Abstract
Babesia is one of the main causes of anemia in cattle, a lot of elucidations have been suggested to explain its pathogenesis. This study was designed to investigate clinical, hemato-biochemical and oxidant/antioxidant status and its relation with the resultant anemia in Babesia-infected calves. Seventeen (17) native breed calves were involved in this study, clinical signs and microscopic findings were recorded, also blood samples were taken to investigate hematologic changes, serum biochemical variations and oxidative stress biomarkers. The most commonly observed clinical signs were fever, emaciation, depression, icterus and hemoglobinuria. Significant reduction in PCV, HB, RBCs, MCHC, Total protein, and albumin along with significant increase in MCV, WBCs, monocytes and BUN were the most consistent hemato-biochemical changes. Oxidant/antioxidant and trace mineral assessment showed significant reduction in Superoxide dismutase “SOD”, Glutathione peroxidase “GPx”, Zn, Cu along with significant increase in malondialdehyde (MDA) activities. In the current investigation, oxidant/antioxidant imbalance along with the synchronized alterations in antioxidant trace minerals was detected in Babesia-infected calves. These findings support notion that Babesia infection associated with oxidative stress and this process may be linked to the resultant anemia.
Keywords: Babesia, Clinical, Hemato-biochemical, Anemia, Oxidative stress
1. Introduction
Livestock in Egypt plays a considerable role in country economy. Cattle is one of the main livestock population raised in Egypt, according to FAO [1], cattle population in 2009 ranged from 3.5 to 5 million head.
Cattle industry can be plagued with abundant diseases, among which tick-borne ailments are of considerable importance. These diseases have a negative influence on animal health and subsequently the economy [2]. Bovine babesiosis is an important tick-borne ailment all over the world but mostly in subtropical and tropical territories [3]. Babesia is an intraerthrocytic hemoprotozoan affecting animal erythroctytes [4]. In Egypt, bovine babesiosis are caused by B. bovis and B. bigemina [5], [6].
Babesiosis common presentation is pyrexia, anemia, icterus, emaciation, inappetence and hemoglobinuria, in pregnant cattle, diarrhea and even abortion may occur [7], [8]. Bovine babesiosis was known to cause anemia, the type of anemia usually hemolytic, though, ranged from hypochromic to normochromic, this change is associated with reduction in erythrogram parameters and alterations in serum biochemical values [3]. The microscopic examination of blood smear considered the most applicable and the cheapest method for diagnosis, presence of the protozoan inside red cells considered confirmative especially during acute stage of the disease, this ability decrease sharply in other situations like carrier animals [9], [10].
Oxidative stress is a disproportion between scavenging mechanism and radical generating mechanism [11]. The erythrocyte peroxidation occurs in hemo-protozoan infection may be correlated to disease pathogen [12]. Free radicals can harm tissue and cells, when Reactive oxygen species “ROS” exceed the ability of antioxidant system, oxidative process ensues [13]. Recently in blood parasites investigations, strong accumulated evidences were found to link the resultant anemia with oxidative damage due to lipid peroxidation of red cells [12]. Anti-oxidant elements such as SOD and GPx are vital in counteract ROS damage [11].
The purpose of this study is to describe the clinical and laboratory alterations in Babesia-infected calves with special emphasis on oxidant-antioxidant status associated with this disease.
2. Materials and methods
2.1. Animals
Seventeen (17) clinically-ill native breed male calves aged 1 year, located at Alexandria-Cairo desert road, Egypt were involved in this study. Criteria of inclusion were presence of tick infestation, clinical signs and positive microscopic blood smear. Giemsa-stained thin blood films were inspected under the light microscope for identification of intraerythrocytic stages of the hemoparasite [14]. Control calves (n = 6) from same farm with the same age and sex were involved. Calves were thoroughly clinically examined, fecal samples were taken and blood smears from each control calf was examined microscopically to ensure they are parasitological free. The criteria for inclusion in control group were also depended on no former or existing history of tick exposure, and absence of clinical signs.
