Abstract
We characterized the establishment of an Epidermal Growth Factor Receptor (EGFR) organizing center (EOC) during leg development in Drosophila melanogaster. Initial EGFR activation occurs in the center of leg discs by expression of the EGFR ligand Vn and the EGFR ligand-processing protease Rho, each through single enhancers, vnE and rhoE, that integrate inputs from Wg, Dpp, Dll and Sp1. Deletion of vnE and rhoE eliminates vn and rho expression in the center of the leg imaginal discs, respectively. Animals with deletions of both vnE and rhoE (but not individually) show distal but not medial leg truncations, suggesting that the distal source of EGFR ligands acts at short-range to only specify distal-most fates, and that multiple additional ‘ring’ enhancers are responsible for medial fates. Further, based on the cis-regulatory logic of vnE and rhoE we identified many additional leg enhancers, suggesting that this logic is broadly used by many genes during Drosophila limb development.
Author summary
The EGFR signaling pathway plays a major role in innumerable developmental processes in all animals and its deregulation leads to different types of cancer, as well as many other developmental diseases in humans. Here we explored the integration of inputs from the Wnt- and TGF-beta signaling pathways and the leg-specifying transcription factors Distal-less and Sp1 at enhancer elements of EGFR ligands. These enhancers trigger a specific EGFR-dependent developmental output in the fly leg that is limited to specifying distal-most fates. Our findings suggest that activation of the EGFR pathway during fly leg development occurs through the activation of multiple EGFR ligand enhancers that are active at different positions along the proximo-distal axis. Similar enhancer elements are likely to control EGFR activation in humans as well. Such DNA elements might be ‘hot spots’ that cause formation of EGFR-dependent tumors if mutations in them occur. Thus, understanding the molecular characteristics of such DNA elements could facilitate the detection and treatment of cancer.
Introduction
cis-regulatory modules (CRMs) are critical for the development and evolution of all organisms. CRMs integrate the information that a single cell or group of cells receives and, in response, trigger changes in cellular and tissue fate specification [reviewed in 1]. The Drosophila melanogaster leg imaginal disc, which gives rise to the entire leg and ventral body wall of the adult fly, provides an attractive model system for studying the molecular mechanisms of cellular fate integration at the CRM level and the consequent execution of developmental programs that pattern an entire appendage [reviewed in 2]. Leg imaginal discs are initially specified early during embryonic development through the activation of the transcription factors Distal-less (Dll) and Sp1 in distinct groups of cells in each thoracic segment [3–5]. These groups of cells segregate from the embryonic ectoderm to become the leg imaginal discs, the precursors of the adult legs and ventral thorax. During larval stages, the leg discs proliferate, and defined expression domains of the signaling molecules Wg (ventrally expressed) and Dpp (dorsally expressed) activate Dll through the DllLT CRM in the center of the leg imaginal disc, where the wg and dpp expression patterns abut each other (Fig 1A) [6–9]. Slightly later in development, medial leg fates are established by the feed-forward activation of dachshund (dac) by Dll through the dacRE CRM [2, 10]. During subsequent growth of the leg disc, partially overlapping Dll and dac expression domains are maintained by autoregulation. These Dll and dac expression domains, together with the most proximal domain marked by homothorax (hth) expression, create a rudimentary proximal-distal (PD) axis [2] (Fig 1A).
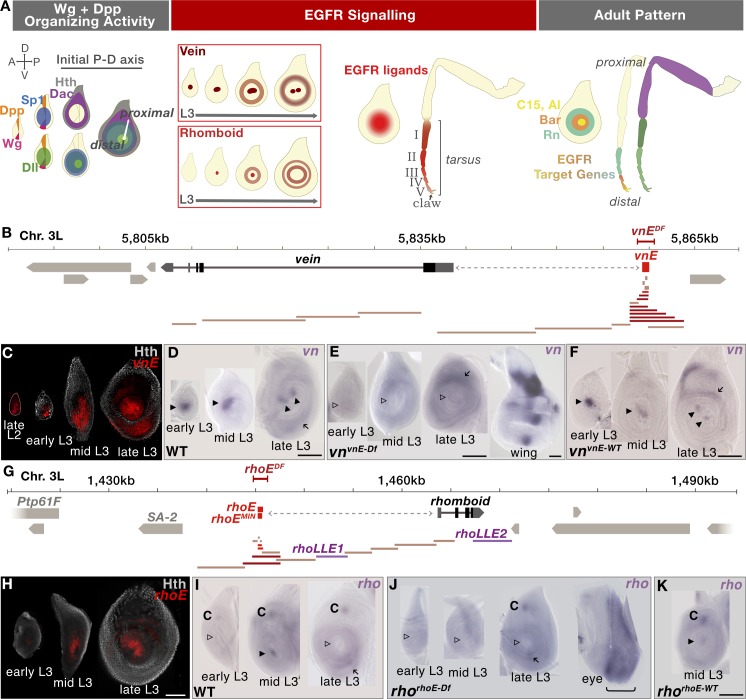
Fig 1. EOC generation in third instar leg discs.
(A) Schematic representation of the establishment of an initial PD axis and EGFR signaling events in the center of leg discs (the number of rings depicted is not meant to be accurate). (B) Schematic representation of the vn genomic locus on chromosome 3L; enhancer bashing fragments for identification of vnE represented in tan did not drive expression in leg discs, dark red drove expression in leg discs and bright red designates the minimal enhancer used for further analysis; the vnvnE-Df CRISPR deletion is represented by red bracketed bar. (C) Time-course analysis of the expression pattern of vnE-lacZ reporter gene. In young discs, expression is limited to the EOC, while in late L3 additional expression appears in medial rings. (D-F) In situ analysis of vn expression in 3rd instar leg discs with genotypes: WT (D), vnvnE-Df (E; a wing disc from the same genotype serves as a positive control). vnvnE-WT, in which Recombination mediated cassette exchange (RMCE) was used to re-introduce the wild type CRM (F). Arrowheads indicate the presence (filled) or absence (open) of EOC vn expression, arrows indicate non-EOC medial expression. (G) Schematic representation of the rho genomic locus on chromosome 3L; enhancer bashing fragments for identification of rhoE represented in tan did not drive expression in leg discs, dark red drove expression in leg discs and bright red designates the minimal enhancers used for further analysis; rhoEMIN was only used for enhancer mutagenesis; rhorhoE-Df CRISPR deletion is represented by the red bracketed bar. (H) Time-course analysis of the expression pattern of rhoE-lacZ reporter gene. In young discs, expression is limited to the EOC, while in late L3 additional expression appears in medial rings. (I-K) In situ analysis of the rho expression pattern in 3rd instar leg disc with genotype: WT (I), rhorhoE-Df (J, an eye disc from the same genotype serves as a positive control), rhorhoE-WT, in which RMCE was used to re-introduce the wild type CRM (K). Arrowheads indicate presence (filled) or absence (open) of EOC rho expression, arrows indicate medial expression and “C” indicates chordotonal organ precursor expression. Scale bar = 100μm in all figures.
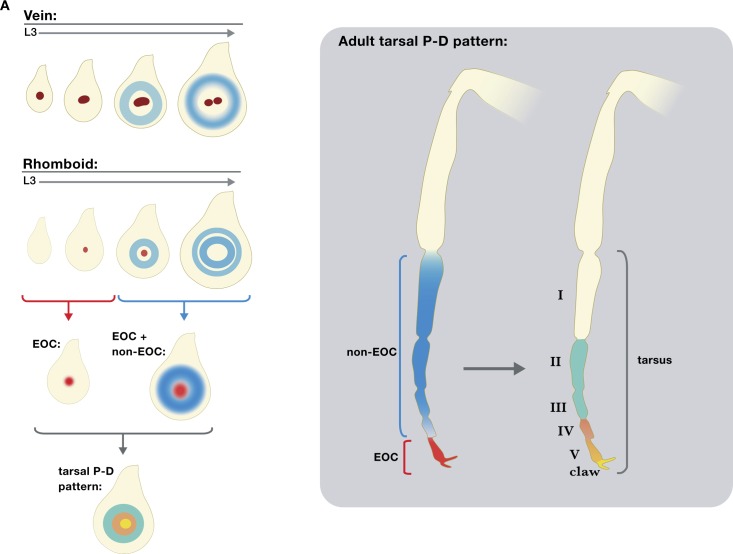
The initial PD axis defined by Dll, Dac, and Hth is further refined by an additional signaling cascade mediated by the Epidermal Growth Factor Receptor (EGFR) pathway [11, 12]. Like Dll, the EGFR pathway is initially triggered by Wg and Dpp, which activate two types of EGFR ligands in the center of the leg disc [11, 12]. One is the neuregulin-related ligand Vein (Vn) and the second is the TGF-α-like ligand Spitz (Spi), which requires metalloproteases of the Rhomboid (Rho) family for processing and secretion [reviewed in 13]. The local activation of vn and rho family members in the center of the leg disc creates an EGFR organizing center (EOC), a local source of secreted Vn and Spi that activate EGFR signaling in neighboring cells. EGFR signaling in turn results in the activation of a series of downstream target genes that are expressed in nested concentric domains that pattern the future tarsus, the distal-most region of the adult leg [11, 12, 14, 15] (Fig 1A).
The mechanism by which EGFR signaling patterns the distal leg is not fully understood. One model suggests that EGFR ligands, produced in the EOC, function as morphogens, acting on neighboring cells to generate distinct transcriptional outputs in a concentration-dependent manner. Consistent with this idea is the observation that gradually reducing EGFR activity by raising flies carrying a temperature-sensitive Egfr allele (Egfrtsla) at increasing temperatures results in gradually more severe leg truncations [11]. However, although consistent with a morphogen model, this result is complicated by the fact that in addition to the EOC, there are other sources of EGFR ligands expressed in rings that appear later in leg development [14]. This additional EGFR activation would also be compromised in Egfrtsla experiments, leaving open the question of the degree to which tarsal PD patterning is due solely to EOC activity. An alternative model posits that the activation of EGFR in the center of the leg disc triggers only local transcriptional outputs, and that alternative sources of EGFR ligands, in combination with indirect transcriptional cascades, are responsible for specifying fates that are further from the EOC.
The EOC/morphogen model predicts that eliminating the production of EGFR ligands from the EOC will have long-range consequences. In contrast, if alternative, non-EOC sources of EGFR ligands play a role in leg patterning, eliminating only the EOC would produce only local defects in distal leg patterning. To distinguish between these models, we searched for CRMs responsible for the expression of EGFR ligands and ligand-processing proteases in the EOC, with the idea that we could specifically eliminate EOC expression by deleting these CRMs. We identified EOC CRMs for vn and rho (vnE and rhoE, respectively) and showed that they are necessary for EOC expression of these genes, respectively. However, although EOC expression is eliminated, simultaneous deletion of these CRMs causes only local PD patterning defects and tarsal truncations comparable to mild Egfr perturbations in the distal tarsus. These results suggest that the EOC is required for activating local EGFR responses in the center of the leg disc, implying that other sources of EGFR ligands, controlled by non-EOC CRMs, further elaborate the tarsal PD pattern. Finally, we also performed rigorous genetic and biochemical analysis of the vn and rho EOC CRMs, and used the discovered regulatory logic to predict additional CRMs, many of which are active in the Drosophila leg. Together, these data reveal a common regulatory logic for gene activation in the distal leg that is used by many genes, in addition to vn and rho.
