Abstract
Background and objectives
Microneedling therapy is a widely used technique in dermatology. However, little is known about the underlying molecular effects of this therapy on extracellular matrix remodeling, wound healing, and inflammation. The aim of this study was to examine morphological and molecular changes caused by microneedling treatment in a standardized in vitro full-thickness 3D model of human skin.
Materials and methods
A microneedling device was used to treat full-thickness 3D skin models. Specimens were harvested at specified time points and qRT-PCR and microarray studies were performed. Frozen sections were examined histologically.
Results
Microneedling treatment caused morphological changes in the skin model resulting in an almost complete recovery of the epidermis five days after treatment. Microarray analysis identified an upregulation of genes that are associated with tissue remodeling and wound healing (e.g. COL3A1, COL8A1, TIMP3), epithelial proliferation and differentiation (KRT13, IGF1), immune cell recruitment (CCL11), and a member of the heat shock protein family (HSPB6). On the other hand, we detected a downregulation of pro-inflammatory cytokines (e.g. IL1α, IL1β, IL24, IL36γ, IL36RN), and antimicrobial peptides (e.g. S100A7A, DEFB4). These data were confirmed by independent RT-PCR analyses.
Conclusion
We present for the first time the direct molecular effects of microneedling therapy on epidermal keratinocytes and dermal fibroblasts using a standardized 3D skin model. Treatment resulted in histological alterations and changed the expression of various genes related to epidermal differentiation, inflammation, and dermal remodeling. This data suggests that skin microneedling plays a role in dermal remodeling, increases epidermal differentiation, and might also have a direct effect on collagen synthesis. These findings may increase our understanding of the molecular mechanisms of human skin repair induced by microneedling therapy and will allow comparisons with competing applications, such as ablative laser therapies.
Introduction
Skin microneedling therapies are growing in popularity for the treatment of a wide variety of dermatological conditions [1]. This technique has most commonly been used to treat atrophic acne scars [2], striae distensae [3], melasma [4], and to promote skin rejuvenation [5]. Skin microneedling is also used in combination with skin cell transplantation to treat burn victims and for transdermal drug delivery [6–8]. Due to its low cost and easy handling compared to ablative and non-ablative laser therapies, this method is becoming increasingly popular and research in this field recently intensified [9, 10]. Skin treatment procedures vary broadly from chemical techniques, to laser treatments, to surgical interventions [11]. However, most of these treatments are invasive and can cause secondary problems like hyper- or hypopigmentation, especially in patients with darker skin types [12]. Clinical results for potential skin rejuvenation showed promising results with microneedling (1–1.5 mm needle length) similar to those with medical needling (3 mm needle length) [10]. A major advantage of microneedling is that it is less invasive and can be applied under local anesthesia. In microneedling therapy, the epidermis remains relatively intact, which helps to limit post procedural adverse events, such as bleeding, swelling, and pain [13].
Human organotypic 3D skin models have established themselves as a standard method for studying human skin and have revealed interesting and reproducible results after laser induced microwounding [14–17]. We previously developed a standardized human 3D skin model for studying morphological and molecular modifications during wound healing after laser treatment [18]. Until now, little is known about the underlying molecular and histomorphological effects of microneedling treatment on human skin, because changes in the expression of various growth factors (TGFβ1–3, FGF, EGF, VEGF, TNF-α) that promote collagen synthesis have only been described in animal skin biopsies [7]. Therefore, the aim of the present study was to investigate the time-dependent histological and molecular alterations following microneedling treatment in an established human 3D skin model.
Materials and methods
Isolation and culture of normal human epidermal keratinocytes (NHEK) and normal human dermal fibroblasts (NHDF)
NHDF and NHEK were isolated from biopsies of four different donors after cutaneous surgery. The epidermis was separated from the dermis by digestion with dispase (BD Biosciences, Franklin Lakes, NY) and trypsin (Lonza, Basel, Switzerland). Trypsin Neutralization Solution (Lonza) was used for pH-neutralization. The dermal portion of the biopsy was incubated in collagenase 1A (Sigma, Taufkirchen, Germany) to yield a single cell suspension of NHDF. This study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of the University Hospital, RWTH Aachen, Germany. Written informed consent was obtained from the skin donor. Cultivation of NHEK and NHDF was performed as described previously [18].
