Abstract
Dehydroepiandrosterone (DHEA) reacted with m-chloroperoxybenzoic acid (m-CPBA) to form 3β-hydroxy-5α,6α-epoxyandrostan-17-one (1), but it did not react with 30% H2O2. 1,4,6-Androstatrien-3,17-dione (2) was obtained from DHEA and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in dioxane. Compound 2 was reacted with 30% H2O2 and 5% NaOH in methanol to give 1α,2α-epoxy-4,6-androstadien-3,17-dione (3), which was stereoselectively reduced with NaBH4 to form 1α,2α-epoxy-4,6-androstadien-3β,17β-diol (7) and reacted with Li metal in absolute ethanol-tetrahydrofuran mixture to give 2-ethoxy-1,4,6-androstatrien-3,17-dione (8). Compound 2 was also epoxidized with m-CPBA in dichloromethane to afford 6α,7α-epoxy-1,4-androstadien-3,17-dione (4), which was reacted with NaBH4 to synthesize 6α,7α-epoxy-4-androsten-3β,17β-diol (9). Compound 4 was reduced with Li metal in absolute ethanol-tetrahydrofuran mixture to form 7β-ethoxy-6α-hydroxy-1,4-androstadien-3,17-dione (10). Compound 2 was reduced with NaBH4 in absolute ethanol to form 4,6-androstadien-3β,17β-diol (5), which was reacted with 30% H2O2 to give the original compound, but which reacted with m-CPBA to give 4β,5β-epoxy-6-androsten-3β,17β-diol (6).
Keywords: Dehydroepiandrosterone; position selective and reagent selective epoxidation and reduction; H2O2; m-chloroperoxybenzoic acid; sodium borohydride, lithium
Introduction
Dehydroepiandrosterone (3β-hydroxyandrost-5-en-17-one, DHEA) is an endogenous steroid synthesized in the adrenal cortex, gonads, brain, and gastrointestinal tract [1,2]. It is known to have chemopreventive and anti-proliferative actions on tumors [3,4,5]. DHEA is the most abundant steroid in human blood; it is not only an intermediate in the biosynthesis of testosterone and estrogens but it also exerts several physiological effects independent of the sex hormones. It is also an anti-obesity agent for genetically obese and normal animals, but does not affect food intake [6]. It decreases blood cholesterol concentration in several species [7,8,9], lessens the severity of diabetics in genetically predisposed mice [10], enhances the immune system [11,12], and improves memory in aged mice [13].
Many naturally occurring polyhydroxylated sterols and oxysterols exhibit potent biological activities [14,15]. Some polyhydroxylated sterols showed potent cytotoxicity to cancer cells [16,17], but oxygenated and hydroxylated analogs of DHEA had apparently not been tested for biological activity in animals until recently [18,19,20,21]. Several monohydroxylated derivatives of DHEA [4α,5α,7α,7β,11β,16α,19] and of 5-androsten-3β,17β-diol were prepared. Among them, 7-hydroxy-DHEA was fully as active as DHEA and 7-oxo-DHEA was more active [18,20]. To search for possible derivatives of DHEA that might have greater biological activity and specificity, we sought to synthesize the corresponding epoxy and hydroxy-DHEA derivatives.
Epoxides are among the most frequently occurring functional groups found in natural products and their synthetic analogues. Not only are these compounds easily prepared from variety of starting materials, but the inherent polarity and the strain of their three-membered ring makes them susceptible to reaction with a large number of reagents – electrophiles, nucleophiles, acids, bases, reducing agents, and oxidizing agents [22,23]. Whilst the balance between the various stereoelectronic factors that control the stereochemistry of epoxidation of steroidal alkenes has been thoroughly investigated, there have been relatively few studies of the epoxidation of conjugated steroidal dienes and trienes [24].
In order to investigate the reagent selectivity and position selectivity of different epoxidizing agents towards 1,4,6-androstatrien-3,17-dione and 4,6-androstadien-3β,17β-diol as examples of trienes and dienes, 30% H2O2 and m-chloroperoxybenzoic acid were used. These reagents are easily handled and inexpensive materials that did not require complicated procedures or expensive metal-catalysts, which were mostly used to control the asymmetric epoxidation. In addition, and in order to study the reagent and position selectivity of the resulting epoxy derivatives towards reducing agents, we selected NaBH4 and Li metal in absolute ethanol-tetrahydrofuran mixture and we tried to compare the selectivity of these reagents for the cleavage of epoxide ring, and reduction of carbonyl groups and double bonds of epoxy unsaturated DHEA derivatives.
