Graphical abstract
Keywords: Acute toxicity, Oxidative stress, Pathology, Thymoquinone, Titanium dioxide nanoparticles, Ultrastructure
Abstract
Although the nanoparticles had a beneficial activity, it had also adverse effects as a result of generation of oxidative stress. The current study aimed to assess the ameliorative effect of thymoquinone (TQ) on titanium dioxide nanoparticles (TiO2 NPs) induced acute toxicity in male rats. Forty-eight male rats were distributed into four equal groups (12 rats each). Group (1) received single oral dose of TiO2 NPs (300 mg/kg), Group (2) received TiO2 NPs and TQ (20 mg/kg), Group (3) received TQ and group (4) received only the vehicle and served as control group. TiO2 NPs intoxicated group showed increased the level of lipid peroxidation product (LPO), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and decreased the level of antioxidants and testosterone. Vascular and degenerative changes in the liver and testes were observed by light microscopy as well as presence of TiO2 NPs in the lysosomes by electron microscopy. Treatment with TQ revealed improvement of the biochemical parameters, histology and ultrastructure of the liver and testes. It was concluded that acute intoxication of rats with TiO2 NPs induced adverse effect in the liver and testes. Administration of TQ has an ameliorative effect against oxidative stress induced by TiO2 NPs intoxication.
1. Introduction
Titanium dioxide nanoparticles (TiO2 NPs) are commonly used in many applications as household necessities, industrial, medicinal products as well as food colorant and white pigment [1], [2]. Titanium dioxide nanoparticles induced genotoxicity, mutagenicity and cytotoxicity and potentially carcinogenicity [3]. TiO2 NPs are used in industrial field as cosmetics in two crystalline forms anatase and rutile [4].
Nanoparticles induced cytotoxicity, apoptosis and oxidative stress to the liver cells [5], [6]. TiO2 NPs were observed in the hepatocytes and Kupffer cells in mice [5]. Jain et al. [3] reported that TiO2 NPs induced mitochondrial swelling and disruption of the nuclear membrane in mammalian lung fibroblast cells. TiO2 NPs might distribute from the lung tissue to other body tissue and induced systemic effects [7].
Black cumin seed (Nigella sativa) is used in traditional medicine in many countries as Greece, Turkey, Egypt and many other countries in Asia and Africa [8]. Thymoquinone (TQ) is the main component of the Nigella sativa [9]. TQ has a powerful anti-inflammatory and antioxidant effects [10], [11]. Moreover, TQ has anticancer activities through targeting the carcinogenic signaling molecules and immununomodulatory activities [12].
In the present study we aimed to estimate the oxidative stress, histopathology and ultrastructure of the liver and testis following oral administration of TiO2 NPs to male rats and to assess the ameliorative effect of thymoquinone.
2. Materials and methods
2.1. Experimental design
Forty-eight male Sprague-Dawley (SD) rats of 200–230 g weight obtained from the animal house of Faculty of Medicine, Assiut University. The experiment was ethically approved by the committee of research ethics, Jazan University. Rats were acclimatized for 2 wks then divided into 4 even groups (12 rats each). Group (1) received single oral dose of TiO2 NPs (300 mg/kg b.w.) in 1% Tween 80 by stomach tube, Group (2) received Tio2 NPs and TQ daily oral dose (20 mg/kg b.w. in corn oil by stomach tube), Group (3) received TQ daily oral dose and group (4) received single dose of the vehicle (1% Tween 80) by stomach tube. All chemical in this study were purchased from Sigma-Aldrich, Germany.
2.2. Biochemical analysis
Serum samples were collected for estimation of lipid peroxidation (LPO), total antioxidants, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and testosterone levels by using commercial kits.
2.3. Histopathological analysis
Tissue samples from the liver and testis of male rats were collected after 14 days. The samples were fixed in 10% buffered formalin then routine processing, stained by hematoxylin and eosin (HE) and examined by light microscope [13].
2.4. Electron microscopy analysis
Samples from the liver and testis were obtained and immersed in gluteraldehyde solution for transmission electron microscopy (TEM). Specimens were then processed and ultrathin sections stained with uranyl acetate and lead citrate were examined by TEM (JEOL100 CXII, Japan) in the EM Unit, King Khalid University, KSA [13].
