Abstract
Many studies have been done over the years to assess the effectiveness of Echinacea as an immunomodulator. We have assessed the potential bioavailability of alkylamides and caffeic acid conjugates using Caco-2 monolayers and compared it to their actual bioavailability in a Phase I clinical trial. The caffeic acid conjugates permeated poorly through the Caco-2 monolayers. Alkylamides were found to diffuse rapidly through Caco-2 monolayers. Differences in diffusion rates for each alkylamide correlated to structural variations, with saturation and N-terminal methylation contributing to decreases in diffusion rates. Alkylamide diffusion is not affected by the presence of other constituents and the results for a synthetic alkylamide were in line with those for alkylamides found in an ethanolic Echinacea preparation. We examined plasma from healthy volunteers for 12 hours after ingestion of Echinacea tablets manufactured from an ethanolic liquid extract. Caffeic acid conjugates could not be identified in any plasma sample at any time after tablet ingestion. Alkylamides were detected in plasma 20 minutes after tablet ingestion and for each alkylamide, pharmacokinetic profiles were devised. The data are consistent with the dosing regimen of one tablet three times daily and supports their usage as the primary markers for quality Echinacea preparations.
Keywords: Alkylamides, Echinacea, pharmacokinetics, bioavailability, Caco-2
Introduction
Echinacea has been used for many years as an agent to enhance immune system function and many clinical studies have been carried out to assess its efficacy as an immunomodulator [1,2,3,4]. There are two major difficulties in comparing these studies. The first is that the actual bioavailable components were not determined and the second is that the phytochemical profile of the Echinacea used in each study was not provided. There are three major