Abstract
Some new (naphthalen-1-yl-selenyl)acetic acids derivatives 7a‑d have been synthesized by two different methods, using naphthylselenols or naphthylselenocyanates. The structures of the products were investigated by spectroscopic methods.
Keywords: Selenol, selenocyanate, naphthalene, selenium, (naphthalen-1-yl-selenyl)–acetic acid
Introduction
Selenium is an element which resembles sulfur in terms of its chemical properties. It has been successfully introduced into organic compounds either as an electrophile or as a nucleophile. Humans and animals need selenium for various biological functions which involve some organoselenium compounds [1]. It is also know that many selenium containing organic molecules are antibacterial and antifungal agents [2]. Organoselenium compounds are commonly employed as useful and powerful reagents in organic synthesis and many different organoselenium compounds have been synthesized [3,4]. Arylselenyl acetic acid derivatives are of interest due to their utility in some syntheses [5]. There are different approaches to the synthesis of arylselenyl acetic acid derivatives [6,7]. Two of these approaches are based on: i) arylselenols and ii) arylselenocyanates. The arylselenols are synthesized using Grignard reagents [8], whereas the arylselenocyanates are obtained from the reaction of aryldiazonium salts with potassium selenocyanate [9,10]. In this paper the naphtyl moiety has been introduced as the aryl group in the abovementioned compounds.
Results and Discussion
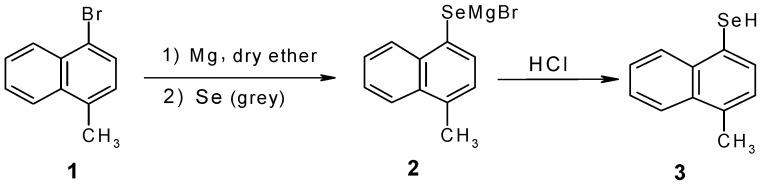
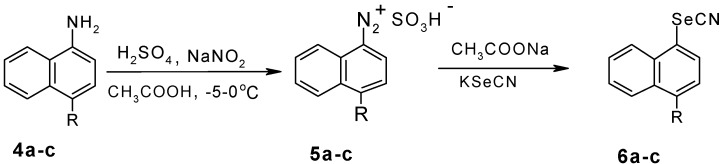
In the present study, compounds 7a-d were synthesized starting from the corresponding naphtylselenol 3 or naphthylselenocyanates 6a-c. Scheme 1 and Scheme 2 outline the syntheses of these intermediates in the syntheses of the selenoacetic acids.
Scheme 1.
Scheme 2.
R= a: H, b: NO2, c: Br
In the second method (Scheme 2) the yields are generally low unless the medium is buffered (pH= 5.5) by the addition of NaOAc. Most probably due to the hyperacidity, potassium selenocyanate decomposes to release HCN.
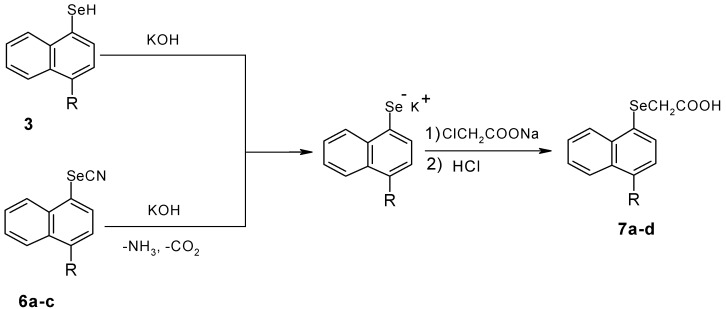
The target (naphthalen-1-yl-selenyl)acetic acid derivatives are then synthesized either from the naphthalylselenol or naphthylselenocyanates with neutralized chloroacetic acid (Scheme 3).
Scheme 3.