Results
Identification and genomic manipulation of the vn and rho EOC enhancers
To understand the molecular mechanism by which the EGFR signaling pathway is activated in the center of leg imaginal discs during larval stages, we searched for leg disc enhancer elements controlling the expression of EGFR ligands and ligand-processing proteases implicated to function in this process [11, 14]. We scanned the genomic regions of vn and rho using in vivo lacZ reporter assays (Fig 1B and 1G and S1 Table) and defined minimal enhancers (vnE– 654 bp and rhoE– 544 bp) that recapitulate the expression pattern of these genes in the center of leg discs during development (Fig 1C and 1H), as well as in the serially homologous antennal discs (S1A and S1B Fig). The vnE- and rhoE-lacZ transgenes exhibited earlier expression (starting at ~71h PEL for vnE and ~82h PEL for rhoE; Fig 1C and 1H) than detected by in situ for vn and rho (Fig 1D and 1I), perhaps because of the greater sensitivity of the anti-ßgal staining, and suggest that the genes might be expressed earlier than previously thought [11, 12, 14, 15]. Our search for leg disc enhancers across the vn locus uncovered only vnE, while in rho we identified two additional rho leg disc enhancers (rhoLLE1 and rhoLLE2 (Fig 1G, LLE stands for ‘late leg enhancer’) that drive expression in ring patterns starting in mid-third instar leg discs (90-92h PEL) (S1C and S1D Fig). Although these enhancers do not participate in EOC formation, they are active at later developmental stages and drive expression in medial/proximal ring patterns and are thus likely to be additional sources of EGFR activity (S1C and S1D Fig).
We also re-examined the expression pattern of additional EGFR ligands and proteases using enhancer-reporter assay (S1E, S1I and S1L Fig; S1 Table), in situ hybridization (S1F, S1J, S1M and S1O Fig; S2 Table) and available enhancer trap lines (S1G Fig) and found that roughoid (ru) (as previously reported [11]) and spitz (spi) (S1G and S1J Fig), but not Keren (Krn) or gurken (grk) (S1M and S1O Fig), were expressed in leg discs during third larval instar. Curiously, ru expression was only detected by an enhancer trap (ruinga-lacZ) and by a newly identified enhancer, ruLLE, that recapitulates the ruinga expression pattern (S1H Fig) but was not detected by in situ hybridization (S1F Fig) (see also Campbell 2002). spi was expressed broadly in leg discs (S1J Fig), and this pattern was recapitulated by a ~10 kb region that includes its promoter and introns (S1K Fig). Although there are five additional rho-family proteases in Drosophila [16], previous genetic analysis suggests that rho and ru are the most relevant [11, 14]. Further, because ru did not show expression in early L3 leg discs (and see below for additional genetic tests), and spi expression was ubiquitous, we focused on vnE and rhoE as the primary CRMs active in the leg disc EOC.
To assess the requirement of the vnE and rhoE CRMs for vn and rho expression, we deleted them from their native genomic loci using CRISPR/Cas9-mediated genome editing ([17–19]; see Materials and Methods) and assessed the phenotypes of these alleles (vnvnE-Df and rhorhoE-Df). We found that these deficiencies abolished the expression of these genes, respectively, only in the EOC of the legs (Fig 1E and 1J). The lack of expression in the enhancer deletion alleles was restored when the wild type enhancers were resupplied in their native genomic positions (Fig 1F and 1K). Therefore, we conclude that vnE and rhoE are necessary and sufficient for vn and rho expression in the EOC, respectively.
Genetic analysis of vnvnE-Df and rhorhoE-Df mutants
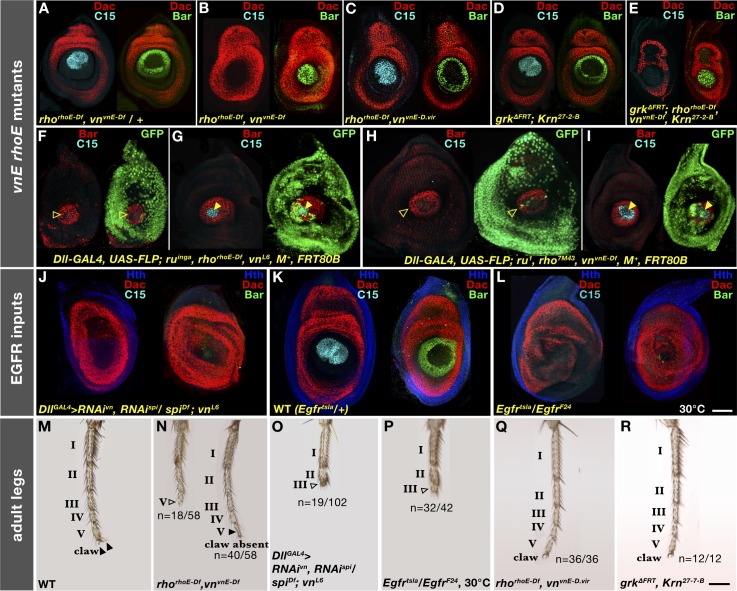
Individually, both vnvnE-Df and rhorhoE-Df are viable as homozygotes, exhibit normal leg disc patterning (S2A and S2C Fig), and form morphologically normal and functional legs (S2B and S2D Fig), consistent with previous reports that vn and rho single mutants do not affect the leg disc or adult leg pattern [11, 12]. However, when we examined the combined effect of these deficiencies in rhorhoE-Df vnvnE-Df double mutant flies we found that the expression of EGFR downstream genes C15 and aristaless (al) was abolished in these animals (Figs 2A, 2B, S2E and S2F), and the expression of BarH1/H2, a pair of more proximally expressed PD genes [20], collapsed from a ring pattern to a central circular domain in the leg disc (Fig 2B). In agreement with the leg disc pattern changes, adult rhorhoE-Df vnvnE-Df double mutants exhibited distal leg truncations that lack a pretarsus and parts of tarsal segment 5 (Fig 2N). rhorhoE-Df vnvnE-Df double mutant flies die in late pupal stages most likely because of an inability to exit the pupal case.
Fig 2. vnE and rhoE requirement for PD patterning of leg discs and adult legs.
(A-L) Effects on distal PD genes (C15 and Bar) in third instar leg discs in: (A) rhorhoE-Df vnvnE-Df /+; (B) rhorhoE-Df vnvnE-Df; (C) rhorhoE-Df vnvnE-D.vir; (D) grkΔFRT; Krn27-7-B; (E) grkΔFRT; rhorhoE-Df vnvnE-Df Krn27-7-B; (F,G) ruinga rhorhoE-Df vnL6 mutant clones; (H,I) ru1 rho7M43 vnvnE-Df mutant clones; (J) spi vn double RNAi; (K) Egfrtsla/+ WT leg disc at restrictive temperature; and (L) Egfrtsla mutant leg discs at restrictive temperature. (M-R) Adult leg morphology of: (M) WT; (N) rhorhoE-Df vnvnE-Df mutant; (O) spi vn double RNAi driven by Dll-Gal4 (legs with tarsal segments I-II: n = 21/102, I-III: n = 19/102, I-IV: n = 35/102, I-V: n = 27/102); (P) Egfrtsla mutant at restrictive temperature (remaining 10/42 legs less severely truncated); (Q) rhorhoE-Df vnvnE-D.vir; and (R) grkΔFRT; Krn27-7-B mutant. n refers to number of individual legs with a given number of tarsal segments present. Arrowheads indicate intact (filled) or perturbed (open) tarsal segments.
A sequence comparison between D. melanogaster and D. virilis, two Drosophila species that diverged from each other ~50 million years ago [21], revealed that vnE is well conserved (45.8% identity over 0.65 kb) and at a similar location upstream of the D. virilis vn transcription start site. In contrast, rhoE could not be identified by sequence homology in D. virilis. These observations prompted us to ask if the orthologous D. virilis vnE (vnE-D.vir) could substitute for the function of D. melanogaster vnE and rescue the rhorhoE-Df vnvnE-Df phenotype. We performed the swap of enhancers (see Materials and Methods) and we found that, indeed, the leg imaginal discs of rhorhoE-Df vnvnE-D.vir flies had normal PD patterning (Fig 2C) and normal adult legs (Fig 2Q). This result suggests that the function of vnE has been maintained over tens of millions of years and this enhancer element plays a conserved role in limb development.
Spi and Vn are the relevant EGFR ligands for tarsal leg patterning
Rho is an EGFR ligand-processing metalloprotease that has the potential to cleave the membrane-bound ligands Spi, Krn, and Grk in order to convert them into active secreted forms, while Vn is expressed as a secreted form that does not require Rho function [reviewed in 13]. Although we did not detect any expression of Krn and grk in leg discs (S1M and S1O Fig), this does not exclude the possibility that these genes function in leg disc development at a level of expression below what is detected in our in situ hybridization experiments. To address this possibility, we performed genetic experiments and found that the single null mutants Krn27-7-B [22] and grkΔFRT [23], and the double mutant grkΔFRT; Krn27-7-B, do not exhibit any leg disc patterning defects (Fig 2D) or adult leg phenotypes (Fig 2R). In addition, rhorhoE-Df vnvnE-Df Krn27-7-B triple mutant (S2G and S2H Fig) and grkΔFRT; rhorhoE-Df vnvnE-Df Krn27-7-B quadruple mutant (Fig 2E) leg discs had similar defects as rhorhoE-Df vnvnE-Df double mutants (Fig 2B), even though the quadruple mutant larvae died at late L3, just before pupation. These results support our conclusion that Krn and Grk are unlikely to be involved in leg development.
The remaining rho-dependent EGFR ligand, Spi, is expressed broadly in leg discs (S1J and S1K Fig) and is a good candidate for participating in EOC activity under the temporal and spatial control of Rho. To confirm the role of Spi, we used RNAi (see Materials and Methods) to examine the phenotypes of animals depleted for both spi and vn in leg discs. We found that, indeed, Spi is the EGFR ligand processed by Rho in the center of leg discs, because spi vn double RNAi (driven by Dll-Gal4) caused loss of expression of the downstream EGFR gene C15, and the near elimination of Bar expression (Fig 2J). This phenotype is stronger than any other combination of EGFR pathway components, similar to Egfrtsla mutants grown at the restrictive temperature of 30°C (Fig 2L and 2P). In addition, in animals depleted for spi and vn using RNAi we observed leg truncations (Fig 2O) similar to those observed in Egfrtsla mutants at 30°C (Fig 2P). Taken together, these results suggest that Vn and Spi are likely the only ligands that activate EGFR signaling during fly leg development.
