Highlights
-
•
Subclinical infection with Eimeria spp. was detected in sheep (57.7%) and goats (60%).
-
•
Infected sheep serum levels of protein and sodium showed a significant decrease.
-
•
Extracted DNA from sporulated oocysts was successfully amplified at 100 bp band using PCR.
Keywords: Egypt, Eimeria, PCR, Small ruminants, Subclinical
Abstract
Coccidiosis is a disease of high economic importance caused by Eimeria species that show ubiquitous distribution among several species including small ruminants. The prevalence of Eimeria infection in sheep and goats in Geneffe village, Suez Governorate, Egypt was determined during the period from March 2015 to February 2016. Total of 277 animals (142 sheep and 135 goats) were clinically examined and fecal samples were collected and tested both microscopically and by PCR. Sera samples of sheep and goats under 1 year were collected for biochemical analysis. Results revealed that (60%) of goats and (57.70%) of sheep were suffering from subclinical coccidiosis. Adult female goats were significantly (P < 0.05) more infected (82.2%) than adult male goats (40%). Eimeria infection was significantly prevalent in summer (75%) and autumn (74.2%) in sheep than winter (38.2%) and spring (43.2%), while goats did not show significant seasonal variations of infection. The Eimeria species were identified as E. crandallis, E. granulosa, E. ovina, E. parva, E. faurei, E. ovinoidalis, E intricate, E. pallida, E. arloingi, and E. ahasta in sheep, and E. ninakohlyakimovae, E. hirci, E. caprina, E. christenseni, E. jolchijevi, E. apsheronica and E. arloingi in goats. Although animals were subclinically infected with coccidia, some significant biochemical changes were observed in serum samples of sheep and goats. The molecular detection of Eimeria oocysts did not yield any positive results but after sporulation, Eimeria oocysts were detected at zone 100 bp. Our results showed a moderate prevalence of Eimeria infection among adult and yearling sheep and goats in Geneffe village. Suez governorate, Egypt.Hence, good control and prevention programs are necessary.
1. Introduction
Coccidiosis (Eimeriosis sensu stricto) is a protozoan infection caused by parasites of the genus Eimeria that develop and propagate in the small and the large intestines of animals and affect young age particularly [1]. Coccidiosis is a serious disease of small ruminants in Egypt [2] as well as in various parts of the world and in different animal species [3], [4], [5] which emphasize the importance of further studies for better control and prevention programs.
Small ruminants from all ages and breeds are susceptible to Eimeria infection, however, lambs from 3 weeks to 5 months of age are most severely affected by outbreaks of coccidial infection, while the rest of the flock might act as carriers [6]. Coccidiosis is clinically characterized by diarrhea which can be hemorrhagic in adult sheep [7] while kids or lambs suffer from watery diarrhea with clumps of mucous and occasional changes in the color of feces to yellow or brown [8]. In the subclinical form, impairment of growth is the main sign, and reduced milk production in dairy goats has also been recorded [9]. Under intensive breeding systems which accompanied by high animal density and high productivity, coccidiosis may become an infection of significant economic importance which might lower thriftiness and productivity of small ruminants [7].
Surveys based on the examination of ruminant feces have shown that most animals are infected with a wide variety of Eimeria species from an early age [10], [11]. Eimeria diagnosis usually depends on morphological detection of the oocysts by light microscope. That method encompasses several shortcomings including time and effort. It depends on the skill and experience of the examiner. Recently, modern molecular techniques have been deployed for Eimeria identification including PCR amplification technique [12]. Nevertheless, the main issue with the PCR procedures is that the Eimeria oocyst wall is extremely robust in addition to the low DNA concentration obtained especially if the oocysts numbers are low in subclinical cases, thus a successful DNA extraction from oocysts is imperative for reliable PCR amplification and detection of Eimeria [13].
Subclinical coccidiosis is common among small ruminants [14] and the adverse effects of subclinical coccidiosis on animal health and productivity justify the need for screening. Based on our knowledge there is no study of prevalence of this infection in the rural sector of Suez Governorate, Egypt. Therefore, the purposes of this study were to investigate the prevalence of Eimeria infection in sheep and goat in Geneffe village, one of the most important and biggest villages in Suez Governorate, Egypt, and to identify the Eimeria species. Attempts were also made to determine the prevalence of infection in relation to age, sex and season, and to elucidate the effect of infection itself on the health condition and biochemical parameters. Finally, to adopt a recent approach for the molecular detection of Eimeria oocysts.
2. Materials and methods
2.1. Study sites
The study was conducted in Geneffe village at the Northern border of Suez Governorate (30°14′52.4″ N 32°24′55.1″ E), Egypt which constitutes the main village of the rural sector of Suez Governorate. This one-year survey was conducted from the beginning of March 2015 till the end of February 2016. The study was conducted according to ethical guidelines approved by ethics of scientific research committee, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
2.2. Clinical examination and sample collection
The study was targeting apparently healthy sheep and goats in unorganized farms at different locations in Geneffe village. Animals were routinely released for grazing during the day time and sheltered at night in small buildings (Partially covered system). Animals were given ad Lib. access to water. Sheep and goats of more than 6 months old were randomly selected using random sampling procedure from the different farms in Geneffe village from March 2015 to February 2016. Sampling was carried out during the four seasons (Spring, Summer, Autumn, and Winter) throughout the year. Apparently diseased animals, animals receiving treatment or animals in the withdrawal period were excluded.
