Abstract
Quinazolinone and quinazolinedione derivatives are of considerable interest due to their wide array of pharmacological properties. In this paper we report the synthesis of ten quinazolinediones. The previous isolation of two of these compounds, namely 1-methyl-3-(2'-phenylethyl)-1H,3H-quinazoline-2,4-dione and 1-methyl-3-[2'-(4'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione, from the seed husks of Mexican Zanthoxylum species has been reported
Keywords: Quinazoline-2,4-dione; alkaloids; triphosgene; anti-hypertensive activity
Introduction
Quinazolinone and quinazolinedione derivatives are of considerable interest because of their wide array of pharmacological properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. We have been specifically interested in the synthesis of novel heterocycles containing the quinazoline-2,4-dione backbone, which are known to exhibit potential anti-hypertensive properties [16,17,18,19,20]. A previous report from this laboratory described an efficient synthetic route to Pelanserine (TR 25-15), a potent anti-hypertensive [21], structurally similar to the clinically used anti-hypertensive agent Ketanserine [22,23]. We have also extended this methodology to the synthesis of other quinazoline-2,4-diones with amino acids and dipeptide side chains which when tested showed mild to no antihypertensive properties [24]. In our search for other lead compounds containing the quinazoline-2,4-dione ring system, we encountered two simple natural alkaloids, 1-methyl-3-(2'-phenylethyl)-1H,3H-quinazoline-2,4-dione and 1-methyl-3-[2'-(4'-methoxy-phenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1c and 1f), both isolated from the seed husks of Zanthoxylum arborescens [25].
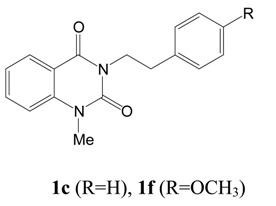 |
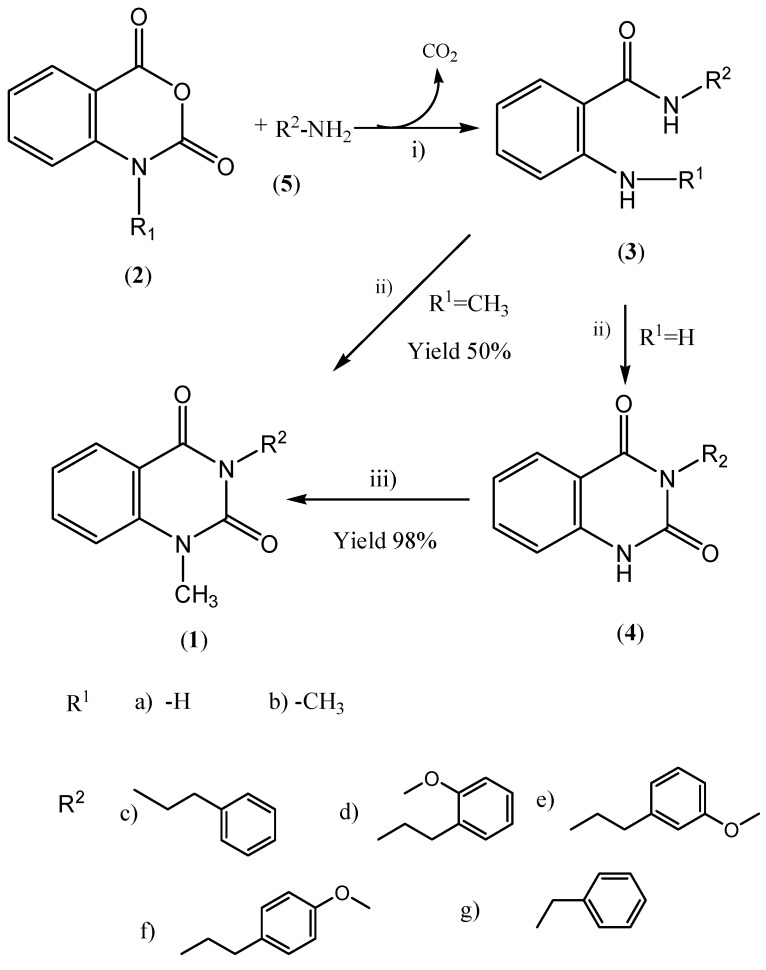
In order to study their antihypertensive properties in more detail, we embarked on a large scale synthesis of 1c and 1f, and their analogues 1d,e and g (Scheme 1).
