Abstract
A facile, high yielding synthesis of 9,10-dihydro-9,10-ethano- anthracene-11-carboxylic acid methyl ester using a modified commercial domestic microwave oven is reported.
Keywords: 9,10-Dihydro-9,10-ethanoanthracene-11-carboxylic acid methyl ester; modified microwave reactor
Introduction
A considerable amount of research has been devoted to the synthesis of 9,10-dihydro-9,10-ethanoanthracene-11-carboxylic acid methyl ester, a starting material used in many synthetic routes [1]. In 1986, Gedye et al. [2,3] and Giguere et al. [4,5,6] demonstrated that a wide variety of organic reactions can be conducted very rapidly using microwave irradiation. Since then, several other groups have described accelerated organic reactions [7,8,9], but no study of the microwave-assisted synthesis of 9,10-dihydro-9,10-ethanoanthracene-11-carboxylic acid methyl ester has been reported. We wish to report herein a facile high yielding synthesis of this compound in a short time using a modified commercial domestic microwave oven controlled via software developed in this laboratory.
Results and Discussion
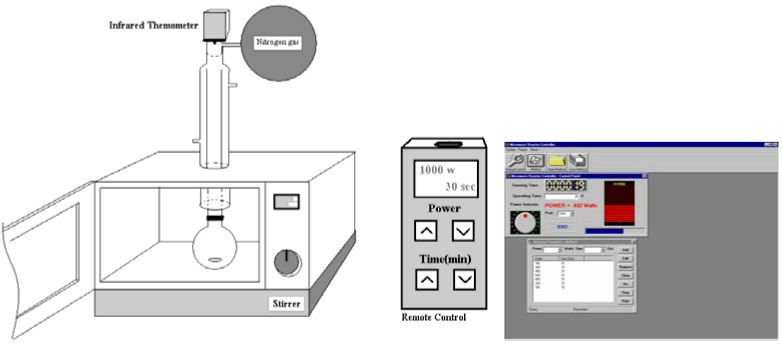
In the reaction reported herein we have used an apparatus devised by our group. It consists of a modified domestic microwave oven perforated on the top to accommodate a reflux condenser and a 10-cm pipe to avoid microwave leakage. Additional modifications include: replacement of the turntable with a magnetic stirrer and addition of an infrared temperature detector and a computer-driven remote for the control of time and incident power. For automatic control, special software was developed using Microsoft® Visual Basic® (version 5) to control the step power and time by program setting via the micro controllers [10]. The software can create timer programs, save methods, name and precisely control the reaction conditions (Figure 1).
Figure 1.
A modified commercial domestic microwave oven, control box and software.
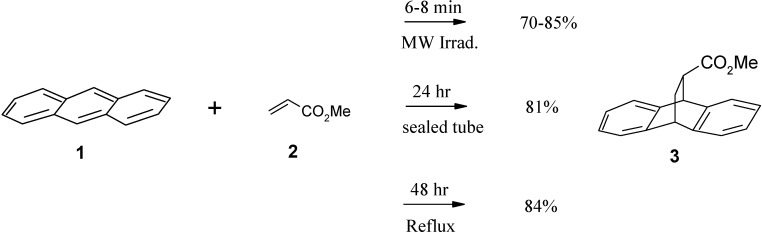
It was found that when a mixture of anthracene (1) and methyl acrylate (2) in xylene (an excellent energy transfer medium) was irradiated (1000 W) in a microwave oven for 6-8 min, compound 3 was obtained in 70-85% yield. The temperature during microwave irradiation was recorded using an infrared thermometer (266-280°C). The classical methods using a sealed tube and general refluxing were also performed using the same molar reagent ratios. The sealed tube was dipped in an oil bath (120°C) and left stirring for 24 hrs, while 48 hrs (120°C) was used for the refluxing method. After purification, compound 3 was obtained in 81 and 84% yield, respectively (Scheme 1). Comparisons between times, temperature and yields are summarized in Table 1. The product 3 was identified by comparing its physical and spectroscopic properties with literature values [11].
Scheme 1.
Table 1.
Comparison between methods, times, temperatures and yields.
| Microwave irradiation | 6-8 | 266-280 | 70-85 |
| Sealed tube | 1440 | 120 | 81 |
| General refluxing | 2880 | 120 | 84 |
[a]: Measured by infrared thermometry.
Conclusions
In summary, we have developed a facile synthesis in a short time and with high yield of the useful starting material 9,10-dihydro-9,10-ethanoanthracene-11-carboxylic acid methyl ester using a modified commercial domestic microwave oven.
Experimental
General
1H-NMR spectra (400 MHz) were obtained in CDCl3 solution using a Bruker AM 400 spectrometer. Proton chemical shifts (δ) are reported in parts per million relative to Me4Si (δ= 0). The IR spectrum was recorded on a Perkin Elmer FT-IR spectrometer. All reagents and chemicals were obtained from Aldrich Chemical Company.