Fecal samples were collected from 142 sheep and 135 goats. The number of sampled animals was calculated using Solvin's depending upon the exact number of animals in each population. The fecal samples were collected once directly from the rectum using gloves and stored at 4 °C until being examined.
2.3. Recovery and species morphological detection and identification of Eimeria oocysts
The fecal samples were examined by flotation technique using saturated saline and oocysts per gram (OPG) quantified using modification of McMaster [15]. Each sample was performed in triplicates, and the oocysts number of every sample was expressed using McMaster slide multiplied by the dilution factor (×100) to express the OPG. The final results of each sample were obtained using the mean value of three independent examinations. After examination, the purified oocysts recovered from each sample were transferred into 2.5% (w/v) aqueous potassium dichromate solution to be sporulated at 26–33 °C in a wet chamber. The species identification of oocysts was performed based on the time of sporulation Coudert’s key, and oocysts sizes and morphology (shape, color, form index, presence or absence of micropyle and its cap, presence or absence of residual, polar and stieda bodies) of the oocysts and sporocysts under 400X magnifications [8], [16].
2.4. Serum analysis
Five milliliter blood were collected from the jugular vein of the young sheep and goats (less than one year) that were divided into 2 groups; infected group with Eimeria species and control group which included Eimeria-free animals. Samples were centrifuged at 3000 rpm for 20 min to obtain serum which were stored at −20 °C and analyzed for total protein, albumin, sodium, potassium, calcium and inorganic phosphorous using colorimetric kits (Millipore, Sigma, USA) and measured by spectrophotometer (sp-756 spectrum instruments, Shanghai Spectrum Instruments Co., Ltd., Shanghai, China).
2.5. Molecular detection of the Eimeria oocysts
2.5.1. Fecal samples
A total of 8 positive fecal samples were further processed for molecular detection, which were previously examined under light microscope. Samples were kept at −20 °C until processed for DNA extraction.
2.5.2. DNA extraction
2.5.2.1. DNA Extraction from the fecal samples
DNA was extracted from the fecal samples using Genomic DNA Purification kit (Applied biotechnology, USA) following manufacturer’s instructions. Briefly, fecal samples were incubated for 20 min at 65 °C in 200 µL of Nuclei Lysis Solution, plus 40 µL of a 20 mg/mL Proteinase K solution, and for the optimal extraction of DNA from, the fecal samples were homogenized utilizing the ceramic beads, tubes were centrifuged at 10,000×g to eliminate debris. Supernatants were collected and 100 µL of isopropanol were added to each sample. The mixture was run through filter columns at 13,000×g for 1 min. DNA bound to filter was washed and eluted following manufacturer instructions. The extracted DNA concentration and purity were measured using a NanoDrop® spectrophotometer (NanoDrop Technologies, Inc. Wilmington, DE, USA) as described above and stored at −20 °C until use.
2.5.2.2. DNA Extraction from the sporulated oocyst
The Eimeria oocysts were sporulated by potassium dichromate 2.5% as mentioned before, pelleted and washed then the sporulated oocytes were centrifuged. DNA was extracted from the pellets as previously described (check Section 2.5.2) using Genomic DNA Purification kit (Applied biotechnology, USA) following manufacturer’s instructions.
2.5.3. Molecular detection of the Eimeria oocysts from fecal samples based on ITS-1 rDNA gene
The ITS-1 DNA from each sample was amplified using conventional PCR and the cycling conditions and primer sequences of the forward and reverse primers were as follows: Forward 5′-GCAAAAGTCGTAACACGGTTTCC-3′, Reverse: 5′-CTGCAATTCACAATGCGTATCG-3′ [12], cycling conditions were: initial denaturation at 94 °C for 5 min followed by 35 cycles of 94 °C for 45 s, 55 °C for 1 min and 72 °C for 1 min, this was followed by a final extension at 72 °C for 7 min. The amplification products from ITS-1 rDNA were separated on 1.6% agarose gel containing 0.4 µg/mL of ethidium bromide at 90 V for 40–60 min, and then imaged.
2.6. Statistical analysis
SPSS 22.00 software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) was used for the statistical analysis. Chi-square test was employed to test the significance of differences among proportions. Independent t-test was performed to test the significance between infected and control groups. The level of significance at which the null hypothesis was rejected was α = 0.05.
3. Results
3.1. Detection and morphological identification of coccidian oocysts
In the present study, the total number of sheep examined was 142 (66 less than 1 year and 76 adult) and for goats the total number was 135 (60 less than 1 year and 75 adult). The prevalence of Eimeria sp. infection in Geneffe village was found in 81 goats (60%) and 82 sheep (57.7%). Mixed infections were found in 25 of 36 examined lambs (69.4%) and 31 of 46 examined adult sheep (67.4%) as well as in 20 of 27 examined kids (74%) and 43 of 54 examined adult goats (79.4%) of the positive specimens while the rest of positive samples of each category of sheep and goats were single infection as shown in Fig. 1. Table 2 shows that prevalence of infection was significantly higher in adult does (82.2%) rather than bucks (40%) (P < 0.05). Although the prevalence of infection was relatively higher in ewes compared to rams, there was no significant difference between them. Meanwhile there was no significant difference between males and females in the prevalence of Eimeria infection in sheep and goats less than 1 year.