Scheme I.
i) Triethylamine/DMF ii) Triphosgene/CH2Cl2 iii) Tetramethylguanidine/Iodomethane
Results and Discussion
Amines 5c-g [R2-NH2] were reacted with isatoic anhydride (2a) to give the corresponding 2-aminobenzamides 3 in excellent yields (84-93%). Following the methodology developed for the synthesis of Pelanserine, we used triphosgene to ring close the amides 3 to give quinazolinediones 4c-g in good yields [21]. Use of ethyl chloroformate instead of triphosgene was limited to an analytical scale and led to a complex mixture of products in a scaled up reaction. Synthesis of N-methylquinazoline-2,4-diones 1 from N-methylisatoic anhydride (2b) gave less than a 50% yield of the desired compounds, however, using tetramethylguanidine as base, amides 4 were methylated with methyl iodide to give quinazolinediones 1 in good yields (83-96%). Using this method we also synthesized analogues 1c-g, for further biological evaluation. The methylene protons of 1 are magnetically non-equivalent and display a AA'XX’ spin system, with the hydrogens on the same carbon splitting each other, thus leading to a more complicated pattern than expected.
Conclusions
We have synthesized ten quinazoline-2,4-diones for biological evaluation as potential antihypertensive agents. While up to now most of the structural modifications have been confined to the amine R2-NH2 fragment, the basic backbone 4 now provides a target for introduction of different heterocyclic rings on the amide –NH to diversify the quinazoline-2,4-dione structural system.
Experimental Section
General
Melting points were measured on an Electrothermal 88629 apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Perkin Elmer FT-IR 1600 spectrometer. 1H- and 13C-NMR spectra were recorded in CDCl3 at 200 MHz and 50.289 MHz, respectively, on a Varian Mercury 200 Spectrometer with TMS as internal standard. Mass spectra were obtained on a Agilent 1100 series LC/MSD Trap, SL Spectrometer by electrospray insertion.
General Method for Preparing o-Aminobenzamides 3: 2-Amino-N-(2-phenylethyl)-benzamide (3ac).
A solution of phenylethylamine (5c, 1mmol) in DMF (30 mL) was stirred while isatoic anhydride (2a, 1.3 mmol) was added portionwise over a period of 30 minutes and then the temperature was maintained at ~50°C for an additional 30 minutes as CO2 was evolved. The mixture was stirred at room temperature for 2 hours more, hot water (100 mL) was added and then it was kept at room temperature without stirring for 6 hours. The solids were collected by filtration, dried under high vacuum and purified by silica gel chromatography column using dichloromethane as eluant. Finally, the excess of solvent was removed under reduced pressure to give compound 3ac as a pale brown solid. Yield 85%; mp 83-85°C; IR (KBr): 3291, 1631, 1596, 1303, 1155, 1030 cm-1; 1H-NMR: δ 7.40-7.13 (m, 7H, Ar-H), 6.67 (br, 1H, NH-CO), 6.07 (brs, 2H, NH-Ar), 5.50 (brs, 1H, NH-CO), 3.66 (q, 2H, J=6.8 Hz, CH2-NH), 2.91 (t, 2H, J=6.8 Hz, CH2-Ar) ppm; 13C NMR: δ 164.5, 148.6, 138.9, 132.2, 128.9, 128.8, 127.0, 126.6, 117.3, 116.6, 40.8, 35.8 ppm; ESI-MS (m/e): 263[M+Na]+.