groups of compounds found in Echinacea extracts that are thought to be responsible for its immunomodulatory activity. Of these, polysaccharides are not found in a high ethanolic Echinacea extract [5] (which is the traditional method of preparation of the herb for therapeutic purposes) due to low solubility and as such are not relevant to this study. Caffeic acid conjugates (caftaric acid, cichoric acid and echinacoside) and alkylamides are present in the ethanolic extracts used in this study. Despite many in vitro studies ascribing immunomodulatory activity to both the caffeic acid conjugates and alkylamides [6], this activity is only possible if they are bioavailable. In this two-tiered study we have assessed the potential bioavailability of these two groups of compounds from Echinacea using Caco-2 monolayers (an in vitro assessment of a compounds ability to diffuse across the intestinal barrier [7]) and compared this with their actual bioavailability in a Phase I clinical trial.
Results and Discussion
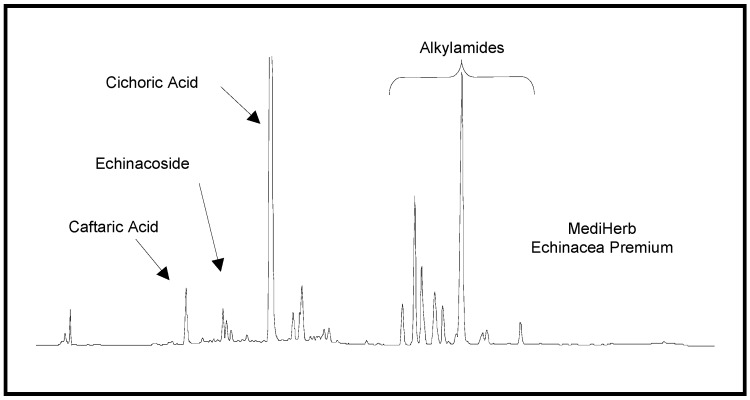
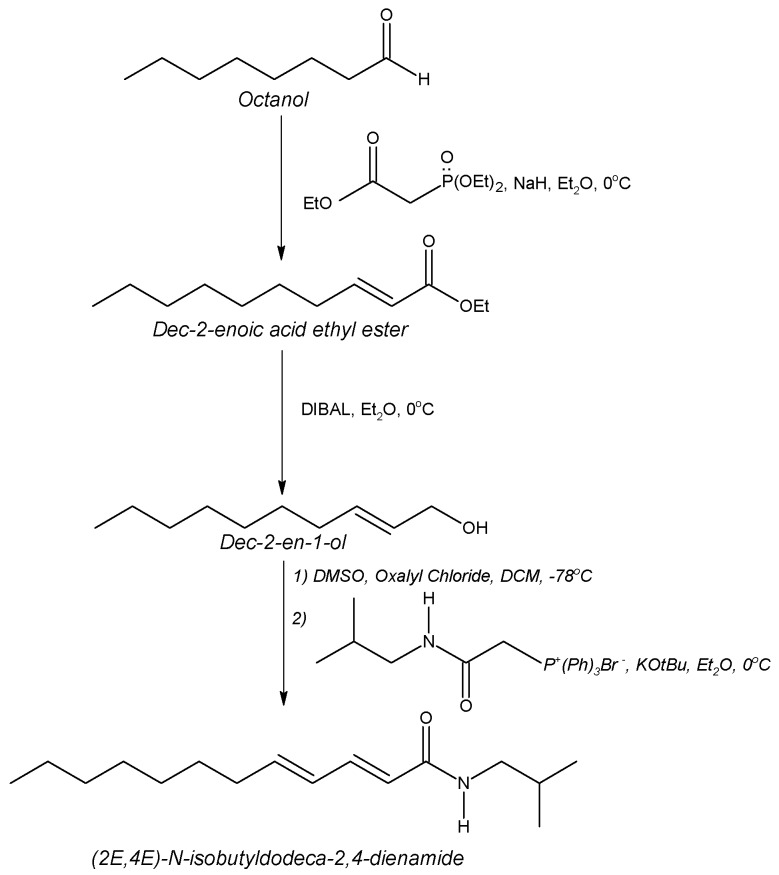
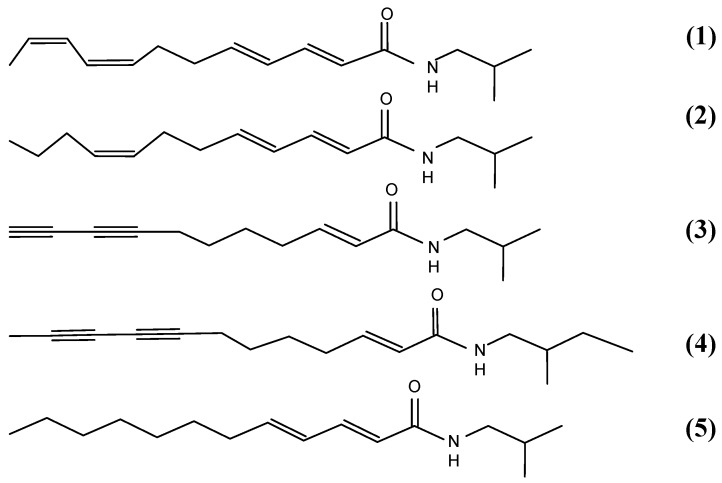
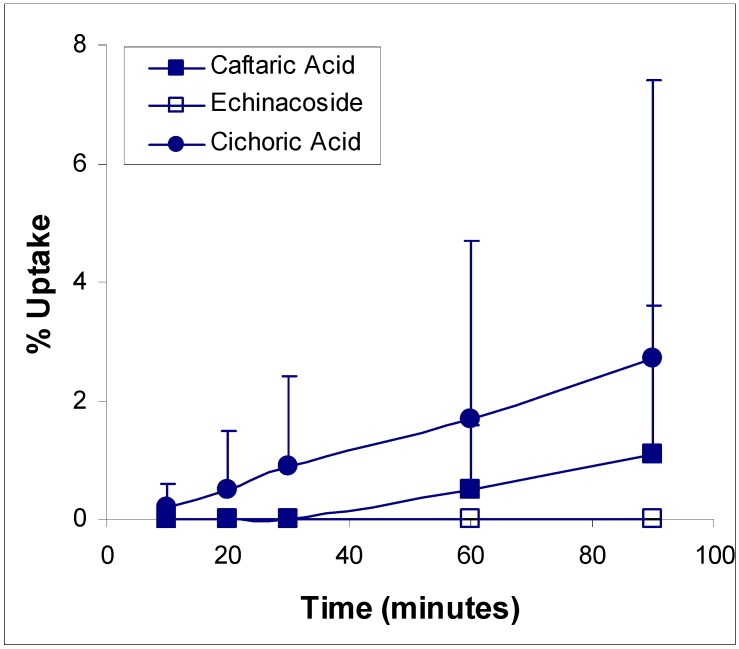
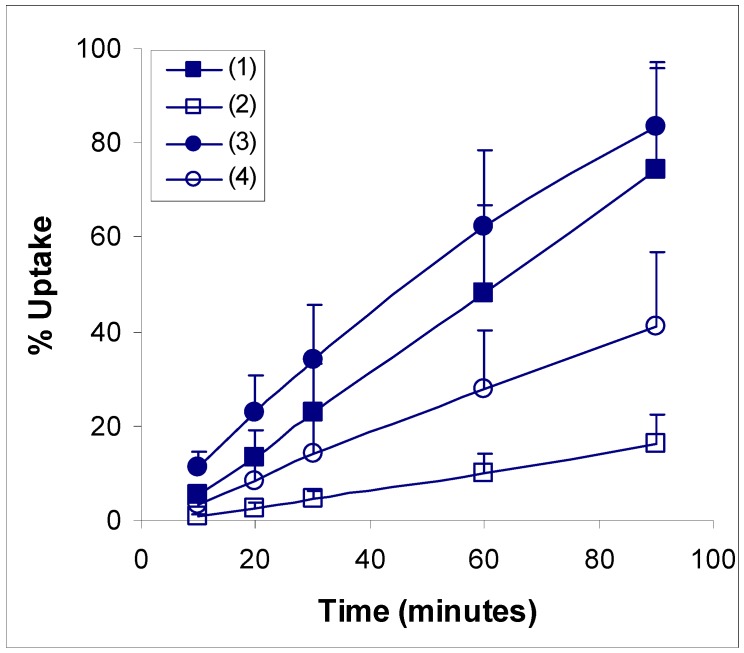
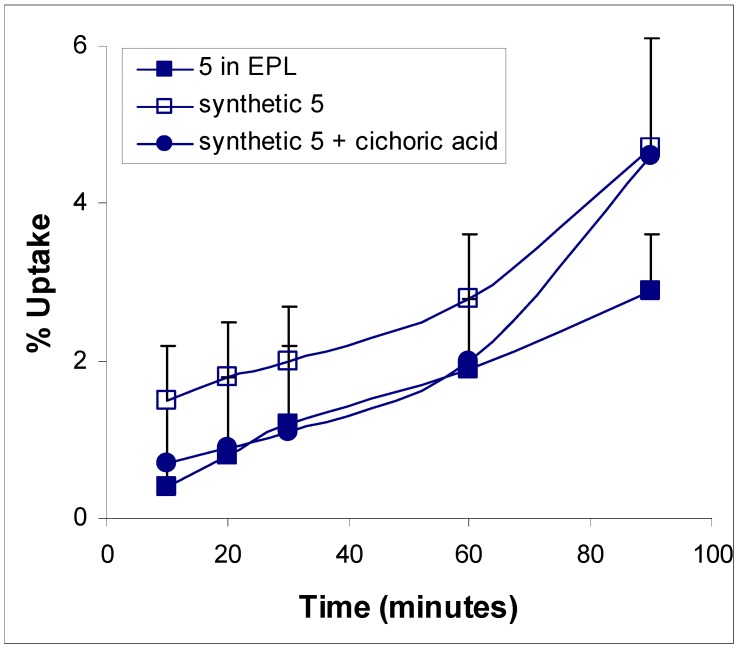
Ethanolic Echinacea preparations contain both caffeic acid conjugates and alkylamides [5]. Herbal preparations are a complex mixture of many different compounds as exemplified in Figure 1 for the Echinacea preparation, a mixture from Echinacea angustifolia and Echinacea purpurea roots, examined in these two studies. The caffeic acid derivatives – caftaric acid, echinacoside and cichoric acid – are individually identified in Figure 1 together with the alkylamide grouping. Isolation of a certain alkylamide from an Echinacea mix is difficult. One alkylamide (5) was synthesised using a novel procedure (Figure 2) to investigate its ability to