2.5. Statistical analysis
All values were presented as mean ± SE for the studied parameters and analyzed using GraphPad Prism software program.
3. Results
3.1. Biochemical results
The levels of lipid peroxidation (LPO) and liver enzyme markers (AST & ALT) were significantly increased (P ≤ 0.001) in TiO2 NPs intoxicated group compared to other groups. Conversely, testosterone and total antioxidants levels were decreased significantly. Administration of TQ recovered the serum levels of LPO, total antioxidants, AST, ALT and testosterone (Table 1).
Table 1.
Biochemical analysis of oxidative stress, liver enzymes and testosterone in the serum of rat of all groups.
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| LPO | 9.76 ± 0.14a | 6.23 ± 0.17b | 4.73 ± 0.37b | 4.90 ± 0.05b |
| TBARS | 0.38 ± 0.08b | 0.66 ± 0.03a | 0.91 ± 0.05a | 0.90 ± 0.01a |
| AST | 40.10 ± 0.4a | 27.10 ± 0.9b | 20.56 ± 0.41b | 20.36 ± 0.2b |
| ALT | 82.60 ± 2.3a | 40.8 ± 1.7b | 34.10 ± 1.06b | 32.46 ± 0.4b |
| Testosterone | 1.35 ± 0.06b | 2.60 ± 0.2a | 3.77 ± 0.1a | 3.60 ± 0.2a |
The values are presented as mean ± SE, different superscripts in the same raw were significantly different at (P ≤ 0.001).
3.2. Histopathology
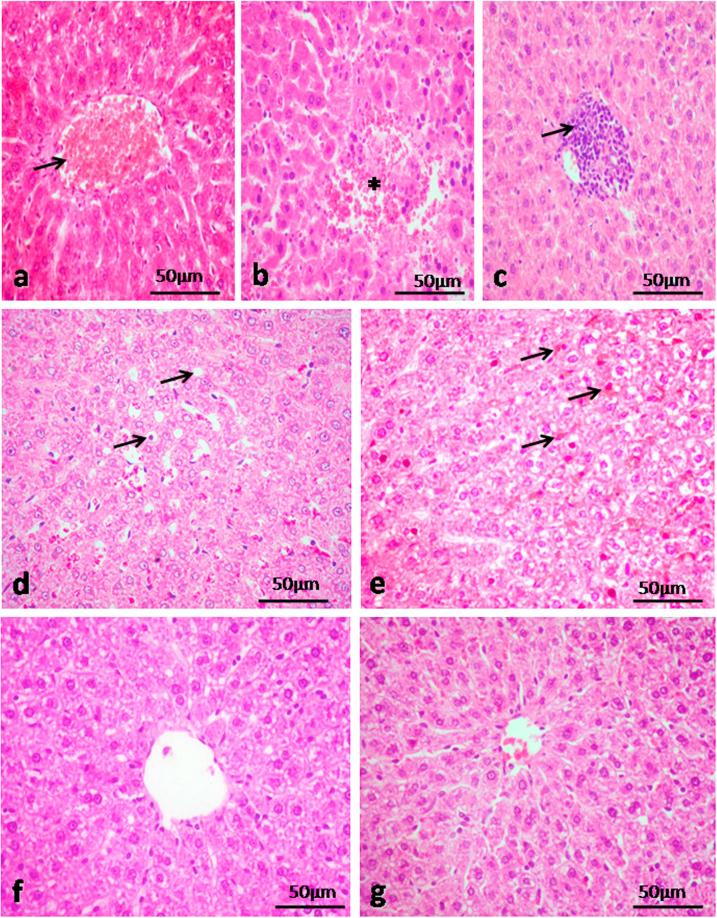
The lesion score of the histopathology of the studied groups were summarized in Table 2. Histopathological examination of the liver tissue of the rat intoxicated with TiO2 NPs revealed pathological alterations. Congestion of the central veins and dilatation of the hepatic sinusoids were observed (Fig. 1a). Focal hemorrhagic areas were observed in the hepatic parenchyma as well as coagulative necrotic changes in the hepatocytes. Proliferation of Kupffer cells were also seen (Fig. 1b). Peri-central aggregations of mononuclear cells especially lymphocytes were consistent phenomena in the examined liver in group 1 (Fig. 1c). Vacuolar degeneration of some hepatocytes was observed (Fig. 1d). Some examined sections revealed TiO2 NPs in Kupffer cells which appeared as eosinophilic particles (Fig. 1e). Examined liver section from TQ treated group showed normal architecture with dilatation of the central veins (Fig. 1f). The liver of control group showed normal histological appearance (Fig. 1g).