The following compounds were similarly prepared:
2-Amino-N-[2-(2-methoxyphenyl)ethyl]benzamide (3ad). Yield 92%; mp 80-82°C; IR (KBr): 3468, 3032, 2937, 1664, 1596, 1255, 1093 cm-1; 1H-NMR: δ 7.21-7.16 (m, 4H, Ar-H), 6.92-6.85 (m, 2H, Ar-H), 6.65-6.59 (m, 2H, Ar-H), 6.42 (brs, 2H, NH-Ar), 5.20 (brs, 1H, NH-CO), 3.81 (s, 3H, CH3O), 3.60 (q, 2H, J=6.8Hz, CH2N), 2.93 (t, 2H, J=6.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.4, 157.4, 148.6, 132.1, 130.0, 127.9, 127.0, 120.94, 117.2, 116.4, 110.4, 55.3, 40.5, 29.9 ppm; ESI-MS (m/e) 263[M+ Na]+.
2-Amino-N-[2-(3-methoxyphenyl)ethyl]benzamide (3ae). Yield 84%; mp 69-71°C; (KBr): 3453, 3350, 1635,1587, 1255, 1041 cm-1; 1H-NMR: δ 7.27-7.14 (m, 3H, Ar-H), 6.84-6.71 (m, 3H, Ar-H), 6.67-6.55 (m, 3H, Ar-H), 6.11 (brs, 1H, NH-Ar), 4.5 (brs, 2H, NH-CO), 3.77 (s, 3H, CH3-O), 3.66 (q, 2H, J1=J2=6.8 Hz, NH-CH2), 2.88 (t, 2H, J1= 6.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.4, 159.9, 148.6, 140.6, 132.3, 129.7, 127.1, 121.2, 117.3, 116.6, 116.2, 114.4, 112.1, 55.2, 40.7, 35.7 ppm; ESI-MS (m/e): 293[M+Na]+, 271[M+H]+.
2-Amino-N-[2-(4-methoxyphenyl)ethyl]benzamide (3af) Yield 88%; mp 98-100°C; IR (KBr): 3483, 3230, 2922, 1671, 1244, 1030 cm-1; 1H-NMR: δ 7.25-7.11 (m, 4H, Ar-H), 6.89-6.55 (m, 2H, Ar-H), 6.68-6.55 (m, 2H, Ar-H), 6.07 (brs, 2H, NH-Ar), 5.0 (brs, 1H, NH-CO) 3.79(s, 3H, CH3-O), 3.62 (q, 2H, J1=J2=6.8 Hz, CH2-N), 2.85 (t, 2H, J=6.8 Hz, CH2-Ar) ppm; 13C-NMR: δ 169.3, 158.3, 148.6, 132.2, 129.7, 129.0, 117.2, 116.6, 114.13, 55.3, 40.9, 34.9 ppm; ESI-MS (m/e): 293[M+Na]+.
o-Amino-N-benzylbenzamide (3ag) Yield 93%; mp 104-106°C; IR (KBr): 3468, 3030, 2937, 1664, 1251, 1063 cm-1; 1H-NMR: δ 7.31-7.14 (m, 7H, Ar-H), 6.67-6.60 (m, 2H, Ar-H), 6.45 (brs, 2H, NH-Ar), 5.45 (brs, 2H, NH-CO) 4.57 (d, 2H, J=5.4 Hz, CH2-Ar) ppm; 13C-NMR: δ 169.1, 148.7, 138.2, 132.4, 128.7, 127.8, 127.5, 127.1 117.3, 116.7, 43.7 ppm; ESI-MS (m/e): 263[M+Na]+..
General Method for Preparing Quinazolinediones 4: 3-Phenylethyl-1H-quinazoline-2,4-dione (4c).