Scaffold skin equivalents
Matriderm (Medskin Solutions, Suwelack A.G., Billerbeck, Germany) is a 3D bovine collagen–elastin matrix consisting of bovine collagen types I, III, and V. In this study, matrices of 148 x 105 x 1 mm were used. The collagen–elastin matrix was sliced into circular 22 mm punches and transferred into six-well cell culture inserts (BD Falcon, Bedford, MA, USA), then stored under sterile conditions in six-well plates until use. Matriderm scaffolds were inoculated with 3 x 105 NHDF per cm2 in Tisseel (Baxter, Derfield, IL, USA) and submersed with fibroblast growth medium used for dermal scaffold skin equivalents. After three days, 3 x 106 NHEK were seeded on top of each dermal equivalent. Skin equivalents were submersed in equal volumes of DMEM and keratinocyte growth medium with 5% fetal calf serum (FCS), 50 μg ascorbic acid, and 5 μg/ml aprotinin (Applichem, Chicago, IL, USA). On the following day, skin equivalents were lifted to the air–liquid interface. The calcium concentration of the culture medium was increased to 1.0 mM and medium was changed every other day [18].
Skin needling
3D skin models were treated with an eDermastamp (Dermaroller GmbH, Wolfenbüttel, Germany) using a six needle plate (1.5 NM615LS16309, Amiea Med). One hundred insertions were made per second at a penetration depth of 1.0 mm and with three passes, according to the manufacturer´s and clinical treatment recommendations. After treatment, the models were cultivated in fresh culture medium and harvested on day 5 for histological analysis and detection of gene expression. Untreated models were maintained as negative controls. All experiments were performed in triplicate for every time point.
RNA isolation
Total RNA was isolated using the Nucleo Spin RNA Kit (Macherey and Nagel, Düren, Germany) according to the manufacturer’s instructions. RNA isolation included on-column digestion of DNA with RNase-free DNase I. The RNA was quantified by photometric measurement (NanoDrop Technologies, Wilmington, DE, USA) and its integrity was analyzed on a 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Purified RNA was reversed transcribed with SS VILO Mastermix (Life Technologies) according the manufacturer’s instructions. TaqMan experiments were carried out on an ABI Prism 7,300 sequence detection system (Applied Biosystems, Weiterstadt, Germany) using Assays-on-Demand gene expression products for CCL11 (Hs00237013_m1), IGF1 (Hs01547656_m1), TIMP3 (Hs00165949_m1), KRT13 (Hs00357961_g1), COL3A1 (Hs00943809_m1), HSPB6 (Hs00328933_m1), COL8A1 (Hs00156669_m1), IL1α (Hs00174092_m1), IL1β (Hs00174097_m1), IL24 (Hs01114274_m1), IL36γ (Hs00219742_m1), IL36RN (Hs_00202179_m1), S100A7A (00752780_s1), and DEFB4 (Hs00823638_m1), according to the manufacturer’s recommendations. An Assay-on-Demand product for HPRT (Hs99999909) was used as an internal reference to normalize the target transcripts. All measurements were performed in triplicate in separate reaction wells.
Gene expression analysis using exon expression arrays
Purified mRNA was analyzed on GeneChip Human Gene 2.0 ST arrays as reported previously [18] using Gene-SpringGX software, version 14.9 (Agilent Technologies, Frankfurt am Main, Germany). Gene ontology (GO) analysis was performed using http://www.gene-ontology.org/.
Light microscopy
For light microscopy, 4 μm cryosections of skin equivalents were embedded in Tissue Tec OCT and stained with hematoxylin and eosin. Sections were examined by a photomicroscope (DMIL, Leitz, Wetzlar, Germany).
Statistical analysis
Data are given as arithmetical means ± standard deviation and were analyzed with the Mann-Whitney U test using GraphPad PRISM, version 7 (La Jolla, CA, USA). P values <0.05 were considered statistically significant.
Results
To investigate the effects of microneedling therapy on skin morphology, we established full-thickness human 3D skin equivalents containing dermal and epidermal structures, including a functional stratum corneum, a basal layer, and a basal membrane. Fig 1 depicts representative images of 3D skin models directly after microneedling and five days later compared with untreated controls. Histological examination revealed clearly defined lesions of the epidermis and dermis immediately after microneedling treatment, whereas dermal and epidermal structures were almost totally restored after five days.
