Abstract
Background
The reported prevalence of pain after stroke varies considerably, depending on how pain is measured, time after stroke, and characteristics of the selected population. The aims of this study were to investigate the prevalence and distribution of new-onset pain initially and three months after stroke in a general Norwegian cohort, and to examine whether symptoms of anxiety or depression were associated with new-onset pain after stroke.
Material and methods
Stroke patients were included from eleven different hospitals within 14 days after stroke onset. Pain was assessed at inclusion and three months after stroke, and the distribution of pain was marked on a body map. New-onset pain was defined as pain reported by the patients to have occurred after the stroke. Symptoms of anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale.
Results
A total of 390 patients were included. Pain data were available in 142 patients at both inclusion and follow-up, while 245 patients had available data for the regression analysis. In patients with follow-up data, new-onset pain occurred in 14 (9.9%) patients at inclusion and in 31 (21.8%) patients three months later, P=0.005. New-onset pain in the affected upper limb and bilaterally in the lower limbs was more common at three months than initially after stroke. Symptoms of anxiety were associated with new-onset pain (OR=1.13, 95% CI 1.01–1.27, P=0.030).
Conclusion
This study shows that new-onset pain occurs in one out of ten patients initially after stroke and in one out of five patients three months after stroke, and it was associated with symptoms of anxiety. This raises the question of whether easing symptoms of anxiety might help to prevent or treat new pain after stroke.
Keywords: stroke, pain, cerebral infarction, complication, anxiety
Introduction
The reported prevalence of pain after stroke varies considerably, and range from 11% to 54%, depending on how pain is measured, time after stroke, and selection and characteristics of the population.1–3 Despite some inconsistencies, several studies have found that pain in stroke patients is associated with female gender, younger age, and more severe strokes.4–6
As pain is always subjective, the most obvious way of having it assessed is by structured interviews.3,7,8 Structured questionnaires exist,9 and different scales such as Numeric Rating Scale (NRS), Visual Analog Scale, and Verbal Rating Scale make it possible to describe pain with some accuracy.10
The number of patients experiencing pain seems to increase during follow-up, at least within the first months after stroke.2,3 Previous research also indicates different patterns for different kinds of post-stroke pain.3,5,11 While headache mainly develops as an acute problem declining with time, pain related to the muscular skeletal system seems to increase gradually and over time.3
The nature of post-stroke pain is intricate, and it is difficult to grasp all aspects involved in its development and its influence on the lives of the affected patients. Even though pain does not seem to affect functional outcome three months after stroke,12 the affected patients appear to have an increased risk of experiencing emotional distress.4,7,13 Studies have found an association between pain and depression in stroke patients, but there are some inconsistencies indicating that this relationship is complex and not properly accounted for.4,7,14,15
The association between anxiety disorders and pain is established in several populations, indicating a bidirectional relationship between the two.16–19 Although anxiety is fairly common in stroke patients,20 little is known about how it influences the occurrence of pain after stroke. One study has found that anxiety was more common in patients with pain than no pain. However, pain was not specified as new after the stroke, and the analyses were not adjusted for confounders.21
Pain is also common in the general population.22 It is therefore difficult to evaluate whether the pain existed prior to or occurred after the stroke, and even more difficult to establish whether the pain was caused by the stroke. Previous research has focused on the total burden of post-stroke pain,2,4 and even though some studies differentiate between pre-stroke and post-stroke pain, few studies follow the same group of patients over time to see how new pain evolves after the stroke. In the current study, we used data from a Norwegian multicenter study (the LEAST study)23 to investigate the occurrence of pain initially and three months after stroke. We also wanted to distinguish between pre-stroke and post-stroke pain.
The primary aim of this study was to report the prevalence and distribution of new-onset pain in the early phase and three months after stroke in a group of stroke patients. Secondary aims were to examine whether symptoms of anxiety and depression were associated with new-onset pain after stroke and to describe how patients reported the pain to influence on activities of daily living and enjoyment of life.