Fig. 1.
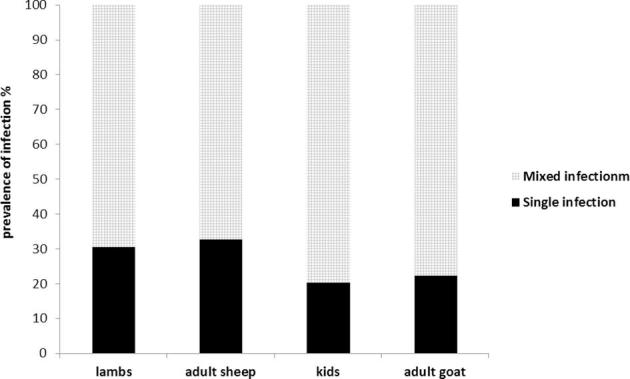
Prevalence of single and mixed infection in relation to the age of sheep and goats.
Table 2.
The prevalence of Eimeria infection in the examined sheep and goats in relation to age and sex.
| Animals groups | Examined animals | Positive No. | Prevalence% | Males |
Females |
||
|---|---|---|---|---|---|---|---|
| Positive no. | Prevalence % | Positive no. | Prevalence % | ||||
| Lambs | 66 | 36 | 54.5 | 17/30 | 56.6% | 19/36 | 52.7% |
| Adult sheep | 76 | 46 | 60.5 | 13/32 | 40.6% | 37/45 | 73.3% |
| Kids | 60 | 27 | 45 | 15/28 | 73.3% | 12/35 | 37.5% |
| Adult goats | 75 | 54 | 72 | 12/30 | 40% | 37/45* | 82.2%* |
Superscript indicates the significant difference P < 0.05.
Fecal examination and McMaster method revealed that infection of sheep and goats was about 700 oocysts/g (±200) which indicated a subclinical infection except in 8 cases of goats which revealed higher infestation of 11,000 oocysts/g (±300). According to oocysts sizes and morphological characteristics of the oocysts and sporocysts, ten species of Eimeria were found (E. crandallis, E. granulosa, E. ovina, E. parva, E. faurei, E. ovinoidalis, E intricate, E. pallida, E. arloingi, and E. ahasta) in sheep. While in goat seven species have been found and identified as E. ninakohlyakimovae, E. hirci, E. caprina, E. christenseni, E. jolchijevi, E. apsheronica, and E. arloingi. In sheep, the most common Eimeria species were E. ahasta (30.48%), E. crandalis (30.48%) and E. ovina (26.82%). In goats, E. arloingi (37.04%), E. ninakohlyakimovae (30.86%) and E. hirci (24.69%) were the most common [Table 1 and Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6].
Table 1.
The prevalence of the different Eimeria spp. in positive sheep and goats.
| Eimeria species | Sheep (n = 82) |
Goats (n = 81) |
||
|---|---|---|---|---|
| Positive samples | Prevalence % | Positive samples | Prevalence % | |
| Eimeria ovina | 22 | 26.82% | – | – |
| E. parva | 15 | 18.29% | – | – |
| E. pallida | 11 | 13.41% | – | – |
| E. granulosa | 12 | 14.63% | – | – |
| E. ahasta | 25 | 30.48% | – | – |
| E. ovinoidalis | 10 | 12.19% | – | – |
| E. faurei | 15 | 18.29% | – | – |
| E. crandalis | 25 | 30.48% | – | – |
| E. intricate | 6 | 7.31% | – | – |
| E. ninakohlyakimovae | – | – | 25 | 30.86% |
| E. hirci | – | – | 20 | 24.69% |
| E. caprina | – | – | 14 | 17.28% |
| E. christenseni | – | – | 13 | 16.05% |
| E. jolchijevi | – | – | 10 | 12.35% |
| E. apsheronica | – | – | 13 | 16.04% |
| E. arloingi | – | – | 30 | 37.04% |
Fig. 2.
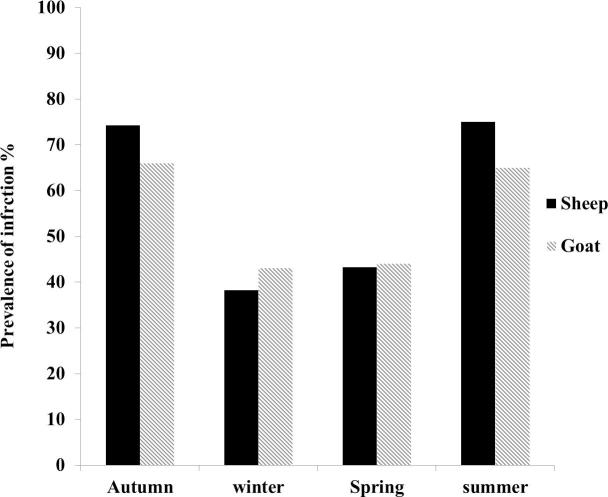
Prevalence of Eimeria infection in relation to season in sheep and goats.