Genetic dissection of rhomboid and roughoid in leg development
The triple ru1 rho7M43 vnL6 mutant, but not the rho7M43 vnL6 double mutant, produces a strong leg truncation phenotype, similar to Egfrtsla animals grown at 30°C, suggesting that Ru is involved in patterning the adult leg together with Vn and Rho [11]. vnL6 is a nonsense mutation and a null by genetic criteria [24, 25]. rho7M43 is also a null allele [16], although we, as well as previous studies [16], were unable to identify any amino acid changes in the rho coding sequence of this allele. ru1 is a nonsense mutation that leads to a premature stop codon after residue 55, prior to the Rhomboid domain, suggesting that it is also a null allele [26]. A potential caveat to this conclusion is that ru1/Df (including Dfs ruPLLb and ruPLJc) results in a stronger ‘rough-eye’ phenotype than the ru1 homozygote, implying that ru1 is a hypomorph [16, 26]. However, ru, together with several other genes, is located in the intron of the protein tyrosine phosphatase encoding gene, Ptp61F, which plays a role in EGFR/MAPK signaling (S1E Fig) [27]. Consequently, deficiencies that remove ru could also affect MAPK/EGFR signaling by reducing Ptp61F expression, and could potentially lead to stronger phenotypes compared to the cleaner ru1 allele. Taken together, these observations suggest that ru1 is likely to be a null mutation.
Notably, rho and ru are physically close to each other on chromosome 3L, with rhoE ~55 kb away from the ru promoter, raising the possibility that rhoE could also regulate ru (Figs 1G and S1E). To test this possibility, we examined the lacZ expression pattern driven by the ruinga enhancer trap [28] in the background of the homozygous rhorhoE-Df (see Materials and Methods). We did not detect any effect of rhorhoE-Df on ruinga-lacZ expression in leg discs (S2K and S2L Fig), suggesting that ru is not regulated by rhoE.
Because the triple ru1 rho7M43 vnL6 mutant produces adult leg truncations [11] that are stronger than those observed in our rhorhoE-Df vnvnE-Df double mutant, we carried out additional experiments to address a potential role for ru in leg disc patterning. In the first experiment, instead of examining adult legs we examined ruinga rhorhoE-Df vnL6 triple mutant clones in leg discs (see Materials and Methods). Notably, leg disc patterning in these mutant discs was similar to the pattern observed in the rhorhoE-Df vnvnE-Df double mutant (Fig 2F), and even a small patch of WT tissue in the center of the leg disc could restore a normal PD pattern (Fig 2G). In a second test, we generated ru1 rho7M43 vnvnE-Df triple mutant clones and, as in the previous experiment, we observed the loss of C15 and collapse of BarH1 expression (Fig 2H), similar to the rhorhoE-Df vnvnE-Df double mutant, and a rescue of C15 expression if some distal cells remain wild type (Fig 2I).
Together, these results suggest that ru does not contribute significantly to EOC activity in the early L3 stage to pattern the L3 imaginal disc. Instead, these results suggest a model in which EOC activity is mediated primarily by vnE and rhoE, while the later rings of EGFR activation are controlled by a distinct set of enhancers (e.g. rhoLLE1, rhoLLE2, and ruLLE) (S1C, S1D and S1H Fig), and that this second wave of EGFR activity is important for patterning medial regions of the adult leg. In addition, these data suggest that ru, and perhaps other rho-like family members, plays a role later in leg development through its ring-like expression pattern to ultimately impact adult leg patterning.
Genetic regulation of vnE and rhoE
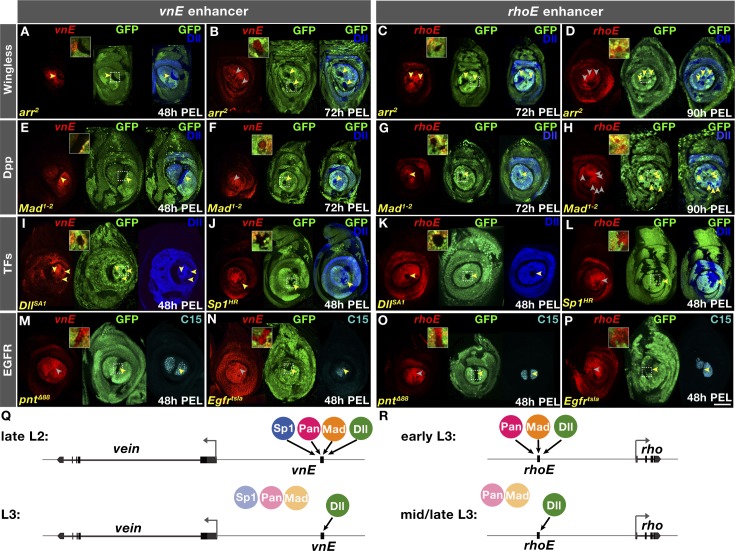
Previous studies have underscored the importance of the Wg and Dpp pathways for EGFR activation in the center of leg discs [11, 12]. Using the vnE and rhoE enhancer elements, we have been able to address this question in greater detail. We generated mutant clones of arrow (arr), an obligate co-receptor in Wg signaling, and Mothers against dpp (Mad), a downstream effector of Dpp signaling, at different time points, and assessed the requirement of these pathways for vnE and rhoE activation. Both Wg and Dpp pathways are necessary for the initiation of vnE-lacZ expression in late L2 larval stage (Fig 3A and 3E), while clones made early in L3 stage did not affect vnE-lacZ expression (Fig 3B and 3F). rhoE-lacZ expression was lost when either Wg or Dpp activity was removed during L2 or early L3 (Fig 3C and 3G) but became independent of these pathways later in mid-L3 (Fig 3D and 3H).
Fig 3. Genetic analysis of inputs into vnE and rhoE.
(A-P) lacZ reporter gene expression driven by vnE or rhoE in mutant clones as indicated. Absence of GFP marks the clone; 2X-magnified insets showcasing specific disc regions (designated with squares) are provided in each case. (A) vnE in arr2 clones generated 48h PEL; (B) vnE in arr2 clones generated 72h PEL; (C) rhoE in arr2 clones generated 72h PEL;(D) rhoE in arr2 clones generated 90h PEL; (E) vnE in Mad1-2 clones generated 48h PEL; (F) vnE in Mad1-2 clones generated 72h PEL; (G) rhoE in Mad1-2 clones generated 72h PEL; (H) rhoE in Mad1-2 clones generated 90h PEL; (I) vnE in DllSA1 clones generated 48h PEL. (J) vnE in Sp1HR clones generated 48h PEL; (K) rhoE in DllSA1 clones generated 48h PEL; (L) rhoE in Sp1HR clones generated 48h PEL; (M) vnE in pntΔ88 clones generated 48h PEL; (N) vnE in Egfrtsla Minute+ clones generated 48h PEL; (O) rhoE in pntΔ88 clones generated 48h PEL; (P) rhoE in Egfrtsla Minute+ clones generated 48h PEL. (Q, R) Schematics summarizing vnE and rhoE regulation by inputs, respectively.
In addition to Wg and Dpp, at the early larval stages of leg disc development there are two other factors that are crucial for leg specification and growth–the homeodomain transcription factor Distal-less (Dll) [29] and the Zn finger transcription factor Sp1 [4, 5]. Dll mutant clones induced at any larval stage abolished vnE-lacZ expression (Figs 3I and S3A). In addition, ectopic expression of Dll activated vnE not only in leg discs but in other imaginal discs (S3C, S3D and S3E Fig), as long as Wg and Dpp were available in these tissues at the time of clone induction (S3C, S3D and S3E Fig). These results suggest that Dll is required for vnE activity. Similarly, rhoE-lacZ expression also required Dll at all developmental times (Figs 3K and S3B).
We also examined the requirement of Sp1 for vnE and rhoE activation. We found that vnE activation requires Sp1, either when the entire animal was mutant or in clones (Figs 3J and S3F). This requirement is not mediated by Dll because Dll expression remained intact in mutant clones (Fig 3J) and in leg discs from Sp1 homozygous animals (S3F Fig). In contrast, Sp1 was dispensable for rhoE-lacZ expression (Figs 3L and S3H). In addition, although Sp1 is required for the activation of vnE at L2 larval stage (Figs 3J and S3F), at the beginning of L3 larval stage Sp1 was no longer required for vnE (S3G Fig). We also assessed if vnE and rhoE are regulated by buttonhead (btd), an Sp1 paralog that is co-expressed with Sp1 in leg discs [5]. We found that neither EOC enhancer requires btd (S3I and S3J Fig) and it is unlikely that rhoE requires both Sp1 and Btd redundantly since we did not detect Sp1/Btd binding sites or in vivo binding at rhoE for Sp1 (see below). Together, these results support a model in which vnE activation requires Wg and Dpp together with Dll and Sp1; later, vnE activity becomes independent of Wg, Dpp and Sp1, but still requires Dll (Fig 3Q). Similarly, although the timing differs, rhoE requires initial input from Wg, Dpp, and Dll but later only requires Dll for its maintenance (Fig 3R). The differential onset of expression between the two enhancers might depend on the differential requirement for Sp1.
To investigate if EGFR activity is required for vnE and rhoE, we examined the expression driven by these CRMs in the background of mutants for EGFR pathway components. vnE- and rhoE-driven expression was normal in pntΔ88 [30] mutant clones or Egfrtsla [31] mutant clones at the restrictive temperature (Fig 3M, 3N, 3O and 3P). Capicua (Cic), another downstream component of EGFR [32], is expressed in leg discs (S3K Fig) but was also not required for vnE and rhoE activity (S3L and S3M Fig).
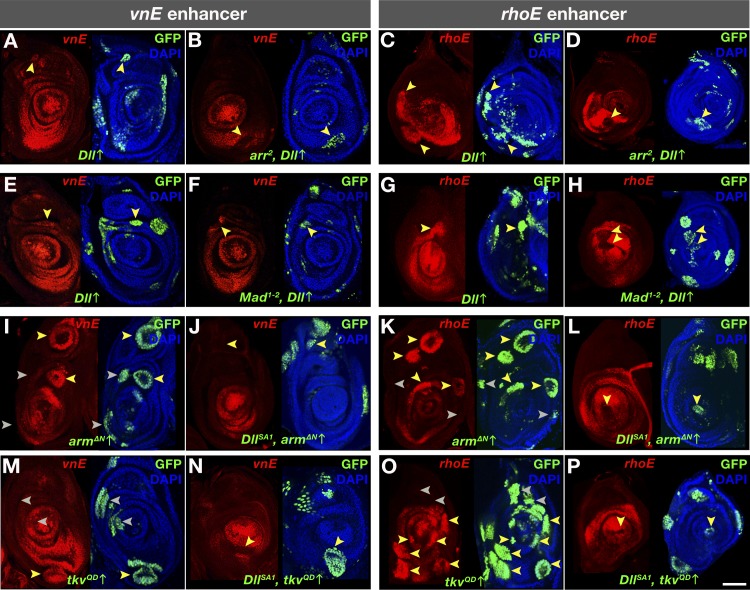
We next carried out epistasis experiments using the MARCM technique [33] in which we overexpressed one vnE or rhoE input and removed another. We excluded Sp1 from this analysis because Sp1 sometimes affects Dll expression making results difficult to interpret [5]. For both vnE and rhoE, we found that while ectopic activation of Dll induced the activity of these enhancers in wildtype tissue (Fig 4A, 4E, 4C and 4G), in clones compromised for either Wg or Dpp signaling neither vnE nor rhoE were activated (Fig 4B, 4F, 4D and 4H). Dll was also unable to induce vnE-lacZ expression in ectopic clones in other imaginal discs when Wg and Dpp signaling was compromised (S3C, S3D and S3E Fig). Further, consistent with previous results [6, 7, 34], ectopic Wg and Dpp pathway activity induced vnE- and rhoE-lacZ expression and created additional EOCs in leg discs when these clones were located close to an endogenous source of Dpp and Wg, respectively (Fig 4I, 4M, 4K and 4O). However, when these clones were also mutant for Dll, these pathways were not able to activate either vnE or rhoE, and hence EGFR signaling (Fig 4J, 4N, 4L and 4P).