Material and methods
Study design and setting
This was a prospective observational study recruiting stroke patients admitted to stroke units in 11 Norwegian hospitals. The participating hospitals were two university hospitals, seven middle-sized hospitals (treating 100–400 stroke patients per year), and two small hospitals (treating less than 100 patients per year). Hospitals were contacted once every fortnight, and inclusion was carried out if there were two or more eligible patients. Trained assessors performed the follow-up assessment three months later, mainly by telephone or face-to-face.23
Participants
Patients were recruited from December 2011 to June 2013. Patients were eligible if they had been diagnosed with acute stroke according to the WHO’s definition24 within the last 14 days, were aged more than 18 years, not receiving palliative care, were able to understand the Norwegian language, and willing to sign informed consent. They had to stay in the hospital for the whole process of inclusion. Patients unable to consent for themselves were included if their next of kin consented to their inclusion. The study was approved by the Regional Committee for Medical and Health Research Ethics in Norway (REK no 2011/1428).
Baseline assessment
Baseline characteristics including gender and age were recorded immediate after inclusion. Stroke was classified as infarction or hemorrhage. Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) at inclusion.25 Premorbid and post-stroke functional level was registered using the modified Rankin Scale (mRS).26
Measures
Pain was assessed at three months after stroke, with a later protocol adjustment and implementation of pain assessment at inclusion to the study as well. At inclusion, patients were asked if they had any pain. Pain was defined according to the definition used in the Brief Pain Inventory, that is, ignoring pain that most of us have from time to time, such as minor headache, sprains, and toothache. Furthermore, patients were asked to locate the pain on the body map from the same questionnaire.9 To decide if the pain was new after the stroke, patients were asked if it had occurred after the stroke.
Additionally to these pain questions, an extended questionnaire was implemented at three months after the stroke. Patients were asked if the pain required regular (>2 times per week) use of analgesics. NRS (0–10) was used to rate pain intensity. Patients with new-onset pain were asked to rate on the NRS how the pain influenced their daily activities and enjoyment of life. A score of 3 or less on NRS was categorized as “mild”, a score from 4 to 7 as “moderate”, and a score of more than 7 as “severe”.27
Symptoms of anxiety and depression were measured at three months after stroke using the Hospital Anxiety and Depression Scale (HADS),28 which has been validated for use in stroke patients.29 It consists of seven questions related to depression and seven questions related to anxiety, and it generates a score for each of the two subscales (0–21) as well as a total score (0–42). It is recommended that the sub-scales are used separately.30 Dichotomization of the score is possible,31 but in this study, we used the total score for both subscales, symptoms of depression and anxiety.
Statistics
Baseline data were analyzed using the Pearson chi-squared test for dichotomous proportions, while the Mann–Whitney U test and Student’s t-test were used to compare groups on scale variables. Descriptive statistics were used to report the prevalence and distribution of new pain according to the body map. Distribution of pain on the body map was defined according to the following categories: head/neck, upper limb on the affected or unaffected side of the body, lower limb on the affected or unaffected side of the body, back, chest, and abdomen. The McNemar mid-P-test was used to analyze differences in new-onset pain in the acute phase and after three months.
Univariable logistic regression, with new-onset pain three months after stroke as the dependent variable, was used to analyze associations. Independent variables were gender, age, stroke severity assessed with the NIHSS, symptoms of anxiety, and symptoms of depression three months after stroke. A multivariable logistic regression analysis was performed with symptoms of anxiety and depression as independent variables. Adjustments for gender, age, and NIHSS were made as these variables are known to be associated with pain after stroke.5,6 This regression analysis was repeated three times, once with both “anxiety” and “depression” included in the model, once with “anxiety” alone and once with “depression” alone included in the model, in addition to gender, age, and NIHSS. A two-sided P-value <0.05 was considered significant. Statistical analyses were performed in SPSS 24 and Microsoft Excel 2016.