cross biological barriers such as the Caco-2 cell monolayers in the absence of other Echinacea components. The structures of all the alkylamides investigated in this study, both the synthetic 5 and others found in Echinacea preparations, are shown in Figure 3. In vitro, in the Caco-2 assays, the caffeic acid conjugates were not found to diffuse across the monolayers (Figure 4). The apparent small amount of uptake shown in Figure 4 is equal to or less than the diffusion seen with mannitol (data not shown) which is known to have very poor intestinal absorption. This poor passage across the monolayers suggests that these compounds will not cross the intestinal barrier and so will not be bioavailable. This therefore invalidates the concept that caffeic acid conjugates are responsible for the immunomodulatory activity of Echinacea. In contrast to this, most alkylamides readily crossed the Caco-2 monolayers as seen in Figure 5. As demonstrated with these four alkylamides, different alkylamides diffused across the monolayers at different rates. This variability was found to correlate with differences in the alkylamide structures. Decreased ability of the alkylamides to cross the monolayers was attributed to increased saturation, as seen in the differences between uptake rates for alkylamides 1 and 2, and methylation of the N-terminal, as seen in differences between uptake rates for alkylamides 3 and 4. The transport of the alkylamides was not influenced by the presence of other constituents, as shown in Figure 6. Diffusion across the Caco-2 cell monolayer by 5 was not significantly affected by the presence of either the non-diffusing cichoric acid or the other components present in the Echinacea ethanolic extract (EPL). These data suggest that alkylamides, but not caffeic acid conjugates, are likely to cross the intestinal barrier and thus be available to influence an immune response.
