Abstract
The heart is an organ where primary malignant tumors rarely develop. In particular, the incidence of cardiac rhabdomyosarcoma (RMS) is extremely low. It has been reported that the risk of second malignant tumors in mediastinum is increased by radiotherapy in women with breast cancer. However, little is known about the association between irradiation to heart and cardiac RMS. Here, we report a case of a 68-year-old woman with primary cardiac RMS. She suddenly presented syncope at a workplace, and was taken to the emergency room at our hospital. Several imaging tests, including echocardiogram and cine magnetic resonance imaging, detected two tumors in the right ventricle (RV) and its outflow tract, which had almost obstructed the main trunk of the pulmonary artery (PA). To avoid sudden PA occlusion by the tumor, we emergently performed surgical excision of the tumors from the RV. Pathological analysis revealed that these tumors were embryonal type RMS. She had received radiotherapy after mastectomy for left breast cancer 18 years previously, and no recurrence of breast cancer had been detected. This cardiac RMS is considered as a second malignant tumor related to radiotherapy for breast cancer.
<Learning objective: We experienced a 68-year-old woman having two tumors of the RMS in RV, who had received radiotherapy for left breast cancer 18 years previously and had previously presented no recurrence. There is literature suggesting that radiotherapy may increase the risk of soft-tissue sarcoma in women with breast cancer. We should be aware of cardiac RMS as a second malignant tumor related to radiotherapy for breast cancer, although its incidence is extremely low.>
Keywords: Primary cardiac tumor, Rhabdomyosarcoma, Radiotherapy, Breast cancer
Introduction
Primary cardiac tumors are rare and the incidence is estimated to be 0.0017% [1]. Of these, 10–25% are malignant [1], [2]. The incidence of rhabdomyosarcoma (RMS) within primary malignant cardiac tumors is approximately 10% [2]. Thus, the overall incidence of primary cardiac RMS is extremely low. However, it has been reported that radiotherapy increases the risk for soft-tissue sarcoma in women with breast cancer [3], [4]. Here, we report a patient with primary cardiac RMS that developed in the right ventricle (RV), who had received radiotherapy for left breast cancer 18 years previously. The association between irradiation to the heart and the development of a cardiac malignant tumor is discussed herein.
Case report
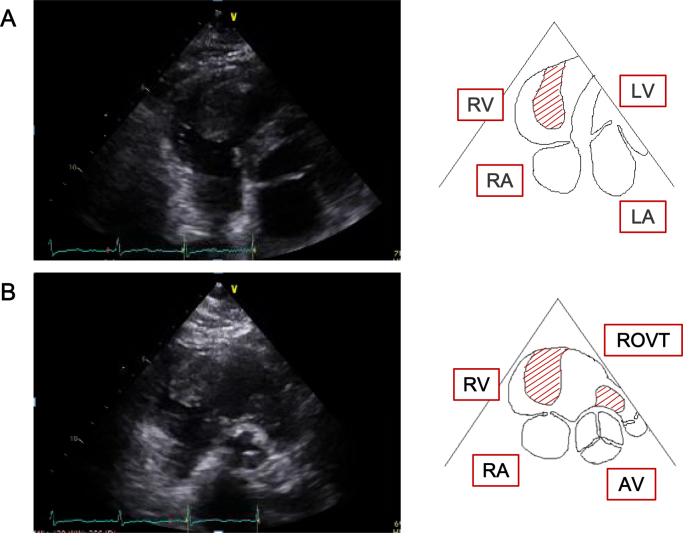
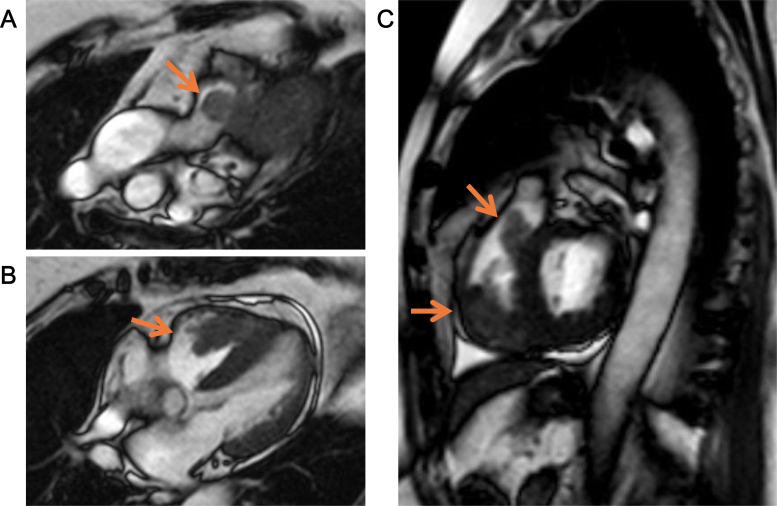
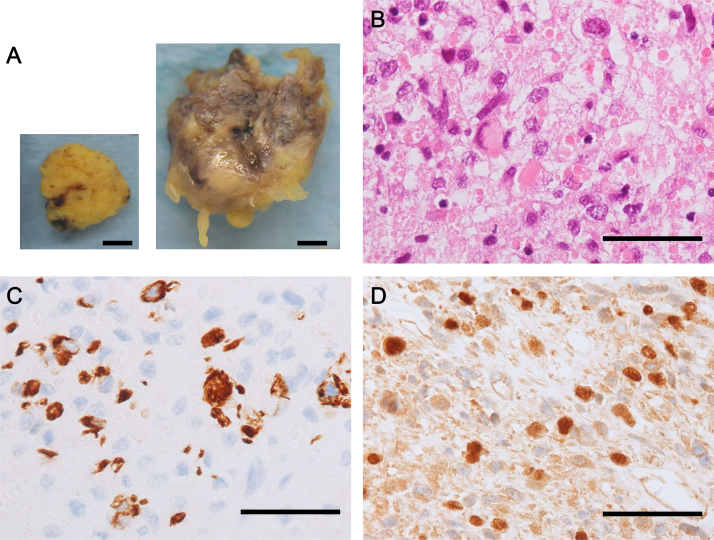