Triphosgene (10 mmol) in dichloromethane (10 mL) was added with stirring at room temperature to a solution of 2-amino-N-phenyl-ethylbenzamide (3ac, 5mmol) in dichloromethane (50 mL) and the resulting mixture was stirred for an additional 30 minutes. This was followed by the addition of triethylamine (30 mmol) and the mixture was refluxed for 2 hours. The reaction was quenched by addition of aqueous acid (5% HCl, 50 mL), and the mixture was extracted into dichloromethane. The organic phase was washed successively with saturated Na2CO3 solution (2 x 40 mL) and water (2 x 40 mL) and then dried over anhydrous Na2SO4 to give, after removal of excess solvent under reduced pressure, compound 4c as a yellow solid. Yield 75%; mp 173-175°C; IR (KBr): 2937, 1719, 1653, 1590, 1266 cm-1; 1H-NMR: δ 10.40 (s, 1H, NH-CO), 8.15 (dd, 1H, J1=1.6, J2=8.2 Hz, H-Ar), 7.67-7.59 (ddd, J1=1.6, J2=J3= 7.4 Hz, 1H, H-Ar), 7.34-7.15 (m, 7H, H-Ar) 4.33 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-NH), 3.05 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 162.26, 151.9, 139.3, 138.6, 134.9, 128.95, 128.7, 128.6, 126.6, 115.3, 114.6, 42.3, 34.1 ppm; ESI-MS (m/e): 264.9[M- H]-.
The following compounds were similarly prepared:
3-[2-(2-Methoxyphenyl)ethyl]-lH-quinazoline-2,4-dione (4d). Yield 73%; mp 174-176°C; IR (KBr): 2937, 2800, 1719, 1654, 1477, 1243, 1028 cm-1; 1H-NMR: δ 10.65 (s, 1H, NH-Ar), 8.18 (dd, 1H, J1=1.6, J2=8.2 Hz, H-Ar), 7.53 (ddd, J1=1.6, J2=J3=7.4 Hz, 1H, H-Ar), 7.21-6.69 (m, 6H, H-Ar), 4.34 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, NH-CH2), 3.77 (s, 3H, CH3), 3.02 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 162.4, 157.4, 151.0, 138.8, 134.3, 130.3, 127.7, 127.4, 126.9, 122.4, 120.1, 114.8, 114.2, 109.9, 55.1, 40.6, 28.5 ppm; ESI-MS (m/e): 295 [M –H]-.
3-[2-(3-Methoxyphenyl)ethyl]-lH-quinazoline-2,4-dione (4e). Yield 73%; mp 165-167°C; IR (KBr): 2928, 2593, 1728, 1671, 1604,1152 cm-1; 1H-NMR: δ 10.65 (s, 1H, NH-Ar), 7.93 (dd, J1=1.6 Hz, J2=8.2 Hz, 1H, H-Ar), 7.42 (ddd, J1=1.6, J2=J3= 7.4 Hz, 1H, H-Ar), 7.01-6.58 (m, 6H, H-Ar), 4.12 (ddd, 2H, J1=5.6, J2=J3=7.8 Hz, CH2-NH), 3.80 (s, 3H, CH3-O) 2.82 (ddd, J1=5.6 Hz, J2=J3=7.8 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 159.4, 157.8, 135.2, 133.9, 130.6, 128.3, 127.7, 127.1, 126 0, 125.3, 120.9, 120.5, 118.8, 110.2, 55.5, 43.3, 28.5 ppm; ESI-MS (m/e): 295 [M –H]-.
3-[2'-(4'-Methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (4f). Yield 78%; mp 76-78°C; IR (KBr): 1741, 1675, cm-1; 1H-NMR: δ 9.80 (s, 1H, NH-Ar) 8.13 (dd, J1=1.6 Hz, J2=8.2 Hz, 1H, H-Ar) 7.61 (ddd, J1=1.6, J2=J3=7.4 Hz, 1H, H-Ar) 7.26-6.81 (m, 6H, H-Ar), 4.25 (ddd, J1=5.6, J2=J3=7.4 Hz, 2H, CH2-NH), 3.76 (s, 3H, CH3-O), 2.95 (ddd, J1=5.6, J2=J3=7.4 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 161.3, 159.1, 151.2, 138.1, 134.6, 130.2, 129.6, 128.1, 123.1, 114.6, 113.6, 55.1, 42.1, 32.8 ppm; ESI-MS (m/e): 295 [M- H]-.