Results
Study population
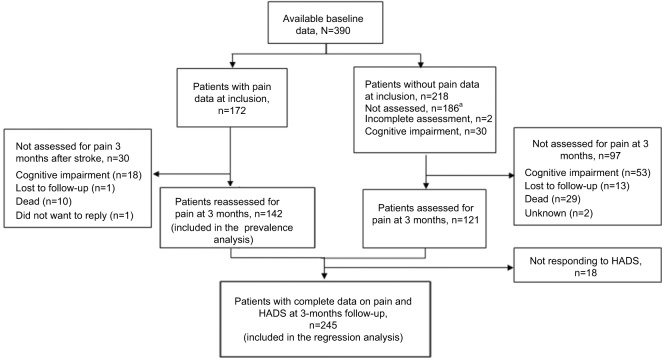
A total of 547 patients were screened for inclusion from December 2011 to June 2013. Fifty-one patients did not meet the inclusion criteria, while 60 patients were excluded due to early discharge and 26 patients did not want to par ticipate. Sixteen patients were later excluded because they did not have a stroke diagnosis, and one withdrew. Hence, 393 patients had available baseline data. Furthermore, three patients were excluded due to a lack of follow-up data, leaving a total of 390 patients to be included in the study. The flow of patients with and without pain data is described in Figure 1. Assessment of pain at inclusion was implemented after the study had been in progress for a while. Hence, pain data were available in 174 patients at inclusion, while 142 patients had available pain data on both occasions, giving a sample of 142 patients available for the prevalence analysis. Out of 263 patients with available pain data at three months, 18 were lacking data on HADS, leaving a total of 245 patients available for the regression analysis. For patients alive, the main reasons for not responding when asked about pain were aphasia or dysarthria, other cognitive impairment, or loss to follow-up.
Figure 1.
Illustration of patients assessed for pain at inclusion and three months after stroke.
Note: a Patients included before implementation of pain assessment at inclusion to the study.
Abbreviation: HADS, Hospital Anxiety and Depression Scale.
Baseline information of the patients included in prevalence analysis and the regression analysis is listed in Table 1.
Table 1.
Baseline characteristics of all patients, patients responding to pain questionnaire, and patients with new-onset pain three months after stroke
| Characteristics | All patients n=390 | Patients available for prevalence analysis n=142 | Patients available for regression analysis n=245 |
|---|---|---|---|
| Women, N (%) | 201 (51.5) | 71 (50) | 113 (46.1) |
| Age (years) | |||
| Mean (SD) | 76.8 (11.3) | 73.9 (12.1) | 74.4 (11.6) |
| Median (25–75 percentile) | 79.0 (70.5–84.7) | 77.3 (68.4–82.8) | 76.6 (68.1–83.3) |
| Infarction, n (%) | 334 (85.6) | 119 (83.8) | 213 (86.9) |
| Hemorrhage, n (%) | 56 (14.4) | 23 (16.2) | 32 (13.1) |
| NIHSS | |||
| Mean (SD) | 7.9 (7.7) | 4.9 (4.7) | 4.7 (4.6) |
| Median (25–75 percentile) | 5.0 (2.0–12.0) | 4.0 (2.0–6.3) | 3.0 (2.0–6.0) |
| mRS at inclusion | |||
| Mean (SD) | 3.6 (1.2) | 3.3 (1.1) | 3.1 (1.1) |
| Median (25–75 percentile) | 4.0 (3.0–5.0) | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) |
| Premorbid mRS | |||
| Mean (SD) | 1.75 (1.22) | 1.6 (1.1) | 1.4 (1.0) |
| Median (25–75 percentile) | 1.0 (1.0–3.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Comorbidity | |||
| Atrial fibrillation, n (%) | 104 (28.7)a | 30 (21.1) | 54 (22.0)b |
| Previous stroke/TIA, n (%) | 78 (21.5)a | 32 (22.5) | 48 (19.6)b |
| Dementia, n (%) | 29 (8.0)a | 3 (2.1) | 6 (2.4)b |
| Heart attack, n (%) | 63 (17.4)a | 25 (17.6) | 45 (18.4)b |
| Hypertension, n (%) | 247 (68.2)a | 104 (73.2) | 158 (64.5)b |
Notes:
n=362;
n=228.
Abbreviations: NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale; TIA, transient ischemic attack.
Prevalence and distribution of pain
The prevalence analysis included 142 patients with complete pain data at both inclusion and three-month follow-up. Out of these, 45 patients (31.7%) reported pain at inclusion and 59 patients (41.5%) reported pain three months after stroke. Fourteen (9.9%) patients who reported pain at inclusion also reported that the pain had occurred after the stroke. Four of these patients still reported pain three months later, and 27 additional patients developed new pain, resulting in a total of 31 patients (21.8%) with new pain three months after stroke (Table 2), which constitutes a significant increase since inclusion, P=0.005. Of the 31 patients with new-onset pain three months after stroke, 18 (58.1%) used analgesics regularly.
