate analogs. The first set of mechanism-based inhibitors were designed with sulfur or an aziridine function and they were considered to contain latent reactive functions that will be specifically activated by the SMT. The effectiveness of sulfur and aziridine-containing steroid derivatives on inhibition of enzyme activity is related to the structure of the sterol nucleus [7,41,46] whereas the mode of action is related to the structure and position of the functional group in the side chain. Two hypothetical mechanisms have been considered for the mode of action of 36 and 40 (Scheme 5). Compounds 36 and 40 have been tested with SMT1 from fungi and plants and protozoa [23,44,45,46]. Compound 36 is a tight-binding reversible inhibitor Ki of 10 nM lacking any time-dependent inhibition of activity thereby suggesting that it is a high energy intermediate. It follows that the inhibition of SMT by treatment with 36 occurs by path a in Scheme 5. Alternatively, compound 40 was a competitive inhibitor of the soybean SMT1 with a Ki value of 2 μM and exhibited time-dependent inhibition of activity, the rate for irreversible inactivation of the enzyme, kinact of 0.3 min-1. The binding of 24-thaicycloartenol to soybean SMT was site-directed, since time-dependent inhibition can be blocked or competed for by increasing the substrate concentration during the preincubation phase. The exact mechanism of inhibition has not been resolved, however, C-methylation of 24-thiacycloartenol is proposed to occur to generate the charged intermediate 41. Compound 41 can serve as a methyl donor to inactivate the enzyme according to path d (Scheme 5). Compound 41 was tested as an inhibitor of soybean SMT1 and found to be a noncompetitive inhibitor with a Ki value of 55 nM and when added to the SMT assay it was not time-dependent [45]. These results suggest that 41 can inhibit SMT via ionic interactions according to path c (Scheme 5). Similar findings were obtained with a related sulfonium analog assayed with the yeast SMT1 [46]. When the salt 41 is tested with SMT separately from 40 which is not significantly basic the charged methylated species apparently behaves in a manner similar to the protonated 36 species (or compound 37) and this may be because 41 can enter a different subsite from 40 depending on the enzyme conformation. Another possibility is that thioether 40 can be methylated in the active site to yield sulfonium ion 41 and this reaction prompts a change in the enzyme conformation to spatially direct 41 to react with an active site base or a nearby nucleophilic amino acid side chain to covalently inactivate the enzyme. The promise that specific sulfur derivatives can serve as leads to new antifungal drugs warrants further investigation.
Scheme 5.
Hypothetical mechanisms for ionic versus covalent interaction of azridinium- and sulfonium-type inhibitors with the SMT. Nu, sterol nucleus.
As a basis for rational design to generate a new class of mechanism-based inhibitors, we focused on the steric-electric plug model (Figure 4) and the reaction progress proceeding by way of an ionic mechanism (Scheme 2). We envision the sterol acceptor molecule to bind to the donor site of SMT via a combination of polar and hydrophobic interactions with the flexible side chain restricted to a single conformer such that inhibitors designed as substrate analogs with a correctly positioned olefin structure can undergo C-methylation by SMT but will not be catalyzed by enzymes in the cholesterol pathway. Cation-π interactions are considered to stabilize the ionic intermediate(s) formed during SMT catalysis and to prevent alkylation that could presumably inactive the enzyme during normal turnover [6]. It is expected that cation formation is triggered by C-methylation of an isolated double bond or related olefinic structure in the terminal segment of the side chain and catalysis proceeds stereoselectively in a manner similar to, for example, zymosterol conversion to fecosterol. In contrast to the native intermediate formed during catalysis, these inhibitors are designed to undergo rearrangement or delocalization during catalysis to place a positive charge in the region of the SMT active site that does not normally encounter electrophilic species. Elimination of the positive charge on the inhibitor side chain by covalent modification of the SMT active site is expected to render inhibition or “suicide” of the enzyme. These compounds are proposed to bind to the yeast SMT with an affinity comparable to that of the substrate and thus represents a starting point for further study.