3-Benzyl-1H,3H-quinazoline-2,4-dione (4g). Yield 89%; mp 163-165°C; IR (KBr): 2956, 1709, 1657, 1266, cm-1; 1H-NMR: δ 11.15 (s, 1H, NH), 7.92(d, 1H, J=8.2 Hz, H-Ar), 7.46-7.03 (m, 8H, H-Ar), 5.12(s, 2H, CH2-Ar) ppm; 13C-NMR: δ 169.9, 155.3, 144.1, 141.7, 139.2, 132.7, 132.3, 131.7.8, 127.1, 119.8, 118.6, 48.3 ppm; ESI-MS (m/e) 251 [M- H]-.
General Method for Preparing N-Methylquinazolinediones 1: 1-Methyl-3-(2'-phenylethyl)-1H,3H-quinazoline-2,4-dione (1c).
Tetramethylguanidine (0.064 g, 0.56 mmol) was added to a solution of 3-phenylethyl-1-H-quinazoline-2,4-dione (4c, 0.015g, 0.56 mmol) in CHCl3 (1 mL) and the mixture was stirred at room temperature for 15 min., then methyl iodide (0.15 g, 12 mmol) was added and the reaction was heated to 55°C for 45 minutes. Activated carbon was added and the mixture was stirred for 10 min. and then filtered. Removal of the solvent under reduced pressure gave the title compound 1c as a yellow solid. Yield >96%; mp 99-101°C (Lit. [25], mp. 100-102°C); IR (KBr): 3042, 2929, 1701, 1654, 1610, 1481 cm-1; 1H-NMR: δ 8.29 (dd, 1H, J1=2.6, J2=8.4 Hz, Ar-H), 7.69 (ddd, 1H, J1= 5.4, J2= J3=7.4 Hz, Ar-H), 7.34-7.23 (m, 7H, Ar-H), 4.31 (ddd, J1= 5.0, J2= J3=7.6 Hz, 2H, N-CH2), 3.62 (s, 3H, N-CH3), 2.98 (ddd, J1= 5.0, J2= J3=7.6 Hz, Ar-CH2) ppm; 13C-NMR: δ 161.3, 150.5, 140.2, 138.3, 134.8, 129.9, 128.7, 128.6, 128.2, 126.2, 122.7,120.9, 113.25, 43.3, 33.9, 30.6 ppm; ESI-MS (m/e): 289.1[M+ H]+.
The following compounds were prepared in similar fashion:
1-Methyl-3-[2'-(2'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1d). Yield >98%; mp 155-163°C; IR (KBr): 2943, 1703, 1651, 1608, 1484, 1243, 1028 cm-1; 1H-NMR: δ 8.20 (dd, 1H, J1=2.2, J2=8.4 Hz, Ar-H), 7.63 (ddd, 1H, J1= 5.4, J3= J2=7.4 Hz, 1H, Ar-H), 7.25-6.75 (m, 6H, Ar-H), 4.33 (ddd, J1= 4, J2=J3=5.8 Hz, 2H, N-CH2), 3.62 (s, 3H, N-CH3), 2.98 (ddd, J1= 4, J2=J3=5.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 161.3, 157.5, 140.2, 134.6, 130.3, 128.6, 127.48, 126.87, 122.56, 120.17, 113.16, 110.0, 55.2, 41.8, 30.6, 28.7 ppm; ESI-MS (m/e): 310.9[M+ H]+; 332 [M +Na]+.