Fig 4. Genetic interactions of inputs into vnE and rhoE.
(A-P) vnE- or rhoE-directed lacZ reporter gene expression in MARCM clones of: (A and C) Dll ectopic expression in WT background; (B and D) Dll ectopic expression in arr2 mutant background; (E and G) Dll ectopic expression in WT background; (F and H) Dll ectopic expression in Mad1-2 mutant background; (I and K) armΔN ectopic expression in WT background; (J and L) armΔN ectopic expression in DllSA1 mutant background; (M and O) tkvQD ectopic expression in WT mutant background; (N and P) tkvQD ectopic expression in DllSA1 mutant background.
Dissection of vnE and rhoE molecular inputs
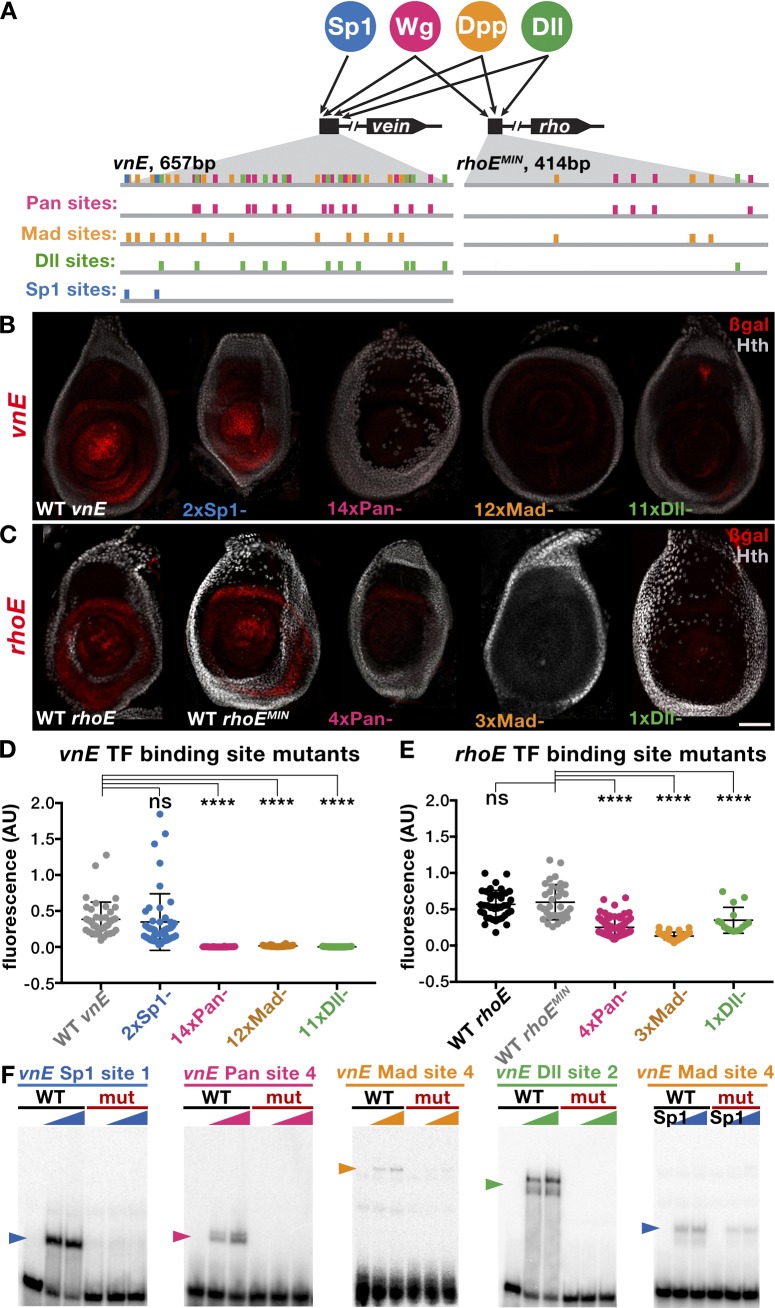
Our genetic analysis suggests a complex interplay between the signaling pathways Wg and Dpp and the transcription factors Dll and Sp1 on the vnE and rhoE enhancers. To investigate the configuration of binding sites and the transcription factor grammar of these CRMs, we searched for putative binding motifs using available position weighted matrices (PWMs) [35] and computational methods for identifying consensus Pan (downstream effector of Wg signaling), Mad (downstream effector of Dpp signaling), Dll and Sp1 binding motifs [36]. We performed a comprehensive in vivo mutagenesis analysis for both enhancers (Fig 5A). We mutagenized the enhancer elements by progressively adding (one at a time) mutations (S3 Table) in putative binding sites for each transcription factor (Fig 5A), starting with those that best match consensus binding sites and proceeding to more degenerate binding sites. Because the information from the enhancer bashing experiments (Fig 1B and 1G; S1 Table) revealed that parts of the enhancers containing multiple sites for each of the TFs can not drive intact expression patterns, we inferred that only having the full set of binding sites gives full expression patterns. Based on the combined analysis between the mutagenesis and the enhancer bashing data we found that there are a large number of binding sites important for vnE activation—14 Pan binding sites, 12 Mad sites, and 11 Dll sites (Figs 5A, 5B, 5D, S4A and S4B); mutagenesis of subsets of these binding sites leads only to reduction of enhancer-driven expression (S4A and S4B Fig). In contrast, for each TF, there were fewer binding sites important for rhoE activation—4 Pan, 3 Mad and a single Dll binding site (Fig 5A, 5C and 5E). Curiously, in the case of Dll we found 5 additional putative sites in rhoE that were not required for enhancer activity in optimal laboratory conditions (S4E and S4F Fig; S3 Table). In general, the identified binding sites for the two enhancers had an additive effect on the expression levels of vnE and rhoE because partially mutated enhancers drove patchy expression and progressively diminished levels of reporter expression (S4A, S4B, S4C and S4D Fig). We also confirmed the binding of the TFs involved in vnE and rhoE regulation by in vitro binding assays, suggesting that they act directly to regulate these enhancers (Fig 5F).
Fig 5. Dissection of Pan, Mad, Dll and Sp1 inputs into vnE and rhoE.
(A) Schematic representation of binding sites in vnE and rhoE. Putative binding sites for each TF were mutagenized one at a time leading to progressive increase in the number of mutant binding sites until the expression driven by a mutant enhancer was lost. Sites in each TF category that were mutagenized either first or last are not sufficient for full enhancer-driven expression since fragments of vnE and rhoE (Fig 1B and 1G; S1 Table) that contain multiple such sites from each TF input cannot drive correct expression pattern. (B) Expression pattern of WT vnE-driven expression and mutant vnE enhancer-driven expression. (C) Expression pattern of WT rhoE-driven expression and mutant rhoE enhancer-driven expression. (D) Quantification of WT and mutant vnE-driven expression levels in third instar leg discs (WT vnE n = 41, 2xSp1 n = 48, 14xPan n = 24, 12xMad n = 32, 11xDll n = 49 where n indicates number of leg discs analyzed). For normalization, fluorescence was calculated as a ratio of β-gal:Dll intensity in the center of the leg disc (see Methods for details).(E) Quantification of reduction of WT and mutant rhoE-driven expression in third instar leg discs (WT rhoE n = 39, WT rhoEMIN n = 35, 4xPan n = 78, 3xMad n = 41, 1xDll n = 15 where n indicates number of leg discs analyzed). For normalization, fluorescence was calculated as a ratio of β-gal:Dll intensity in the center of the leg disc (see Methods for details).(F) EMSA analysis of selected WT vs mutant binding sites.
It is striking that vnE contains many more binding sites for each TF compared to rhoE. In addition to the differential requirement for Sp1, this difference may also contribute to the earlier timing of vnE activation compared to rhoE, because the larger number of binding sites might render vnE more sensitive to lower TF concentrations.
Consistent with the genetic requirement for Sp1, we identified two putative Sp1 binding sites in vnE. However, when we mutagenized them reporter gene expression was unaltered (Fig 5B and 5D). Therefore, we scanned the enhancer by EMSA using overlapping fragments (S2 Table) in order to identify additional Sp1 binding sites in an unbiased manner. We found that Sp1 binds with low affinity to some Mad binding sites (Fig 5F). Because both Sp1 and Mad can bind to some of the same binding sites, loss of vnE-lacZ expression when Mad sites are mutated may be a consequence of eliminating all Mad and some of the Sp1 inputs.
Because Sp1 and Dll are co-expressed during leg development, we also scanned all of vnE using overlapping oligos (S2 Table) to determine if these proteins might bind cooperatively to DNA. For these experiments we used full-length Dll and nearly full-length Sp1 proteins (see Materials and Methods). Although these experiments confirmed Dll binding to its binding sites, we failed to detect any cooperative binding between Dll and Sp1. Taken together, our results suggest that Sp1 regulates vnE through two Sp1 binding sites and some shared binding sites with Mad.
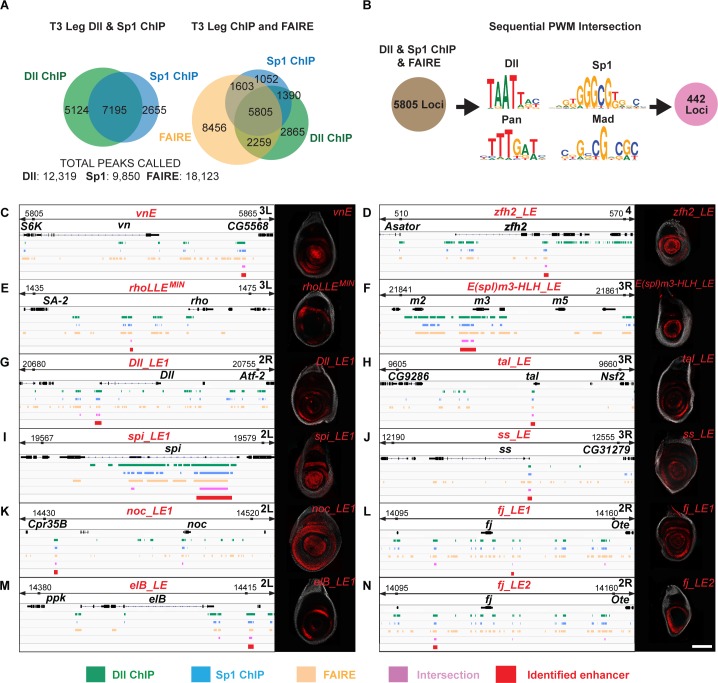
The vnE and rhoE regulatory logic is widely used among leg CRMs
The vnE and rhoE regulatory inputs that we discovered here resemble one previously characterized in DllLT [9], in that they are all activated by the combinatorial input of Wg, Dpp, Dll and/or Sp1 [5]. These findings prompted us to test if there might be a battery of CRMs that is regulated in the leg disc by these same inputs. To test this idea, we first determined the genome-wide in vivo binding profiles of Dll and Sp1 using chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) in third instar leg discs (Fig 6A). We used either anti-Dll antibody or anti-GFP antibody to ChIP an Sp1-GFP fusion protein expressed from an engineered ~80 kb BAC construct (see Materials and Methods) that drives Sp1-GFP expression identically to Sp1, and can rescue an Sp1 null mutant. Here we focus on genomic loci that show an intersection between 1) Sp1 and Dll binding events, 2) putative Dll, Sp1, Mad and Pan binding sites, and 3) have accessible chromatin as revealed by FAIRE-seq data for leg discs [37]. We found 442 genomic regions that satisfy all six criteria, many of which were close to genes that are expressed in leg discs (S4 Table). In addition, two regions correspond to vnE and DllM, another previously defined CRM of Dll (Fig 6C and 6G). As expected, rhoE was not identified because there was no consensus Sp1 binding site in rhoE. However, this approach identified a fragment that is within rhoLLE1 (rhoLLE1MIN) that, when tested in a reporter gene, drove expression in similar ring pattern as rhoLLE1 (Fig 6E).