Each calf was subjected to comprehensive clinical examination and clinical signs were recorded.
2.2. Blood sampling and microscopic detection
Blood samples were collected from jugular vein in EDTA-containing tubes and plain tube to separate serum samples; the blood in plain tubes were preserved in slanted spot for approximately 2 h and then refrigerated at 4 °C overnight for serum separation. Clinical hematology was performed within 2 h after the sample collection. Three Giemsa stained blood films from each animal were examined under oil-immersion lens, to confirm the Babesia infection.
2.3. Hematologic investigations
For hematological analysis, Haemo-cytometric method was used to determine erythrocyte and leukocyte counts. Hemoglobin was estimated using Drabkin colorimetric method. Hematocrit values were evaluated by micro-hematocrit centrifugation. Differential leukocyte counts were determined from smears stained by the Giemsa method. The erythrocyte indices of MCV and MCHC values were also estimated by appropriate formulas.
2.4. Estimation of oxidant-antioxidant status
EDTA whole blood samples were used to formulate erythrocytes lysates for estimation levels of glutathione peroxidase (GPX) and superoxide dismutase (SOD) enzymes using respective test kits (Bio-Diagnostic Company-Egypt). While resultant plasma was used to estimate catalase using respective test kit (Bio-Diagnostic Company-Egypt). Estimation of these parameters was done manually using spectrophotometer (APEL, PD-303S, Japan) according to manufacturer instructions.
2.5. Estimation of serum biochemical parameters
Serum samples were used to determine total protein, albumin, cholesterol, triglycerides, BUN, Malondialdehyde (MDA), zinc (Zn) and copper (Cu) (Bio-Diagnostic Company-Egypt, Spectrum Diagnostic, and Egypt). Estimation of these parameters was done manually using spectrophotometer (APEL, PD-303S, Japan) according to manufacturer instructions.
2.6. Statistical analysis
Results of diseased calves were compared to control data, calculation of mean ± SE, and data comparison using student t-test (STATISTICA for Windows, version 5.1., StatSoft, Inc.). P ⩽ 0.05 considered significant.
3. Results
The most consistent clinical signs recorded in diseased calves were fever, tachypnea, decreased weight, anorexia, inappetence and depression; icterus and hemoglobinuria were also observed. Tick infestation was detected on affected calves.
The examination of blood films revealed the presence of pyriform merozoites of B. bigemina at an acute angle inside the erythrocytes, and only the calves that showed positive on microscopic examination were included in this study.
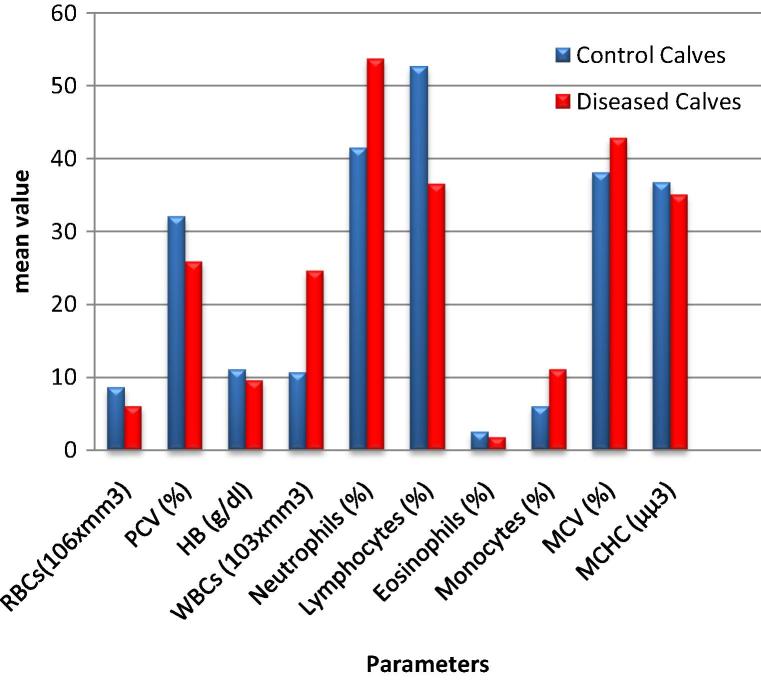
Hematologic variations in Babesia infected calves are shown (Table 1 and Fig. 1). Significant decrease (P ⩽ 0.05) in RBCs, HB, PCV, MCHC, platelet count along with significant increase in MCV was observed in diseased calves compared to control data, while platelet count showed decrease in diseased calves compared to control data. In leucogram, significant increase (P ⩽ 0.05) in WBCs and monocytes was observed in diseased calves while lymphocyte was significantly decreased. Neutrophils in diseased calves although numerically higher than control data but this elevation was considered to be not quietly statistically significant.