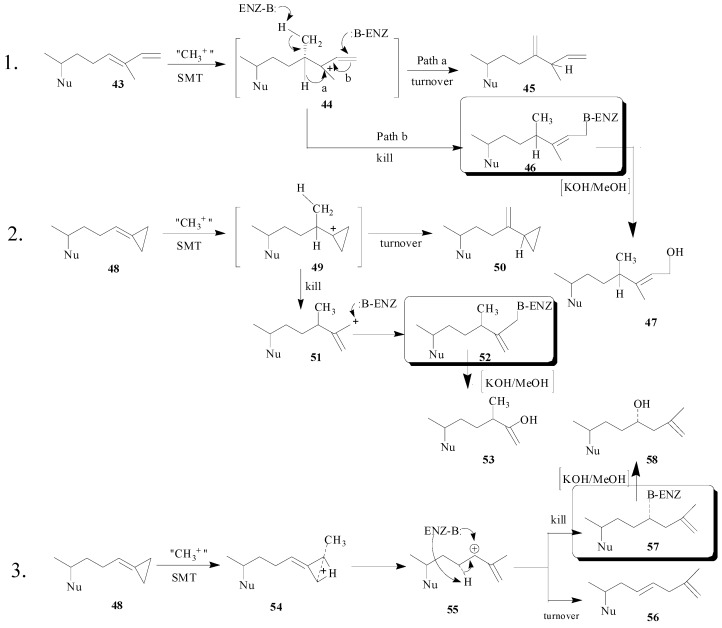
A similarity of binding to the SMT1 from fungi was found in the series of zymosterol-type substrates e.g., zymosterol 14 (Kd, 5 μM), 26, 27-dehydrozymosterol 48 (Kd, 10 μM), zymosta-8, 24,26-trienol 43 (Kd, 4 μM) [19,20,43] and fecosterol 15 (Kd,10 μM) but a 10 fold or greater difference in catalytic competence was found in modifications of the 24,25-double bond to form the structures 24(28)-exocyclic methylene 15, methylenecyclopropane 48 and substrate vinyl analog 43 shown in Scheme 6 (Table 2) [43,44].
Scheme 6.
Hypothetical mechanisms for inhibition of SMT by substrate analogs designed as mechanism-based inactivators. Two pathways are considered for the turnover (product generation) or kill (covalent attachment of inhibitor to enzyme) of the SMT. Boxed structures represent the putative ligand-enzyme adduct.
Table 2.
Catalytic competence of sterols to the recombinant wild-type sterol methyltransferase from S. cerevisiae (yeast) and Glycine max (soybean).
| Substrate | Parameters | Reference | ||||
|---|---|---|---|---|---|---|
| Structure | Source | Km (µM) | kcat (min-1) | kcat/Km (normalized to 100%) | ||
| Cycloartenol | 2 | Soybean | 30 | 0.60 | 0.02 (100%) | [17] |
| Fecosterol | 15 | Yeast | Not Active | Not Active | 0.00 (0%) | [15] |
| 24(28)Methylenelophenol | 6 | Soybean | 22 | 0.06 | .003 (2%) | [17] |
| 26,27-Dehydrozymosterol | 48 | Yeast | 15 | 0.05 | .003 (2%) | [19] |
| 26,27-Dehydrocycloartenol | 48 | Soybean | 10 | 0.02 | .002 (1%) | [49] |
| Zymosta-24,26-dienol | 43 | Yeast | 10 | 0.02 | .002 (1%) | [41] |
| Cycloarta-24,26-dienol | 43 | Soybean | 5 | 0.03 | .006 (3%) | [48] |
Since there are no three-dimensional structures of a SMT, probing SMTs with mechanism-based inactivators can reveal comparative information of their active site topography. In the case of Prototheca wickerhamii, 2 but neither 43 or 48 were acceptable substrates for the SMT1 [50], suggesting the structure of the side chain is important to catalysis. Alternatively, different catalytic efficiencies of the native substrates for SMT1 and SMT2, fecosterol 15 and 24(28)-methylenelophenol 6 were observed (Table 2) that result from minor differences in the sterol nucleus. Thus, these preliminary findings suggest that species differences exist in the active-sites of SMT1 from difference sources.
The position and structure of the olefin undergoing C-methylation as well as the source of SMT were found to be critical to the potency of the inhibitor and to the reaction mechanism. Kinetic analysis suggested that all the compounds tested in Table 3 are competitive inhibitors of the SMT and each one can generate a cation to interact with a nucleophile from the SMT, suggesting in principle that any one of these compounds can act as a mechanism-based inactivator. Compounds 43 and 48 were found to exhibit time-dependent inhibition of activity and co-incubation with the native substrate afforded protection against inactivation. Interestingly, 48 was not found to be a time-dependent inhibitor of the P. wickerhamii SMT1 [50] whereas it is in the case of soybean and yeast SMT1 [49], confirming the potential application of using these compounds to probe for species differences in this class of catalyst.