Fig 1. Representative hematoxylin and eosin stained sections of 3D skin models zero and five days after microneedling treatment.
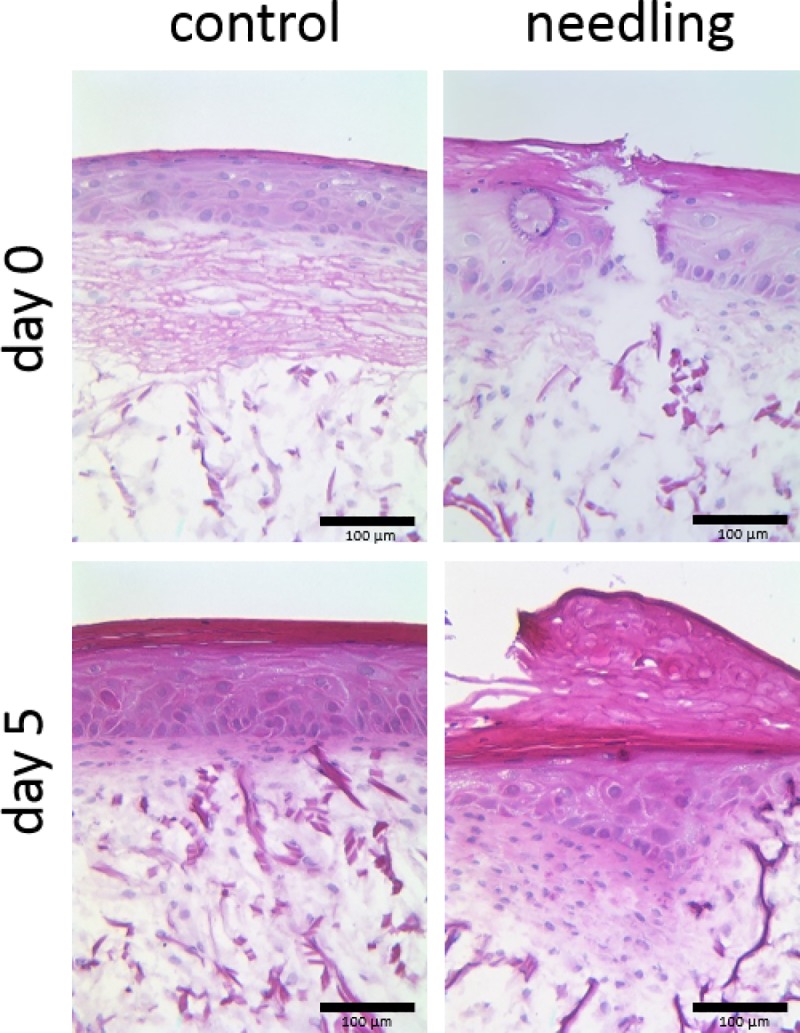
Untreated models are shown as controls. Magnification = 100 x, scale bar = 100 μm.