Figure 1.
HPLC trace of an ethanolic Echinacea preparation containing Echinacea angustifolia and Echinacea purpurea root. The elution gradient was ramped from 30 to 100% acetonitrile:50 mM phosphoric acid over 55 minutes.
Figure 2.
Novel synthetic pathway for (2E,4E)-N-isobutyldodeca-2,4-dienamide mw = 251 (5).
Figure 3.
Echinacea alkylamide structures.
Figure 4.
Caffeic acid derivative transport kinetics in Caco-2 monolayers. Values are means ± sd for 3 - 5 monolayers.
Figure 5.
Echinacea alkylamide transport kinetics in Caco-2 monolayers. ■ (2E,4E,8Z,10Z)-N-isobutyldodeca-2,4,8,10-tetraenamide mw = 247 (1); □ (2E,4E,8Z)-N-isobutyldodeca-2,4,8-trienamide mw = 249 (2); ● (2E)-N-isobutylundeca-2-ene-8,10-diynamide mw = 231 (3); ○ (2E)-N-(2-methylbutyl)dodeca-2-ene-8,10-diynamide mw = 259 (4). Values are means ± sd for 5 monolayers.
Figure 6.
Effect of other compounds on the potential bioavailability compound 5 using Caco-2 monolayers. Data is expressed as means ± sd for n = 3. (EPL – ethanolic Echinacea preparation liquid).
(1) (2E,4E,8Z,10Z)-N-isobutyldodeca-2,4,8,10-tetraenamide mw = 247;
(2) (2E,4E,8Z)-N-isobutyldodeca-2,4,8-trienamide mw = 249;
(3) (2E)-N-isobutylundeca-2-ene-8,10-diynamide mw = 231;
(4) (2E)-N-(2-methylbutyl)dodeca-2-ene-8,10-diynamide mw = 259;
(5) (2E,4E)-N-isobutyldodeca-2,4-dienamide mw = 251.
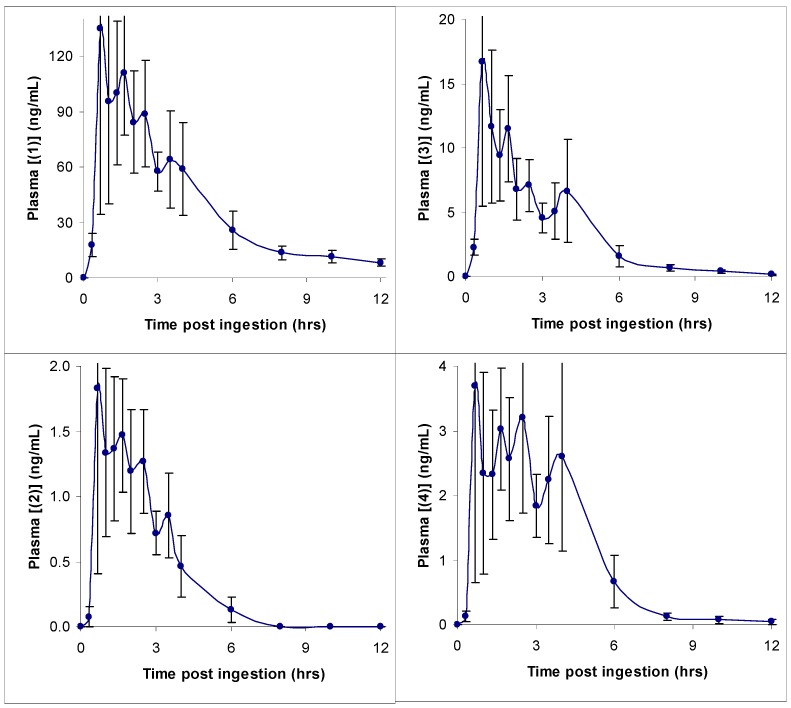
In the Phase I clinical trial, alkylamides were found to be rapidly absorbed and were present in blood at the first sampling time of 20 minutes after ingestion of the Echinacea tablets. Alkylamides remained present in blood for up to 12 hours. Caffeic acid conjugates were not detected at any of the sampling times. This is in agreement with the results for the Caco-2 monolayers and further supports the idea that, although caffeic acid conjugates may exhibit activity in vitro, they are unlikely to have any effect in vivo as they are not bioavailable.