Table 3.
Inhibition of SMT1 from different sources by substrate analogs.
| Inhibitor | Parameters2 | Partition ratio | Reference | |||
|---|---|---|---|---|---|---|
| Structure | Source | Ki (µM) | kinact (min-1) | kcat/kinact | ||
| Fecosterol | 14 | Yeast | 55 | None | None | [28] |
| 24(28)Methylenecycloartanol | 4 | Soybean | 171 | None | None | [15] |
| 26,27-Dehydrozymosterol | 48 | Yeast | 1 | 1.52 | 0.03 | [19] |
| 26,27-Dehydrocycloartenol | 48 | Soybean | 42 | 0.29 | 0.01 | [49] |
| Cycloarta-24,26-dienol | 43 | P. wickerhamii | 30 | 0.30 | None | [50] |
| Cycloarta-24,26-dienol | 43 | Soybean | 17 | 0.24 | 0.02 | [48] |
The covalent nature of the inhibitor can be detected by radiofluorography of the protein bound to the radiolabeled steroid. In addition, following tryptic digestion of the ligand-protein adduct and Edman sequencing of the resulting HPLC pure radiolabeled peptide fraction the amino acid covalently bound to the inhibitor can be revealed. These findings coupled with the characterization of the enzyme-generated turnover product will reveal key amino acids in the active site and mechanistic information. For 26,27-deydrozymosterol, the E68 residue was determined to be the amino acid bound to the inhibitor and careful structural characterization of the monol and diol (generated by saponification) products of catalysis indicated structures 56 and 58 rather than 50 and 53 [19]. The hypothetical mechanisms of SMT inhibition by 43 and 48 are shown in Scheme 6.
The proposed mechanism of 26,27-dehydrozymosterol catalysis that leads to turnover or kill of the enzyme proceeds by backside nucleophilic attack of a methyl group on the methylenecyclopropane carbon-26 leading to ring opening and ring expansion. The termination step gives rise to either olefinic (elimination) or hydroxyl (water addition) products. The unique aspect of the catalysis is the C‑methylation of C-26 rather than C-24 which was anticipated [6] and which occurs in the case of 43 [48].
Conclusions
The remarkable fidelity in olefin product formation by SMTs from plants and fungi may reflect the inherent positional selectivity of the normal deprotonation step of the reaction and with mechanism-based inhibitors to trap these bases we may be able to fingerprint species differences in the active site. Unfortunately, the X-ray structures of this class of catalysts are not available, only a limited few SMTs have been cloned and purified and only a few inhibitors have been tested in vivo or in vitro. Nonetheless, there is an increasing awareness of the potential for the development of species-specific inhibitors of sterol biosynthesis and there are therapeutic benefits. Undoubtedly, as a greater variety of inhibitors and SMTs are tested, additional comparative information of their active sites will be determined as well as the efficacy of the compounds.
References and Notes
- 1.This work was supported by the National Institutes of Health (GM63477). National Science Foundation (MCB-0115401) and the Welch Foundation (D-1276).