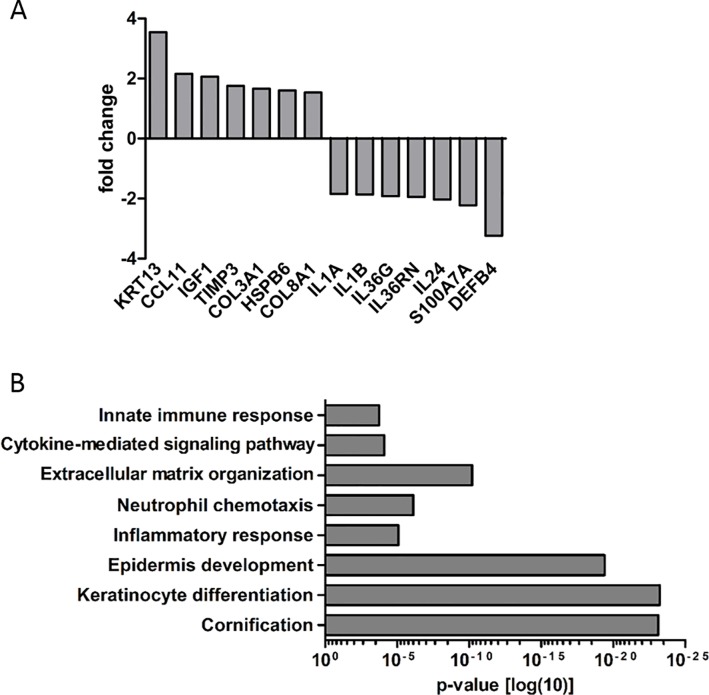
To our knowledge, the molecular effects of microneedling on human skin are poorly defined. In the present study, we conducted a transcriptomic microarray profiling of 3D skin models (n = 3) five days after microneedling (Fig 2A). Gene array analysis revealed a >1.5-fold upregulation of genes associated with tissue remodeling and wound healing (TIMP3, COL3A1, COL8A1), epithelial proliferation and differentiation (KRT13, IGF1), immune cell recruitment (CCL11) and an upregulation of heat shock protein (HSP) B6 in microneedling-treated skin models compared with untreated controls. On the other hand, we detected a downregulation of different cytokines (IL1A, IL1B, IL36G, IL36RN, IL24) as well as antimicrobial peptides (S100A7A, DEFB4) in microneedling-treated skin models compared with untreated controls. Additionally, GO analysis confirmed an impact of microneedling treatment on biological processes such as “cornification”, “keratinocyte differentiation”, “epidermis development”, “inflammatory response”, and “extracellular matrix organization” (Fig 2B). To verify these findings, we used RT-PCR analysis to measure the expression of selected genes in four independent approaches (Fig 3). In general, RT-PCR analysis confirmed up- and downregulation of all genes. The downregulation of IL36G, S100A7A, and DEFB4 in microneedling-treated models was particularly significant.
Fig 2. Gene expression profiling in microneedling-treated 3D skin models (microarray analysis).
(A) 3D skin models were harvested five days after microneedling treatment. Gene expression was measured using the Affymetrix Gene Chip Human Exon 2.0 ST array. (B) Gene ontology (GO) analysis of microarray results.
Fig 3. TaqMan real-time PCR analysis of four independent approaches displaying the expression of selected genes five days after microneedling treatment.Ctrl = control.
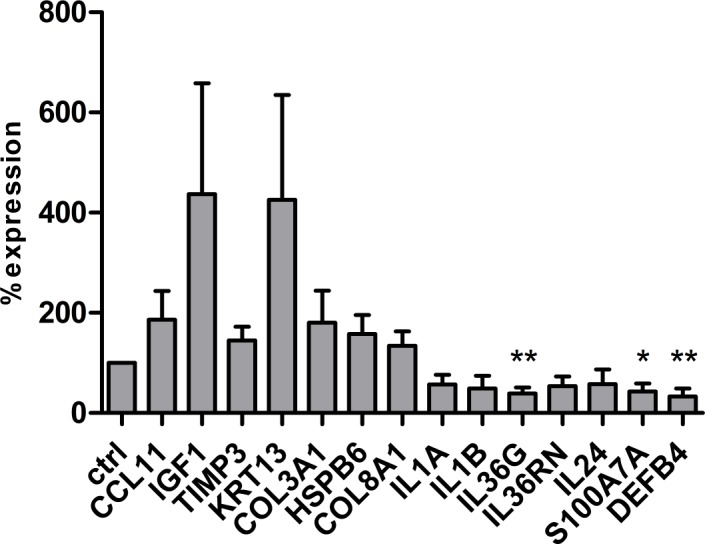
*p < 0.05, **p < 0.01.
Discussion
Skin microneedling has been used to successfully treat acne scars [2] and striae distensae [3], and to promote skin rejuvenation [8]. It has also been used in combination with skin cell transplantation, in transdermal drug delivery, and during pretreatment with photodynamic therapy (PDT) [6, 7, 19]. In a case of melasma, upper dermal neocollagenesis, restoration of basal membrane, and epithelial acanthosis were observed after two sessions of gentle microneedling, reinforcing the hypothesis that microneedling can induce repair [20]. This evolving technique is considered minimally invasive with low costs and a high safety profile [9]. Nevertheless, little is known about the underlying molecular effects of microneedling therapy on the skin.