Fig 6. Genome-wide analysis of combinatorial inputs of Dll, Sp1, Wg, and Dpp in leg discs.
(A) Venn diagram representing the intersection between Dll ChIP-Seq, Sp1 ChIP-Seq and FAIRE data from third instar leg discs. (B) Schematic representation of bioinformatic intersection between Dll/Sp1 binding events and FAIRE data together with PWMs for Dll, Sp1, Pan and Mad. (C-N) Schematic representation of the binding events at selected genomic loci, the intersections and the expression pattern of tested intersection fragments for: vnE (C); zfh2_LE (D); rhoLLEMIN (E); E(spl)m3-HLH_LE (F); Dll_LE1 (G); tal_LE (H); spi_LE1 (I); ss_LE (J); noc_LE1 (K); fj_LE1 (L); elB_LE (M); fj_LE2 (N).
To validate the larger set of predicted CRMs, we picked 20 additional genomic fragments (23 together with vnE, DllM and rhoLL1MIN, ~5% of the total 442 intersections) near 11 genes [Antennapedia (Antp), four-jointed (fj), spitz (spi), disconnected (disco), tarsal-less (tal), spineless (ss), Zn finger homeodomain 2 (zfh2), elbow B (elB), no-ocelli (noc), Enhancer of split m3 (E(spl)m3-HLH), and Distal-less (Dll)]. Using this approach, we discovered at least one leg disc enhancer element with a PD bias for each of the genes we tested (Figs 6 and S5; S5 Table), except for disco. In some cases (Antp, fj, spi, Dll, noc) multiple fragments generated leg disc expression patterns. Interestingly, we uncovered two leg disc enhancers for the EGFR ligand Spi. Overall, 18 of the 23 tested fragments (78%) are leg enhancers, suggesting that there is a battery of leg disc gene CRMs that drive expression differentially along the leg disc PD axis and are regulated by the direct input of Wg, Dpp, Dll and Sp1.
We also used genome-wide intersection criteria that excluded Sp1 as a factor, thus following the rhoE regulatory logic. Not surprisingly, this dataset was much larger (3809 loci), making it difficult to validate experimentally. Nevertheless, it also seems to predict enhancer loci because, in addition to rhoE, some of the identified regions corresponded to previously identified leg CRMs such as Dll DKO [38], Dll LL [39], and enhancer elements identified in genome-wide tiling studies [40].
Discussion
Multiple sources of short-range EGFR signaling during fly leg development
The EGFR signaling pathway is widely used in animal development, and is frequently a target in human disease and developmental abnormalities [reviewed in 41]. Yet despite its importance in animal biology, many questions remain about how this pathway functions. Among these questions is whether secreted ligands that activate this pathway can induce distinct cell fates in a concentration-dependent manner. Here, we test this idea by specifically eliminating a single source of EGFR ligands from the center of the Drosophila leg imaginal disc, which fate maps to the distal-most region of the adult leg. One plausible scenario is that this single source of secreted EGFR ligands, which we refer to as the EOC, activates distinct gene expression responses at different distances from this source. Alternatively, eliminating ligands secreted from the EOC might only affect gene expression locally, close to or within the EOC. Taken together, our data are most consistent with the second scenario (Fig 7). This conclusion is largely supported by our observations that CRM deletions that eliminate vn and rho expression from the EOC have mild developmental consequences, both in the L3 leg imaginal discs and adult legs. These phenotypes are significantly weaker than those generated when the entire EGFR pathway is compromised using a temperature sensitive allele of the EGFR receptor. The difference between these two phenotypes is most likely explained by removing only a single source of EGFR ligands in the enhancer deletion experiments versus affecting EGFR signaling throughout the leg disc in the Egfrtsla experiments. This explanation is further supported by our observation that there are indeed additional CRMs, some of which we define here, that drive EGFR ligand production in more medial ring-like patterns during the L3 stage.
Fig 7. Summary of PD axis patterning by EGFR.
Schematic representation of EOC and non EOC sources of EGFR activation along the PD axis in leg discs (A) and adult legs (B).
One possible caveat to these conclusions is that there are a total of seven rho-like protease genes in the Drosophila genome that could, in principle, play a role in distal leg development. We focused on rho and ru, based on previous results [11, 14] showing that triple rho ru vn clones generate severe leg truncations that phenocopy strong Egfrtsla truncations. In addition, we note that if other rho family proteases were active in the EOC, we would not expect to see leg truncations and patterning defects in the leg discs of the rhorhoE-Df vnvnE-Df double mutant, because those proteases should be able to produce active Spi. These observations suggest that the remaining five rho-like protease genes play a minor (or no) role in leg development. However, this conclusion will ultimately benefit from further genetic and expression analysis of these additional rho-like genes.
An additional previous observation that contrasts with the suggestion that EOC activity has only a limited role in specifying distal leg fates is the partial rescue of the PD axis when only a small number of distal leg cells were wild type in legs containing large rho ru vn clones [11]. However, we note that even in these ‘rescued’ legs, medial defects in PD patterning were apparent. It is also noteworthy that in these earlier experiments, only adult legs were examined. When we repeated the same experiment, but analyzed L3 discs, we found that rho ru vn clones generated phenotypes that were very similar to those produced by our double vnE rhoE enhancer deletions. Taken together, these observations suggest that timing must be considered in the interpretation of these experiments. When assayed at the late L3 stage, both our enhancer deletion and rho ru vn clone experiments argue that EOC activity is limited to specifying only the most distal fates, marked by the expression of al and C15. Starting in mid L3, and perhaps continuing into pupal development, there are additional sources of EGFR ligands [14] that, when compromised, can affect adult leg morphology. Nevertheless, at least at the L3 stage, these data suggest that EGFR ligands produced from the EOC have a limited and local role in specifying distal leg fates (Fig 7).
cis-regulatory networks during leg development
Integration of inputs from signaling pathways and organ selector genes at CRMs in order to execute distinct developmental programs is a recurrent theme during animal development (reviewed in [42]). Here, we identified two leg EGFR ligand CRMs that integrate the inputs from the Wg and Dpp signaling pathways and the leg selector genes Dll and/or Sp1 in a manner that is very similar to a previously characterized leg enhancer DllLT [9]. In addition, when we applied the same regulatory logic to the whole genome, we identified a battery of leg enhancer elements (Fig 6). Interestingly, each of these enhancers drives expression in a specific manner with slightly different timing despite the fact that many of the inputs are shared. It is conceivable that the different expression patterns directed by these enhancers are in part a consequence of additional inputs and/or the difference in the TF binding site grammar. In support of this idea, vnE and rhoE differ in the number of binding sites for many inputs and vnE requires Sp1 while rhoE does not. Both of these differences may contribute to the earlier onset of vnE expression compared to rhoE. The remaining enhancer elements identified in this study direct a plethora of PD-biased leg expression patterns–ranging from ubiquitous, to central and ‘ring’ patterns (Fig 6), which likely integrate inputs in addition to the ones described here. Future studies of these CRMs would help reveal the complex network of regulation that orchestrates leg development in the fruit fly. Such detailed understanding of the cis-regulatory architecture of fly leg development would likely give insights into organogenesis and evolution in other animals as well.
cis-regulation of EGFR signaling and cancer
The EGFR signaling pathway has tremendous oncogenic potential and understanding the various mechanisms regulating its activation is not only interesting from the point of view of animal development but also has important practical implications. While the core components of the EGFR pathway have been thoroughly studied because of their potent tumorigenic capability in humans [reviewed in 43], little is known about the transcriptional regulation of EGFR ligands that bind the receptor and activate the pathway. The reiterative use of EGFR signaling in many developmental processes implies that different cis-regulatory elements are likely utilized by each EGFR ligand in different organs and tissues in order to correctly read the diverse cues in any specific developmental context. It is conceivable that genomic variation in EGFR pathway CRMs might lead to a predisposition to different types of EGFR-dependent tumors in humans, since such CRMs may respond to potent growth-promoting signaling pathways, such as Wnt and BMP.
In this study, we characterized in detail two Drosophila EGFR CRMs, vnE and rhoE, and showed how they integrate the cues from two transcription factors, Dll and Sp1, and two signaling pathways, Wg and Dpp, in order to execute a leg patterning developmental program. Analogous EGFR CRMs are likely to exist in mammals, especially because complex interactions between BMP, Wnt, Shh, multiple Dlx paralogs and other factors, are implicated in the induction of FGF signaling in mammalian limb development. Consistent with this idea, specific single nucleotide polymorphisms (SNPs) in humans in non-coding loci of genes encoding EGFR ligands have been shown to be associated with different types of cancer [44–46]. Such loci may be enhancer elements analogous to vnE and rhoE. We also note that the regulatory logic uncovered here is likely to be relevant to many CRMs and genes that share spatial and temporal expression programs. Exploiting this regulatory logic in other systems might streamline the identification of enhancer elements that will aid in the discovery of mechanisms that are relevant to EGFR-related human disease and developmental birth defects.
Materials and methods
Drosophila genetics
The following mutant alleles and enhancer trap alleles were used in this study: arr2, btdXA, cicQ474X, cicP[PZ]08482, dacp7d23 (dac-Gal4), DllSA1, Dllem212 (Dll-Gal4), Egfrtsla, Egfrf24, grkΔFRT, Krn27-7-B, Mad1-2, pntΔ88, rho7M43, ru1, ruinga, Sp1HR (shared ahead of publication, [47]), spiSC1, spiDf(2L)Exel8041, vnL6, vnGAL4. Transgenic alleles used for in vivo clonal ectopic expression of genes were: UAS-armΔN, UAS-tkvQD, UAS-Dll.
To perform RNAi knockdown of vein and spitz the following strains were used: UAS-vnRNAi (TRiP.HMC04390)/CyO, Dfd:EYFP; UAS-spiRNAi (TRiP.HMS01120) crossed to either Dll-GAL4 (Dllem212), spiDf(2L)Exel8041/ CyO, Dfd-EYFP; vnL6/TM6B, or spiDf(2L)Exel8041/CyO, Dfd-EYFP; vnGAL4/TM6B (vnGAL4 is a null allele [48]). Crosses were raised at 18°C, then shifted to >25°C at the start of L3. For assessment of larval phenotypes, crosses remained at 25°C until fixation and dissection as wandering larvae. For assessment of adult leg phenotypes, crosses were returned to 18°C after 24h until eclosion.