Table 2.
The histopathological lesions observed by light microscopy and electron microscopy in the studied groups.
| Lesions | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Liver lesions observed by Light microscope | ||||
| Hemorrhage | ++ | – | – | – |
| Congestion | ++++ | + | – | – |
| Vacuolar degeneration | ++++ | + | – | – |
| Mononuclear infiltration | +++ | – | – | – |
| Kupffer cell proliferation | +++ | – | – | – |
| Liver lesions observed by EM | ||||
| Mitochondrial swelling | ## | # | – | – |
| Fatty globules | ### | # | – | – |
| Nanoparticles in Kupffer cells and hepatocytes | ## | – | – | – |
| Testicular lesions observed by Light microscope | ||||
| Interstitial edema | ++++ | + | – | – |
| Congestion | ++++ | – | – | – |
| Degenerative changes | ++++ | – | – | – |
| Testicular lesions observed by EM | ||||
| Presence of nanoparticles | ### | – | – | – |
Presence of lesions by light microscopy expressed by; – (0 rats), + (1–3 rats), ++ (4–6 rats), +++ (7–9 rats), ++++ (10–12 rats). Presence of lesions by EM expressed by; – (0 rats), #(1 rats), ## (2 rats), ### (3 rats).
Fig. 1.
Histopathological micrographs of liver lesions intoxicated with TiO2 NPs. a) Congestion of the central vein (arrow). b) Focal area of hemorrhage (asterisk). c) Peri-central lymphocytic infiltration (arrow). d) Hepatocytes with vacuolar degeneration (arrows). e) Kupffer cells loaded with TiO2 NPs (arrows). f) Liver of treated group with TiO2 NPs and TQ revealed normal architecture. g) Liver of control group showing normal histological appearance. H&E.
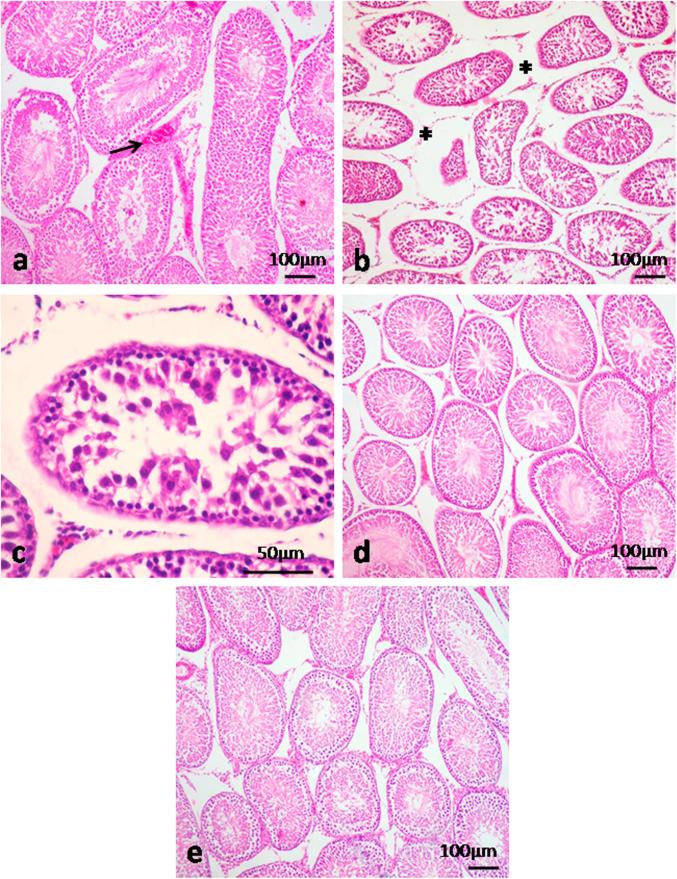
Examination of testis in rats intoxicated with TiO2 NPs revealed congestion of the blood vessels (Fig. 2a). Interstitial edema was observed and the seminiferous tubules appeared atrophied. The seminiferous tubules showed degenerative changes in which Sertoli cells appeared necrosed with few germinal cells (Figs. 2b, c). TQ reduced the histopathological alteration induced by TiO2 NPs (Fig. 2d). No histopathological changes could be seen in control group (Fig. 2e).