So far, microneedling has been clinically evaluated in animal skin [7] or in individual human skin biopsies [21] only revealing changes in growth factor expression associated with the de novo synthesis of collagen (e.g. TGFβ1–3, FGF, EGF, VEGF, TNF-α) [7]. To our knowledge, the present study is the first report of the effects of microneedling therapy in a standardized in vitro human 3D skin model. In previous studies, we could reveal that human skin equivalents are a suitable standardized in vitro tool for detecting the ex vivo effects of various laser systems on skin physiology, skin morphology, and gene regulation [14, 18, 22]. Full-thickness skin models seemed to be suitable for studying the effects of microneedling therapy, particularly on deeper dermal layers. Therefore we applied this model system to systematically analyze time-dependent histological and molecular alterations in the skin following microneedling therapy.
Using microarray and RT-PCR analyses, our findings revealed a downregulation of pro-inflammatory cytokines such as IL1α, IL1β, IL24, IL36RN, and IL36γ as well as antimicrobial peptides (AMPs) such as S100A7A and DEFB4 in microneedling-treated skin models. The wound healing process consists of an acute inflammatory phase, reepithelization, and remodeling of the dermal extracellular matrix [23]. Initiating and perpetuating the inflammatory response involves many molecules, including cytokines and AMPs [24]. Acute inflammation usually lasts for 2–5 days and ceases once the harmful stimuli have been removed [24], which is consistent with our finding that pro-inflammatory cytokines and AMPs are downregulated in 3D skin models five days after microneedling. This downregulation may act as a wound-related signal to stimulate reepithelization.
On the other hand, we detected an upregulation of the known chemokine eotaxin (CCL11). CCL11 regulates cell activation and contributes to angiogenesis [25]. Overall, changes in interleukin and chemokine expression may modify the formation and structural integrity of the epidermis after needling therapy and thereby contribute to neocollagenesis and wound healing.
Matrix metalloproteinases (MMPs) are involved in the remodeling of abnormal scars and also influence other wound healing responses, such as inflammation and reepithelization [26–28]. Tissue inhibitors of metalloproteinase (TIMPs) are downregulated in hypertrophic scars [29]. Interestingly, we detected an upregulation of TIMP3 after microneedling therapy.
A study by Dohi et al. showed that downregulation of TIMP2 contributes to the progression and development of keloids suggesting that higher levels of TIMPs may be beneficial to the reduction of the thick dermis and collagen bundles seen in keloids [30]. In this context, microneedling induced expression of TIMP3 could suggest a positive effect of this therapy in the treatment of hypertrophic scars.
Furthermore, we detected an upregulation of insulin-like growth factor 1 (IGF-1) in our skin model five days after microneedling. Lewis et al. [31] showed that IGF-1 expression in human dermal fibroblasts is triggered by stress, such as UVB-induced DNA damage, which alters the protective stress response of epidermal keratinocytes. In our study, IGF-1 expression was upregulated after microneedling treatment. This may represent a response to stress-induced cell damage that induces DNA-repair mechanisms. The highly ordered process of wound healing comprises the coordinated regulation of cell proliferation and migration as well as tissue remodeling. These processes are predominantly regulated by polypeptide growth factors, such as members of the insulin growth factor family [32]. The importance of IGF-1 in wound healing has already been shown in several studies [33, 34]. Our findings are in line with those of studies that show increased IGF-1 expression during wound repair processes and support the hypothesis that IGF-1 signaling is required for efficient re-epithelialization and wound healing [24, 34].
It has already been clinically demonstrated that microneedling can induce collagen synthesis following aesthetic surgery and may represent an alternative to laser surgery [35]. Matrix remodeling following the inflammatory phase of wound healing is characterized by collagen synthesis [36]. In this context, we detected an upregulation in the expression of genes that are related to collagen synthesis (COL3A1, COL8A1) five days after microneedling.
Moreover, expression of HSPs, such as HSPB6, was higher after microneedling therapy in treated skin models than in untreated controls. HSPs were first described as proteins that protect cells following heat stress-related damage [37, 38]. Since then, it has been hypothesized that HSPs also modulate wound contraction in fibroblast cell lines after wound infliction [39]. Previous studies have shown increased HSP expression after ablative fractional resurfacing treatment, suggesting that the heat shock response was most likely due to the thermal effects of the laser treatment [37, 40]. Beside their protective functions, accumulating evidence indicates that HSPs are also involved in tissue remodeling and wound healing [40–42]. Our findings support the theory that HSPs play an important role in dermal remodeling after microneedling treatment.