Table 1.
Hematologic evaluation of erythrogram and leukogram of diseased calves compared with the apparently healthy calves.
| Parameter | Control Calves (A) | Diseased Calves (B) | P value |
|---|---|---|---|
| RBCs(106 × mm3) | 8.59 ± 0.51 | 6.026 ± 0.224 | 0.001 |
| PCV (%) | 32 ± 1.140 | 25.892 ± 1.099 | 0.004 |
| HB (g/dl) | 11.05 ± 0.31 | 9.56 ± 0.366 | 0.021 |
| WBCs (103 × mm3) | 10.640 ± 0.928 | 24.630 ± 0.566 | 0.0252 |
| Neutrophils (%) | 41.40 ± 5.01 | 53.67 ± 5.61 | 0.173 |
| Lymphocytes (%) | 52.60 ± 4.2 | 36.50 ± 4.75 | 0.049 |
| Eosinophils (%) | 2.50 ± 0.5 | 1.75 ± 0.37 | 0.259 |
| Monocytes (%) | 6.00 ± 0.71 | 11.10 ± 1.23 | 0.028 |
| MCV (%) | 38 ± 0.860 | 42.80 ± 0.77 | 0.016 |
| MCHC (μμ3) | 36.70 ± 0.33 | 35 ± 0.32 | 0.006 |
| Platelets (103 × mm3) | 450.200 ± 90.481 | 290.777 ± 21.195 | 0.0451 |
Fig. 1.
Hematologic alterations in diseased group compared to control group.
Serum biochemical changes are recorded (Table 2). Significant decrease (P ⩽ 0.05) in total protein and albumin along with significant increase in BUN was recorded in diseased calves, where no statistical changes were observed in cholesterol and triglycerides.
Table 2.
Serum biochemical evaluation in diseased calves compared with the apparently healthy calves.
| Parameter | Control Calves (A) | Diseased Calves (B) | P value |
|---|---|---|---|
| Total Protein (g/dl) | 6.97 ± 0.21 | 5.286 ± 0.5 | 0.001 |
| Albumin (g/dl) | 3.65 ± 0.155 | 2.71 ± 0.20 | 0.013 |
| Cholesterol (mg/dl) | 70.75 ± 7.94 | 65.36 ± 7.11 | 0.652 |
| Triglycerides (mg/dl) | 10.0880 ± 1.112 | 8.374 ± 0.9570 | 0.2857 |
| BUN (mg/dl) | 10.0600 ± 1.045 | 19.460 ± 3.4958 | 0.0424 |
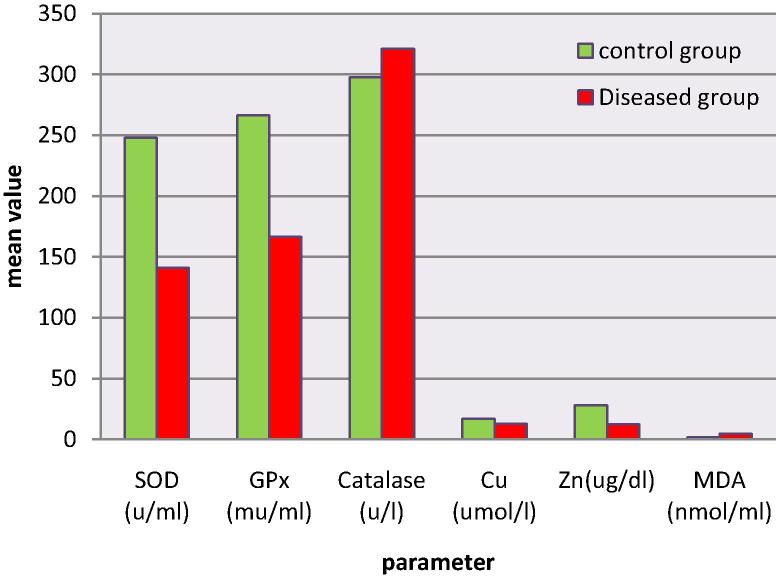
Results of oxidative stress biomarkers are shown (Table 3 and Fig. 2). Significant increase (P ⩽ 0.05) in MDA accompanied by significant decrease in SOD, GPx, Zn and Cu in diseased calves compared to control data was reported, whereas catalase showed increase in diseased calves though this increase considered not statistically significant.
Table 3.
Oxidant/antioxidant changes in diseased calves compared with the apparently healthy calves.
| Parameters | Control Calves (A) | Diseased Calves (B) | P value | |
|---|---|---|---|---|
| Enzymatic anti-oxidant | SOD (u/ml) | 247.795 ± 20.171 | 141 ± 12.20 | 0.0013 |