Fig. 2.
Histopathological micrographs of testicular lesions intoxicated with TiO2 NPs. a) Congested bl.vs (arrow). b) Interstitial edema (asterisks), testicular degeneration. c) Higher magnification showing few germ cells and degenerated Sertoli cells. d) Testis of treated group with TiO2 NPs and TQ revealed normal seminiferous tubules. e) Testis of control group showing normal seminiferous tubules. H&E.
3.3. Transmission electron microscopy
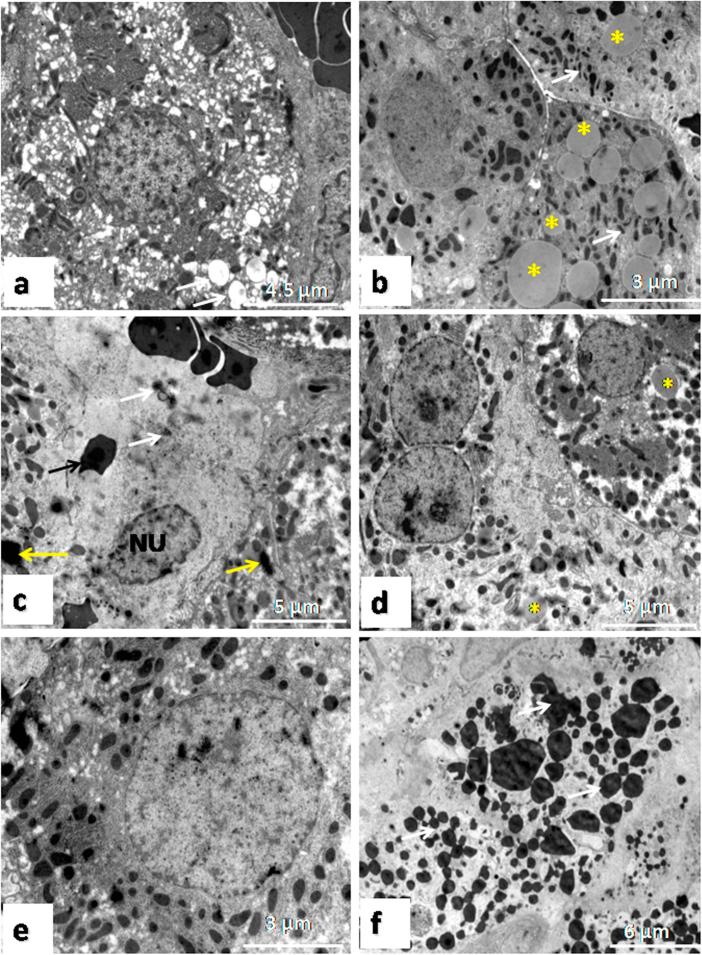
The lesion score of EM examination in the studied groups were summarized in Table 2. With ultrathin sections, the cytoplasm of hepatocytes intoxicated with TiO2 NPs revealed mitochondrial swelling (Fig. 3a). Numerous intracytoplasmic fatty globules of variable sizes were observed associated with pyknotic mitochondria with intense metrical condensation (Fig. 3b). Kupffer cells revealed several lysosomes loaded with TiO2 NPs which appeared electron dense granules either minute or compact particles. These particles were also seen in the cytoplasm of hepatocytes (Fig. 3c). Examined hepatocytes from TQ treated group showed normal nucleus and cyptoplasmic organelles as well as few intracytoplasmic fatty globules (Fig. 3d). The hepatocytes from control group showed normal nucleus and cytoplasmic organelles (Fig. 3e). Examination of the ultrathin section of seminiferous tubules in TiO2 NPs group showed abundant lysosomes loaded with TiO2 NPs which appeared as electron dense material of variable sizes (Fig. 3f).