For generation of mutant clones that encompass the entire Dll-expressing leg disc region a yw; Dll-Gal4 (Dllem212), UAS-Flp; Ubi-GFP M- y+ FRT80B/C(2L;3R)Tb strain was crossed to a corresponding FRT80B-containg mutant strain (ruinga rhorhoE-Df vnL6 or ru1 rho7M43 vnvnE-Df). For Flp-FRT inducible mitotic recombination and subsequent mosaic clonal analysis fly larvae were heat-shocked at 48h post egg laying (PEL), 72h PEL or 90h PEL and dissected for staining as crawling stage larvae at around 120h PEL. For generation of Flp-FRT mitotic recombination clones, larvae were heat-shocked for 40 minutes at 37°C. Mitotic recombination clones were generated using the following strains: w hs-Flp1.22 Ubi-RFP FRT19A, yw hs-Flp1.22; Ubi-GFP FRT40A /CyO; E/TM6B, yw hs-Flp1.22; FRT42D Ubi-GFP/CyO; E/TM6B, yw hs-Flp1.22; E/CyO; Ubi-GFP FRT80B /TM6B, yw hs-Flp1.22; E/CyO; FRT82B Ubi-GFP/TM6B, yw hs-Flp1.22; FRT42D M- hs-GFP/CyO; E/TM6B, yw hs-Flp1.22; E/CyO; Ubi-GFP M- FRT80B/TM6B. The corresponding strains carrying mutant alleles were used in crosses for generation of mutant clones in the resulting progeny. E in these genotypes designates either vnE-lacZ or rhoE-lacZ inserted in landing sites 51D or 86Fa on chromosome II and III, respectively. To induce GFP expression in larvae marked with hs-GFP, an additional heat-shock was given 1 h before dissection for 20 min to 1 hour at 37°C.
The following strains were used for MARCM experiments where E designates either vnE-lacZ or rhoE-lacZ inserted in site 86Fa: yw hs-Flp1.22 tub-Gal4 UAS-GFP; tub-Gal80 FRT40A/CyO; E/TM2, yw hs-Flp1.22 tub-Gal4 UAS-GFP; FRT42D tub-Gal80/CyO; E/TM2, yw; Mad1-2 FRT40A; UAS-Dll/C(2L;3R)Tb, yw; FRT42D arr2; UAS-Dll/C(2L;3R)Tb, yw; FRT42D DllSA1; UAS-armΔN/C(2L;3R)Tb, yw; FRT42D DllSA1; UAS-tkvQD/ C(2L;3R)Tb, yw; y+ FRT40A; UAS-Dll/C(2L;3R)Tb, yw; FRT42D y+; UAS-Dll/C(2L;3R)Tb, yw; FRT42D y+; UAS-armΔN/C(2L;3R)Tb, yw; FRT42D y+; UAS-tkvQD/ C(2L;3R)Tb.
For all in vivo clonal experiments, at least 20 examples of discs with clones of the correct genotype were examined, which is typical for experiments of this type, and more than one independent experiment was carried out for each tested genotype.
Plasmids and transgenes
All wildtype and mutagenized enhancer-reporter transgenic constructs were made using the lacZ reporter vector pRVV54 as an acceptor vector [49]. Coordinates of the genomic fragments PCR-amplified in the enhancer bashing experiments are listed in S1 and S5 Tables. The ΦC31 system was used for transgenesis and plasmids were introduced in landing sites 51D or 86Fa [50].
Site-directed mutagenesis of the vnE and rhoE enhancers was performed according to the QuikChange II protocol (Agilent Technologies). vnE and rhoE enhancers were first introduced in pBluescript SK+ vector for site-directed mutagenesis and the resulting mutated enhancers were consequently transferred to pRVV54 for in vivo analysis in the fruit fly. Primers used for mutagenizing of putative binding site are listed in S3 Table.
Plasmids for recombinant protein production were made by introducing cDNA sequences into pET21 series vectors (Novagen-EMD Millipore) and their derivatives, resulting in C-terminally tagged His proteins. Primers used to generate Dll-His (full-length Dll), Sp1Zn-finger-His (only the Zn-finger domains; used for confirming in vitro binding to Sp1 sites), Sp1424AA-His (used to examine cooperativity with Dll), MadMH1-His (only the MH1 domain) and PanHMG-His (only the HMG domain) vectors are listed in S2 Table.
CRISPR/Cas9 alleles
The vnE and rhoE CRISPR/Cas9 alleles were generated by using pCFD4 vector for driving gRNA expression [18] and a germline-expressing Cas9 donor strain for plasmid mix injection [19]. The following sequences were used as gRNAs for generation of the vnEDf allele: CGATTTTAATGCGAAAGCTA and TTTGGCTTTCAACGCTTAAT. The following sequences were used as gRNAs for generation of the rhoEDf allele: GAGCCGAGGGCACAAATTGA and ATGATGATGATGTATTGCCC. We created a vector containing a cassette with P3-RFP [50] and FRT(F5)-hs-neo-FRT(F5) selectable markers flanked by minimal inverted ΦC31 [51] attP sites (pRVV613) [52]. This vector was used for insertion of upper and lower homologous arms for generation of donor vectors for creation of platforms for cassette-exchange. Primers used for PCR-amplification of the homologous arms are listed in S2 Table. vnE and rhoE pCFD4-based gRNA vectors (250ng/μl) were co-injected with the corresponding vnE and rhoE homologous arm donor cassette vectors (500ng/μl) and resulting flies were screened for P3-RFP expression. To generate rhoE deletion allele in the background of ruinga, injections to generate the rhoEDf were repeated in a nos-Cas9/CyO; ruinga/TM3 strain. Positive fly lines were verified by PCR for correct insertion of the donor cassettes. Deletion alleles without P3-RFP were generated through RMCE by injection with an empty multiple cloning site vector containing inverted ΦC31 attB sites (pRVV578) [52]. The P3-RFP-containing and -non-containing enhancer deletion alleles exhibited identical expression patterns and phenotypes. The WT vnE, rhoE and the D. virilis vnE enhancers were cloned into pRVV578 and resupplied by RMCE in a similar manner (primers are listed in S2 Table).
Protein assays
Recombinant proteins were expressed in BL21 (DE3) cells (Agilent Technologies) through IPTG induction for 4h. Proteins were subsequently purified through Cobalt chromatography with TALON Metal Affinity Resin (Clontech, #635501). EMSA gels were performed as previously described [53].
Immunohistochemistry and adult leg analysis
Immunostainings of fly imaginal discs was performed by standard protocol. The following antibodies were used in this study: rabbit anti-β-galactosidase (Cappel), mouse anti-β-galactosidase (Sigma-Aldrich, #G4644), guinea pig anti-Dll [9], rat anti-Sp1, guinea pig anti-Hth [54], mouse-anti-GFP (ThermoFisher Scientific, #A11121), rat anti-C15 [15], rat anti-Al [6], rat anti-BarH1 [55], rabbit anti-BarH1 [56], mouse anti-Dac [57]. AlexaFluor488-, AlexaFluor555-, and AlexaFluor647-conjugated secondary antibodies from ThermoFisher Scientific or Jackson ImmunoResearch Laboratories were used at 1⫶500 dilution.
Adult legs were dissected, mounted, and analyzed by light microscopy. All adults of the relevant genotype that eclosed within an 8-hour period were scored. Roman numerals in the figure legends indicate the tarsal segments present in each phenotypic class (with the distal most segment perturbed). For example, a truncation designated as I-III means that tarsal segments I, II and III were present, with segment III partially defective (e.g. Fig 2P). n refers to the number of individual legs scored. The number of legs examined for each genotype is reported in the figures and figure legends.
In situ hybridization
To generate vectors for in situ probes vn, ru, spi, Krn, and grk DNA sequences were amplified from genomic DNA and rho DNA sequence was amplified from cDNA clone (LD06131; DGRC clone #3528) using primers listed in S2 Table. DNA fragments were cloned into pBluescript SK+ (Agilent Technologies).
RNA antisense probes were transcribed with either T3 or T7 RNA polymerase (depending on the cDNA sequence orientation in the vectors listed in S2 Table) and labeled using DIG UTP mix (Sigma, #11175025910). Sense RNA probes were used as negative controls. rho probes were then hydrolized for 30 minutes at 60°C as previously described [58]. Third instar larvae were dissected in cold 1xPBS and fixed for 16h at 4°C in 4% PFA + 2mM EGTA. In situ hybridization was then performed as previously described [58] and signal was developed in BM-Purple AP substrate (Sigma #11442074001) after staining with anti-DIG -AP antibody at a concentration of 1:2000 (Roche #1093274). Multiple (≥10) discs were examined for each time point, probe, and genotype.
Fluorescence quantification
Mid-third instar larvae carrying wild-type or mutant vnE- or rhoE-lacZ reporter constructs were raised, fixed, stained and imaged in parallel according to standard immunohistochemical protocols. Average fluorescence was measured for the area within the central/tarsal domain of all unobstructed leg imaginal discs using ImageJ software (http://rsb.info.nih.gov/ij) and reported as the ratio of β-gal:Dll (staining control) in arbitrary units (AU). Ordinary one-way ANOVA adjusted for multiple comparisons (Dunnett's test) were performed and graphed in Prism software (graphpad.com) to compare wild-type fluorescence to mutant enhancer genotypes where ns = not significant, * = p≤ 0.0332, ** = p≤ 0.0021, *** = p≤ 0.0002 and **** = p<0.0001 (adjusted p-values). n refers to the number of individual leg discs scored. The number of leg discs scored for each genotype is reported in the figure legends.
Chromatin IPs
Triplicate pools of 100 yw and 100 Sp1-GFPBAC L3 wandering larvae were used to perform independent chromatin IPs as previously described [59]. The Sp1-GFPBAC is a GFP-tagged Sp1 in BAC clone CH321-64M02 inserted in landing site VK00033 (gift from Dr. Rebecca Spokony). All 6 leg discs from each larva were used as material for each IP. Chromatin from the yw larvae pools was immuno-precipitated with goat anti-Dll antibody (sc-15858, Santa Cruz Biotechnology, 1.5 μg/ml for IP) while chromatin from the Sp1-GFPBAC larvae pools was immuno-precipitated with rabbit anti-GFP antibody (ab290, Abcam, 1∶300 dilution for IP). DNA from non-immunoprecipitated 10% chromatin input was isolated from each pool as reference control. Both control and immunoprecipitated DNA samples were prepared for Illumina sequencing using the Epicentre Nextera DNA Sample Preparation Kit and sequenced on an Illumina HiSeq 2000 according to the manufacturer's specifications. Experiments were performed in duplicate and peak calling was based on merged reads for duplicate ChIPs. Sequences were aligned to the Drosophila genome using the Burrows-Wheeler Aligner and ChIP-seq peaks were called using MACSv2 [60, 61]. Peak regions were defined using a p-value cutoff of 1.00e-02, but only those peaks passing a more stringent q-value cutoff of 1.00e-04 were used for further analysis. Datasets generated in this study are available at the Gene Expression Omnibus (GEO): accession number GSE113574.