- 2.Nes W. R., McKean M. L. Biochemistry of Steroids and Other Isopentenoids. University Park Press; MD: 1977. pp. 300–375. [Google Scholar]
- 3.Nes W. D., Janssen G. G., Crumley F. G., Kalinowska M., Akihisa T. The structural requirements of sterols for membrane function in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1993;300:724. doi: 10.1006/abbi.1993.1100. [DOI] [PubMed] [Google Scholar]
- 4.Bloch K. E. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 1983;14:47. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- 5.Hartman M-A. Plant sterols and the membrane environment. Trends. Plant Sci. 1988;3:170. [Google Scholar]
- 6.Nes W. D. Sterol methyltransferase: enzymology and inhibition. Biochim Biophys. Acta. 2000;1529:63. doi: 10.1016/s1388-1981(00)00138-4. [DOI] [PubMed] [Google Scholar]
- 7.Guo D., Nichols D., Zhou W., Lopez M, Mangla A. T., Nes W. D. Antifungal sterol biosynthesis inhibitors. Subcellular Biochem. 1997;28:89. doi: 10.1007/978-1-4615-5901-6_4. [DOI] [PubMed] [Google Scholar]
- 8.Rahier A., Taton M. Fungicides as tools in studying postsqualene sterol synthesis in plants. Pest. Biochem. Physiol. 1997;57:1. [Google Scholar]
- 9.Kaneshiro E. S. Sterol biosynthesis in Pneumocystis: unique steps that define unique targets. Drug Resistance Update. 2002;5:259. doi: 10.1016/s1368-7646(02)00122-x. [DOI] [PubMed] [Google Scholar]
- 10.Georgopapadakou N. H., Walsh T. J. Antifungal agents: Chemotherapeutic targets and immunological strategies. Antimicrobiol. Agents Chemother. 1996;40:279. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bammert G. F., Fostel J. M. Genome-wide expression patterns in Saccharomyces cerevisiae: Comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrobiol. Agents Chemother. 2000;44:1255. doi: 10.1128/aac.44.5.1255-1265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen-pergakes K. L., Kennedy M. A., Lees N. D., Barbuch R., Koegel C., Bard M. Sequencing, disruption, and characterization of the Candida albicans sterol methyl transferase (ERG6) gene: Drug susceptibility studies in erg6 mutants. Antimicrobiol. Agents Chemother. 1998;42:1160. doi: 10.1128/aac.42.5.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clouse S. D. Arabidopsis mutants reveal multiple roles for sterol in plant development. Plant Cell. 2002;14:1995. doi: 10.1105/tpc.140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvier-Nave P., Husselstein T., Beveniste P. Two families of sterol methyltransferase are involved in the first and second methylation steps of plant sterol biosynthesis. Eur. J. Biochem. 1998;256:88. doi: 10.1046/j.1432-1327.1998.2560088.x. [DOI] [PubMed] [Google Scholar]
- 15.Nes W. D., Song Z., Dennis A. L., Zhou W., Nam J., Miler M. B. Biosynthesis of phytosterols. Kinetic mechanism for the enzymatic C-methylation of sterols. J. Biol. Chem. 2003;278:34505. doi: 10.1074/jbc.M303359200. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W., Nes W. Sterol methyltransferase2: purification, properties and inhibition. Arch. Biochem. Biophys. 2003;420:18. doi: 10.1016/j.abb.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Nes W. D., McCourt B.S., Zhou W., Ma J., Marshall J.A., Peek L-A., Brennan M. Overexpression, purification and stereochemical studies of the recombinant (S)-adenosyl-L-methionine: Δ24(25) – to Δ24(28) - sterol methyl transferase enzyme from Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1998;353:297. doi: 10.1006/abbi.1998.0665. [DOI] [PubMed] [Google Scholar]
- 18.Nes W. D., McCourt B. S, Marshall J. A., Ma J., Dennis A. L., Lopez M., Li H., He L. Site-directed mutagenesis of the sterol methyl transferase active site from Saccharomyces cerevisiae results in formation of novel 24-ethyl sterols. J. Org. Chem. 1999;64:1535. doi: 10.1021/jo9819943. [DOI] [PubMed] [Google Scholar]
