Abstract
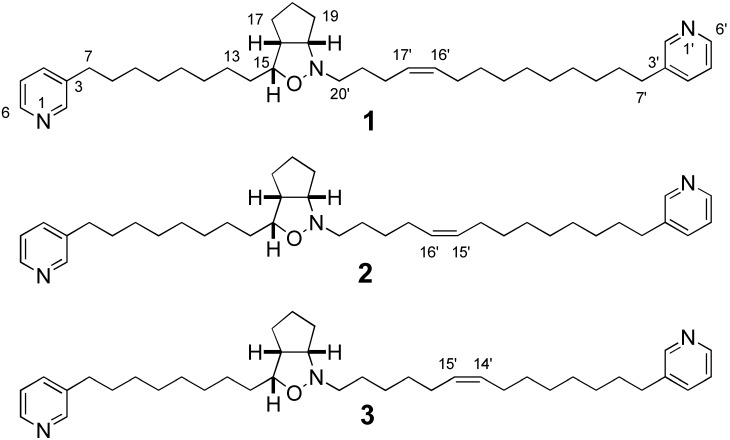
Asymmetric synthesis of double bond isomers (+)-2 (Δ15’,16’) and (+)-3 (Δ14’,15’) of the structure (1) (Δ16’,17’) proposed for pyrinodemin A, a cytotoxic bis-pyridine alkaloid with a unique cis-cyclopent[c]isoxazolidine moiety from a marine sponge, has been accomplished. Pyrinodemin A was indicated to be a 1:1 racemic mixture of 2 from comparison of C18 and chiral HPLC analysis for pyrinodemin A and the synthetic compounds as well as ESIMS data of oxidative degradation products of pyrinodemin A.
Keywords: Pyrinodemin A, Amphimedon sp., asymmetric synthesis, structural revision
Pyrinodemin A, a cytotoxic bis-pyridine alkaloid with a unique cis-cyclopent[c]isoxazolidine moiety, has been isolated from a marine sponge Amphimedon sp., and its relative stereostructure was proposed as 1 (Δ16’,17’) on the basis of spectral data [1]. The unique structure of pyrinodemin A has prompted synthetic chemists to its total synthesis of 1 as well as syntheses of the double bond isomers 2 (Δ15’,16’) and 3 (Δ14’,15’) [2,3,4] followed by different proposals of the structural revision of pyrinodemin A to be 2 [2] or 3 [3,4].
In order to examine the correct structure of pyrinodemin A, we have synthesized (+)-2 and (+)-3, the double bond isomers of 1, as an optically active form, and compared HPLC profiles of the synthetic compounds and pyrinodemin A. In addition, oxidative degradation experiments were performed for a remaining small amount of pyrinodemin A to determine the position of a double bond. In this paper, we describe asymmetric synthesis of (+)-2 and (+)-3, and indication of the structure of pyrinodemin A to be (±)-2.
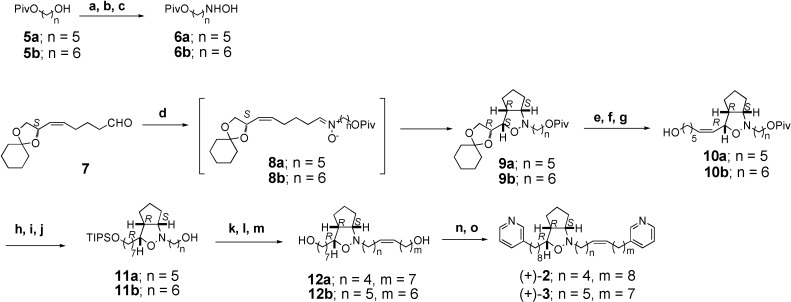
The Δ15’,16’ double bond isomer (+)-2 was synthesized as follows (Scheme 1). The synthesis of hydroxylamine 6a commenced with known pivaloate 5a [5]. Oxidation of alcohol 5a with 2-iodobenzoic acid (IBX) [6] in DMSO and THF afforded its aldehyde. Treatment of the aldehyde with NH2OH·HCl and NaOAc in MeOH provided oxime which was reduced with NaBH3CN in MeOH to afford hydroxylamine 6a [7,8]. Condensation of 6a and optically active aldehyde 7 [8] in CHCl3 containing Na2SO4 at r.t. gave the nitrone 8a, which was followed by heating to afford cis-cyclopent[c]isoxazolidine [9] 9a in 58% yield.