Table 2.
Prevalence and distribution of new-onset pain for patients with pain data at inclusion and three-month follow-up (n=142)
| Pain at inclusion/three months later | Yes/Yes | Yes/No | No/Yes | No/No | P-valuea |
|---|---|---|---|---|---|
| All new-onset pain | 4 | 10 | 27 | 101 | 0.005 |
| Head/neck | 1 | 6 | 6 | 128 | 1 |
| Affected shoulder/arm/hand | 0 | 0 | 13 | 129 | 0.0001 |
| Unaffected shoulder/arm/hand | 0 | 3 | 5 | 134 | 0.51 |
| Affected leg/hip | 0 | 0 | 13 | 129 | 0.0001 |
| Unaffected leg/hip | 0 | 1 | 7 | 134 | 0.039 |
| Back | 1 | 2 | 5 | 133 | 0.29 |
| Chest | 0 | 1 | 1 | 139 | 1 |
| Abdomen | 0 | 1 | 2 | 138 | 0.63 |
Notes: One patient did not report where the pain was located, and hence the total number of patients reporting distribution of pain was 141. Patients could report pain from more than one location.
McNemar mid-P-test.
The anatomical distribution of new-onset pain is listed in Table 2. Patients could report pain from more than one location. New pain in the upper limb on the affected side of the body was significantly more common three months after stroke than at inclusion. New pain in the lower limb was also more common at three months, both in affected and unaffected parts of the body.
Associations between symptoms of anxiety or depression and new-onset pain three months after stroke
The regression analysis included all the 245 patients who responded to both the pain questionnaire and HADS three months after stroke. In the univariable regression analyses, female gender, higher score on the NIHSS, symptoms of anxiety, and depression were significantly associated with new-onset pain three months after stroke (Table 3).
Table 3.
Univariable and multivariable logistic regression with new-onset pain at three months after strokea as dependent variable, n=245
| Variables | OR | 95 % CI | P-value |
|---|---|---|---|
| Univariable regression | |||
| Female gender | 2.67 | 1.39–5.14 | 0.003 |
| Age (years) | 0.98 | 0.95–1.0 | 0.104 |
| NIHSS | 1.08 | 1.02–1.15 | 0.015 |
| Anxiety | 1.18 | 1.08–1.18 | <0.001 |
| Depression | 1.14 | 1.06–1.23 | 0.001 |
| Multivariable regressionb | |||
| Female gender | 2.63 | 1.29–5.35 | 0.008 |
| Age (years) | 0.97 | 0.94–1 | 0.042 |
| NIHSS | 1.08 | 1.01–1.15 | 0.020 |
| Anxiety | 1.13 | 1.01–1.27 | 0.030 |
| Depression | 1.04 | 0.94–1.16 | 0.45 |
Notes:
Number of participants reporting new-onset pain at three-month followup: n=49.
Each variable is adjusted for all other variables.
Abbreviation: NIHSS, National Institute of Health Stroke Scale.
Results from the multiple regression model showed that anxiety, but not depression, was significantly associated with new pain, with an increased likelihood of concurrent pain as the score on the anxiety subscale of HADS increased. In a separate multivariable regression analysis (not shown in the table) not adjusted for depression, the association between anxiety and new-onset pain was slightly more pronounced (OR=1.16, 95% CI=1.06–1.27, P=0.001). When the analysis was not adjusted for anxiety, there was a significant association between depression and new-onset pain (OR=1.12, 95% CI=1.03–1.22, P=0.007).
Patients’ perception of how new-onset pain influences activities of daily living and enjoyment of life
A description of the scores from patients ranging the intensity of new-onset pain and how they perceived this pain to influence their daily activities and enjoyment of life is listed in Table 4. A total of 51% found the pain to have a moderate to severe influence on daily activities, while 53.5% of patients reported pain to have moderate to severe influence on enjoyment of life.
Table 4.