Results and Discussion
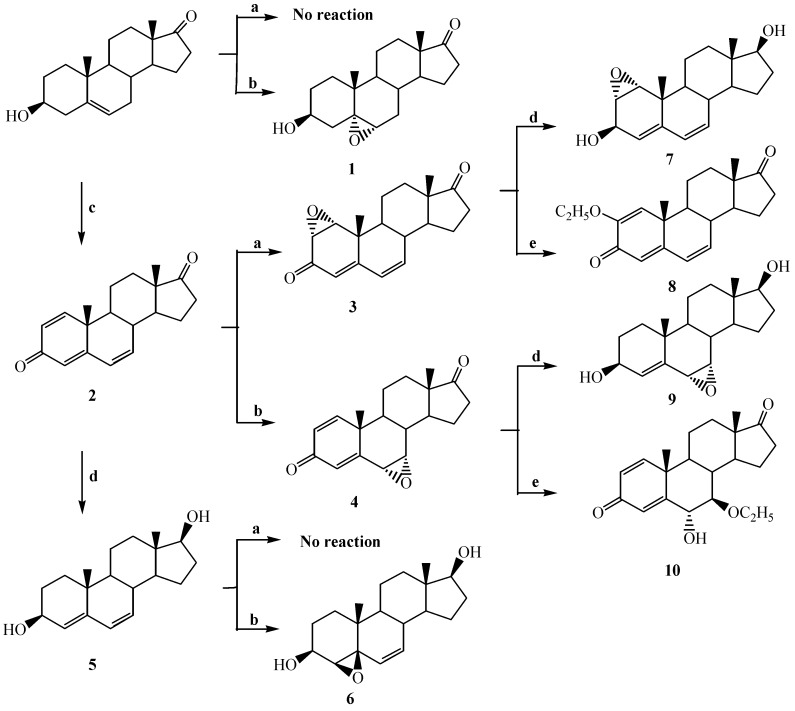
For the synthesis of dehydroepiandrosterone (DHEA) derivatives, commercially available DHEA was used as the starting material. The planned synthetic routes from DHEA are shown in Scheme 1.
Scheme 1.
Synthesis of dehydroepiandrosterone derivatives.
a: H2O2, 5%NaOH/MeOH, MeOH, rt, b: m-CPBA, CHCl3, rt, c: DDQ, dioxane, reflux, d: NaBH4, absolute ethanol, rt, e: Li, absolute ethanol, THF, rt.
First, DHEA was treated with 30% H2O2 and 5% NaOH in MeOH solution at room temperature, but it did not react. DHEA was then reacted with m-chloroperoxybenzoic acid to give 5α,6α-epoxyandrostan-3β-ol (1), which was formed by α-face attack of the peracid to the 5,6-double bond. The initial axial downside attack on the 5-androsten-3β-ol involves an interaction between the π-HOMO of the alkene and the σ*-LUMO of the O-O bond of the peracid. The peracid may be directed to the α-face of the steroid by unfavourable interaction with C-10 angular methyl group on the β-face [25].
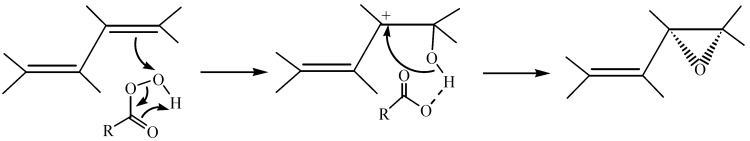
1,4,6-Androstatrien-3,17-dione (2) was synthesized from DHEA and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone [26]. Compound 2 was epoxidized with 30% H2O2 and 5% NaOH in MeOH solution at room temperature, the only 1,2-double bond of 2 was epoxidized to form 1α,2α-epoxy-4,6-androstadien-3,17-dione (3) regioselectively [27,28]. The stereochemistry of 3 was identified from the corresponding NOESY spectrum, where a cross peak of H-1 and H-19 was observed, meaning that the 1β-hydrogen and C-10 methyl hydrogens (H-19) are in a cis configuration relationship and thus the 1,2-epoxide is in the α-configuration. Compound 2 was epoxidized with m-chloroperoxybenzoic acid in chloroform at room temperature, the 6,7-double bond was epoxidized to form 6α,7α-epoxy-1,4-androstadien-3,17-dione (4). Initial attack by a peracid should to be directed to a pseudo-axial site on the face opposite to the C-10 methyl group to form α-epoxide. Allylic interaction between the adjacent double bond and the intermediate in the epoxidation might favour a particular site of attack by stabilizing an adjacent carbocationic intermediate [24] (Scheme 2).
Scheme 2.
Formation of stable allylic carbocationic intermediate by mCPBA.