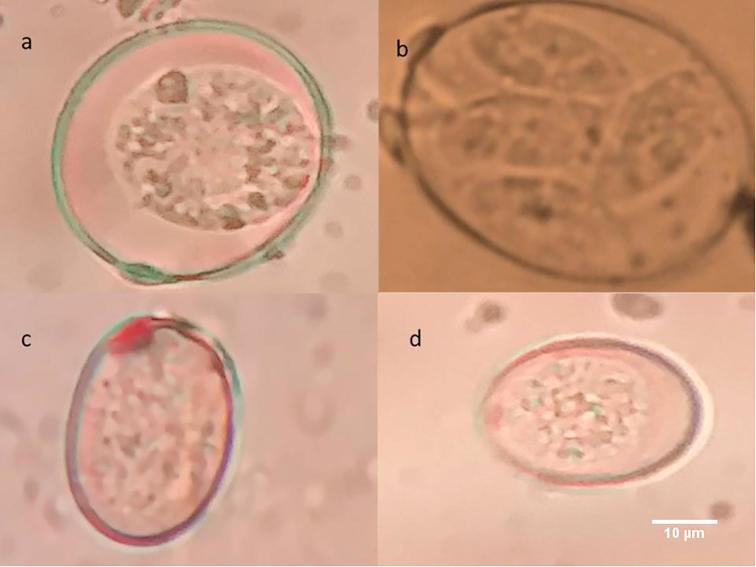
Fig. 3.
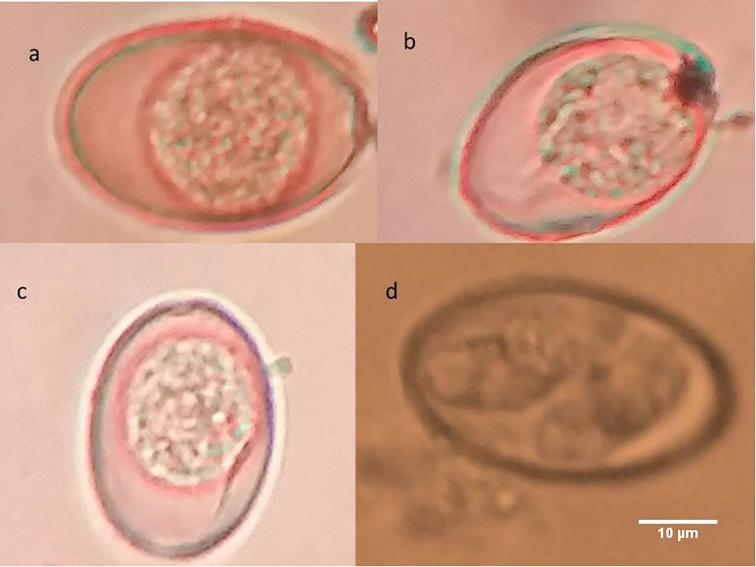
Eimeria oocysts from sheep. (a) E. ahsata; (b) E. ahsata sporulated oocyst; (c) E. crandallis; (d) E. ovinoidalis.
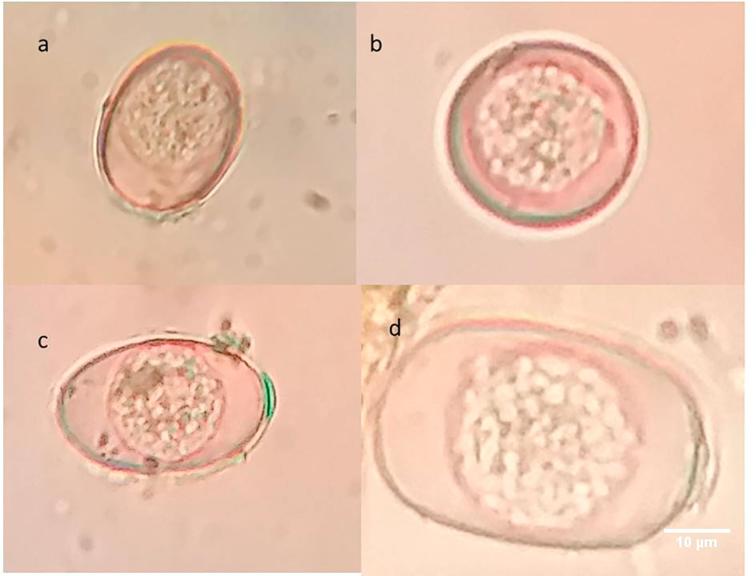
Fig. 4.
Eimeria oocysts from sheep. (a) E. pallida; (b) E. parva; (c) E. granulosa; (d) E. arloingi.