Interestingly, we found an increased expression of the known epithelial cell differentiation markers keratin 13 (KRT13) after microneedling treatment. Wound healing is not fully completed five days after microneedling; therefore the upregulation of this marker in our model may reflect the ongoing differentiation of epithelial cells.
It is worth mentioning that microneedling treatment in 3D models causes similar changes in the expression of differentiation markers as ablative laser therapy using a Er:YAG laser [22, 43]. This may be explained by the fact that both treatments completely remove the epidermis, which then needs to be fully re-developed. In agreement with the observed changes in gene expression, we found an impact of microneedling therapy on biological processes like “cornification”, “keratinocyte differentiation”, “epidermis development”, “inflammatory response”, and “extracellular matrix organization”. These data support the impact of microneedling on wound healing, re-epithelialization, and skin rejuvenation.
The human 3D model system used in the present study is a useful tool for studying physiology, morphology, and time-dependent gene expression after microneedling treatment. The corium is fully developed and allows the effects of microneedling on the collagen structure in deeper skin layers to be examined. Molecular mechanisms underlying the proliferative effect of e.g. pantothenate or laser treatments were previously investigated by global gene expression analysis (microarray analysis) in cultured human dermal fibroblasts (in vitro) and in a clinical trial (in vivo) [18, 22, 44]. A limitation of this simplified human in vitro 3D skin model, which contains only two cell types (keratinocytes and fibroblasts), is that it is not able to mimic the complex requirements of in vivo conditions. However, the advantage is that the observed changes in gene expression can be specifically attributed to changes in keratinocytes and fibroblasts. In summary, our findings have revealed that microneedling therapy in a 3D human skin model induces histological alterations and changes the expression of various genes related to epidermal differentiation, inflammation, and dermal remodeling. Based on our results, we assume that microneedling therapy stimulates collagen synthesis, which may be beneficial for skin rejuvenation or the treatment of atrophic scars. Further in vitro studies with novel 3D skin models which additionally also contain macrophages appear to be useful in order to take into account the indirect effects of the microneedling therapy on inflammatory cells.
Supporting information
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119425.
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bonati LM, Epstein GK, Strugar TL. Microneedling in All Skin Types: A Review. J Drugs Dermatol. 2017;16(4):308–13. . [PubMed] [Google Scholar]
- 2.Cachafeiro T, Escobar G, Maldonado G, Cestari T, Corleta O. Comparison of Nonablative Fractional Erbium Laser 1,340 nm and Microneedling for the Treatment of Atrophic Acne Scars: A Randomized Clinical Trial. Dermatol Surg. 2016;42(2):232–41. 10.1097/DSS.0000000000000597 . [DOI] [PubMed] [Google Scholar]
- 3.Nassar A, Ghomey S, El Gohary Y, El-Desoky F. Treatment of striae distensae with needling therapy versus microdermabrasion with sonophoresis. Journal of cosmetic and laser therapy: official publication of the European Society for Laser Dermatology. 2016;18(6):330–4. 10.1080/14764172.2016.1175633 . [DOI] [PubMed] [Google Scholar]
- 4.Lima Ede A. Microneedling in facial recalcitrant melasma: report of a series of 22 cases. An Bras Dermatol. 2015;90(6):919–21. 10.1590/abd1806-4841.20154748 ; PubMed Central PMCID: PMC4689089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Yadav S. Microneedling: Advances and widening horizons. Indian dermatology online journal. 2016;7(4):244–54. 10.4103/2229-5178.185468 ; PubMed Central PMCID: PMC4976400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch KH, Bender R, Walezko N, Aziz H, Altintas MA, Aust MC. Combination of medical needling and non-cultured autologous skin cell transplantation (renovacell) for repigmentation of hypopigmented burn scars in children and young people. Annals of burns and fire disasters. 2016;29(2):116–22. ; PubMed Central PMCID: PMC5241190. [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitter S, Sikora Z, Jahn S, Stahl F, Strauss S, Lazaridis A, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns: journal of the International Society for Burn Injuries. 2014;40(5):966–73. 10.1016/j.burns.2013.12.008 . [DOI] [PubMed] [Google Scholar]