Compound 2 was reduced with NaBH4 in absolute ethanol to form 4,6-androstadien-3β,17β-diol (5) as 1,2- and 1,4 addition product [29]. Metallic hydrides, such as LiAlH4 and NaBH4 , do not in general reduce carbon-carbon double bond, but these reagents may also double bond in conjugated with C=O bonds, as well as reducing the C=O bonds [30,31]. The attack of hydride anion of NaBH4 to the axial direction of the carbonyl group produced the 3β- and 17β-hydroxy configuration and only the 1,2-double bond of the 1,4,6-triene 2 was reduced. The stereochemistry was established from the NOESY spectrum. No correlation between the hydrogens of the C-10 methyl group on the β-face and H-3, and C-13 methyl hydrogens and H-17 was observed in the NOESY spectrum of 5, therefore both OH groups on C-3 and C-17 are directed in the β-position. On attempted epoxidation of compound 5 in the presence of 30% H2O2 and 5% NaOH in methanol, only starting material was recovered. Compound 5 reacted with m-chloroperoxybenzoic acid to give 4β,5β-epoxy-androst-6-en-3β,17β-diol (6). In this case, the presence of the 3β-hydroxy group of the allylic alcohol directed the epoxidation exclusively to the β-face of the double bond of the molecule [32,33].
Compounds 3 and 4 were reduced with NaBH4 and Li metal in absolute ethanol-THF mixture, respectively. Compound 3 reacted with NaBH4 to give 1α,2α-epoxy-4,6-androstadien-3β,17β-diol (7). NaBH4 selectively attack the two carbonyl groups of compound 3 to give the corresponding 3β,17β-diol. This result was similar to the observation by Benn et al [34] that 1α,2α-epoxyandrostan-3β,17β-diol was obtained from 1α,2α-epoxyandrostan-3,17-dione with NaBH4. Compound 3 was also reacted with Li metal in absolute ethanol-tetrahydrofuran mixture to form 2-ethoxy-1,4,6-androstatriene-3,17-dione (8). In this case, ethoxide anion, formed from Li metal in the solvent, attacked the 2-position of the epoxide ring to form 2β-ethoxy-1α-hydroxy-4,6-androstadien-3-one as intermediate, and then H2O was eliminated to give compound 8. In the 1H-NMR spectrum of 8 four double bond hydrogens were identified at 5.93(1H), 6.11(2H), 6.33(1H) ppm and six double bond carbons at 161.8, 150.3, 136.1, 128.1, 124.1, 120.3 ppm and two carbonyl carbons at 219.1, 181.2 ppm were identified in the 13C-NMR spectrum. Compound 4 was reacted with NaBH4 to afford 6α,7α-epoxy-4-androsten-3β,17β-diol (9), obtained by 1,2- and 1,4-addition of hydride to the α,β-unsaturated carbonyl group and α-attack of hydride on the 17-carbonyl group. 7β-Ethoxy-6α-hydroxy-1,4-androstadien-3,17-dione (10) was obtained from the reaction of 4 with Li metal in absolute ethanol-tetrahydrofuran mixture at room temperature. The β-face attack of ethoxide ion to the 7-position of epoxide ring might be directed to cause cleavage of the epoxide ring. In the 1H-NMR spectrum of 10 three double bond hydrogens were observed at 6.16, 6.25, 7.15 ppm and four double bond carbons at 161.7, 157.3, 130.6, 126.8 ppm and two carbonyl carbons at 220.5, 185.9 ppm were identified in 13C-NMR spectrum, respectively. The structure for 10 was further confirmed by FABMS showing an (M+H-C2H5OH)+ at 299.1 m/z and (M+H-C2H5OH-H2O)+ at 281.1 m/z.
Conclusions
On treatment of 1,4,6-androstatrien-3,17-dione (2) with 30% H2O2, of the double bonds conjugated with the carbonyl group at position 3 only the 1,2-double bond was selectively transformed into the corresponding 1α,2α-epoxide in 5% NaOH in methanol solution, whereas m-CPBA epoxidized the 6,7-double bond of the trienone to form the 6α,7α-epoxide derivative in the presence of the carbonyl group at position 3. On reduction in ethanol in the presence of NaBH4, only the 1,2-double bond and the carbonyl of 2 were reduced to give 4,6-androstadien-3β,17β-diol (5). The 3β-hydroxy group of the allylic alcohol of compound 5 directed the subsequent epoxidation by m-CPBA exclusively to the β-face of the 4,5-double bond. 30% H2O2 did not react in the presence of hydroxyl group at position 3. The carbonyl groups at positions 3 and 17 of 1α,2α-epoxy- and 6α,7α-epoxyandrostadien-3,17-dione (3 and 4) were selectively reduced by NaBH4 to form the 3β,17β-diol. And reaction of epoxy-dienone in absolute ethanol-tetrahydrofuran mixture in the presence of Li metal resulted in the cleavage of epoxide ring by ethoxide anion without reduction of double bonds and carbonyl groups.