Bioinformatic intersection analysis
PWMs for Dll, Sp1, Pan, and Mad were extracted from The Fly Factor Survey Database using the command grep within the MotifDb Bioconductor/R package. To generate BED files containing position information for each of the above PWMs, the matchPWM command from the Biostrings Bioconductor/R package was used. In-house code was used to run the command iteratively through the chromosomes (using DM3 build). Only hits above a minimum score of 80% were retained. IGVtools within the Integrative Genomics Viewer (IGV) was used to sort and index the BED files prior to intersection. Intersections of all BED files (derived from PWM analysis and ChIP-seq and FAIRE peak calling analysis) were done using Bedtools2 run locally from the command line. ChIP-seq peaks for Dll and Sp1 were first intersected with the FAIRE peaks. The product of this intersection was then sequentially intersected with each of the PWM files, always returning the peak coordinates from the initial file. The command intersectBed was used with options: -wa, -F 1.0, -u. To determine the gene nearest to each of the intersected ChIP peaks, packages within R/Bioconductor were used. The annotation package TxDb.Dmelanogaster.UCSC.dm3.ensGene was downloaded and annotated transcripts extracted. The distanceToNearest function was used to find the nearest annotated transcript to each of the ChIP Peaks. In-house R script was then used to generate the table containing the coordinates of the ChIP peaks, as well as the nearest annotated gene (S4 Table).
Supporting information
(A-B) Expression pattern of vnE (A) and rhoE (B) in third instar eye-antennae discs. (C-D) Expression pattern of (C) rhoLLE1 and (D) rhoLLE2 throughout leg disc development. (E) Schematic representation of ru genomic locus with enhancer bashing results. Fragments represented in tan did not drive expression in leg discs. (F-H) Expression pattern of ru from in situ (F), ruinga (G) and ruLLE (H). (I) Schematic representation of spi genomic locus with enhancer bashing results. (J-K) Expression pattern of spi from in situ (J) and spi-lacZ reporter construct (K). (L) Schematic representation of Krn genomic locus with enhancer bashing results and Krn27-7-B mutant. Fragments represented in tan did not drive expression in leg discs. (M) Expression pattern of Krn from in situ. (N) Schematic representation of grk genomic locus with grkΔFRT mutant. (O) Expression pattern of grk from in situ.
(TIF)
(A) Expression pattern of C15 and Al in vnvnE-Df mutant. (B) Adult leg of vnvnE-Df mutant. Filled arrowhead indicates intact pretarsal claw. (C) Expression pattern of C15 and Al in rhorhoE-Df mutant. (D) Adult leg of rhorhoE-Df mutant. Filled arrowhead indicates intact pretarsal claw. (E) Expression pattern of Al and BarH1 in WT (rhorhoE-Df vnvnE-Df/+) and (F) rhorhoE-Df vnvnE-Df double mutant. (G) Expression pattern of C15/Bar/Dac in WT (rhorhoE-Df vnvnE-Df Krn27-7-B/+) and (H) rhorhoE-Df vnvnE-Df Krn27-7-B triple mutants. (I-J) spi vn double RNAi driven by vn-GAL4. Expression pattern of C15 and BarH1 in third instar leg discs (I) and adult leg (J). Open arrowhead indicates absent pretarsal claw). (K-L) Expression pattern of ruinga-lacZ in ruinga rhorhoE-Df/+ (K) and ruinga rhorhoE-Df/ rhorhoE-Df (L) leg imaginal discs.
(TIF)
(A) vnE- or (B) rhoE-driven lacZ expression in DllSA1 mutant clones generated at 90h PEL. (C-E) vnE-driven lacZ expression in wing discs in Dll ectopic overexpression clones in WT background (C); Dll ectopic overexpression clones in arr2 mutant background (D); Dll overexpression clones in Mad1-2 mutant background (E); (C-E) clones were generated at 48h PEL. (F-G) vnE-driven lacZ expression in leg discs of Sp1HR mutant animals (F); Sp1HR mutant clones generated at 72h PEL (G). (H) rhoE-driven lacZ expression in leg discs of Sp1HR mutant animals. (I-J) vnE- (I) or rhoE- (J) driven lacZ expression in btdXA mutant clones generated at 48h PEL. (K) cic-lacZ expression in leg discs. (L-M) vnE- (L) or rhoE- (M) driven lacZ expression in leg discs with cicQ474X mutant clones generated at 48h PEL.
(TIF)
(A, C, E) vnE- (A) and rhoE- (C, E) driven expression of WT and intermediately mutant CRMs. (B and D) schematic representation of binding sites in vnE and rhoE, respectively. Mutated sites for the CRM-reporter genes shown in A, C, and E are indicated by the *, † and ‡. (F) Quantification of expression levels; fluorescence was calculated as a ratio of β-gal:Dll intensity in the center of the discs (see Methods for details). WT rhoEMIN n = 23, 6xDll n = 14, 1xDll n = 18, 5xDll n = 27 where n indicates number of leg discs analyzed.
(TIF)
Schematic representation of identified genomic loci and the expression patterns they drive in reporter genes from Dll_LE2 (A); spi_LE2 (B); noc_LE2 (C); Antp_LE1 (D); Antp_LE2 (E); Ote/fj_LE (F).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to Drs. Myriam Zecca, Yannis Mavromatakis, Gerard Campbell, Gary Struhl, Amanda Simcox, Benny Shilo, Andrew Tomlinson, Carlos Estella, Jessica Treisman, Josepha Steinhauer, Gerardo Jimenez, Rebecca Spokony and Trudi Schupbach for sharing numerous reagents that greatly facilitated this study. We thank members of the Mann lab, Gary Struhl, and Harmen Bussemaker for comments and suggestions during the course of these studies.
Data Availability
The ChIP-Seq data files are available at NCBI/GEO (accession number GSE113574): https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113574. All other relevant data are available within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by National Institutes of Health grants R35GM118336 and RO1GM058575 awarded to RSM; by the National Institutes of Health training grant 5T32DK007328 awarded to SN; and by Leukemia and Lymphoma Society grant 5250-12 awarded to RV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20(17):R754–63. 10.1016/j.cub.2010.06.070 ; PubMed Central PMCID: PMCPMC4280268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estella C, Voutev R, Mann RS. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr Top Dev Biol. 2012;98:173–98. 10.1016/B978-0-12-386499-4.00007-0 ; PubMed Central PMCID: PMCPMC3918458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SM. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature. 1990;343(6254):173–7. Epub 1990/01/11. 10.1038/343173a0 . [DOI] [PubMed] [Google Scholar]
- 4.Estella C, Rieckhof G, Calleja M, Morata G. The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development. 2003;130(24):5929–41. Epub 2003/10/17. 10.1242/dev.00832 . [DOI] [PubMed] [Google Scholar]
- 5.Estella C, Mann RS. Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS Genet. 2010;6(6):e1001001 Epub 2010/06/30. 10.1371/journal.pgen.1001001 ; PubMed Central PMCID: PMC2891808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74(6):1113–23. . [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372(6502):175–9. 10.1038/372175a0 . [DOI] [PubMed] [Google Scholar]
- 8.Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388(6638):139–45. 10.1038/40563 . [DOI] [PubMed] [Google Scholar]
- 9.Estella C, McKay DJ, Mann RS. Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev Cell. 2008;14(1):86–96. 10.1016/j.devcel.2007.11.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgianni MW, Mann RS. Establishment of medial fates along the proximodistal axis of the Drosophila leg through direct activation of dachshund by Distalless. Dev Cell. 2011;20(4):455–68. Epub 2011/04/19. doi: S1534-5807(11)00123-7 [pii] 10.1016/j.devcel.2011.03.017 ; PubMed Central PMCID: PMC3087180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418(6899):781–5. 10.1038/nature00971 . [DOI] [PubMed] [Google Scholar]
- 12.Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297(5579):256–9. 10.1126/science.1072311 . [DOI] [PubMed] [Google Scholar]
- 13.Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284(1):140–9. . [DOI] [PubMed] [Google Scholar]
- 14.Galindo MI, Bishop SA, Couso JP. Dynamic EGFR-Ras signalling in Drosophila leg development. Dev Dyn. 2005;233(4):1496–508. 10.1002/dvdy.20452 . [DOI] [PubMed] [Google Scholar]
- 15.Campbell G. Regulation of gene expression in the distal region of the Drosophila leg by the Hox11 homolog, C15. Dev Biol. 2005;278(2):607–18. 10.1016/j.ydbio.2004.12.009 . [DOI] [PubMed] [Google Scholar]
- 16.Wasserman JD, Urban S, Freeman M. A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 2000;14(13):1651–63. . [PMC free article] [PubMed] [Google Scholar]
- 17.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194(4):1029–35. 10.1534/genetics.113.152710 ; PubMed Central PMCID: PMCPMC3730909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A. 2014;111(29):E2967–76. Epub 2014/07/09. doi: 1405500111 [pii] 10.1073/pnas.1405500111 ; PubMed Central PMCID: PMC4115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195(3):715–21. Epub 2013/09/05. doi: genetics.113.156737 [pii] 10.1534/genetics.113.156737 ; PubMed Central PMCID: PMC3813859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima T, Sato M, Saigo K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development. 2000;127(4):769–78. Epub 2000/01/29. . [DOI] [PubMed] [Google Scholar]
- 21.Powell J. Progress and Prospects in Evolutionary Biology: The Drosophila Model: Oxford University Press; 1997. September 4, 1997. [Google Scholar]
- 22.McDonald JA, Pinheiro EM, Kadlec L, Schupbach T, Montell DJ. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 2006;296(1):94–103. 10.1016/j.ydbio.2006.04.438 . [DOI] [PubMed] [Google Scholar]
- 23.Lan L, Lin S, Zhang S, Cohen RS. Evidence for a transport-trap mode of Drosophila melanogaster gurken mRNA localization. PLoS One. 2010;5(11):e15448 10.1371/journal.pone.0015448 ; PubMed Central PMCID: PMCPMC2980492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simcox AA, Grumbling G, Schnepp B, Bennington-Mathias C, Hersperger E, Shearn A. Molecular, phenotypic, and expression analysis of vein, a gene required for growth of the Drosophila wing disc. Dev Biol. 1996;177(2):475–89. 10.1006/dbio.1996.0179 . [DOI] [PubMed] [Google Scholar]
- 25.Donaldson T, Wang SH, Jacobsen TL, Schnepp B, Price J, Simcox A. Regulation of the Drosophila epidermal growth factor-ligand vein is mediated by multiple domains. Genetics. 2004;167(2):687–98. 10.1534/genetics.103.019588 ; PubMed Central PMCID: PMCPMC1470887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Lee T, Lin N, Wolf MJ. Affecting Rhomboid-3 function causes a dilated heart in adult Drosophila. PLoS Genet. 2010;6(5):e1000969 10.1371/journal.pgen.1000969 ; PubMed Central PMCID: PMCPMC2877733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchankouo-Nguetcheu S, Udinotti M, Durand M, Meng TC, Taouis M, Rabinow L. Negative regulation of MAP kinase signaling in Drosophila by Ptp61F/PTP1B. Mol Genet Genomics. 2014;289(5):795–806. 10.1007/s00438-014-0852-2 . [DOI] [PubMed] [Google Scholar]