A 68-year-old female suddenly lost consciousness at a workplace. Her blood pressure was below 60 mmHg at that time. She was immediately taken to the emergency room at our hospital. After a rapid infusion of saline, her blood pressure was raised to 117/66 mmHg, and her consciousness was restored and clear. On admission, no remarkable physical finding was observed. Electrocardiogram showed a regular sinus rhythm (heart rate 70 beats/min) and no remarkable change in ST segment and T wave. Transthoracic echocardiogram showed two masses swinging in the right ventricle (RV) and its outflow tract, which were 31 × 24 mm and 22 × 17 mm in size, respectively (Fig. 1). Contrast-enhanced computed tomography showed that these tumors seemed to take root in the septal wall of the RV. There was no metastatic lesion in other organs including liver and lung. Cine magnetic resonance imaging of the heart revealed that one of the high-intensity masses on T2 STIR had almost obstructed the main trunk of the PA (Fig. 2). From these examinations, we inferred that her syncope was caused by an obstructive shock that temporarily occurred due to a transient obstruction of PA flow by the tumor. To avoid a subsequent sudden PA occlusion by the tumor, we emergently performed surgical excision of the tumors from the RV that was not a complete removal. In order to preserve RV function, we could not remove all of the tumors from the RV wall and the papillary muscles. The excised tumors were yellowish, lustrous, elastic, and had processes. In histological findings, the tumors were composed predominantly of primitive mesenchymal cells and rhabdomyoblasts. Massive necrosis was also seen. In immunohistochemical analysis, the tumor cells were positively stained for myogenin as well as desmin, muscle actin, and vimentin. These histopathological analyses provided the tumor diagnosis as embryonal type RMS (Fig. 3). This patient was additionally treated with a series of chemotherapy and radiotherapy after the surgery, and is still free of fatal tumor growth or metastasis eight months later.
Fig. 1.
Transthoracic echocardiogram and schematic illustration. (A) Four-chamber view. (B) Short-axis view (aortic valve level). The tumors are indicated by the hatched area.
RV, right ventricle; RA, right atrium; LV, left ventricle; LA, left atrium; ROVT, right ventricular outflow tract; AV, aortic valve.
Fig. 2.
Magnetic resonance imaging of the heart. (A) Sagittal section (pulmonary artery level). (B) Sagittal section (four-chamber view). (C) Sagittal section (short-axis view, interventricular septum level). The tumors are indicated by the arrows.
Fig. 3.
Macroscopic and pathological observations of the tumors. (A) Macroscopic images of the tumors in the pulmonary artery (left panel) and the right ventricle (right panel). Bar = 1 cm. (B) Hematoxylin and eosin-staining of the tumor. Bar = 50 μm. (C) Immunohistochemical staining for desmin. Bar = 50 μm. (D) Immunohistochemical staining for myogenin. Bar = 50 μm.
Eighteen years previously, this patient had left breast cancer and received radiotherapy, with chemotherapy after a mastectomy. A radiation dose of 50 Gy was delivered with 25 fractions on her mediastinum including the heart. Tamoxifen and tegafur–uracil were used as adjuvant chemotherapy. No recurrence of breast cancer had been detected until now.