Experimental
General
Reactions were performed under nitrogen gas. Solvents were purified and dried prior to use. Melting points were measured on Thomas-Hoover melting point apparatus in open capillary tubes and are not corrected. 1H- and 13C-NMR spectra were obtained on Gemini 200 MHz or 50MHz spectrometers in CDCl3, chemical shifts δ are in ppm are relative to tetramethylsilane, coupling constants J in Hz. NOESY spectra were measured on Bruker AM-500MHz spectrometer (Rheinstetten, Germany). IR spectra were determined on a Jasco FT-IR 300E spectrometer as KBr pellets. FAB mass spectra were recorded on a tandem mass spectrometer made by Jeol Ltd (Japan). Analytical TLC was performed on Merck precoated silica gel 60 F254 plates. Column chromatography was carried out on Merck silica gel 9385 (230-400 mesh).
5α,6α-Epoxy-anrostan-3β-ol-17-one (1)
To a solution of androst-5-en-3β-ol (dehydroepiandrosterone, 1 g, 3.47 mmol) in CHCl3 (50 mL) m-chloroperoxybenzoic acid (2 g, 6.93 mmol) was added at room temperature. The mixture was stirred at room temperature for 24 hours. The solution was diluted with CHCl3, treated with aqueous sodium sulfite and the aqueous layer was extracted with several portions of CHCl3. The combined organic extracts were washed with water, dried with anhydrous MgSO4, filtered and evaporated to leave a crude solid, which was column chromatographed with 1:2 ethyl acetate/n-hexane as eluent to afford white crystals. Yield: 897 mg (85 %); mp: 208.5-212 °C; IR (cm-1): 3520, 2946, 1721, 1440; 1H-NMR δ: 0.82 (3H, s, H-18), 1.26 (3H, s, H-19), 2.94 (1H, d, J=3.0Hz, H-6), 3.79-3.96 (1H, m, H-3); 13C-NMR δ: 220.7, 68.4, 65.9, 58.9, 51.9, 47.7, 42.9, 39.8, 35.8, 35.1, 32.5, 31.2, 31.1, 29.6, 27.8, 21.8, 20.1, 16.1, 13.7; FABMS (M+H-H2O)+: 287.0.
1,4,6-Androstatrien-3,17-dione (2)
A solution of DHEA (5 g, 17.36 mmol) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (11.8 g, 52.08 mmol) in dioxane (100 mL) was refluxed for 24 hours according to the procedure reported by Furst et al. [35]. The reaction mixture was cooled down and the resulting precipitate was filtered off and washed with dichloromethane. The filtrate was rotary evaporated to dryness and the dark brown residue was purified by column chromatography on silica gel (ethyl acetate/n-hexane=1:3) to afford white crystals. Yield: 2.98 g (61 %); mp : 165-166 °C; IR (cm-1): 2977, 2937, 1739, 1649, 1602; 1H-NMR δ: 1.01 (3H, s, H-18), 1.23 (3H, s, H-19), 6.08 (2H, t, H-6, H-7), 6.24-6.36 (2H, m, H-2, H-4), 7.06 (1H, d, J=10.0Hz, H-1); 13C-NMR δ: 186.1, 161.7, 152.4, 135.7, 128.6, 128.4, 124.3, 49.0, 48.6, 48.0, 41.2, 37.7, 35.7, 31.4, 21.5, 21.8, 20.9, 14.0; FABMS (M+H)+: 283.0.
1α,2α-Epoxy-4,6-androstadien-3,17-dione (3)
According to a procedure of Pelc et al. [27], to a solution of the trienone 2 (600 mg, 2.1 mmol) in methanol (30 mL) was added a mixture of 5 % NaOH-MeOH (0.4 mL) and 30 % H2O2 (1.2 mL). The reaction mixture was stirred at room temperature for 22 hours, at which time TLC indicated reaction completion, and then extracted with dichloromethane. The organic layers were combined and washed with brine and H2O, dried over anhydrous MgSO4, filtered, and concentrated to leave a crude solid. Column chromatography on silica gel, eluting with 1:2 ethyl acetate/n-hexane gave pure white crystals of epoxide 3. Yield: 1.65 g (78 %); mp: 220-223 °C; IR (cm-1): 2946, 1740, 1671, 1664, 1615, 1440; 1H-NMR δ: 1.00 (3H, s, H-18), 1.23 (3H, s, H-19), 3.45-3.49 (1H, m, H-1), 3.61 (1H, d, J=4.0Hz, H-2), 5.69 (1H, s, H-4), 6.09-6.23 (2H, m, H-6, H-7); 13C-NMR δ: 194.7, 158.1, 137.7, 128.9, 120.3, 63.2, 62.5, 59.6, 55.0, 48.9, 48.1, 46.5, 37.1, 35.9, 31.4, 21.8, 20.9, 18.8,14.0; FABMS (M+H)+: 299.0.