Fig. 3.
Electron micrographs of liver lesions intoxicated with TiO2 NPs. a) Mitochondrial swelling (arrows). b) Numerous fatty globules (asterisks), mitochondrial pyknosis (white arrow). c) Kupffer cell phagocytozed RBCs (black arrow), TiO2NPs (white arrow) and the particles in the hepatocytes (yellow arrow). d) A hepatocyte of treated group with TiO2 NPs and TQ revealed normal nucleus and cytoplasmic organelles and presence of fatty globules (asterisks). e) A hepatocyte of control group showing normal nucleus and cytoplasmic organelles. f) Testicular lesions intoxicated with TiO2 NPs showing numerous nanopaticles in the seminiferous tubule (arrows).
4. Discussion
The nanoparticles might be valuable in drug delivery due to it's highly penetration to the cells. Conversely, detrimental effects of nanoparticles induced oxidative stress and impaired antioxidant activity. Several studies revealed that TiO2 NPs can be accumulated in the parenchymatous organs generating inflammatory reactions [14], [15].
In the current study, the group of rats intoxicated with TiO2 NPs showed significant increased of LPO, AST and ALT and decreased the total antioxidant compared to the treated and control group. Many authors reported that TiO2 NPs increased the oxidative stress indices and liver enzyme markers [4], [6], [16].
Histopathological examination of the liver showed congestion, hemorrhage and degenerative changes in the hepatocytes as well as mononuclear cell infiltration. Similar results obtained by Hassanein and El-Amir [11] and Xu et al. [17]. The hepatocelluar changes were confirmed by EM which revealed mitochondrial swelling, mitochondrial pyknosis, fatty globules and presence of TiO2 NPs in the lysosomes of Kupffer cells and hepatocytes. Similarly, Jeon et al. [5] found that TiO2 NPs in the Kupffer cells as electron dense granular mass. The presence of the nanoparticles within the lysosomes may be due to nonspecific defense mechanism against foreign substance [18]. Also, Jain et al. [3] found nuclear membrane disruption and mitochondrial swelling following administration of TiO2 NPs to the lung fibroblast cells. Cheng [19] reported that acute toxicity with zinc oxide nanoparticles (ZnO NPs) induces mitochondrial pyknosis and damage as a result of cell death. Moreover, mitochondrial pyknosis was observed in copper-induced toxicity in sheep. This occurs due to mitochondrial oxidative injury [20].
In this study, TiO2 NPs intoxicated group showed low serum level of testosterone compared to control group. Light microscopic examination of the testes revealed congestion of the blood vessels and testicular degeneration and presence of nanoparticles by EM. In parallel, Hong et al. [21] found that TiO2 NPs decreased the process of spermatogenesis and testosterone levels. Shi et al. [22] suggested that the presence of TiO2 NPs in the testis resulted from its penetration of the blood-testis barrier.
The group of rats treated with TQ revealed significantly decrease in the level of LPO, AST and ALT as well as significantly increase the level of testosterone and total antioxidants. Minimal histopathological and ultrastructural changes were observed in the liver and testicular tissues. Similarly, Hassanein and El-Amir [11] demonstrated that TQ improved the levels of liver enzymes, oxidative stress as well as improvement of the histology of the liver and testis in rats chronically intoxicated with TiO2 NPs. TQ is a free radical scavenger because of its strong antioxidant activity [23].
5. Conclusions
The rats intoxicated with TiO2 NPs for 14 days revealed oxidative stress associated with hepatocelluar and testicular damage. Thymoquinone restored the adverse effects resulted from TiO2 NPs induced toxicity. Hence, the ameliorative effect of TQ may be related to powerful antioxidant capability.
Competing interests
All authors declare no competing interests.
Acknowledgements
The present study was financially supported by Jazan University, KSA (research project number: JU6-3747).