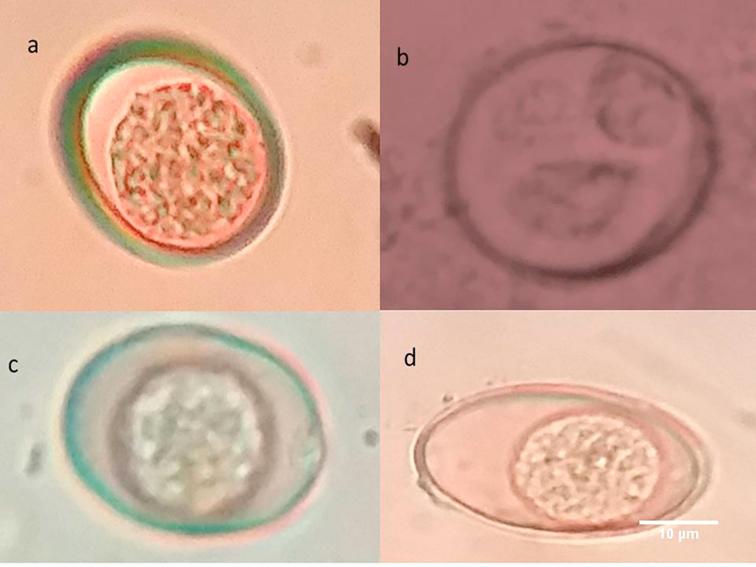
Fig. 5.
Eimeria oocysts from goat. (a) E. ninakohlyakimovae; (b) E. ninakohlyakimovae sporulated oocyst; (c) E. hirci; (d) E. caprina.
Fig. 6.
Eimeria oocysts from goat. (a) E. christenseni; (b) E. jolchijevi; (c) E. apsheronica; (d) E. apsheronica sporulated oocyst.
About (74.2%), (38.2%), (43.2%), (75%) of sheep (Fig. 2) and (66%), (43%), (44%) and (65%) of goats were infected with Eimeria in autumn, winter, spring and summer, respectively. Investigation of seasonal dynamic of infection with Eimeria species showed that the proportion of infection in sheep and goats in autumn and summer were greater than in winter and spring especially in sheep which recorded a significant difference at P < 0.05 (Fig. 2).
3.2. Clinical signs and serum biochemical findings
Although no specific clinical signs of acute coccidiosis were exhibited by the infected animals, impairment of growth, especially in goats, was the main sign of subclinical coccidiosis. Sheep were apparently healthy, alert, with good appetite, but some cases showed erect, dull and lusterless wool coat.
Serum findings of sheep and goats less than 1 year are shown in Table 3, Table 4. Table 3 displays a significant decrease in the serum levels of total protein (P = 0.004) and sodium (P = 0.005) in the infected sheep than in the control group, meanwhile infected goat showed a significant increase in the potassium serum levels (P = 0.049) and a significant decrease in calcium levels (P < 0.001). Other serum biochemical parameters did not reveal any significant difference between infected and control neither in sheep nor in goats less than 1 year.
Table 3.
Serum biochemical findings in infected and control sheep less than one year.
| Biochemical parameters | Infected | Control | t value | P value |
|---|---|---|---|---|
| Total protein (g/dL) | 6.28 ± 0.21a | 7.40 ± 0.27b | 3.195 | 0.004 |
| Albumin (g/dL) | 5.86 ± 0.60a | 5.59 ± 0.55a | 0.316 | 0.755 |
| Sodium (mEq/L) | 107.63 ± 9.97a | 142.95 ± 5.54b | 3.097 | 0.005 |
| Potassium (mmol/L) | 9.78 ± 1.29a | 10.81 ± 1.46a | 0.528 | 0.603 |
| Calcium (mg/dL) | 7.50 ± 1.00a | 7.02 ± 1.15a | 0.311 | 0.759 |
| Inorganic phosphorus (mg/dL) | 9.60 ± 0.66a | 9.77 ± 1.24a | 0.128 | 0.90 |
Means within the same row with different superscripts are significant at P ≤ 0.05.
Table 4.
Serum biochemical findings in infected and control goats less than one year.
| Biochemical parameters | Infected | Control | t value | P value |
|---|---|---|---|---|
| Total protein (g/dL) | 7.66 ± 0.36a | 8.08 ± 0.07a | −0.70 | 0.49 |
| Albumin (g/dL) | 4.10 ± 0.19a | 4.64 ± 0.90a | −0.86 | 0.40 |
| Sodium (mEq/L) | 135.00 ± 3.75a | 134.52 ± 0.50a | 0.09 | 0.93 |
| Potassium (mmol/L) | 6.75 ± 0.58b | 4.87 ± 0.23a | 2.22 | 0.049 |
| Calcium (mg/dL) | 8.33 ± 0.10a | 9.14 ± 0.10b | −5.88 | 0.00 |
| Inorganic phosphorus (mg/dL) | 6.74 ± 0.72a | 7.06 ± 0.65a | −0.29 | 0.78 |
Means within the same row with different superscripts are significant at P ≤ 0.05.