| GPx (mu/ml) | 266.196 ± 31.5 | 166.40 ± 21.870 | 0.027 | |
| Catalase (u/l) | 297.6667 ± 19.35 | 321.298 ± 47.116 | 0.775 | |
| Anti-oxidant-Trace Element | Cu (umol/l) | 16.837 ± 0.489 | 12.65 ± 0.74 | 0.004 |
| Zn (ug/dl) | 27.901 ± 3.23 | 12.497 ± 1.574 | 0.006 | |
| Oxidant | MDA (nmol/ml) | 1.566 ± 0.173 | 4.664 ± 0.415 | 0.004 |
Fig. 2.
Oxidant/antioxidant alterations in Babesia infected calves.
4. Discussion
Tick-borne diseases have a negative effect on livestock health [11], and responsible for major economic losses.
In this study, Babesia infection was confirmed via light microscopy examination. Presence of pear shape piroplasms inside RBCs is confirmative of diagnosis especially in acute stages of the disease [9].
Fever, emaciation, tachypnea, anorexia, depression, icterus and hemoglobinuria were observed, similar manifestations were reported [15], [16]. The major modus operandi by which the protozoan operates is hemolysis which leads to anemia and subsequent inflammatory process in kidney and liver [7]. It has been speculated that in the face of every clinical condition, there are tens of latent undiscovered conditions [17]. Correlation between Babesia and release of “pharmacologic-active mediators” was found [18] that stimulates certain mediators causing circulatory failure with coma and death eventually [19].
Significant decrease in RBCs, HB, PCV, MCHC and platelets values along with significant increase in MCV was observed in diseased calves, the erythrogram results advocates the presence of macrocytic hypochromic anemia.
Anemia is a feature of Babesia; the parasite is capable of destroying RBCs when it exists [19]. In B. bigemina; parasitemia is adept to cause a major alteration in total RBCs osmotic fragility even intact unaffected RBCs, these red cells become easily destroyed creating hemolysis [20]. In other animal models, auto-antibodies against circulating red cells have been suggested [21]. The antibody-coated red cells are attacked by macrophages and complements bound to membrane via Ag–Ab reaction creating hemolysis [22].
The resultant decrease in platelets is attributed to decrease the production or increase destruction [23]. Thrombocytopenia is usually correlated with immune-mediated process and hemolysis [24].