In Figure 7, representative concentration-time profiles for four different alkylamides (two 2,4-dienes and two 2-enes) are given. These alkylamides were found to exhibit similar classic single dose pharmacokinetic profiles, although maximal plasma concentrations differed. As can be seen from these graphs, the best indication from these data for the approximate time for ingestion of subsequent doses is the 6 hour time point when the plasma concentration for each alkylamide has decreased markedly. The data are in fact consistent with the dosing regimen already recommended for Echinacea - that of one dose three times daily [10,11]. A multiple dose protocol study would have to be undertaken to find the steady state plasma concentration and hence the exact time for ingestion of subsequent doses. This study was only the first step in the investigation of the bioavailability of compounds present in Echinacea preparations.
Figure 7.
Human plasma alkylamide concentration time curves for compounds 1-4. Values are means ± se for 9 subjects.
These differences in plasma concentrations appeared to be determined by the ingested amount of each alkylamide, with the relative amounts of the alkylamides being similar in both the starting material and plasma. This suggested an equal uptake and metabolism for all alkylamides. In agreement with this is a recent study investigating P450-mediated metabolism of alkylamides [9]. In this study, it was found that the presence of alkylamide 3 prevented the rapid metabolism of 1 that occurred in its absence. With alkylamide 3 present in the ingested material and its ability to rapidly diffuse across the Caco-2 monolayers, it was probable that it would be bioavailable and hence present in plasma to help prevent first pass liver metabolism of 1. This hypothesis appears correct since both these alkylamides were easily detected in plasma after ingestion of the Echinacea tablets used in this study. These data – the fact that alkylamides are bioavailable and caffeic acid conjugates are not - support the use of alkylamides as the primary quality markers for Echinacea preparations.
Conclusions
The compounds present in an Echinacea preparation that were found to cross the Caco-2 monolayers were found to be the same as those detected in plasma. Alkylamides, but not caffeic acid conjugates, were detected in plasma from subjects ingesting an Echinacea preparation as predicted from the Caco-2 studies. This indicates that the Caco-2 cell system is a good pre-clinical method for assessing the potential bioavailability of compounds from complex mixtures such as herbal preparations.
Experimental
Alkylamide Synthesis: (2E,4E)-N-isobutyldodeca-2,4-dienamide (5) was synthesised using the novel method described below.
Dec-2-enoic acid ethyl ester: Triethyl phosphonoacetate (13 g, 58 mmol) was added dropwise to a suspension of NaH (2.79 g, 58 mmol as a 50% dispersion in mineral oil) in diethyl ether (Et2O) (250 mL) at 0°C. After hydrogen evolution was complete (5 minutes), octanal (6.4 g, 50 mmol) was added dropwise with vigorous stirring. Fifteen minutes later the reaction was quenched by the addition of saturated ammonium chloride solution (100 mL aq). The ether layer was separated and washed twice with water, once with brine, dried over MgSO4 and evaporated to yield a colourless oil, which was purified by flash chromatography (silica gel (230-400 mesh), 5% EtOAc in hexane), yielding dec-2-enoic acid ethyl ester (9.5 g, 96%) as a colourless oil. 1H-NMR (400 MHz, CDCl3) δ = 0.86 (t, 3H, 7.08Hz, RCH2CH3), 1.20-1.36 (m, 8H, RCH2R), 1.27 (t, 3H, 6.84Hz, -OCH2CH3), 1.39-1.47 (m, 2H, RCH2CH2CH=CH-), 2.16 (dtd, 2H, 7.08, 7.08, 1.56Hz, RCH2CH=CH), 4.15 (q, 2H, 7.12Hz, -OCH2CH3), 5.78 (dt, 1H, 15.6, 1.6Hz, RCH=CHCOOR), 6.94 (dt, 1H, 16.64, 6.96Hz, RCH=CHCOOR); 13C-NMR (100 MHz) δ = 14.0, 14.21, 22.57, 27.96, 29.0, 29.05, 31.69, 32.14, 60.02, 121.15, 149.40, 166.70; mass spec: m/z (E/I) 41 (96), 43 (100), 55 (92), 69 (32), 84 (24), 101 (29), 110 (15), 127 (7), 141 (2), 152 (11), 153 (19), 169 (1), 198 (0.5), 199 (0.5).