6α,7α-Epoxy-1,4-androstadien-3,17-dione (4)
m-Chloroperoxybenzoic acid (1.95 g, 11.33 mmol) was added to a solution of trienone 2 (1.6 g, 5.66 mmol) in CHCl3 (80 mL) at room temperature. The reaction mixture was stirred at the same temperature until no starting material could be detected by TLC. The mixture was stirred with H2O and extracted with dichloromethane. The extract was dried over anhydrous MgSO4, filtered, and evaporated to obtain pale yellow precipitate. Column chromatography on silica gel, eluting with 1:5 ethyl acetate/n-hexane gave the pure white crystals of epoxide 4. Yield: 1.35 g (64 %); mp: 199-202 °C; IR (cm-1): 2942, 1743, 1662, 1454; 1H-NMR δ: 1.00 (3H, s, H-18), 1.25 (3H, s, H-19), 3.50 (1H, d, J=2.2Hz, H-6), 3.72 (1H, d, J=3.8Hz, H-7), 6.25 (1H, dd, J=1.8Hz, 10.0Hz, H-4), 6.51 (1H, d, J=1.2Hz, H-2), 7.02 (1H, d, J=10.2Hz, H-1); 13C-NMR δ: 219.0, 185.2, 159.2, 153.1, 131.4, 128.0, 53.0, 51.8, 47.9, 46.7, 41.1, 38.7, 35.7, 35.2, 31.2, 21.5, 21.4, 20.8, 13.9; FABMS (M+H)+: 299.0.
1,4-Androstadien-3β,17β-diol (5)
To a solution of compound 2 (1 g, 3.55 mmol) in absolute ethanol (100 mL), NaBH4 (537 mg, 14.2 mmol) was added at room temperature. The resulting mixture was stirred overnight at same temperature, and then was concentrated by rotary evaporation to afford white solid. It was stirred with water for 30 minutes, then extracted with dichloromethane from the aqueous layer. The organic layer was washed with aqueous sodium hydrogen carbonate, water and dried with anhydrous MgSO4, filtered, and concentrated to give a residue, which was recrystallized from 1:2 ethyl acetate/n-hexane to afford white crystals of the title compound. Yield: 1.37 g (63 %); mp: 155-158.5 °C; IR (cm-1): 3400, 2960, 1670, 1460; 1H-NMR δ: 0.83 (3H, s, H-18), 1.01 (3H, s, H-19), 3.62-3.66 (1H, m, H-3), 4.36 (1H, m, H-17), 5.58 (1H, s, H-4), 5.60 (1H, d, J=9.8Hz, H-6), 5.96 (1H, dd, J=2.4Hz, 10.0Hz, H-7); 13C-NMR δ: 159.1, 148.3, 126.7, 123.2, 58.7, 54.5, 48.5, 47.7, 40.5, 36.2, 34.7, 30.5, 29.5, 27.8,25.8, 22.0, 21.2, 19.0, 13.8; FABMS (M+H-H2O)+: 271.1.
4β,5β-epoxyandrost-6-en-3β,17β-diol (6)
m-Chloroperoxybenzoic acid (611 mg, 3.55 mmol) was added to a solution of dienol 5 (500 mg, 1.77 mmol) in CHCl3 (50 mL) at room temperature and the reaction mixture was then treated in a manner similar to that described for the synthesis of compound 1. The pure compound 6 was obtained as white crystals by column chromatography on silica gel (elution with 1:3 ethyl acetate/n-hexane=). Yield: 678 mg (64 %); mp: 146-149 °C; IR (cm-1): 3405, 2933, 1455; 1H-NMR δ: 0.85 (3H, s, H-18), 1.22 (3H, s, H-19), 3.57-3.80 (2H, m, H-3, H-17), 4.17 (1H, d, J=3.2Hz, H-4), 5.32 (1H, dd, J=2.2Hz, 6.4Hz, H-7), 5.64 (1H, d, J=2.2Hz, H-6); 13C-NMR δ: 125.3, 121.7, 69.3, 63.6, 58.2, 53.2, 47.5, 46.2, 39.9, 36.2, 35.1, 30.7, 30.0, 28.8, 27.5 22.0, 21.1, 19.0, 14.0; FABMS (M+H)+: 305.1, (M+H-H2O)+: 287.1
1α,2α-Epoxy-1,4--androstadien-3β,17β-diol (7)
To a solution of compound 3 (500 mg, 1.68 mmol) in absolute ethanol (100 mL) NaBH4 (237 mg, 6.27 mmol) was added at room temperature. The resulting mixture was stirred overnight at the same temperature and then was concentrated by rotary evaporation to afford a white solid which was stirred with water and d-HCl solution for 30 minutes, then extracted with dichloromethane from the aqueous layer. The organic layer was washed with water and dried with MgSO4, filtered, and concentrated to give a white solid, which was purified by column chromatography (1:3 ethyl acetate/ n-hexane). Yield: 706 mg (69 %); mp : 139-142 °C; IR (KBr) cm-1 : 3407, 2931, 2859, 1672, 1453; 1H-NMR (CDCl3) δ: 0.81 (3H, s, H-18), 1.00 (3H, s, H-19), 3.33 (1H, m, J=9.6Hz, H-1), 3.54 (1H, t, H-2), 3.63-3.75 (1H, m, H-3), 4.53 (1H, br s, H-17), 5.21 (1H, s, H-4), 5.60 (1H, d, J=6.2Hz, H-6), 5.95 (1H, d, J=9.6Hz, H-7); 13C-NMR (CDCl3) δ: 140.0, 130.7, 128.1, 121.1, 81.2, 65.7, 58.9, 56.0, 48.8, 45.8, 43.5, 36.9, 36.6, 36.4, 30.2, 23.6, 21.0, 17.4, 11.2; FABMS (M+H)+: 303.1.