R= a: H, b: NO2, c: Br, d: CH3
(Naphthalen-1-yl-selenyl)acetic acid (7a) was obtained both by Grignard synthesis [7] or via the corresponding diazonium salt. In each case the same product was obtained in comparable yields.
Conclusions
The intermediate products obtained in these syntheses are novel. They were isolated and their molecular structures identified. The final compounds 7a-d have some further synthetic potential in the direction of some heterocyclic compounds, which could be obtained by means of ring closure reactions such as Friedel Crafts reactions.
Experimental
General
Substituted naphthylamines, bromonaphthalenes and other chemicals were obtained from Merck. All mps were determined in sealed capillaries and are uncorrected. FT-IR spectra were recorded on a Mattson 1000 spectrometer as KBr pellets. 1H-NMR spectra were recorded on a Varian Gemini 300 (300 MHz) NMR spectrometer in CDCl3 and CCl4. Mass spectra measurements were recorded on a Thermo Finnigan Trace DSQ.
4-Methyl-1-naphthylselenol (3)
The Grignard reagent of 1-bromo-4-methyl naphthalene (3.9 mL; 0.025 mole) was prepared under a nitrogen atmosphere using dry ether as solvent. Selenium (grey, 1.975 g, 0.025 mole) was added and the mixture refluxed for 2 hours. Dilute HCl solution was then added into the mixture. The ether layer was separated and evaporated under vacuum to yield the title compound as a viscous liquid (4.365 g, 0.020 mole, yield: 79%); IR νmax/cm-1: 2048 (Se-H), 3075 (Ar-H), 2973(C-H); 1H-NMR (CCl4) δH: 1.8(s, 1H); 2.5(s, 3H); 7.1-8.2 (m, 6H); MS for C11H10Se (M+.): 222.1
1-Naphthylselenocyanate (6a). NaNO2 (0.17 g; 0.0025 mole) was dissolved in cooled H2SO4. 1‑Naphthylamine (0.357g; 0.0025 mole) was dissolved in glacial acetic acid and cooled below 20 oC. The solution of H2SO4-NaNO2 was dropwise added into the solution of α-naphthylamine. The pH of the medium was adjusted to 5.5 by the addition of sodium acetate solution. Then KSeCN (0.36g; 0.0025 mole) was added portionwise. A brown precipitate formed, which was filtered by suction, dried and purified by crystallization. Yield: 44%; m.p. 66-67 oC (ethanol) (lit. [11]: m.p. 69-70 oC); IR νmax/cm-1 2158 (SeCΞN), 3055 (Ar-H); 1H-NMR (CCl4) 7.3-8.5 (m, Ar-H); MS for C11H7NSe (M+.): 232.9
4-Nitro-1-naphthylselenocyanate (6b). Yield: 46%; m.p. 88-89oC (ethanol); IR νmax/cm-1: 2156 (SeCΞN), 3083 (Ar-H); 1H-NMR (CCl4) δH: 7.1-8.6 (m, Ar-H); MS for C11H6N2O2Se (MH+): 279.0
4-Bromo-1-naphthylselenocyanate (6c). Yield: 38%; m.p.78-80 oC (ethanol); IR νmax/cm-1: 2152 (SeCΞN), 3075 (Ar-H); 1H-NMR (CCl4) δH: 7.2-8.4 (m, Ar-H)
(Naphthalen-1-yl-selenyl)acetic acid (7a). KOH (3M, 30 mL) was added to 6a. This mixture was stirred under reflux for 2 hours in a water bath. Neutralized chloroacetic acid was added to the cooled mixture. The final mixture was boiled for 30 minutes. Then HCl was added to the cooled mixture until the precipitation was complete. The precipitate obtained was dried and purified by crystallization. Yield: 28%; m.p. 65-66 oC (CCl4) (lit. [7]: m.p. 66-67 oC; IR νmax/cm-1: 1703 (C=O), 2816 (C-H), 3040 (Ar-H), 3140 (O-H); 1H-NMR (CDCl3) δH: 3.5 (s, 2H); 7.2-8.5 (m, 7H); 9.6 (s, 1H); MS for C12H10O2Se (M+.): 265.9