Leukocytosis and monocytosis are observed in clinically infected calves, and these findings were observed in Babesia infected animals [25], [26]. The ability of the protozoan to trigger lymphoid system linked to increase in WBCs count during infection [27]. Monocytosis is predictable in hemolytic diseases [28].
Significant decrease in Total protein and albumin with elevated BUN levels was observed in diseased calves. Babesia can cause disruption in liver function that leads to decrease albumin synthesis and consequently affect total protein levels [29], [30]. Babesia can cause degeneration and necrosis in kidney convoluted tubules, consequently a rise in BUN is expected [7].
In recent years, there were cumulative evidences suggest the pathogenesis of anemia in babesiosis is correlated with lipid peroxidation and oxidative process [31]. Our data showed increase in oxidant system along with decrease in antioxidant system. Significant rise in MDA was observed, MDA is a profound index of lipid peroxidation process [32]; this increase leads to buildup of oxidative ions in RBCs causing its lyses [33].
RBCs membrane structure is rich in polyunsaturated fatty acids, Babesia is capable of making the red cell membrane highly prone to per oxidative impairment [11], [34], [35]. It appears that Babesia has a great influence on the oxidant/antioxidant system resulting in ROS production in substantial quantities among which is MDA, which subsequently overload the antioxidant system and cause direct damage to red cells [11], [34], [35].
The increase in oxidant markers was coupled with exhaustion of antioxidant markers (GPx and SOD), important indicators of oxidative stress. GPx is an important antioxidant enzyme that is widely known to metabolize lipid peroxidation products [36]. GPx plays a part in catabolism of ROS, it has been suggested that “peroxide-mediated deactivation” of GPx can occur [37]. The role GPx played in protection against toxic effect of ROS can explain the decrease in its activity in infection.
SOD an antioxidant enzyme known for its ability to scavenge toxins from body [38]. In this study SOD activity showed a significant decrease compared to control data, SOD plays an integral part in RBCs protection system against oxidative damage [39]. Presence of hemo-protozoan inside RBCs considerably affects the key anti-oxidant system of red cells, leading to a state in which red cells bump into oxidant mediators’ assault which eventually will cause cell injury and hemolysis [40]. It appears the protozoan- prompted modulation of anti-oxidant enzymes cause a speed up in RBCs removal via phagocytosis [41].
In contrary to SOD and GPx pattern, Catalase showed non-significant increase in diseased calves. Similar results were found in T. annulate infected cows and Thelieria-infected sheep [42], [43]. Catalase is accountable for H2O2 removal and thus it provides protection against damaging effect of H2O2 leading to rise RBCs lifetime, so the increase in its activity may be contributed to the indirect compensatory reaction to oxidant encounter [43].
The enzymatic antioxidant mechanism activities rely primary on certain trace minerals among them Zinc and Copper [44]. Cu and Zn play an important role in body antioxidant mechanism by preventing radical-induced injury [40]. Zn can antagonize the redox active agents and decreases cellular injury, though the properties of zinc as antioxidant have been already established, the mechanism is unclear [45]. Copper has been recorded to decrease in bovine babesiosis [11], [46]. Cu–Zn SOD catalyzing the alteration of O2− to H2O2 [41]. Copper can perform as both a pro-oxidant and an antioxidant, Cu removes free radicals and helps in preventing them from exerting some of their damaging effect [47].
5. Conclusion
In the current investigation, oxidant/antioxidant imbalance along with the synchronized alterations in antioxidant trace minerals was detected in Babesia-infected calves. These findings support notion that Babesia infection associated with oxidative stress and this process may be linked to the resultant anemia.
Declaration
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.FAO; c2011, http://www.fao.org/ag/agp/agpc/doc/counprof/egypt/egypt.html#ruminant/; 2011 [accessed 07.11].