2-Ethoxy-1,4,6-androstatrien-3,17-dione (8)
To a solution of 3 (500 mg, 1.68 mmol) in absolute ethanol (50 mL) lithium metal (400 mg) was added slowly and the resulting mixture was vigorously stirred at the room temperature for 18 hours. The reaction mixture was cooled down to 0°C and water was slowly added with stirring until a clear solution was obtained. The mixture was evaporated to remove the ethanol, and then the aqueous layer was extracted several times with ethyl acetate. The combined organic layer was washed with H2O and dried over anhydrous MgSO4, filtered and evaporated to give a crude oil, which was purified by column chromatography on silica gel (1:3 ethyl acetate/n-hexane). Yield: 372 mg (73 %); mp :175-178 °C; IR (KBr) cm-1: 2930, 2870, 1735, 1649, 1456, 1191; 1H-NMR (CDCl3) δ: 1.01 (3H, s, H-18), 1.24 (3H, s, H-19), 1.44 (3H, t, OCH2CH3), 3.86 (2H, q, OCH2CH3), 5.93 (1H, s, H-4), 6.11 (2H, d, J=12.4Hz, H-6, H-1), 6.33 (1H, dd, J=1.2Hz, J=9.8Hz, H-7); 13C-NMR (CDCl3) δ: 219.1, 181.2, 161.8, 150.3, 136.1, 128.1, 124.1, 120.3, 63.4, 49.6, 49.0, 48.0, 41.3, 37.7, 35.8, 31.4, 29.9, 22.2, 21.8, 21.5,14.5, 14.0; FABMS (M+H)+: 327.1.
6α,7α-Epoxy-androst-4-en-3β,17β-diol (9)
To a solution of 4 (500 mg, 1.68 mmol) in absolute ethanol (100 mL) NaBH4 (177 mg, 4.70 mmol) was added and the mixture stirred at room temperature until the starting material had disappeared in a TLC test. The reaction mixture was evaporated to a dry solid and H2O was added to the residue with stirring. The aqueous layer was extracted with ethyl acetate, and the organic layer was dried over anhydrous MgSO4, filtered, concentrated to give a crude precipitate. The purified product was obtained by column chromatography on silica gel (1:3 ethyl acetate/n-hexane). Yield: 390 mg (76 %); mp: 138-141 °C; IR (KBr) cm-1: 3401, 2935, 1452; 1H-NMR (CDCl3) δ: 0.78 (3H, s, H-18), 1.16 (3H, s, H-19), 3.70 (1H, t, H-7), 3.83 (1H, s, H-6), 4.19-4.28 (1H, m, H-3), 5,44 (1H, s, H-4); 13C-NMR (CDCl3) δ: 151.3, 145.4, 68.9, 66.3, 59.2, 53.3, 46.7, 45.4, 43.1, 42.2, 39.5, 37.8, 34.9, 30.4, 22.2, 21.3, 19.4, 14.0; FABMS (M+H)+ : 305.1.
7β-Ethoxy-6α-hydroxy-1,4-androstadien-3,17-dione (10)
To a solution of 4 (500 mg, 1.68 mmol) in absolute ethanol (50 mL) lithium metal (300 mg) was added slowly and the mixture vigorously stirred at room temperature until the starting material had disappeared in a TLC test. The workup was as described in the preparation of compound 8. The purified product was obtained as white crystals by column chromatography on silica gel (1:3 ethyl acetate/n-hexane). Yield: 450 mg (78 %); mp: 210-211 °C; IR (KBr) cm-1: 3407, 2922, 1739, 1657, 1453, 1105; 1H-NMR (CDCl3) δ: 0.96 (3H, d, J=5.4Hz, H-18), 1.19 (3H, t, OCH2CH3), 1.76 (3H, s, H-19), 3.35-3.54 (2H, m, OCH2CH3), 3.91 (1H, d, J=13.0Hz, H-6), 4.05 (1H, m, H-7), 6.19 (1H, d, J=1.8Hz, H-4), 6.24 (1H, s, H-2), 7.05 (1H, d, J=9.4Hz, H-1); 13C-NMR (CDCl3) δ: 220.5, 185.8, 161.7, 157.3, 130.6, 126.8, 85.2, 71.3, 64.6, 47.6, 45.2, 43.7, 43.5, 35.8, 35.3, 31.1, 21.7, 21.6, 19.3, 15.2, 13.8; FABMS (M+H)+: 345.1, (M+H-C2H5OH)+: 299.1, (M+H-C2H5OH-H2O)+: 281.1.