1-Methyl-3-[2'-(3'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1e). Yield 89%; mp 131-133°C; IR (KBr): 2945, 2833, 1699, 1656,1604 cm-1; 1H-NMR: δ 8.21 (dd, 1H, J1=2.1, J2=8.3 Hz, Ar-H), 7.63 (ddd, 1H, J1= 5.4, J3= J2=7.5 Hz, 1H, Ar-H), 7.25-6.62 (m, 6H, Ar-H), 4.29 (ddd, J1= 5.2, J2=J3=8.2 Hz, 2H, N-CH2), 3.79 (s, 3H, O-CH3), 3.61 (s, 3H, N-CH3), 2.98 (ddd, J1=4, J2=J3=5.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.5, 159.9, 148.5, 140.5, 132.8, 132.4, 129.7, 127.0, 126.4, 122.7, 117.4, 116.8, 116.3, 112.1, 55.1, 40.7, 35.7 ppm; ESI-MS (m/e): 332.9 [M +Na]+.
1-Methyl-3-[2'-(4'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1f). Yield >83%; mp. 134-136°C. (Lit. [25] mp. 133-134°C); IR (KBr): 3301, 2928, 1700, 1647, 1600, 1400, 1261. cm-1; 1H-NMR : δ 8.210 (dd, 1H, J1=1.4, J2=7.8 Hz, Ar-H), 7.69 (ddd, 1H, J1= 1.4, J3= J2=7.4 Hz, 1H, Ar-H), 7.30-6.85 (m, 6H, Ar-H), 4.26 (ddd, J1= 5.2, J2=J3=7.8 Hz, 2H, N-CH2), 3.79 (s, 3H, O-CH3), 3.61 (s, 3H, N-CH3), 2.91 (ddd, J1= 5.2, J2=J3=7.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 161.8, 158. 2, 140.5, 130.1, 129.2, 124.2, 114.0, 113.6, 55.5, 43.7, 33.4, 31.0 ppm; ESI-MS (m/e): 332.9 [M +Na]+.
1-Methyl-3-(benzyl)-1H,3H-quinazoline-2,4-dione (1g). Yield 93%; mp.103-106°C; IR (KBr): 3416, 2918, 1700, 1652, 1604, 1480, 1266, cm-1; 1H-NMR: δ 7.95 (d, 1H, J=8.0 Hz, H-Ar), 7.51 (t, 1H, J=7.9 Hz, H-Ar), 7.25-6.90 (m, 7H, H-Ar) 5.04 (s, 2H, CH2-Ar), 3.05 (m, 3H, CH2-NH) ppm; 13C-NMR: δ 161.2, 150.2, 139.8, 136.5, 134.9, 128.1, 127.9, 127.8, 126.9, 122.5, 114.7, 44.5 ppm; ESI-MS (m/e) 288.9 [M+Na]+.
Acknowledgments
We gratefully acknowledge support for this project by the Consejo Nacional de Ciencia y Tecnología, México (CONACyT, GRANT No. 28488-E) and the Consejo Nacional de Educación Tecnológica, México (COSNET, GRANT No. 623.97-P). Karla Espinoza thanks CONACyT for a graduate fellowship. The authors are indebted to Dr. Charles Perrin for encouragement.