Description of how patients with new-onset pain three months after stroke reported pain to influence their lives
| Question | Na | No or mild, n (%) | Moderate, n (%) | Severe, n (%) | Mean (SD) |
|---|---|---|---|---|---|
| Describe the pain you feel when you are not moving about. | 47 | 21 (44.7) | 18 (38.3) | 8 (17.0) | 3.9 (3.1) |
| Describe the pain you feel when you are moving about. | 47 | 16 (34.0) | 19 (40.4) | 12 (25.5) | 5.0 (3.0) |
| How much has the pain influenced daily activities during the last 24 hours? | 47 | 23 (49.9) | 19 (40.4) | 5 (10.6) | 3.7 (3.1) |
| How much has the pain influenced enjoyment of life for the last 24 hours? | 43 | 20 (46.5) | 14 (32.6) | 9 (20.9) | 3.9 (3.4) |
Notes: No or mild: NRS 0–3, moderate: NRS 4–7, severe: NRS >7.
Not all patients completed rating of the pain.
Abbreviation: NRS, Numeric Rating Scale.
Discussion
This study has shown that pain occurred in about 30% of patients initially after stroke, and one-third of these reported the pain to have occurred after the stroke. Three months later, pain was reported by 40% of the patients, and half of these found that the pain had developed after the stroke. New pain in the affected limbs and the unaffected lower limb was more frequently reported at three months compared to initially after the stroke. Anxiety was significantly associated with new-onset pain three months after stroke. Finally, half of the patients found new-onset pain to have a moderate to severe influence on their daily activities and quality of life.
Only one out of ten patients experienced new-onset pain early after stroke. This contrasts with other research reporting a prevalence ranging from 20% to 37.8% in the early phase after stroke.3,32 New-onset pain in 20% of patients at three months after stroke is similar to what was found by Sommerfeld et al, but lower than reported by Hansen et al who found that new pain occurred in 41.8% at three months after stroke.3,32 Hansen et al asked specifically for different types of pain, which might be one reason for the higher number of patients reporting pain. They also performed a sensory examination, and it is hypothesized that more abnormalities are found when physical examination is performed.33 On the other hand, examinations were also performed in the study by Sommerfeld et al, and they still found a lower prevalence of pain. Differences in the selection of patients between the studies might also explain some of the inconsistencies.
The trend toward more new-onset pain at three months than initially after stroke was more prominent in our study than in the studies by Sommerfeld et al and Hansen et al. Only four patients who reported pain at inclusion to our study still experienced pain at the follow-up. This could imply that a different type of pain develops over time after stroke. We found an increase in new-onset pain in the limbs affected by the stroke, which is in accordance with previous studies on painful shoulder.3,11 We also found that the prevalence of new pain in the hip and leg on both the affected and unaffected sides had increased at three months after stroke. We did not differentiate between nociceptive and neuropathic pain, which would have provided a deeper understanding of the pain that evolves after stroke. However, pain in the affected lower extremity could be caused by altered muscle force and tone,32,34 while increased pain in the unaffected lower extremity could possibly be caused by an alteration in balance or gait pattern.
A relatively small number of patients, 4.9%, experienced new-onset pain in the head/neck. This is less than what is described in the literature, as previous studies have found the occurrence to be around 30%,35–37 and frequency in later stages is found to range from 15.3% to 7.2%.3,4,35 Patients were told to ignore light pain that can occur from time to time, which possibly could explain our low numbers of headache.
Slightly more than half of all patients with new-onset pain at three months after stroke used analgesics regularly. Similar consumption was found in one previous study,3 while others have reported that only 20% of stroke patients with pain use analgesics.4 Comparing this to patients with other chronic conditions, 55% of patients with low back pain in the US had analgesic claims.38 Among community-dwelling elderly people with chronic pain in Finland, analgesics were used daily by approximately 15%, and as required by approximately 60%.39 Hence, the consumption in our group was quite similar to other groups with chronic pain. However, it would have been interesting to know what types of analgesics were used.
Experiencing symptoms of anxiety three months after stroke was associated with simultaneously occurring new-onset pain. Symptoms of depression were also associated with new-onset pain, but the association disappeared after adjusting for anxiety in the multivariable regression model. Previous research has shown that depression and anxiety are correlated,31 but our results indicate that anxiety has a stronger association with new-onset pain after stroke than depression. Our finding is supported by another study, which found that both anxiety and depression were associated with impaired health-related quality of life, but only anxiety was significantly associated with pain after stroke.40 This is noteworthy, especially since anxiety has been sparsely investigated in relation to pain after stroke.