Acknowledgments
We thank the Catholic University of Daegu for financial support.
Footnotes
Sample Availability: Samples of compounds 1, 2, 3, 4 and 5 are available from MDPI. For other samples contact the authors.
References
- 1.Robel P., Bourreau E., Corpechot C., Dang D. C., Halberg F., Clarke C. Neurosteroids: 3β-hydroxy-Δ5-derivatives in rat and monkey brain. J. Steroid Biochem. 1998;27:649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- 2.Dalla V. L., Couet J., Labrie Y., Simard J., Belvedere P., Simontacchi C. Occurrence of cytochrome P450c mRNA and dehydroepiandrosterone biosynthesis in the rat gastrointestinal tract. Mol. Cell Endocrinol. 1995;111:83–92. doi: 10.1016/0303-7207(95)03553-J. [DOI] [PubMed] [Google Scholar]
- 3.Schulz S., Klann R. C., Schonfeld S., Nyce J. W. Mechanism of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells dehydroepiandrosterone: role of isoprenoid biosynthesis. Cancer Res. 1992;52:1372–1376. [PubMed] [Google Scholar]
- 4.Boros L. G., Puigjancer J., Cascante M., Lee W. N., Brandes J. L., Bassilian S. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57:4242–4248. [PubMed] [Google Scholar]
- 5.Melvin W. S., Boros L.G., Muscarella P., Brandes J. L., Johnson L. A., Fischer W. E. Dehydroepiandrosterone-sulfate inhibits pancreatic carcinoma cell proliferation in vitro and vivo. Surgery. 1997;121:392–397. doi: 10.1016/S0039-6060(97)90308-1. [DOI] [PubMed] [Google Scholar]
- 6.Welle S., Jozefowicz R., Statt M. Failure of dehydroepiandrosterone influence energy and protein metabolism in humans. J. Clin. Endocrinol. Metab. 1990;71:1259–1264. doi: 10.1210/jcem-71-5-1259. [DOI] [PubMed] [Google Scholar]
- 7.Nestler J., Barlascini C., Clore J., Blackard W. G. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter sensitivity in normal men. J. Clin. Endocrinol. Metab. 1988;66:57–61. doi: 10.1210/jcem-66-1-57. [DOI] [PubMed] [Google Scholar]
- 8.Kritchevsky D., Tepper S., Klurfeld D., Schwarz A. Influence of dehydroepiandrosterone (DHEA) on cholesterol metabolism in rats. Pharmacol. Res. Commun. 1983;15:797–803. doi: 10.1016/S0031-6989(83)80087-3. [DOI] [PubMed] [Google Scholar]
- 9.Kurzman I. D., McEwen E. G., Haffa A. Reduction in body weight and cholesterol in spontaneously obese dogs by dehydroepiandrosterone. Int. J. Obesity. 1990;14:95–104. [PubMed] [Google Scholar]
- 10.Colman D. L., Schwizer R., Leiter E. H. Effect of genetic background on the therapeutic effects of dehydroepiandrosterone (DHEA) in diabetes-obesity mutants and in aged normal mice. Diabetes. 1984;33:26–32. doi: 10.2337/diab.33.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Loria R. M., Inge T. H., Cook S. S., Szakal A. K., Regelson W. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA) Med. Virol. 1988;26:301–314. doi: 10.1002/jmv.1890260310. [DOI] [PubMed] [Google Scholar]
- 12.Danenberg H., Ben-Yehuda A., Zakay-Rones Z., Friedman G. Dehydroepiandrosterone (DHEA) treatment reverses the impaired immune response of old mice to influenza vaccination and protects from influenza infection. Vaccine. 1995;13:1445–1448. doi: 10.1016/0264-410X(95)00063-7. [DOI] [PubMed] [Google Scholar]
- 13.Flood J. F., Morley J. E., Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc. Natl. Acad. Sci. USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandutsch A., Chen H. W., Heininger H. J. Biological activity of some oxygenated sterols. Science. 1978;201:498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- 15.Smith L. L., Johnson B. H. Biological activities of oxysterols. Free Radical Biol. Med. 1989;7:285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- 16.Zeng L. M., Li K. Q., Su J. Y., Fu X., Schmitz F. J. A new cytotoxic dihydroxy sterol from the soft coral Alcyonium patagonicum. J. Nat. Prod. 1995;58:296–298. doi: 10.1021/np50116a025. [DOI] [PubMed] [Google Scholar]