Scheme 1.
Reagents and conditions: (a) IBX, DMSO, THF (69%); (b) H2NOH·HCl, AcONa, MeOH (96%); (c) NaBH3CN, MeOH, pH 3, 0 °C; (d) Na2SO4, 6, CHCl3, r.t.~reflux (58% for 2 steps); (e) 3N HCl, dioxane (80%); (f) NaIO4, MeCN, H2O, 0 °C; (g) Br-[Ph3+(CH2)5CH2OH], n-BuLi, THF, 0 °C (51% for 2 steps); (h) TIPSCl, imidazole, CH2Cl2 (75%); (i) H2, Pd-C, MeOH (93%); (j) DIBAL, CH2Cl2, -78 °C (75%); (k) IBX, DMSO (80%); (l) Br-[Ph3P+(CH2)7CH2OH], n-BuLi, THF, 0 °C (81%); (m) 46% HF, MeCN (55%); (n) CBr4, Ph3P (80%); (o) 3-methylpyridine, LDA, DMPU, -40 °C (64%)
Treatment of 9a with 3N HCl in dioxane gave diol, which was converted into its aldehyde by treatment with NaIO4 and then into alcohol 10a by Wittig reaction [10]. Protection of alcohol 10a as its TIPS ether followed by reduction with Pd-C gave its saturated TIPS ether, which was converted into alcohol 11a with DIBAL. IBX oxidation of 11a followed by Wittig reaction [10] afforded its unsaturated alcohol, which was subjected to deprotection with HF to give diol 12a in 55 %. Treatment of diol 12a with CBr4 and PPh3 provided its dibromide, which was coupled with 3-methypyridine using LDA and DMPU [11] in THF to furnish optically active compound (+)-2. This is the first synthesis of optical active form of 2, although its racemic form ((±)-2) has been synthesized [2,3,4]. The Δ14’,15’ double bond isomer (+)-3 was prepared from pivaloate 5b by almost same procedure as described for synthesis of (+)-2 (Scheme 1).
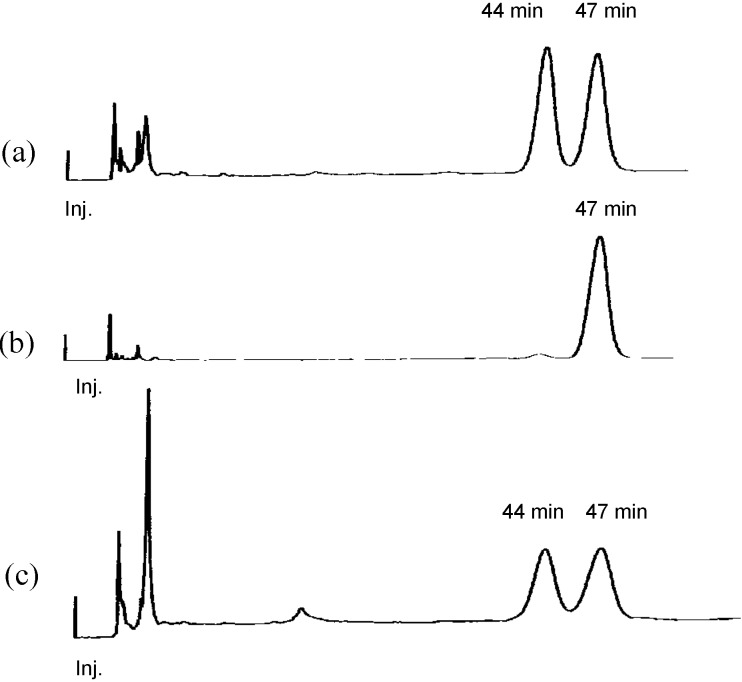
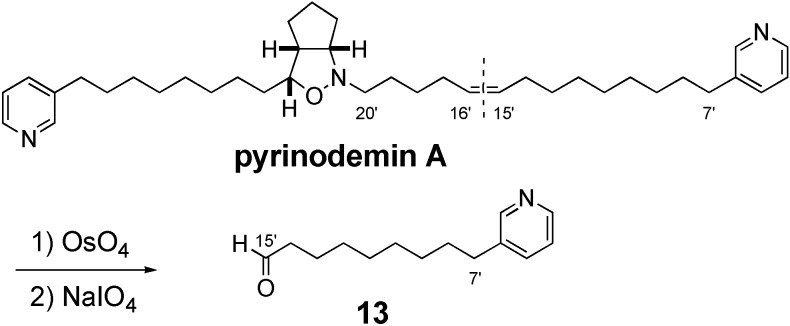
The position of a double bond and the stereochemistry of pyrinodemin A were examined as follows. Compounds (±)-1 [2], (±)-2 [2], and (+)-3 were subjected to C18 HPLC [Wako sil-II 5C18 RS, Wako Ind., Ltd., 4.6 x 250 mm; flow rate 1.0 mL/min: eluent; MeOH/H2O (91:9); UV detection at 263 nm] and found to be separated (1, tR 21.6 mim; 2, tR 17.0 min; 3, tR 15.8 min), while the retention time (tR 17.0 min) of pyrinodemin A was