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Medina C., Santos-Martinez M.J., Radomski A. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Luo M., Tan Z., Dai M., Xie M., Lin J. Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ Toxicol Pharmacol. 2017;49:112–118. doi: 10.1016/j.etap.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Jain A.K., Senapati V.A., Singh D., Dubey K., Maurya R., Pandey A.K. Impact of anatase titanium dioxide nanoparticles on mutagenic and genotoxic response in Chinese hamster lung fibroblast cells (V-79): the role of cellular uptake. Food Chem Toxicol. 2017;105:127–139. doi: 10.1016/j.fct.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 4.De Matteis V., Cascione M.F., Brunetti V., Toma C.C., Rinaldi R. Toxicity assessment of anatase and rutile titanium dioxide nanoparticles: the role of degradation in different pH conditions and light exposure. Toxicol Vitro. 2016;37:201–210. doi: 10.1016/j.tiv.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Jeon J.M., Kim W.J., Lee M.Y. Studies on liver damage induced by nanosized-titanium dioxide in mouse. J Environ Biol. 2013;34:283–287. [PubMed] [Google Scholar]
- 6.Ammendolia M.G., Caiosi F., Maranghi F., Cubadda F., Raggi A., Superti F. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem Toxicol. 2017;102:63–75. doi: 10.1016/j.fct.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Gaté L., Disdier C., Cosnier F., Gagnaire F., Devoy J., Saba W. Biopersistence and translocation to extrapulmonary organs of titanium dioxide nanoparticles after subacute inhalation exposure to aerosol in adult and elderly rats. Toxicol Lett. 2017;265:61–69. doi: 10.1016/j.toxlet.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Salih B., Sipahi T., Dönmez E.O. Ancient nigella seeds from Boyali Höyük in north-central Turkey. J Ethnopharmacol. 2009;124:416–420. doi: 10.1016/j.jep.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Ghosheh O.A., Houdi A.A., Crooks P.A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.) J Pharm Biomed Anal. 1999;19:757–762. doi: 10.1016/s0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 10.Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastricmucosal damage. Arch Toxicol. 2006;80:217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 11.Hassanein K.M.A., El-Amir Y. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol Res Pract. 2017;213:13–22. doi: 10.1016/j.prp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Khan M.A., Tania M., Fu S., Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8:51907–51919. doi: 10.18632/oncotarget.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bancroft T.D., Stevens A., Turner D.R. 4th ed. Churchill Livingstone; New York, USA: 1996. Theory and practice of histological technique; pp. 113–139. [Google Scholar]
- 14.Brown J.S., Zeman K.L., Bennett W.D. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166:1240–1247. doi: 10.1164/rccm.200205-399OC. [DOI] [PubMed] [Google Scholar]
- 15.Attia H.F., Soliman M.M., Abdel-Rahman G.H., Nassan M.A., Ismail S.A., Farouk M. Hepatoprotective effect of N-acetylcystiene on the toxic hazards of titanium dioxide nanoparticles. Am J Pharmacol Toxicol. 2013;8:141–147. [Google Scholar]
- 16.Abdel Azim S.A., Darwish H.A., Rizk M.Z., Ali S.A., Kadry M.O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol. 2015;67:305–314. doi: 10.1016/j.etp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Shi H., Ruth M., Yu H., Lazar L., Zou B. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One. 2013;8:e70618. doi: 10.1371/journal.pone.0070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X., Wu Y., Jin S., Tian Y., Zhang X., Zhao Y. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;22:8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C. St John's College, Department of Engineering, University of Cambridge; 2009. Toxicology of nanoparticles after cellular uptake. Dissertation submitted for the degree of Doctor of Philosophy. [Google Scholar]
- 20.Haywood D., Simpson M., Ross G., Beynon R.J. The greater susceptibility of North Ronaldsay sheep compared with Cambridge sheep to copper-induced oxidative stress, mitochondrial damage and hepatic stellate cell activation. J Comp Pathol. 2005;133:114–127. doi: 10.1016/j.jcpa.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Hong F., Si W., Zhao X., Wang L., Zhou Y., Chen M. TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J Agric Food Chem. 2015;63:7084–7092. doi: 10.1021/acs.jafc.5b02652. [DOI] [PubMed] [Google Scholar]
- 22.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:1–33. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo C.C., Kumar A.P., Sethi G., Tan K.H. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]