- 8.Spencer JM, Freeman SA. Microneedling Prior to Levulan PDT for the Treatment of Actinic Keratoses: A Split-Face, Blinded Trial. J Drugs Dermatol. 2016;15(9):1072–4. . [PubMed] [Google Scholar]
- 9.Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59(4):659–76. 10.1016/j.jaad.2008.05.029 . [DOI] [PubMed] [Google Scholar]
- 10.Hou A, Cohen B, Haimovic A, Elbuluk N. Microneedling: A Comprehensive Review. Dermatol Surg. 2017;43(3):321–39. 10.1097/DSS.0000000000000924 . [DOI] [PubMed] [Google Scholar]
- 11.Fabbrocini G, Fardella N, Monfrecola A, Proietti I, Innocenzi D. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34(8):874–9. 10.1111/j.1365-2230.2009.03291.x . [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Maldonado R, Orozco-Covarrubias ML. Postinflammatory hypopigmentation and hyperpigmentation. Seminars in cutaneous medicine and surgery. 1997;16(1):36–43. . [DOI] [PubMed] [Google Scholar]
- 13.Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26(2):192–9. 10.1016/j.clindermatol.2007.09.006 . [DOI] [PubMed] [Google Scholar]
- 14.Neis MM, Wendel A, Wiederholt T, Marquardt Y, Joussen S, Baron JM, et al. Expression and induction of cytochrome p450 isoenzymes in human skin equivalents. Skin Pharmacol Physiol. 2010;23(1):29–39. 10.1159/000257261 . [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Lüscher-Firzlaff J, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2012;129(2):426–33, 33.e1-8. 10.1016/j.jaci.2011.10.042 . [DOI] [PubMed] [Google Scholar]
- 16.Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev. 2014;69–70:1–18. 10.1016/j.addr.2014.10.006 PubMed PMID: 25451857. [DOI] [PubMed] [Google Scholar]
- 17.Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69–70:81–102. 10.1016/j.addr.2014.10.006 PubMed PMID: 25451857. [DOI] [PubMed] [Google Scholar]
- 18.Marquardt Y, Amann PM, Heise R, Czaja K, Steiner T, Merk HF, et al. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med. 2015;47(3):257–65. Epub 2015/03/15. 10.1002/lsm.22341 . [DOI] [PubMed] [Google Scholar]
- 19.Bay C, Lerche CM, Ferrick B, Philipsen PA, Togsverd-Bo K, Haedersdal M. Comparison of Physical Pretreatment Regimens to Enhance Protoporphyrin IX Uptake in Photodynamic Therapy: A Randomized Clinical Trial. JAMA Dermatol. 2017;153(4):270–8. 10.1001/jamadermatol.2016.5268 . [DOI] [PubMed] [Google Scholar]
- 20.Lima EVA, Lima M, Paixao MP, Miot HA. Assessment of the effects of skin microneedling as adjuvant therapy for facial melasma: a pilot study. BMC dermatology. 2017;17(1):14 10.1186/s12895-017-0066-5 ; PubMed Central PMCID: PMC5706369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helbig D, Mobius A, Simon JC, Paasch U. Heat shock protein 70 expression patterns in dermal explants in response to ablative fractional phothothermolysis, microneedle, or scalpel wounding. Wounds. 2011;23(3):59–67. . [PubMed] [Google Scholar]
- 22.Schmitt L, Amann PM, Marquardt Y, Heise R, Czaja K, Gerber PA, et al. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med Sci. 2017;32(4):805–14. 10.1007/s10103-017-2175-0 . [DOI] [PubMed] [Google Scholar]
- 23.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. 10.1159/000339613 . [DOI] [PubMed] [Google Scholar]
- 24.Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–85. 10.1007/s00018-016-2268-0 ; PubMed Central PMCID: PMC5021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166(12):7571–8. . [DOI] [PubMed] [Google Scholar]