Discussion
Breast cancer is the most frequent cancer among women [5]. Along with the recent progress in operative procedures, adjuvant chemotherapy and radiotherapy, prognosis has improved [5]. However, there is now also concern about the occurrence of a second cancer developed by radiotherapy. Several studies have reported increased risks of a second cancer among breast cancer patients treated with postoperative radiotherapy [3], [4]. In a Danish nationwide study of over 46,000 patients with early breast cancer, the incidence of a second cancer located in radiotherapy-associated sites, including the thorax, was significantly higher than that in non-radiotherapy sites [5]. Although only three patients had radiotherapy-associated second cancer in heart and mediastinum, the standardized incidence ratio (SIR) was calculated as 3.26, which was relatively higher than that in non-irradiated patients (SIR 0.62) in this study [5], suggesting that radiotherapy for breast cancer is associated with an increased risk of second cancer in heart and mediastinum. Considering the high incidence of breast cancer and the improved life prognosis, despite the very low incidence of second cancer in heart, the recent technology of 3D-radiotherapy should be used to avoid irradiation to normal mediastinum organs including the heart for the treatment of breast cancer.
RMS is the most common soft tissue sarcoma in children and adolescents, typically occurring in children younger than 10 years, but rarely in adults [6]. RMS occurs mainly in urogenital organs, head and neck including nasopharynx, and limbs, but very rarely in heart [6]. Embryonal RMS is the most common subtype in adults, accounting for 60% of RMS [6]. This subtype commonly has loss of heterozygosity around 11p15.5 [7], which is chromosomal damage associated with radiation, suggesting that radiotherapy might have strongly predisposed her heart to the development of embryonal RMS, although the natural incidence of RMS is extremely low in an adult heart.
Chemotherapy, especially using alkylating agents, increases the risk for a second cancer [8]. This patient was treated with adjuvant chemotherapy, using tamoxifen and tegafur–uracil, as well as radiotherapy, although these agents have not been proven to increase the risk. Other risk factors for developing soft tissue sarcoma after radiotherapy include a young age at treatment and high radiation dose [9], [10]. These factors may also be involved in the development of cardiac RMS in addition to irradiation to the heart in this patient. We should be more aware of cardiac sarcoma as a second malignant tumor in patients who received radiotherapy for breast cancer, even if they have no recurrence for more than 15 years.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The information reported in the article was presented at the 109th regional meeting of the Japanese Circulation Society in Chugoku district.
References
- 1.Silverman N.A. Primary cardiac tumors. Ann Surg. 1980;191:127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blondeau P. Primary cardiac tumors—French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38(Suppl. 2):192–195. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 3.Huang J., Mackillop W.J. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Grantzau T., Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol. 2015;114:56–65. doi: 10.1016/j.radonc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Grantzau T., Mellemkjaer L., Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiother Oncol. 2013;106:42–49. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Hayes-Jordan A., Andrassy R. Rhabdomyosarcoma in children. Curr Opin Pediatr. 2009;21:373–378. doi: 10.1097/MOP.0b013e32832b4171. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura R., Takita J., Sato-Otsubo A., Kato M., Koh K., Hanada R., Tanaka Y., Kato K., Maeda D., Fukayama M., Sanada M., Hayashi Y., Ogawa S. Characterization of genetic lesions in rhabdomyosarcoma using a high-density single nucleotide polymorphism array. Cancer Sci. 2013;104:856–864. doi: 10.1111/cas.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins M.M., Wilson L.M., Burton H.S., Potok M.H., Winter D.L., Marsden H.B., Stovall M.A. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst. 1996;88:270–278. doi: 10.1093/jnci/88.5.270. [DOI] [PubMed] [Google Scholar]
- 9.Menu-Branthomme A., Rubino C., Shamsaldin A., Hawkins M.M., Grimaud E., Dondon M.G., Hardiman C., Vassal G., Campbell S., Panis X., Daly-Schveitzer N., Lagrange J.L., Zucker J.M., Chavaudra J., Hartman O. Radiation dose, chemotherapy and risk of soft tissue sarcoma after solid tumours during childhood. Int J Cancer. 2004;110:87–93. doi: 10.1002/ijc.20002. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen A., Pukkala E., Auvinen A. Incidence of bone and soft tissue sarcoma after radiotherapy: a cohort study of 295,712 Finnish cancer patients. Int J Cancer. 2006;118:1017–1021. doi: 10.1002/ijc.21456. [DOI] [PubMed] [Google Scholar]