3.3. Molecular detection of the Eimeria oocysts
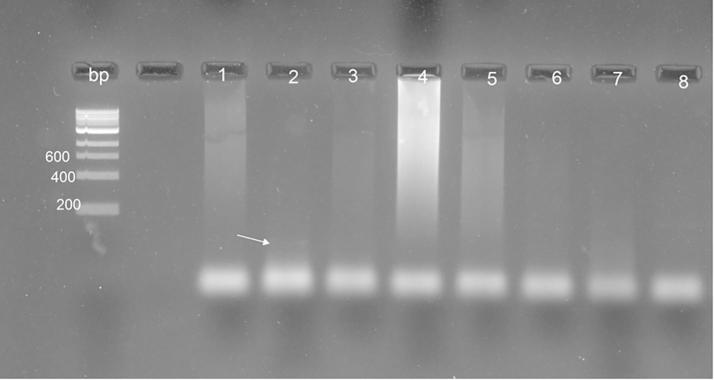
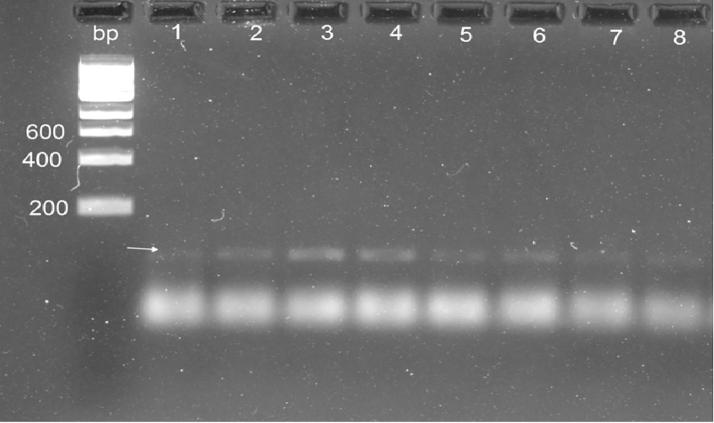
In spite of 8 morphological positive fecal fresh samples were amplified with PCR, only one sample (No. 2) was observed as positive and there was no amplification in the other samples (Fig. 7). After sporulation of the Eimeria oocysts (Fig. 8), all the samples yielded positive bands and were amplified at the size of nearly 100 bp.
Fig. 7.
PCR amplification utilizing fecal samples using the ITS-1PCR reaction DNA ladder is located on the left side of the gel, fragment sizes are represented in base pairs (bp); 1:8 fecal samples.
Fig. 8.
PCR amplification utilizing sporulated Eimeria oocysts using the ITS-1PCR reaction DNA ladder is located on the left side of the gel, fragment sizes are represented in base pairs (bp); 1:8 fecal samples.
4. Discussion
The present study shows moderate prevalence of Eimeria in the Geneffe village, Suez Governorate, Egypt although the arid climatic conditions in this region (Temperature; Min. 15 and Max. 40 °C; humidity Min. 20 and Max. 42%) prevail. To the extent of our knowledge there is no study of prevalence of Eimeria infection in this area, therefore, we conducted this study to determine the most prominent species of Eimeria in sheep and goats in one of the biggest villages in the rural sector of Suez Governorate, and to clarify the effect of age, gender and season on the prevalence of infection. Moreover, knowing the current situation and prevalence of coccidiosis might be beneficial to set an appropriate control strategy and lessen the economic losses.
The current findings revealed that the prevalence of Eimeria species infection reached 57.7% and 60% in inspected sheep and goats respectively referring to a moderate coccidian infection among sheep and goats in this area. Ten species from sheep were identified E. crandallis, E. granulosa, E. ovina, E. parva, E. faurei, E. ovinoidalis, E intricate, E. pallida, E. arloingi, and E. ahasta and seven species from goat including E. ninakohlyakimovae, E. hirci, E. caprina, E. christenseni, E. jolchijevi, E. apsheronica, and E. arloingi. These species were also recorded in Egypt by El-Magdoub et al. [17] as well as in sheep of other countries in the Middle East which have similar climatic conditions such as in Kuwait [5] and North Jordan [18]. We found that the most prevalent Eimeria species in sheep were E. ahasta (30.48%), E. crandalis (30.48%) and E. ovina (26.82%) and these findings are consistent with results of Wang et al. [16] on sheep in China. E. arloingi (37.04%), E. ninakohlyakimovae (30.86%) and E. hirci (24.69%) were the most recognized in goats’ feces. These results are similar to results in Turkey on goats by Deger et al. [19] who identified E. arloingi (47.43%), E. christenseni (45.14%), E. ninakohlyakimovae (36.00%), E. alijevi (26.85%), E. hirci (23.42%), E. caprina (18.28%) and E. caprovina (16.57%) in a previous study. Mixed infections were found in high percentage than single infection with no significant difference in both sheep and goats. That was reported previously [20].
E. ovinoidalis, E. ovina, E. crandallis and E. ahsata have been reported as pathogenic species in sheep [21], [22], and E. christenseni, E. arloingi, E. ninakohliyakimova and E. caprina [15], [23], [8] are pathogenic in goats. In the present survey, no clinical signs of coccidiosis were observed neither in sheep nor goats. We found that sex and age had an influence on the prevalence of infection with coccidiosis in adult female goats, which were significantly higher than adult male goats; which is consistent with the findings of Rehman et al. [6] and Kheirandis et al. [20] from goats in Iran. Also, there was no significant difference in the prevalence of infection between sheep males and females [24]. In this work, summer and autumn are the most seasons where Eimeria infection is more likely to occur. It might be because summer where the climate there is very hot and that might be a stressful factor to the animals that leads to more shedding of the protozoa while autumn shows an increased level of infection due to the humidity which is more favorable for sporulation of oocysts [25]. Infection with Eimeria is considered to be a risk factor when the infected animals are exposed to any stress condition such as transportation or underfeeding and the associated ailments are likely to perturb the immune system, where Eimeria species could propagate intensively in the intestine and result in outbreak [1].