The following compounds were similarly prepared:
(4-Nitronaphthalen-1-yl-selenyl)acetic acid (7b). Yield: 31%; m.p. 91-93 oC (CCl4); IR νmax/cm-1: 1747 (C=O), 2935 (C-H), 3095 (Ar-H), 3147 (O-H); 1H-NMR (CDCl3) δH: 3.7 (s, 2H); 7.8-9.0 (m, 6H); 11.2 (s, 1H); MS for C11H6N2O2Se (C10H6NO2+): 173.0
(4-Bromonaphthalen-1-yl-selenyl)acetic acid (7c). Yield: 25%; m.p. 94-95 oC (formic acid); IR νmax/cm‑1: 1708 (C=O), 2921 (C-H), 3075 (Ar-H), 3317 (O-H); 1H-NMR (CDCl3) δH: 3.2(s, 2H); 7.6-8.8(m, 6H); 10.8(s, 1H)
(4-Methylnaphthalen-1-yl-selenyl)acetic acid (7d). KOH (3M, 30 mL) was added to 3. This mixture was stirred under reflux for 2 hours in a water bath. Neutralized chloroacetic acid was added into the cooled mixture. The final mixture was boiled for 30 minutes, and then HCl was added to the cooled mixture until the precipitation was complete. The precipitate obtained was dried and purified by crystallization. Yield: 38%; m.p.72-73 oC (CCl4); IR νmax/cm-1: 1708 (C=O), 2937 (C-H), 3000 (Ar-H), 3120 (O-H); 1H-NMR (CDCl3) δH: 2.7(s, 3H), 3.5 (s, 2H); 7.3-8.5 (m, 6H); 10.1 (s, 1H); MS for C13H12O2Se (M+.): 279.9
Acknowledgements
We are grateful to the Gazi University Research Foundation for financial support of this work (FEF 05/2002-19). In addition, we would like to thanks to the DPT Project (No: 2001K120590) for NMR and MS spectra measurements.
Footnotes
Sample availability: Samples may be obtained from corresponding author.
References
- 1.Rayman M.P. Brit. Med. J. 2000;356:233. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 2.Günther W.H.H. In: Organic Selenium Compounds Their Chemistry and Biology. Klayman D.L., Günther W.H.H., editors. Wiley; New York: 1973. p. 30. [Google Scholar]
- 3.Xie Y., Short M.D., Cassidy P.B., Roberts J.C. Bioorg. Med. Chem. Lett. 2001;11:2911. doi: 10.1016/S0960-894X(01)00590-X. [DOI] [PubMed] [Google Scholar]
- 4.Overvad K. Bibl. Nutr. Diet. 1998;54:141. doi: 10.1159/000059454. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger N., Cagniant P. C.R. Acad. Sc. Paris. 1969;268:1385. [Google Scholar]
- 6.Morgan G.T., Porrit W.H. J. Chem. Soc. 1925:1755. [Google Scholar]
- 7.Sjöberg B., Herdevall S. Acta Chem. Scand. 1958;12:1347. doi: 10.3891/acta.chem.scand.12-1347. [DOI] [Google Scholar]
- 8.Taboury M. Bull. Soc. Chim. France. 1903;29:762. [Google Scholar]
- 9.Behagel O., Rollmann M. J. Prakt. Chem. 1929;123:336. [Google Scholar]
- 10.Özkan H., Dişli A., Yıldırır Y. Org. Prep. Proc. Int. 2004;36:161. doi: 10.1080/00304940409355388. [DOI] [Google Scholar]
- 11.Murata S., Suzuki C., Inoue H., Andoha Y., Hayashi Y. Heterocycles. 1999;52:621. [Google Scholar]