To our knowledge, this is one of few studies conducted in a stroke unit with follow-up data focusing on pain early after stroke. In addition to assessing follow-up data on new pain after stroke, it shows that anxiety interacts with new-onset pain. In line with previous research,3,14 our study confirms that patients report new-onset pain to influence their daily activities and quality of life.3,14 This emphasize the importance of identifying these patients in order to provide adequate help.
When comparing baseline data of patients responding to pain questionnaires in our study with patients registered in the annual report from the Norwegian Stroke Register 2016,41 they were similar concerning the number of infarctions, median age, and median score on NIHSS at admission to hospital, indicating that patients responding were representative of the general Norwegian stroke population
Invited patients who did not respond to the questionnaires were not a random group; these patients were significantly older, had more severe strokes, and were more likely to be women compared to those responding. Severe strokes may impair the ability to communicate, making patient-reported outcomes difficult to measure. We also found that patients with very mild strokes were less likely to be included due to short length of stay in hospital.
The greatest limitation of this study was the late implementation of pain assessment at inclusion, leaving a limited number of patients with complete pain data from both inclusion and three months later. However, when looking at age, gender, premorbid function and stroke severity, it seems that this was a random selection of patients, and the reported frequencies should be regarded as valid.
Pain is, to some degree, constantly present in any population,22 and as pain is a subjective experience, it is difficult to measure for use in research. It is also challenging to differentiate between pain that occurs in relation to the stroke and pain with other causes. We intended to differentiate between new-onset pain and previous pain by asking specifically if pain occurred after the stroke. However, the patients’ ability to evaluate whether the pain occurred after the stroke may not be reliable, as there is a risk of recall bias, in addition to the difficulties evaluating the causes of pain, which in many cases may be complex. This methodological limitation could possibly have been reduced by doing a more thorough examination of the pain and by evaluating different possible causes in each individual. Some previous studies investigating post-stroke pain have additionally investigated if the pain was caused by wounds, fractures, rheumatoid arthritis and so on, and excluded this type of pain.7,32 In future research, a reference group should be included to be able to distinguish between pain that is stroke related and the pain that is not stroke related.14
A more thorough description of the history of pain, including previous or current treatment, such as physiotherapy or rehabilitation related to the pain would give a more thorough understanding of the types of pain that develop after stroke. In addition, when it comes to depression and anxiety, it would be interesting to know about previous history and current treatment, which is not measured by HADS. It is important to remember that HADS simply measures current symptoms and is not a diagnostic tool.
Conclusion
In this study, we found that new-onset pain occurred in one out of ten patients initially after stroke and one out of five patients three months later. In particular, new pain was more frequent in the upper limb on the affected side of the body and lower limbs on both sides at three-month follow-up. Symptoms of anxiety were associated with new-onset pain, and it would be interesting to investigate if interventions to reduce anxiety could have a therapeutic effect on pain. Finally, the finding that more than half of patients with new-onset pain reported the pain to have a moderate to severe influence on daily activities and enjoyment of life is clinically important. Further research should focus on the prevention and the treatment of pain after stroke, and it would be interesting to know more about how anxiety and the use of analgesics interfere with the relationship between new pain and daily activities and enjoyment of life.
Data availability
Because of Norwegian regulations and conditions for informed consent, the data set is not publicly available.
Acknowledgments
The authors want to thank Mari Gunnes and Christine Sandø Lundemo for their contribution in collecting data. We thank Gitta Rohweder for feedback on the manuscript. Finally, we thank all the hospitals and personnel who helped in recruiting patients to this study. The study was funded by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology and the Research Council of Norway.