In the present study, sheep under 1 year appeared alert, with good appetite but showed lusterless wool coat as well as they were suffering from a decrease in the total protein and sodium levels in the serum, meanwhile infected goats under 1 year showed signs of poor growth rate. These changes could be attributed to the fact that Eimeria usually affects the colon and small intestine of the host and induce histopathological lesions, loss of surface epithelial cells and villous atrophy associated with first-generation schizonts, and crypt destruction or hyperplasia associated with gamonts [26]. These lesions lead to loss of adsorptive surface or capacity of intestine consequently which affect proper absorption of proteins and decrease absorption of electrolytes such as sodium and other essential elements that are necessary for growth and the healthy appearance of lambs and kids [27]. Also, infected goats recorded a decrease in calcium alongside an increase in potassium serum levels. We may attribute these mineral biochemical changes in serum of goats to other than a direct pathological effect of the coccidiosis infection. We can’t find a reasonable link between Eimeria infection and changes that took place in calcium serum levels in goats.
Morphological detection and identification by flotation technique using saturated saline was effective for Eimeria detection despite the need for competent experience, time and effort [13]. This study aimed to detect the molecular amplification of the Eimeria using the ITS-1 rDNA had proven efficient results as well as it reduced time and effort [28].
In our study where most cases were sub-clinically infected, the PCR results were highly sensitive after sporulation and washing of the oocysts that led to removal all the fecal PCR inhibitors, such as fats, glycogen, polysaccharides, minerals and enzymes present in fresh fecal samples which cause inhibition of the PCR reaction [29]. Consequently, PCR amplification was high and most of the examined samples were positive on contrary to using fresh fecal samples because DNA concentration increases after washing these inhibitors and after sporulation. Therefore, it is recommended to perform Eimeria oocysts sporulation before the DNA extraction took place especially in subclinical cases with low numbers of oocysts.
5. Conclusions
In conclusion, the present study revealed that about half of the examined sheep and goat in Geneffe village in Suez Governorate, Egypt are suffering from subclinical coccidiosis as well as those animals are infected with different Eimeria species. Although sheep and goats were not expressing clinical signs of coccidiosis, they were suffering from significant changes in some biochemical parameters. Molecular detection and identification of the Eimeria sporocysts is a valid tool for diagnosis and detection of Eimeria species and best results could be achieved after sporulation of the Eimeria oocysts particularly in subclinical infection. Therefore, further research is needed on this subject in different rural areas in Egypt and more attention must be paid to the molecular identification and characterization of the Eimeria species found. These results provide a relevant baseline data to establish a strategy to control the disease in Egypt.
Acknowledgments
Acknowledgment
The authors would like to thank the agency for veterinary medicine of Suez Governorate for their efforts to accomplish this work. The authors would like to thank the 4 anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Chartier C., Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res. 2012;103(1):84–92. doi: 10.1016/j.smallrumres.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadelhaq S.M., Arafa W.M., Aboelhadid S.M. Molecular characterization of eimeria species naturally infecting Egyptian Baldi chickens. Iran J Parasitol. 2015;10(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- 3.Nuvor S.V., Agyei A.D., Assoku R.K.G. Oocyst counts in crossbred ewes under tree-crop plantation in the forest zone of Ghana. Trop Anim Health Prod. 1998;30(5):279–285. doi: 10.1023/a:1005030601728. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz A., Guedes A.C., Muñoz M.C., Molina J.M., Hermosilla C., Martín S. Control strategies using diclazuril against coccidiosis in goat kids. Parasitol Res. 2012;110(6):2131–2136. doi: 10.1007/s00436-011-2746-0. [DOI] [PubMed] [Google Scholar]
- 5.Majeed Q.A., Alazemi M.S., Henedi A.A., Tahrani L.M. Study on parasites from farm animals in Kuwait. J Egypt Soc Parasitol. 2015;45(1):71–74. doi: 10.12816/0010851. [DOI] [PubMed] [Google Scholar]
- 6.Rehman T.U., Khan M.N., Sajid M.S., Abbas R.Z., Arshad M., Iqbal Z. Epidemiology of Eimeria and associated risk factors in cattle of district Toba Tek Singh, Pakistan. Parasitol Res. 2011;108(5):1171–1177. doi: 10.1007/s00436-010-2159-5. [DOI] [PubMed] [Google Scholar]