identical with that of 2 under the same condition, indicating that the position of a double bond of pyrinodemin A corresponded to that (Δ15’,16’) of 2. To elucidate the stereochemistry of pyrinodemin A, compound (±)-2 was subjected to chiral HPLC [CHIRALCELL OD-H, Daicel Co., Ltd., 4.6 x 250 mm; flow rate 1.0 mL/min: eluent: hexanes/i-PrOH (95:5); UV detection at 263 nm] and found to be separated (tR 44 and 47 min), while the retention time of (+)-2 was 47 min (Figure 1). On the other hand, pyrinodemin A gave the two peaks corresponding to those of (±)-2 in a ratio of 1:1 under the same conditions, indicating that pyrinodemin A is a 1:1 racemic mixture of 2. Furthermore, pyrinodemin A was treated with OsO4 and then NaIO4 to give degradation products, one of which showed an ESIMS fragment ion peak at m/z 242 (M+Na)+, corresponding to an aldehyde (13) of C-7’~C-15’ segment connected to a pyridine ring (Scheme 2). From the results described above, it was indicated that the olefin position of pyrinodemin A was C-15’ and C-16’ (2), as proposed by Snider’s group [2], and that pyrinodemin A was a 1:1 racemic mixture of 2.
Figure 1.
Chiral HPLC profiles of (a) synthetic compounds (±)-2, (b) (+)-2, and (c) pyrinodemin A
Scheme 2.
Acknowledgments
We thank Professor B. B. Snider (Brandeis University) for generous offer of synthetic samples of (±)-1 and (±)-2. This work was supported in part by grants from the Akiyama Foundation and the Takeda Science Foundation and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Sample Availability: Available from the authors.
Experimental
General
Optical rotations were determined on a JASCO P-1030 polarimeter. Infrared spectra were obtained on a JASCO FT/IR-230 spectrometer. Proton and carbon NMR spectra were recorded on a Bruker 600 MHz spectrometer. Chemical shifts are reported in δ values relative to chloroform (δ 7.26 for proton and δ 77.0 for carbon NMR. EI mass spectra were measured on a JEOL JMS-DX303 spectrometer.