- 19.Nes W. D., Marshall J. A., Jia Z., Jaradat T. T., Song Z., Jayasimha P. Active site mapping and substrate channeling in the sterol methyltransferase pathway. J. Biol. Chem. 2002;277:42549. doi: 10.1074/jbc.M204223200. [DOI] [PubMed] [Google Scholar]
- 20.Nes W. D., Jayasimha P., Zhou W., Kanagasabai R., Jin C., Jaradta T. T., Shaw R. W., Bujnicki J. M. Sterol methyltransferase: Functional analysis of highly conserved residues by site-directed mutagenesis. Biochemistry. 2004;43:569. doi: 10.1021/bi035257z. [DOI] [PubMed] [Google Scholar]
- 21.Venkatramesh M., Guo D., Harman J.G., Nes W. D. Sterol specificity of the Saccharomyces cerevisiae ERG6 gene product expressed in Escherchia coli. Lipids. 1996;31:377. doi: 10.1007/BF02522922. [DOI] [PubMed] [Google Scholar]
- 22.Rahier A., Tatin M., Bouvier-Nave P., Schmitt P., Benveniste P., Schuber F., Narula A. S, Cattel L, Anding C., Place P. Design of high energy intermediate analogues to study sterol biosynthesis in higher plants. Lipids. 1986;21:52. doi: 10.1007/BF02534303. [DOI] [PubMed] [Google Scholar]
- 23.Mangla A. T., Nes W. D. Sterol C-methyltransferase from Prototheca wickerhamii. Mechanism sterol specificity and inhibition. Bioorg. Med. Chem. 2002;8:925. doi: 10.1016/s0968-0896(00)00040-7. [DOI] [PubMed] [Google Scholar]
- 24.Nes W. D., Venkatramesh M. Enzymology of phytosterol transformations. CRC Crit. Rev. Rev. Biochem. Mol. Biol. 1999;34:81. doi: 10.1080/10409239991209219. [DOI] [PubMed] [Google Scholar]
- 25.Chappell J., Wolf F., Proulx J., Cuellar R., Saunders C. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl-CoA reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 1995;109:1337. doi: 10.1104/pp.109.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmberg N., Harker M., Gibbard C.L., Wallace A.D., Clayton J.C., Rawlins S., Hellyer A., Safford R. Sterol C-24 methyltransferase type-1 controls the flux of carbon into sterol biosynthesis in tobacco seed. Plant Physiol. 2002;130:303. doi: 10.1104/pp.004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb D.C, Kelly D.E., Venkateswarlu L., Manning N.J., Bligh H.F., Schunck W.H., Kelly S.L. Generation of a complete, soluble and catalytically active sterol 14α-demethylase-reductase complex. Biochemistry. 1999;38:8733. doi: 10.1021/bi9825089. [DOI] [PubMed] [Google Scholar]
- 28.Venkatramesh M., Guo D., Jia Z., Nes W. D. Mechanism and structural requirements for transformation of substrates by the (S)-adenosyl-L-methionine Δ24(25) -sterol methyl transferase from Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1996;1299:313. doi: 10.1016/0005-2760(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 29.Dennis A. L., Nes W. D. Sterol methyltransferase. Evidence for successive C-methyl transfer reactions generating Δ24(28) - and Δ25(27) - olefins by a single plant enzyme. Tetrahedron Lett. 2002;43:7017. [Google Scholar]
- 30.Nes W. D., Janssen G. G., Bergenstrahle A. Structural requirements for transformation of substrates by the (S)-adenosyl-L-methionine: Δ24(25) -sterol methyl transferase. J. Biol. Chem. 1991;266:15202. [PubMed] [Google Scholar]
- 31.Nes W. D., Koike K., Jia Z., Sakamoto Y., Satou T., Nikaido T., Griffin J. F. 9b,19-Cyclosterol analysis by 1H and 13CNMR, crystallographic observations and molecular mechanic calculations. J. Amer. Chem. Soc. 1998;120:5970. [Google Scholar]
- 32.Arigoni D. Stereochemical studies of enzymic C-methylations. Ciba Found. Symp. 1978;60:243. doi: 10.1002/9780470720424.ch14. [DOI] [PubMed] [Google Scholar]
- 33.Nes W. D., Norton R. A., Benson M. Carbon-13 NMR studies on sitosterol biosynthesized from [13C]mevalonates. Phytochemistry. 1992;31:805. [Google Scholar]
- 34.Guo D., Jia Z., Nes W. D. Stereochemistry of hydrogen migration from C-24 to C-25 during phytosterol biomethylation. J. Amer. Chem. Soc. 1996;118:8507. [Google Scholar]
- 35.Zhou W., Guo D., Nes W. D. Stereochemistry of hydrogen migration from C-24 to C-25 during biomethylation in ergosterol biosynthesis. Tetrahedron Lett. 1996;37:1339. [Google Scholar]