- 2.Ghirbi Y.M., Hurtado A., Brandika J., Khlif K., Ketata Z., Bouattour A. A molecular survey of Theileria and Babesia parasites in cattle, with a note on the distribution of ticks in Tunisia. Parasitol Res. 2008;103:435–442. doi: 10.1007/s00436-008-0995-3. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A., Singla L.D., Ashuma Batth B.K., Kaur P. Clinicopath-biochemical alterations associated with subclinical babesiosis in dairy animals. J Arthropod Borne Dis. 2016;10:259–267. [PMC free article] [PubMed] [Google Scholar]
- 4.Zintl A., Mulcahy G., Skerrett H.E., Taylor S.M., Gray J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoud M., Kandil O., Nasr S., Hendawy S.H., Habeeb S.M., Mabrouk D.M. Serological and molecular diagnostic surveys combined with examining hematological profiles suggests increased levels of infection and hematological response of cattle to babesiosis infections compared to native buffaloes in Egypt. Parasit Vectors. 2015;8:319–333. doi: 10.1186/s13071-015-0928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adham E.K., Abd El-Samie E.M., Gabre R.M., El-Hussein H. Detection of tick blood parasites in Egypt using PCR assay I—Babesia bovis and Babesia bigemina. Parasitol Res. 2009;105:721–730. doi: 10.1007/s00436-009-1443-8. [DOI] [PubMed] [Google Scholar]
- 7.Mosqueda J., Olvera-Ramirez A., Aguilar-Tipacamu G., Canto G.J. Current advances in detection and treatment of babesiosis. Curr Med Chem. 2012;19:1504–1518. doi: 10.2174/092986712799828355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decaro N., Larocca V., Parisi A., Losurdo M., Paolo Lia R., Greco M.F. Clinical bovine piroplasmosis caused by Babesia occultans in Italy. J Clin Microbiol. 2013;51:2432–2434. doi: 10.1128/JCM.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A.P., Singla L.D., Singh A. A study on the effects of macroclimatic factors on the seasonal population dynamics of Boophilus micropus (Canes 1888) infesting the cross-bred cattle of Ludhiana district. Int J Anim Sci. 2000;15:29–31. [Google Scholar]
- 10.Almeria S., Castella J., Ferrer D., Ortuno A., Estrada-Pena A., Gutierrez J.F. Bovine piroplasms in Minorca (Balearic Islands, Spain): a comparison of PCR-based and light microscopy detection. Vet Parasitol. 2001;99:249–259. doi: 10.1016/s0304-4017(01)00464-2. [DOI] [PubMed] [Google Scholar]
- 11.Omar A.O.J., Sadat M., Emam F.A. Determination of oxidative stress markers associated with Babesia Bigemina. Afr J Biotechnol Res. 2015;3:150–155. [Google Scholar]
- 12.Nazifi S., Razvi S.M., Kianiamin P., Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzyme lipid perixidations and serum trace element associated with progressive anemia in ovine malignant theileriosis. Parasitol Res. 2011;2:275–281. doi: 10.1007/s00436-010-2248-5. [DOI] [PubMed] [Google Scholar]
- 13.Zaidi S.M., AL-Qirim T.M., Bani N. Effect of antioxidant vitamins on glutathione depletion and lipid peroxidation induced by restrained stress in the rat liver. Drugs R D. 2005;6:157–165. doi: 10.2165/00126839-200506030-00004. [DOI] [PubMed] [Google Scholar]
- 14.Brown A.B. 5th ed. Lea and Feibiger; Philadelphia: 1988. Hematology: principles and procedures. p. 96–97. [Google Scholar]
- 15.Talkhan O.F.A., Radwan M.E.I., Ali M.A. Cattle babesiosis and associated biochemical alteration in Kalubyia governorate. Nat Sci. 2010;12:24–27. [Google Scholar]
- 16.Brown C, Torres A. 7th ed. United States Animal Health Association; Boca Raton (FL): 2008. Foreign animal diseases. [p. 147–85] [Google Scholar]
- 17.Ristic M., Healey G.R. Babesiosis. In: Steel J.H., Arambulo P., editors. CRC Press; Cleveland, OH: 1980. (Parasitic zoonosis - I). [p. 151–65] [Google Scholar]
- 18.Wright I.G., Goodger B.V. Acute Babesiosis bigemina infection: changes in coagulation and kallikrein parameters. Z Parasitenkd. 1977;53:63–73. doi: 10.1007/BF00383116. [DOI] [PubMed] [Google Scholar]
- 19.Wright I.G. Osmotic fragility of erythrocytes in acute Babesia argentina and Babesia bigemina infections in splenectomized Bos taurus calves. Res Vet Sci. 1973;15:299–305. [PubMed] [Google Scholar]
- 20.Kakoma I, Ristic M. Pathogenesis of babesiosis. In: Ristic M, Ambroise-Thomas P, Kreier J, editors. Malaria and Babesiosis: research findings and control measures. Dordrecht: Springer, Netherlands; 1984 [p. 85–93].