General synthetic procedure
Caution: When carrying out microwave-heated reactions in the reactor, sudden boiling of some liquid may occur [12]. Do not heat any flammable liquids or solids, hazardous substances or radioactive materials in any type of microwave oven, whether domestic or laboratory-type. Do not heat sealed containers in the microwave reactor. The reactor must be electrically grounded and connected using a properly rated three-pin cord and plug. It is recommended to carry out the reactions while microwave reactor is kept in an efficient fume hood.
A mixture of anthracene (2.5 g, 14 mmol), methyl acrylate (1.53 g, 17.8 mmol) and xylene (15 mL) contained in a 100 mL round bottomed flask was placed in the modified microwave oven, the flask was connected to a condenser and subjected to 1000W irradiation for the appropriate time, then it was allowed to cool to room temperature. The product was purified according to the simple method previously reported by W. Phutdhawong et al. [1a]. The pure product was recrystallised from MeOH as colourless crystals (1.25 g, 85%). M.p. 117°C (Lit. [11] 117°C); IR (KBr) (νmax, cm-1): 3100 (=C-H arom.), 1725(C=O), 1460(C=C arom.), 1200 (C-O-C); 1H-NMR (CDCl3) 7.42-7.37 (m, 4H, ArH), 7.38-7.00 (m, 4H, ArH), 4.68 (d, 1H, ArCHCHCOOCH3), 4.26 (t, 1H, ArCHCH2), 3.54 (s, 3H, COOCH3), 2.93 (m, 1H, CH2CHCOOCH3), 2.00 (m, 2H, CHCH2CHCOOCH3). The sealed tube and general refluxing methods were also carried out using the same molar ratios of reagents.
Acknowledgements
The financial support of the Thailand Research Foundation (MRG 4780111) is gratefully acknowledged.
Footnotes
Sample availability: Available from the authors.
References and Notes
- 1.(a) Phutdhawong W., Thongban S., Buddhasukh D. ACGC Chem. Res. Commun. 2002;15:2–5. [Google Scholar]; (b) De Meijere A., Faber D., Heinecke U., Walsh R., Muller T., Apeloig Y. Eur. J. Org. Chem. 2001:663–680. [Google Scholar]; (c) Arena P., Brown R. F. C., Eastwood F. W., McNaughton D. Aust. J. Chem. 1999;52:663–672. [Google Scholar]; (d) Weber E., Hens T., Gallardo O., Csoeregh I. J. Chem. Soc., Perkin Trans. 1996:737–45. [Google Scholar]; (e) Gable R. W., Qureshi A., Schiesser C. H. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1996;C52:674–5. [Google Scholar]; (f) Zhang H., Fried J. Synth. Commun. 1996;26:351–5. [Google Scholar]; (g) Pfister-Guillouzo G., Senio A., Gracian F., Khalid M., Ripoll J., Vallee Y. New J. Chem. 1995;19:1071–80. [Google Scholar]; (h) Dembkowski L., Rachon J. Phosphorus, Sulfur Silicon Relat. Elem. 1994;88:27–37. [Google Scholar]; (i) Okada K., Okubo K., Morita N., Oda M. Tetrahedron Lett. 1992;33:7377–80. [Google Scholar]; (j) Baldwin J. E., Bradley M., Turner N. J., Adlington R. M., Pitt A. R., Sheridan H. Tetrahedron. 1991;47:8203–22. [Google Scholar]; (k) Okada K., Okamoto K., Morita N., Okubo K., Oda M. J. Am. Chem. Soc. 1991;133:9401–2. [Google Scholar]
- 2.Gedye R. N., Smith F. E., Westaway K. C. Can. J. Chem. 1988;66:17. [Google Scholar]
- 3.Gedye R., Smith F., Westaway K., Ali H., Baldisera L., Laberge L., Rousell J. Tetrahedron Lett. 1986;27:279. [Google Scholar]
- 4.Giguere R. J., Bray T. L., Duncan S. M., Majetich G. Tetrahedron Lett. 1986;27:4945. [Google Scholar]
- 5.Giguere R. J., Namen A., Lopez B., Arepally A, Ramos D., Majetich G., Defauw J. Tetrahedron Lett. 1987;28:6563. [Google Scholar]
- 6.Giguere R. J. Organic Synthesis: Theory and Application. Vol. 1. JAI Press; Greenwich: 1989. pp. 103–172. [Google Scholar]
- 7.Berlan J., Giboreau P., Lefeuvre S., Merchand C. Tetrahedron Lett. 1991;32:2363. [Google Scholar]
- 8.Chen S.-T., Chiou S.-H., Wang K.-T. J. Chin. Chem. Soc. 1991;38:85. [Google Scholar]
- 9.Maciej S., Skulski L. Molecules. 2005;10:401–406. doi: 10.3390/10020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.See http://www.science.mju.ac.th/chemistry/research/weerachai/reactor_eng.htm, our Research Unit webpage, for details.
- 11.Bartlett P. D., Tate F. A. J. Am. Chem. Soc. 1953;75:91–92. [Google Scholar]
- 12.Laurence P., Andre L. Tetrahedron. 2001;57:9199–9223. [Google Scholar]