- 36.Popják G., Edmond J., Anet F.A.L, Easton N.R., Jr. Carbon-13 NMR studies on cholesterol biosynthesized from [13C]mevalonates. J. Amer. Chem. Soc. 1977;99:931. doi: 10.1021/ja00445a041. [DOI] [PubMed] [Google Scholar]
- 37.Tong Y., McCourt B. S., Guo D., Mangla A. T., Zhou W-X., Jenkins M. D., Zhou W., Lopez M., Nes W.D. Stereochemical features of C-methylations on the path to Δ24(28) -methylene and Δ24(28)-ethylidene sterols: studies on the recombinant phytosterol methyltransferase from Arabidopsis thaliana. Tetrahedron Lett. 1996;38:6115. [Google Scholar]
- 38.Oehlschlager A. C., Angus R. H., Pierce A. M., Pierce H. D., Jr., Srinivasan R. Azasterol inhibition of Δ24(25)-sterol methyltransferase in Saccharomyces cerevisiae. Biochemistry. 1984;23:3582. doi: 10.1021/bi00311a003. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra H. C., Nes W. D. The mechanism of introduction alkyl groups at C-24 of sterols. IV. Inhibition by triparanol. J. Biol. Chem. 1971;246:4934. [PubMed] [Google Scholar]
- 40.Ator M. A., Schmidt S. J., Adams J. L., Dolle R. E., Kruse L. I., Frey C. L., Barone J. M. Synthesis, specificity, and antifungal activity of inhibitors of the Candida albicans Δ24 -sterol methyl transferas. J. Med. Chem. 1995;35:100. doi: 10.1021/jm00079a012. [DOI] [PubMed] [Google Scholar]
- 41.Nes W. D., Guo D., Zhou W. Substrate-based inhibitors of the (S)-adenosyl-L-methionine:Δ24(25) –sterol methyltransferase from Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1997;342:68. doi: 10.1006/abbi.1997.9984. [DOI] [PubMed] [Google Scholar]
- 42.Rahier A., Genot J-C., Schuber F., Benveniste P., Narula A. S. Inhibition of S-Adenosyl-L-methionine sterol C-24-methyltransferase by analogs of a carbocationic ion high energy intermediate. J. Biol. Chem. 1984;259:15215. [PubMed] [Google Scholar]
- 43.Acuna-Johnson A. P., Oehlschlager A. C., Pierce A. M., Pierce H. D., Jr., Czyzewska E.K. Stereochemistry of yeast Δ24 -sterol methyl transferase. Bioorg. Med. Chem. 1997;5:821. doi: 10.1016/s0968-0896(97)00010-2. [DOI] [PubMed] [Google Scholar]
- 44.Rahman M. D., Pascal R. A., Jr. Inhibitors of ergosterol biosynthesis and growth of the trypansomatid protozoan Crithidia fasciculata. J. Biol. Chem. 1990;265:4989. [PubMed] [Google Scholar]
- 45.Zhou W., Song Z., Liu J., Miller M. B., Nes W. D. 24-Thiacycloartanol, a potent mechanism-based inactivator of plant sterol methyltransferase. Tetrahedron Lett. 2004;45:875. [Google Scholar]
- 46.Ator M. A., Schmidt S. J., Adams J. L., Dolle R. E. Mechanism and inhibition of Δ24-sterol methyltransferase from Candida albicans and Candida tropicalis. Biochemistry. 1989;28:9633. doi: 10.1021/bi00451a014. [DOI] [PubMed] [Google Scholar]
- 47.Nes W. D., Xu S., Parish E. J. Metabolism of 24(R,S)-epiminolanosterol to 25-aminolanosterol and lanosterol by Gibberella fujikuroi. Arch. Biochem. Biophys. 1989;272:323. doi: 10.1016/0003-9861(89)90226-9. [DOI] [PubMed] [Google Scholar]
- 48.Using the yeast and soybean recombinant SMT and protocols described in ref. [17] and [19], the compounds fecosterol, 26-homo-zymosta-8,24,26-trienol and 26-homo cycloart-24,26-dienol were tested kinetically for binding by equilibrium dialysis and for substrate and inhibitor efficacy by steady-state kinetic analysis, Song, Z.; Jia, Z.; Marshall, J. A.; Nes, W.D. Unpublished results.
- 49.Song Z., Zhou W., Liu J., Nes W.D. Mechanism-based active site modification of the soybean sterol methyltransferase by 26,27-dehydrocycloartenol. Bioorg. Med. Chem. Lett. 2004;14:33. doi: 10.1016/j.bmcl.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Nes W. D., He L., Mangla A. T. 4,4,14-Trimethyl-9β,19-cyclo-5α-26-homocholesta-24,26-dien-3 β-ol: A potent mechanism-based inactivator of Δ24(25) - to Δ25(27) - sterol methyl transferase. Bioorg. Med. Chem. Lett. 1998;8:3449. doi: 10.1016/s0960-894x(98)00651-9. [DOI] [PubMed] [Google Scholar]