- 17.Cui J. G., Zeng L. M., Su J. Y., Lu W. G. Synthesis of ployhydroxysterols (I): Synthesis of 24-methylene-cholest-4-en-3β,6β-diol, a cytotoxic natural hydroxylated sterol. Steroids. 2001;66:33–38. doi: 10.1016/S0039-128X(00)00132-X. [DOI] [PubMed] [Google Scholar]
- 18.Su C.Y., Lardy H. A. Effect of dehydroepiandrosterone on mitochondrial glycerophosphate dehydrogenase and malic enzyme activities. FASEB J. 1988;2:A581. [Google Scholar]
- 19.Su C.Y., Lardy H. A. Induction of hepatic mitochondrial glycerophosphate dehydrogenase in rats by dehydroepiandrosterone. J. Biochem. 1991;110:201–213. doi: 10.1093/oxfordjournals.jbchem.a123558. [DOI] [PubMed] [Google Scholar]
- 20.Patridge B., Lardy H. A. Enzyme induction by DHEA: Metabolically active derivatives. J. Cell. Biol. 1988;107:203a. [Google Scholar]
- 21.Lardy H. A., Patridge B., Kneer N., Wei Y. Ergosteroids: Induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone. Proc. Natl. Acad. Sci. USA. 1995;92:6617–6619. doi: 10.1073/pnas.92.14.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith J. G. Synthetically useful reactions of epoxides. Synthesis. 1984:629–656. doi: 10.1055/s-1984-30921. [DOI] [Google Scholar]
- 23.Rao A. S., Pakinkar S. K., Kirtane J. G. Recent advances in the preparation and synthetic applications of oxiranes. Tetrahedron. 1983;39:2323–2367. doi: 10.1016/S0040-4020(01)91961-1. [DOI] [Google Scholar]
- 24.Hanson J. R., Hitchcock P. B., Kiran I. The stereochemistry of epoxidation of steroidal 4,6-dienes. J. Chem. Res. 1999:1082–1096. [Google Scholar]
- 25.Woods K. W., Beak P. The endocyclic restriction test: An experimental evaluation of the geometry at oxygen in the transition structure for epoxidation of an alkene by a peroxy acid. J. Am. Chem. Soc. 1991;113:6281–6283. doi: 10.1021/ja00016a061. [DOI] [Google Scholar]
- 26.Turner A. B. Applications of high-potential quinones. Convenient synthesis of steroidal 1,4,6-trien-3-ones. Chem. Commun. 1966;23:845–846. [Google Scholar]
- 27.Pelc B., Hodkova J., Holubek J. Steroid derivatives. XXXVIII. Preparation of 1,2-oxides of androstane derivatives and their reactions. Coll. Czech. Chem. Commun. 1966;31:1363–1370. [Google Scholar]
- 28.Pelc B., Hodkova J. Steroid derivatives. XLIV. Further reactions of 1α,2α-epoxy-3-ketones of androstane series. Coll. Czech. Chem. Commun. 1967;32:410–418. [Google Scholar]
- 29.Kobayashi Y., Taguchi T., Mitsuhashi S., Eguchi T., Ohshima E., Ikekawa N. Studies on organic fluorine compounds. XXXIX. Studies on steroids. LXXIX. Synthesis of 1α,25-dihydroxy-26,26,26,27,27,27-hexafluorovitamin D. Chem. Pharm. Bull. 1982;30:4297–4303. [Google Scholar]
- 30.Brown H. C., Hess H. M. Selective reductions XIII. Reaction of 2-cyclopentenones with representative complex hydrides. Aluminum hydride as a selective reagent for the reduction of the carbonyl group in 2-cyclopentenones. J. Org. Chem. 1969;34:2206–2209. [Google Scholar]
- 31.Meyer R. G. Conjugate and nonconjugate reduction with LiAlH4 and NaBH4. J. Chem. Educ. 1981;58:624–628. doi: 10.1021/ed058p628. [DOI] [Google Scholar]
- 32.Gamoh K., Hirayama M., Ikekawa N. Stereocontrolled synthesis of Withanolide D and related compounds. J. Chem. Soc. Perkin Trans. I. 1984:440–454. [Google Scholar]
- 33.Sharpless K. B., Verhoeven T. R. Metal-catalysed, highly selective oxygenations of olefins and acetylenes with tert-butyl hydroperoxide. Practical considerations and mechanisms. Aldrichimica Acta. 1979;12:63–75. [Google Scholar]
- 34.Benn W. R., Colton F., Pappo R. The structure of ruscogenin. J. Am. Chem. Soc. 1967;79:3920–3920. doi: 10.1021/ja01571a080. [DOI] [Google Scholar]
- 35.Furst A., Labler L., Meier W. An effect procedure for preparing 1,3β-dihydroxy-Δ5-steroids by reduction of 1α,2α-epoxy-4,6-dien-3-ones with lithium in ammonia. Helv. Chim. Acta. 1981;64:1870–1892. doi: 10.1002/hlca.19810640621. [DOI] [Google Scholar]