- 28.Gallio M, Englund C, Kylsten P, Samakovlis C. Rhomboid 3 orchestrates Slit-independent repulsion of tracheal branches at the CNS midline. Development. 2004;131(15):3605–14. 10.1242/dev.01242 . [DOI] [PubMed] [Google Scholar]
- 29.Cohen SM, Bronner G, Kuttner F, Jurgens G, Jackle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338(6214):432–4. 10.1038/338432a0 . [DOI] [PubMed] [Google Scholar]
- 30.Scholz H, Deatrick J, Klaes A, Klambt C. Genetic dissection of pointed, a Drosophila gene encoding two ETS-related proteins. Genetics. 1993;135(2):455–68. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, et al. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125(19):3875–85. . [DOI] [PubMed] [Google Scholar]
- 32.Roch F, Jimenez G, Casanova J. EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development. 2002;129(4):993–1002. . [DOI] [PubMed] [Google Scholar]
- 33.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24(5):251–4. . [DOI] [PubMed] [Google Scholar]
- 34.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72(4):527–40. Epub 1993/02/26. doi: 0092-8674(93)90072-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 35.Zhu LJ, Christensen RG, Kazemian M, Hull CJ, Enuameh MS, Basciotta MD, et al. FlyFactorSurvey: a database of Drosophila transcription factor binding specificities determined using the bacterial one-hybrid system. Nucleic Acids Res. 2011;39(Database issue):D111–7. 10.1093/nar/gkq858 ; PubMed Central PMCID: PMCPMC3013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sosinsky A, Bonin CP, Mann RS, Honig B. Target Explorer: An automated tool for the identification of new target genes for a specified set of transcription factors. Nucleic Acids Res. 2003;31(13):3589–92. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay DJ, Lieb JD. A common set of DNA regulatory elements shapes Drosophila appendages. Dev Cell. 2013;27(3):306–18. 10.1016/j.devcel.2013.10.009 ; PubMed Central PMCID: PMCPMC3866527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay DJ, Estella C, Mann RS. The origins of the Drosophila leg revealed by the cis-regulatory architecture of the Distalless gene. Development. 2009;136(1):61–71. 10.1242/dev.029975 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galindo MI, Fernandez-Garza D, Phillips R, Couso JP. Control of Distal-less expression in the Drosophila appendages by functional 3' enhancers. Dev Biol. 2011;353(2):396–410. Epub 2011/02/16. doi: S0012-1606(11)00092-3 [pii] 10.1016/j.ydbio.2011.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jory A, Estella C, Giorgianni MW, Slattery M, Laverty TR, Rubin GM, et al. A survey of 6,300 genomic fragments for cis-regulatory activity in the imaginal discs of Drosophila melanogaster. Cell Rep. 2012;2(4):1014–24. 10.1016/j.celrep.2012.09.010 ; PubMed Central PMCID: PMCPMC3483442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel). 2017;9(5). 10.3390/cancers9050052 ; PubMed Central PMCID: PMCPMC5447962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtiss J, Halder G, Mlodzik M. Selector and signalling molecules cooperate in organ patterning. Nat Cell Biol. 2002;4(3):E48–51. 10.1038/ncb0302-e48 . [DOI] [PubMed] [Google Scholar]
- 43.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. 10.1038/sj.onc.1210422 . [DOI] [PubMed] [Google Scholar]
- 44.Chaleshi V, Haghighi MM, Savabkar S, Zali N, Vahedi M, Khanyaghma M, et al. Correlation between the EGF gene intronic polymorphism, rs2298979, and colorectal cancer. Oncol Lett. 2013;6(4):1079–83. 10.3892/ol.2013.1481 ; PubMed Central PMCID: PMCPMC3796412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Yang G, Zhang D, Zhang W, Zou H, Zhao H, et al. Association between the epidermal growth factor +61 G/A polymorphism and glioma risk: a meta-analysis. PLoS One. 2014;9(4):e95139 10.1371/journal.pone.0095139 ; PubMed Central PMCID: PMCPMC3989292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kristensen VN, Edvardsen H, Tsalenko A, Nordgard SH, Sorlie T, Sharan R, et al. Genetic variation in putative regulatory loci controlling gene expression in breast cancer. Proc Natl Acad Sci U S A. 2006;103(20):7735–40. 10.1073/pnas.0601893103 ; PubMed Central PMCID: PMCPMC1458617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cordoba S, Requena D, Jory A, Saiz A, Estella C. The evolutionarily conserved transcription factor Sp1 controls appendage growth through Notch signaling. Development. 2016;143(19):3623–31. 10.1242/dev.138735 . [DOI] [PubMed] [Google Scholar]
- 48.Austin CL, Manivannan SN, Simcox A. TGF-alpha ligands can substitute for the neuregulin Vein in Drosophila development. Development. 2014;141(21):4110–4. 10.1242/dev.110171 . [DOI] [PubMed] [Google Scholar]
- 49.Slattery M, Voutev R, Ma L, Negre N, White KP, Mann RS. Divergent transcriptional regulatory logic at the intersection of tissue growth and developmental patterning. PLoS Genet. 2013;9(9):e1003753 10.1371/journal.pgen.1003753 ; PubMed Central PMCID: PMCPMC3764184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104(9):3312–7. 10.1073/pnas.0611511104 ; PubMed Central PMCID: PMCPMC1805588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166(4):1775–82. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voutev R, Mann RS. Robust PhiC31-Mediated Genome Engineering in Drosophila melanogaster Using Minimal attP/attB Phage Sites. G3 (Bethesda). 2018. 10.1534/g3.118.200051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev Cell. 2002;3(4):487–98. Epub 2002/11/01. doi: S1534580702002575 [pii]. . [DOI] [PubMed] [Google Scholar]
- 54.Noro B, Culi J, McKay DJ, Zhang W, Mann RS. Distinct functions of homeodomain-containing and homeodomain-less isoforms encoded by homothorax. Genes Dev. 2006;20(12):1636–50. 10.1101/gad.1412606 ; PubMed Central PMCID: PMCPMC1482483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charlton-Perkins M, Whitaker SL, Fei Y, Xie B, Li-Kroeger D, Gebelein B, et al. Prospero and Pax2 combinatorially control neural cell fate decisions by modulating Ras- and Notch-dependent signaling. Neural Dev. 2011;6:20 10.1186/1749-8104-6-20 ; PubMed Central PMCID: PMCPMC3123624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higashijima S, Michiue T, Emori Y, Saigo K. Subtype determination of Drosophila embryonic external sensory organs by redundant homeo box genes BarH1 and BarH2. Genes Dev. 1992;6(6):1005–18. . [DOI] [PubMed] [Google Scholar]
- 57.Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120(12):3473–86. . [DOI] [PubMed] [Google Scholar]
- 58.Morris CA, Benson E, White-Cooper H. Determination of gene expression patterns using in situ hybridization to Drosophila testes. Nat Protoc. 2009;4(12):1807–19. 10.1038/nprot.2009.192 . [DOI] [PubMed] [Google Scholar]
- 59.Slattery M, Ma L, Negre N, White KP, Mann RS. Genome-wide tissue-specific occupancy of the Hox protein Ultrabithorax and Hox cofactor Homothorax in Drosophila. PLoS One. 2011;6(4):e14686 10.1371/journal.pone.0014686 ; PubMed Central PMCID: PMCPMC3071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 ; PubMed Central PMCID: PMCPMC2705234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9(9):R137 10.1186/gb-2008-9-9-r137 ; PubMed Central PMCID: PMCPMC2592715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-B) Expression pattern of vnE (A) and rhoE (B) in third instar eye-antennae discs. (C-D) Expression pattern of (C) rhoLLE1 and (D) rhoLLE2 throughout leg disc development. (E) Schematic representation of ru genomic locus with enhancer bashing results. Fragments represented in tan did not drive expression in leg discs. (F-H) Expression pattern of ru from in situ (F), ruinga (G) and ruLLE (H). (I) Schematic representation of spi genomic locus with enhancer bashing results. (J-K) Expression pattern of spi from in situ (J) and spi-lacZ reporter construct (K). (L) Schematic representation of Krn genomic locus with enhancer bashing results and Krn27-7-B mutant. Fragments represented in tan did not drive expression in leg discs. (M) Expression pattern of Krn from in situ. (N) Schematic representation of grk genomic locus with grkΔFRT mutant. (O) Expression pattern of grk from in situ.
(TIF)
(A) Expression pattern of C15 and Al in vnvnE-Df mutant. (B) Adult leg of vnvnE-Df mutant. Filled arrowhead indicates intact pretarsal claw. (C) Expression pattern of C15 and Al in rhorhoE-Df mutant. (D) Adult leg of rhorhoE-Df mutant. Filled arrowhead indicates intact pretarsal claw. (E) Expression pattern of Al and BarH1 in WT (rhorhoE-Df vnvnE-Df/+) and (F) rhorhoE-Df vnvnE-Df double mutant. (G) Expression pattern of C15/Bar/Dac in WT (rhorhoE-Df vnvnE-Df Krn27-7-B/+) and (H) rhorhoE-Df vnvnE-Df Krn27-7-B triple mutants. (I-J) spi vn double RNAi driven by vn-GAL4. Expression pattern of C15 and BarH1 in third instar leg discs (I) and adult leg (J). Open arrowhead indicates absent pretarsal claw). (K-L) Expression pattern of ruinga-lacZ in ruinga rhorhoE-Df/+ (K) and ruinga rhorhoE-Df/ rhorhoE-Df (L) leg imaginal discs.
(TIF)
(A) vnE- or (B) rhoE-driven lacZ expression in DllSA1 mutant clones generated at 90h PEL. (C-E) vnE-driven lacZ expression in wing discs in Dll ectopic overexpression clones in WT background (C); Dll ectopic overexpression clones in arr2 mutant background (D); Dll overexpression clones in Mad1-2 mutant background (E); (C-E) clones were generated at 48h PEL. (F-G) vnE-driven lacZ expression in leg discs of Sp1HR mutant animals (F); Sp1HR mutant clones generated at 72h PEL (G). (H) rhoE-driven lacZ expression in leg discs of Sp1HR mutant animals. (I-J) vnE- (I) or rhoE- (J) driven lacZ expression in btdXA mutant clones generated at 48h PEL. (K) cic-lacZ expression in leg discs. (L-M) vnE- (L) or rhoE- (M) driven lacZ expression in leg discs with cicQ474X mutant clones generated at 48h PEL.
(TIF)
(A, C, E) vnE- (A) and rhoE- (C, E) driven expression of WT and intermediately mutant CRMs. (B and D) schematic representation of binding sites in vnE and rhoE, respectively. Mutated sites for the CRM-reporter genes shown in A, C, and E are indicated by the *, † and ‡. (F) Quantification of expression levels; fluorescence was calculated as a ratio of β-gal:Dll intensity in the center of the discs (see Methods for details). WT rhoEMIN n = 23, 6xDll n = 14, 1xDll n = 18, 5xDll n = 27 where n indicates number of leg discs analyzed.
(TIF)
Schematic representation of identified genomic loci and the expression patterns they drive in reporter genes from Dll_LE2 (A); spi_LE2 (B); noc_LE2 (C); Antp_LE1 (D); Antp_LE2 (E); Ote/fj_LE (F).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The ChIP-Seq data files are available at NCBI/GEO (accession number GSE113574): https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113574. All other relevant data are available within the manuscript and its Supporting Information files.