Synthetic Compound (+)-2: [α]25D +5.5°(c 0.6, CHCl3); IR (neat) 1575 cm-1; 1H-NMR (600 MHz, CDCl3) δ 1.25~1.50 (26H, m), 1.50~1.74 (9H, m), 1.75 (1H, m), 2.01 (4H, m), 2.60 (5H, m), 2.91 (2H, m), 3.50 (1H, m), 4.15 (1H, m), 5.33 (2H, m), 7.22 (2H, m), 7.51 (2H, m), 8.44 (4H, m); 13C-NMR (150 MHz, CDCl3) δ 26.3, 26.4, 27.0, 27.1, 27.2, 27.5, 27.8, 29.1, 29.3, 29.4, 29.7, 31.1, 33.0, 34.2, 49.9, 57.1, 72.6, 77.7, 123.2, 129.6, 130.0, 135.7, 137.9, 147.1, 149.9; HREIMS m/z 573.4643 [M+; calcd for C38H59N3O1 573.4658].
Synthetic Compound (+)-3: [α]25D +6.2°(c 0.8, CHCl3); IR (neat) 1575 cm-1; 1H-NMR (600 MHz, CDCl3) δ 1.25~1.50 (26H, m), 1.50~1.74 (9H, m), 1.77 (1H, m), 2.00 (4H, m), 2.58 (5H, m), 2.82 (2H, m), 3.45 (1H, m), 4.04 (1H, m), 5.33 (2H, m), 7.18 (2H, m), 7.47 (2H, m), 8.43 (4H, m); 13C-NMR (150 MHz, CDCl3) δ 26.3, 26.4, 27.1, 28.0, 28.8, 29.1, 31.1, 33.0, 34.3, 49.9, 57.3, 77.7, 123.2, 129.8, 135.7, 137.9, 147.1, 149.9; HREIMS m/z 573.4661 [M+; calcd for C38H59N3O1 573.4658].
References
- 1.Tsuda M., Hirano K., Kubota T., Kobayashi J. Tetrahedron Lett. 1999;40:4819–4820. [Google Scholar]
- 2.Snider B.B., Shi B. Tetrahedron Lett. 2001;42:1639–1642. [Google Scholar]
- 3.(a) Baldwin J.E., Romeril S.P., Lee V., Claridge T.D.W. Org. Lett. 2001;3:1145–1148. doi: 10.1021/ol015646q. [DOI] [PubMed] [Google Scholar]; (b) Romeril S.P., Lee V., Claridge T.D.W., Baldwin J.E. Tetrahedron Lett. 2002;43:327–329. [Google Scholar]
- 4.Morimoto Y., Kitao S., Okita T., Shoji T. Org. Lett. 2003;5:2611–2614. doi: 10.1021/ol034700v. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto K., Kawabata H., Nakamichi N., Hayashi M. Chem. Lett. 2001:934–945. [Google Scholar]
- 6.(a) Frigerio M., Santagostino M. Tetrahedron Lett. 1994;35:8019–8022. [Google Scholar]; (b) Frigerio M., Santagostino M., Sputore S., Palmisano G. J. Org. Chem. 1995;60:7272–7276. [Google Scholar]
- 7.Oppolzer W., Siles S., Snowden R.L., Bakker B.H., Petrzilka M. Tetrahedron. 1985;41:3497–3509. [Google Scholar]
- 8.LeBel N.A., Balasubramanian N. J. Am. Chem. Soc. 1989;111:3363–3368. [Google Scholar]
- 9.Annunziata R., Cinquini M., Cozzi F., Gennari C., Raimondia L. J. Org. Chem. 1987;52:4674–4681. [Google Scholar]
- 10.(a) Browne J.E., Driver M.J., Russell J.C., Sammes P.G. J. Chem. Soc., Perkin Trans. 1. 2000:653–657. [Google Scholar]; (b) Lei H., Atkinson J. J. Org. Chem. 2000;65:2560–2567. doi: 10.1021/jo000029l. [DOI] [PubMed] [Google Scholar]
- 11.Davies-Coleman M.T., Faulkner D.J., Dubouwchik G.M., Roth G.P., Polson C., Fairchild C. J. Org. Chem. 1993;58:5925–5930. [Google Scholar]