Footnotes
Author contributions
The study was conceived by MRB, TA, and BI. TA, BI, and AH conducted the study. MRB contributed in collecting data. SL and MRB performed the statistical analyses. SL, MRB, TA, and BI interpreted the results. MRB and TA drafted the manuscript. All the authors critically revised the manuscript for important intellectual content and gave final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Appelros P. Prevalence and predictors of pain and fatigue after stroke: a population-based study. Int J Rehabil Res. 2006;29(4):329–333. doi: 10.1097/MRR.0b013e328010c7b8. [DOI] [PubMed] [Google Scholar]
- 2.Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical Complications in a Comprehensive Stroke Unit and an Early Supported Discharge Service. Stroke. 2008;39(2):414–420. doi: 10.1161/STROKEAHA.107.489294. [DOI] [PubMed] [Google Scholar]
- 3.Hansen AP, Marcussen NS, Klit H, Andersen G, Finnerup NB, Jensen TS. Pain following stroke: a prospective study. Eur J Pain. 2012;16(8):1128–1136. doi: 10.1002/j.1532-2149.2012.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Post-stroke pain on long-term follow-up: the Bergen stroke study. J Neurol. 2010;257(9):1446–1452. doi: 10.1007/s00415-010-5539-y. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson A-C, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77(5):590–595. doi: 10.1136/jnnp.2005.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell MJ, Diener H-C, Sacco RL, et al. Chronic Pain Syndromes After Ischemic Stroke: PRoFESS Trial. Stroke. 2013;44(5):1238–1243. doi: 10.1161/STROKEAHA.111.671008. [DOI] [PubMed] [Google Scholar]
- 7.Lundström E, Smits A, Terént A, Borg J. Risk factors for stroke-related pain 1year after first-ever stroke. Eur J Neurol. 2009;16(2):188–193. doi: 10.1111/j.1468-1331.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- 8.Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6(3):249. [PubMed] [Google Scholar]
- 9.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 10.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Ratnasabapathy Y, Broad J, Baskett J, Pledger M, Marshall J, Bonita R. Shoulder pain in people with a stroke: a population-based study. Clin Rehabil. 2003;17(3):304–311. doi: 10.1191/0269215503cr612oa. [DOI] [PubMed] [Google Scholar]
- 12.Rohweder G, Ellekjaer H, Salvesen O, Naalsund E, Indredavik B. Functional Outcome After Common Poststroke Complications Occurring in the First 90 Days. Stroke. 2015;46(1):65–70. doi: 10.1161/STROKEAHA.114.006667. [DOI] [PubMed] [Google Scholar]
- 13.van Almenkerk S, Depla MFIA, Smalbrugge M, Eefsting JA, Hertogh CMPM. Pain among institutionalized stroke patients and its relation to emotional distress and social engagement. Int J Geriatr Psychiatry. 2015;30(10):1023–1031. doi: 10.1002/gps.4256. [DOI] [PubMed] [Google Scholar]
- 14.Klit H, Finnerup NB, Overvad K, Andersen G, Jensen TS. Pain Following Stroke: A Population-Based Follow-Up Study. PLoS One. 2011;6(11):e27607. doi: 10.1371/journal.pone.0027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naess H, Lunde L, Brogger J. The Triad of Pain, Fatigue and Depression in Ischemic Stroke Patients: The Bergen Stroke Study. Cerebrovasc Dis. 2012;33(5):461–465. doi: 10.1159/000336760. [DOI] [PubMed] [Google Scholar]
- 16.Gureje O, Simon GE, von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92(1):195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 17.Casten RJ, Parmelee PA, Kleban MH, Lawton PM, Katz IR. The relationships among anxiety, depression, and pain in a geriatric institutionalized sample. Pain. 1995;61(2):271–276. doi: 10.1016/0304-3959(94)00185-H. [DOI] [PubMed] [Google Scholar]
- 18.Bondesson E, Pardo FL, Stigmar K, et al. Comorbidity between pain and mental illness - evidence of a bidirectional relationship. Eur J Pain. 2018;22(7):1304–1311. doi: 10.1002/ejp.1218. [DOI] [PubMed] [Google Scholar]