Footnotes
Sample availability: Available from the authors
References
- 1.Larksarp C., Alper H. J. Org. Chem. 2000;65:2773. doi: 10.1021/jo991922r. [DOI] [PubMed] [Google Scholar]
- 2.Hermecz I., Kokosi J., Podanyi B., Szaz G. Heterocycles. 1994;37:903. [Google Scholar]
- 3.Katritzky A. R., Rees C. W. Comprehensive Heterocyclic Chemistry: The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds, Part 2B. vol. 3 Pergamon Press; New York: 1984. [Google Scholar]
- 4.Pelletier S. W. Alkaloids: Chemical and Biological Perspectives. vol. 1 John Wiley & Sons Ltd.; New York: 1985. [Google Scholar]
- 5.Scovill J., Blank E., Konnick M., Nenortas E., Shapiro T. Antimicrob. Agents Chemother. 2002;46:882. doi: 10.1128/AAC.46.3.882-883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penn J., Mantle P. G., Bilton J. N., Sheppard R. N. J. Chem. Soc. Perkin Trans. I. 1992:1495. [Google Scholar]
- 7.Wong S.-M., Musza L. L., Kydd G. C., Kulnig R., Gillum A. M., Cooper R. J. Antibiot. 1993;46:545. doi: 10.7164/antibiotics.46.545. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto H., Negishi E., Yamaguchi K., Nishi N., Yamazaki M. Chem. Pharm. Bull. 1996;44:1843. [Google Scholar]
- 9.Numata A., Takahashi C., Matsushita T., Miyamoto T., Kawai K., Usami Y., Matsumura E., Inove M., Ohishi H., Shingu T. Tetrahedron Lett. 1992;37:1621. [Google Scholar]
- 10.Takahashi C., Matsushita T., Doi M., Minoura K., Shingu T., Kumeda Y., Numata A. J. Chem. Soc. Perkin Trans I. 1995:2345. [Google Scholar]
- 11.Karwowski J. P., Jackson M., Rasmussen R. R., Humphrey P. E., Poddig J. B., Kohl W. L., Scherr M. H., Kadam S., McAlpine J. B. J. Antibiot. 1993;46:374. doi: 10.7164/antibiotics.46.374. [DOI] [PubMed] [Google Scholar]
- 12.Hochlowski J. E., Mullally M. M., Spanton S. G., Whitten D. N., Hill P., Mc Alpine J. B. J. Antibiot. 1993;46:380. doi: 10.7164/antibiotics.46.380. [DOI] [PubMed] [Google Scholar]
- 13.Larsen T. O., Frydenvang K., Frisvad J. C., Christopherson C. J. Nat. Prod. 1998;61:1154. doi: 10.1021/np980056v. [DOI] [PubMed] [Google Scholar]
- 14.Barrow C. J., Sun H. H. J. Nat. Prod. 1994;57:471. doi: 10.1021/np50106a005. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez F., Buenadicha F. L., Avendaño C., Söllhuber M. Tetrahedron: Asymmetr. 2001;12:3387. [Google Scholar]
- 16.Hayao S., Havera H. J., Strycker W. G., Hong E. J. Med. Chem. 1969;12:936. doi: 10.1021/jm00305a062. [DOI] [PubMed] [Google Scholar]
- 17.Shiau C. V., Chem J. W., Tien J. H., Liang K. C. J. Hetrocycl. Chem. 1989;26:595. [Google Scholar]
- 18.Nishikawa Y., Shindo T., Ishi K., Nakamura H., Kon T., Uno H. J. Med. Chem. 1989;32:583. doi: 10.1021/jm00123a012. [DOI] [PubMed] [Google Scholar]
- 19.Hayao S., Havera H. J., Strycker W. G., Leipzig T. T., Kulp R. A., Hartzle H. E. J. Med. Chem. 1965;8:807. doi: 10.1021/jm00330a017. [DOI] [PubMed] [Google Scholar]
- 20.Hayao S. ((Miles Lab., Inc.)). 3,274,194. US Patent. 1965
- 21.Cortez R., Rivero I. A., Somanathan R., Aguirre G., Rámirez F. Synth. Commun. 1991;21:285. [Google Scholar]
- 22.Leysen J. E., Niemegeers J. E., Neuten J. M., Laduron P. M. Mol. Pharmacol. 1982;21:301. [PubMed] [Google Scholar]
- 23.Darchen F., Scherman D., Laduron P. M., Henry J. P. Mol. Pharmacol. 1988;33:672. [PubMed] [Google Scholar]
- 24.Rivero I. A., Somanathan R. Synth. Commun. 1998;28:2077. [Google Scholar]
- 25.Dreyer D.L., Bayer R.C. Phytochemistry. 1980;19:935. [Google Scholar]