- 21.Irwin P.J. 1st ed. Manson Publishing Ltd.; Barcelona: 2005. Babesiosis and Cytauxzoonsis. Arthropode-borne infectious diseases of dogs and cats. [p. 63–77] [Google Scholar]
- 22.Day M.J. Antigen specificity in canine autoimmune hemolytic anemia. Vet Immuol Immunopathol. 1999;69:215–224. doi: 10.1016/s0165-2427(99)00055-0. [DOI] [PubMed] [Google Scholar]
- 23.Feldman B.F., Zinkl J.G., Schalm O.W. 5th ed. Lippincott Williams and Wilkins; Philadelphia: 2000. Schalm’s veterinary hematology. [p. 561–631] [Google Scholar]
- 24.Riond B.M.M., Braun U., Deplazes P., Joerger K., Thoma R., Lutz H. Concurrent infections with vector-borne pathogens associated with fatal anaemia in cattle: hematology and blood chemistry. Comp Clin Pathol. 2008;17:171–177. [Google Scholar]
- 25.Salem N.Y., Farag H.S. Clinical, hematologic, and molecular findings in naturally occurring Babesia canis vogeli in Egyptian dogs. Vet Med Int [Internet] 2014;2014:270345. doi: 10.1155/2014/270345. https://www.hindawi.com/journals/vmi/2014/270345/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem N.Y., El-sherif M.A. Malondialdehyde status, trace minerals and hematologic results of anemic–T. equi infected Egyptian horses. Int J Vet Sci. 2015;4:118–122. [Google Scholar]
- 27.Ibrahim A.K., Gamil I.S., Abd-El baky A.A., Hussein M.M., Tohamy A.A. Comparative molecular and conventional detection methods of Babesia equi (B. Equi) in Egyptian equine. Glob Vet. 2011;7:201–210. [Google Scholar]
- 28.Raskins R.E., Latimer K.S., Tvedton H. Leucocyte disorder. In: Willard M.D., Tvedten H., editors. Small animal clinical diagnosis by laboratory methods. 4th ed. Saunders; 2004. pp. 63–91. [Google Scholar]
- 29.Werner L.L., Turnwald G.H., Willard M.D. Immunologic and plasma protein disorders. In: Willard M.D., Tvedten H, editors. Small animal clinical diagnosis by laboratory methods. 4th ed. Saunders; 2004. pp. 290–305. [Google Scholar]
- 30.Kerr M.G. 2nd ed. Blackwell Science; Cornwall: 2002. Veterinary laboratory medicine clinical biochemistry and hematology. p. 74–9. [Google Scholar]
- 31.Ambawat H.K., Malhotra D.V., Kumar S., Dhar S. Erythrocyte associated haemato-biochemical changes in Babesia equi infection experimentally produced in donkeys. Vet Parasitol. 1999;85:319–324. doi: 10.1016/s0304-4017(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 32.Chiu D, Lubin B, Shohet SB. Peroxidative reaction in red cell biology. In: Pryor WA, editor. Free radicals in biology. Academic Press; New York: 1982. pp. 115–157. [Google Scholar]
- 33.Mata M.M., Bhardwaj R.M. Possible alterations in erythrocyte metabolism and integrity in post-parturient haemoglobinuria. Ind J Vet Med. 1985;5:67–72. [Google Scholar]
- 34.Sedar D., Yeter B.K., Ozdaland N.A.G. Status of lipid peroxidation, antioxidants and oxidation products of nitric oxide in equine babesiosis: status of antioxidant and oxidant in equine babesiosis. J Equi Vet Sci. 2009;29:743–774. [Google Scholar]