- 19.Castillo RC, Wegener ST, Heins SE, Haythornthwaite JA, Mackenzie EJ, Bosse MJ. Longitudinal relationships between anxiety, depression, and pain: results from a two-year cohort study of lower extremity trauma patients. Pain. 2013;154(12):2860–2866. doi: 10.1016/j.pain.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Burton CAC, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of Anxiety after Stroke: A Systematic Review and Meta-Analysis of Observational Studies. Int J Stroke. 2013;8(7):545–559. doi: 10.1111/j.1747-4949.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang W-K, Lau CG, Mok V, Ungvari GS, Wong K-S. The impact of pain on health-related quality of life 3 months after stroke. Top Stroke Rehabil. 2015;22(3):194–200. doi: 10.1179/1074935714Z.0000000024. [DOI] [PubMed] [Google Scholar]
- 22.Landmark T, Romundstad P, Dale O, Borchgrevink PC, Kaasa S. Estimating the prevalence of chronic pain: Validation of recall against longitudinal reporting (the HUNT pain study. Pain. 2012;153(7):1368–1373. doi: 10.1016/j.pain.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Hokstad A, Indredavik B, Bernhardt J, et al. Hospital Differences in Motor Activity Early after Stroke: A Comparison of 11 Norwegian Stroke Units. J Stroke Cerebrovasc Dis. 2015;24(6):1333–1340. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 25.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 26.Banks JL, Marotta CA. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 27.Collins SL, Moore AR, Mcquay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 29.Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the Beck Depression Inventory, Hospital Anxiety and Depression Scale, SCL-90, and Hamilton Depression Rating Scale as Screening Instruments for Depression in Stroke Patients. Psychosomatics. 2002;43(5):386–393. doi: 10.1176/appi.psy.43.5.386. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale – a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 32.Sommerfeld DK, Welmer A-K. Pain following stroke, initially and at 3 and 18months after stroke, and its association with other disabilities. Eur J Neurol. 2012;19(10):1325–1330. doi: 10.1111/j.1468-1331.2012.03747.x. [DOI] [PubMed] [Google Scholar]
- 33.Dromerick AW, Edwards DF, Kumar A. Hemiplegic Shoulder Pain Syndrome: Frequency and Characteristics During Inpatient Stroke Rehabilitation. Arch Phys Med Rehabil. 2008;89(8):1589–1593. doi: 10.1016/j.apmr.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Tani Y, Otaka Y, Kudo M, Kurayama T, Kondo K. Prevalence of Genu Recurvatum during Walking and Associated Knee Pain in Chronic Hemiplegic Stroke Patients: A Preliminary Survey. J Stroke Cerebrovasc Dis. 2016;25(5):1153–1157. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Hansen AP, Marcussen NS, Klit H, Kasch H, Jensen TS, Finnerup NB. Development of persistent headache following stroke: a 3-year followup. Cephalalgia. 2015;35(5):399–409. doi: 10.1177/0333102414545894. [DOI] [PubMed] [Google Scholar]
- 36.Verdelho A, Ferro JM, Melo T, Canhão P, Falcão F. Headache in Acute Stroke. A Prospective Study in the First 8 Days. Cephalalgia. 2008;28(4):346–354. doi: 10.1111/j.1468-2982.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 37.Tentschert S, Wimmer R, Greisenegger S, Lang W, Lalouschek W. Headache at Stroke Onset in 2196 Patients With Ischemic Stroke or Transient Ischemic Attack. Stroke. 2005;36(2):e1–e3. doi: 10.1161/01.STR.0000151360.03567.2b. [DOI] [PubMed] [Google Scholar]
- 38.Vogt MT, Kwoh CK, Cope DK, Osial TA, Culyba M, Starz TW. Analgesic Usage for Low Back Pain: Impact on Health Care Costs and Service Use. Spine. 2005;30(9):1075–1081. doi: 10.1097/01.brs.0000160843.77091.07. [DOI] [PubMed] [Google Scholar]
- 39.Karttunen NM, Turunen JH, Ahonen RS, Hartikainen SA. Persistence of noncancer-related musculoskeletal chronic pain among community-dwelling older people: a population-based longitudinal study in Finland. Clin J Pain. 2015;31(1):79–85. doi: 10.1097/AJP.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 40.Morris JH, van Wijck F, Joice S, Donaghy M. Predicting health related quality of life 6 months after stroke: the role of anxiety and upper limb dysfunction. Disabil Rehabil. 2013;35(4):291–299. doi: 10.3109/09638288.2012.691942. [DOI] [PubMed] [Google Scholar]
- 41.The Norwegian Stroke Register Annual Report 2016. [Accessed December 14, 2017]. (201720-21). Available from: https://stolav.no/Medisinskekvalitetsregistre/Norsk-hjerneslagregister/%C3%85rsrapport2016-Norsk-hjerneslagregister.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of Norwegian regulations and conditions for informed consent, the data set is not publicly available.