- 26.Shah JM, Omar E, Pai DR, Sood S. Cellular events and biomarkers of wound healing. Indian J Plast Surg. 2012;45(2):220–8. 10.4103/0970-0358.101282 ; PubMed Central PMCID: PMCPMC3495371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–47. Epub 2007/10/26. 10.1016/j.biocel.2007.10.024 ; PubMed Central PMCID: PMCPMC2746915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153(2):295–300. 10.1111/j.1365-2133.2005.06698.x . [DOI] [PubMed] [Google Scholar]
- 29.Tsou R, Cole JK, Nathens AB, Isik FF, Heimbach DM, Engrav LH, et al. Analysis of hypertrophic and normal scar gene expression with cDNA microarrays. The Journal of burn care & rehabilitation. 2000;21(6):541–50. . [DOI] [PubMed] [Google Scholar]
- 30.Dohi T, Miyake K, Aoki M, Ogawa R, Akaishi S, Shimada T, et al. Tissue Inhibitor of Metalloproteinase-2 Suppresses Collagen Synthesis in Cultured Keloid Fibroblasts. Plastic and reconstructive surgery Global open. 2015;3(9):e520 10.1097/GOX.0000000000000503 ; PubMed Central PMCID: PMC4596445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis DA, Travers JB, Somani AK, Spandau DF. The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29(10):1475–85. 10.1038/onc.2009.440 ; PubMed Central PMCID: PMC2837099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bitar MS, Al-Mulla F. ROS constitute a convergence nexus in the development of IGF1 resistance and impaired wound healing in a rat model of type 2 diabetes. Disease models & mechanisms. 2012;5(3):375–88. 10.1242/dmm.007872 ; PubMed Central PMCID: PMC3339831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190(5):589–94. . [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Wang Y, Menendez A, Fong C, Babey M, Tahimic CG, et al. Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired Endochondral Bone Formation During Fracture Healing. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2015;30(9):1572–84. 10.1002/jbmr.2510 ; PubMed Central PMCID: PMC5690481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthetic surgery journal. 2002;22(3):307–9. 10.1067/maj.2002.126195 . [DOI] [PubMed] [Google Scholar]
- 36.Orringer JS, Sachs DL, Shao Y, Hammerberg C, Cui Y, Voorhees JJ, et al. Direct quantitative comparison of molecular responses in photodamaged human skin to fractionated and fully ablative carbon dioxide laser resurfacing. Dermatol Surg. 2012;38(10):1668–77. Epub 2012/07/17. 10.1111/j.1524-4725.2012.02518.x . [DOI] [PubMed] [Google Scholar]
- 37.Helbig D, Mobius A, Simon JC, Paasch U. Heat shock protein 70 expression patterns in dermal explants in response to ablative fractional phothothermolysis, microneedle, or scalpel wounding. Wounds. 2011;23(3):59–67. . [PubMed] [Google Scholar]
- 38.Maytin EV. Heat shock proteins and molecular chaperones: implications for adaptive responses in the skin. J Invest Dermatol. 1995;104(4):448–55. . [DOI] [PubMed] [Google Scholar]
- 39.Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell stress & chaperones. 2004;9(1):29–37. 10.1379/471.1 ; PubMed Central PMCID: PMC1065303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, et al. Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem. 1998;46(11):1291–301. 10.1177/002215549804601109 . [DOI] [PubMed] [Google Scholar]
- 41.Souil E, Capon A, Mordon S, Dinh-Xuan AT, Polla BS, Bachelet M. Treatment with 815-nm diode laser induces long-lasting expression of 72-kDa heat shock protein in normal rat skin. Br J Dermatol. 2001;144(2):260–6. . [DOI] [PubMed] [Google Scholar]
- 42.Zhou JD, Luo CQ, Xie HQ, Nie XM, Zhao YZ, Wang SH, et al. Increased expression of heat shock protein 70 and heat shock factor 1 in chronic dermal ulcer tissues treated with laser-aided therapy. Chin Med J (Engl). 2008;121(14):1269–73. . [PubMed] [Google Scholar]
- 43.Amann PM, Marquardt Y, Steiner T, Hölzle F, Skazik-Voogt C, Heise R, et al. Effects of non-ablative fractional erbium glass laser treatment on gene regulation in human three-dimensional skin models. Lasers Med Sci. 2016;31(3):397–404. Epub 2016/01/21. 10.1007/s10103-015-1863-x . [DOI] [PubMed] [Google Scholar]
- 44.Heise R, Skazik C, Marquardt Y, Czaja K, Sebastian K, Kurschat P, et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol. 2012;25(5):241–8. 10.1159/000341144 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119425.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.