- 7.Foreyt W.J. Coccidiosis and cryptosporidiosis in sheep and goats. Vet Clin North Am Food Anim Pract. 1990;6(3):655–670. doi: 10.1016/s0749-0720(15)30838-0. [DOI] [PubMed] [Google Scholar]
- 8.Koudela B., Bokova A. Coccidiosis in goats in the Czech Republic. Vet Parasitol. 1998;76(4):261–267. doi: 10.1016/s0304-4017(97)00147-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G.H., Lei L.H., Shang C.C., Gao M., Zhao Y.Q., Chen C.X. High prevalence of Eimeria infection in dairy goats in Shaanxi province, northwestern China. Trop Anim Health Prod. 2012;44(5):943–946. doi: 10.1007/s11250-011-9997-8. [DOI] [PubMed] [Google Scholar]
- 10.O'Callaghan M.G., O'Donoghue P.J., Moore E. Coccidia in sheep in South Australia. Vet Parasitol. 1987;24(3–4):175–183. doi: 10.1016/0304-4017(87)90038-0. [DOI] [PubMed] [Google Scholar]
- 11.Amarante A.F., Barbosa M.A. Species of coccidia occurring in lambs in Sao Paulo State, Brazil. Vet Parasitol. 1992;41(3–4):189–193. doi: 10.1016/0304-4017(92)90078-n. [DOI] [PubMed] [Google Scholar]
- 12.Khodakaram-Tafti A., Hashemnia M., Razavi S.M., Sharifiyazdi H., Nazifi S. Genetic characterization and phylogenetic analysis of Eimeria arloingi in Iranian native kids. Parasitol Res. 2013;112(9):3187–3192. doi: 10.1007/s00436-013-3494-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee H.A., Hong S., Chung Y., Kim O. Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res. 2011;27(3):255–258. doi: 10.5625/lar.2011.27.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeton S.T., Navarre C.B. Coccidiosis in large and small ruminants. Vet Clin North Am Food Anim Pract. 2017 doi: 10.1016/j.cvfa.2017.10.009. [in Press] [DOI] [PubMed] [Google Scholar]
- 15.Great Britain . H.M. Stationery Office; 1986. Ministry of agriculture F, Food. Manual of veterinary parasitological laboratory techniques. [Google Scholar]
- 16.Wang C.R., Xiao J.Y., Chen A.H., Chen J., Wang Y., Gao J.F. Prevalence of coccidial infection in sheep and goats in northeastern China. Vet Parasitol. 2010;174(3–4):213–217. doi: 10.1016/j.vetpar.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 17.El-Magdoub A.A., El-Sayed I.A., Mahdy A.E. Relationship between system of raising Egyptian buffaloes and the effect of climate conditions on the helminthic infection rate, middle Delta, Egypt. J Egypt Soc Parasitol. 1999;29(2):505–515. [PubMed] [Google Scholar]
- 18.Abo-Shehada M.N., Abo-Farieha H.A. Prevalence of Eimeria species among goats in northern Jordan. Small Rumin Res. 2003;49(2):109–113. [Google Scholar]
- 19.Deger S., Gul A., Ayaz E., Blcek K. The prevalence of Eimeria species in goats in Van. Turk J Vet Anim Sci. 2003;27:439–442. [Google Scholar]
- 20.Kheirandish R., Nourollahi-Fard S.R., Yadegari Z. Prevalence and pathology of coccidiosis in goats in southeastern Iran. J Parasit Dis. 2014;38(1):27–31. doi: 10.1007/s12639-012-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catchpole J., Gregory M.W. Pathogenicity of the coccidium Eimeria crandallis in laboratory lambs. Parasitol. 1985;91(Pt. 1):45–52. doi: 10.1017/s003118200005650x. [DOI] [PubMed] [Google Scholar]
- 22.Gregory M.W. Boca Raton Coccidiosis of man and domestic animals. CRC Press, Inc.; Florida: 1990. Pathology of coccidial infections; pp. 235–261. [Google Scholar]
- 23.Norton C.C. Coccidia of the domestic goat Capra hircus, with notes on Eimeria ovinoidalis and E. bakuensis (syn. E. ovina) from the sheep Ovis aries. Parasitology. 1986;92(Pt 2):279–289. doi: 10.1017/s0031182000064052. [DOI] [PubMed] [Google Scholar]
- 24.Maingi N., Munyua W.K. The prevalence and intensity of infection with Eimeria species in sheep in Nyandarua district of Kenya. Vet Res Commun. 1994;18(1):19–25. doi: 10.1007/BF01839257. [DOI] [PubMed] [Google Scholar]
- 25.Kumar B., Maharana B.R., Prasad A., Joseph J.P., Patel B., Patel J.S. Seasonal incidence of parasitic diseases in bovines of south western Gujarat (Junagadh), India. J Parasit Dis. 2016;40(4):1342–1346. doi: 10.1007/s12639-015-0686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor M.A., Catchpole J., Marshall J., Marshall R.N., Hoeben D. Histopathological observations on the activity of diclazuril (Vecoxan) against the endogenous stages of Eimeria crandallis in sheep. Vet Parasitol. 2003;116(4):305–314. doi: 10.1016/s0304-4017(03)00256-5. [DOI] [PubMed] [Google Scholar]
- 27.McGavin M.D., Zachary J.F. 5th ed. Elsevier Mosby; London: 2011. Pathologic basis of veterinary disease. [Google Scholar]
- 28.Hatam Nahavandi K., Mahvi A.H., Mohebali M., Keshavarz H., Rezaei S., Mirjalali H. Molecular typing of Eimeria ahsata and E. crandallis isolated from slaughterhouse wastewater. Jundishapur J Microbiol. 2016;9(4):e34140. doi: 10.5812/jjm.34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrader C., Schielke A., Elerboek L., John R. PCR inhibitors – occurrence, properties and removal. Appl Microbiol. 2012;5:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]