- 35.Celi P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol Immunotoxicol. 2011;33:233–240. doi: 10.3109/08923973.2010.514917. [DOI] [PubMed] [Google Scholar]
- 36.Genc S., Gurdol F., Oner-Iyidogan Y., Onaran I. The effect of melatonin administration on ethanol induced lipid peroxidation in rats. Pharmacol Res. 1998;37:37–40. doi: 10.1006/phrs.1997.0263. [DOI] [PubMed] [Google Scholar]
- 37.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signaling. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali H., Yousef C.S., Ali S. Assessment of serum antioxidant enzymes activity in cattle suffering from Theileriosis. Eur J Exp Biol. 2013;3:493–496. [Google Scholar]
- 39.Asri Rezaei S., Dalir-Naghadeh B. Evaluation of antioxidant status and oxidative stress in cattle infected with Theileria annulata. Vet Parasitol. 2006;142:179–186. doi: 10.1016/j.vetpar.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Nazifi S., Razavi S.M., Kianiamin P., Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation, and serum trace elements associated with progressive anemia in ovine malignant theileriosis. Parasitol Res. 2011;109:275–281. doi: 10.1007/s00436-010-2248-5. [DOI] [PubMed] [Google Scholar]
- 41.Razavi S.M., Nazifi S., Rakhshandehroo E., Firoozi P., Farsandaj M. Erythrocyte antioxidant systems, lipid peroxidation and circulating lipid profiles in cattle naturally infected with Theileria annulata. Rev Méd Vét. 2012;163:18–24. [Google Scholar]
- 42.Grewal A., Ahuja C.S., Singh S.P., Chaudhary K.C. Status of lipid peroxidation, some antioxidant enzymes and erythrocytic fragility of crossbred cattle naturally infected with Theileria annuluta. Vet Res Commun. 2005;29:387–394. doi: 10.1007/s11259-005-4682-x. [DOI] [PubMed] [Google Scholar]
- 43.Baghshani H., Razmi G.R., Yaghfouri S., Dezaki A.A. Status of some oxidative stress biomarkers in sheep naturally infected with theileriosis. Res Opin Anim Vet Sci. 2011;1:499–504. [Google Scholar]
- 44.Kleczkowski M., Kluciński W., Sikora J., Zdanowicz M. Role of antioxidants in the protection against oxidative stress in cattle–trace elements and enzymatic mechanisms (Part 3) Pol J Vet Sci. 2004;7:233–240. [PubMed] [Google Scholar]
- 45.Powell S.R. The antioxidant properties of zinc. J Nutr. 2000;130(Suppl):S1447–S1454. doi: 10.1093/jn/130.5.1447S. http://jn.nutrition.org/content/130/5/1447S.long Available from. [DOI] [PubMed] [Google Scholar]
- 46.Abdel Hamid O.M., Radwan M.E.I., Abdel Fatah A. Biochemical changes associated with babesiosis infested cattle. IOSR J Appl Chem. 2014;7:87–92. [Google Scholar]
- 47.Osredkar J., Sustar N. Copper and Zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol. 2011;S3:1–18. http://www.omicsonline.org/copper-and-zinc-biological-role-and-significance-of-copper-zincimbalance-2161-0495.S3-001.php?aid=3055 Available from. [Google Scholar]