Dec-2-en-1-ol: Dec-2-enoic acid ethyl ester (0.93 g, 4.7 mmol) was added to DIBAL (1.47 g, 10.4 mmol) in THF (25 mL) at –30°C. The reaction was warmed to 0°C and stirred at this temperature for another hour. After this the mixture was cautiously quenched with HCl (100 mL 0.5 M aq) and extracted into Et2O. The ether layers were washed with brine, dried over MgSO4 then evaporated to give an oil. Purification by flash chromatography (silica gel (230-400 mesh), 25% EtOAc in hexane) yielded dec-2-en-1-ol (0.57 g, 78%). 1H-NMR (400MHz, CDCl3) δ = 0.86 (t, 3H, 6.84Hz, RCH3), 1.19-1.40 (m, 10H, RCH2R), 1.85 (br s, 1H, -OH), 1.95-2.05 (m, 2H, RCH2CH=CH-), 4.01-4.06 (m, 2H, CH=CHCH2OH), 5.51-5.71 (m, 2H, RCH=CHCH2OH); 13C-NMR (100 MHz) δ = 14.01, 22.59, 29.09(3C), 31.76, 32.16, 63.63, 128.76, 133.36; mass spec: m/z (E/I) 156 (0.5), 138 (1), 127 (1), 110 (3), 109 (4), 96 (6), 95 (7), 83 (5), 82 (11), 81 (11), 68 (17), 67 (19), 57 (100), 55 (44), 43 (83), 41 (96).
(2E,4E)-N-isobutyldodeca-2,4-dienamide: DMSO (0.546 g, 7 mmol) was added dropwise to a solution of oxalyl chloride (0.381 g, 3 mmol) in DCM (20 mL) at –78°C with stirring. After 3 minutes dec-2-en-1-ol (307 mg, 1.97 mmol) in DCM (5mL) was added dropwise over 5 minutes. The mixture was stirred for 1 hour at –78°C before warming to –40°C and stirred for a further 15 minutes. After cooling back to –78°C, triethylamine (1.01 g, 10 mmol) was added and the vigorously stirred mixture allowed to return to room temperature. The resulting solution was diluted with DCM (50mL) and shaken with water (80mL), HCl (80 mL 1M aq), then with water (80 mL) again. After drying the solution over MgSO4 the DCM was evaporated to leave an oil. Next phosphonium, [2-[(2-methylpropyl)amino]-2-oxoethyl]triphenyl- bromide (1.02 g, 2.5 mmol) was suspended in Et2O (15 mL) and KOtBu (257 mg, 2.3 mmol) was added portionwise at room temperature with vigorous stirring. The solution was stirred for a further 20 minutes before addition of the aldehyde prepared earlier diluted with THF (3 mL). After stirring for 2 hours TLC indicated complete conversion of starting material. Water (50 mL) and brine (25 mL) was added to the mixture and extraction into Et2O was performed. The ether layers were washed with brine, dried (MgSO4) and evaporated to give a residue. Purification by flash chromatography (silica gel (230-400 mesh), 20% EtOAc in hexane) yielded (2E,4E)-N-isobutyldodeca-2,4-dienamide (5) (61%, 297 mg) as a white solid. 1H-NMR (500 MHz, C6D6) δ = 0.86 (d, 6H, 6.7Hz, CH2CH(CH3)2), 0.99 (t, 3H, 7.02Hz, RCH2CH3), 1.22-1.43 (m, 10H, RCH2R), 1.74 (sept, 1H, 6.74Hz, CH2CH(CH3)2), 2.05 (m, 2H, -CH2CH=CHCONH), 3.17 (t, 2H, 6.28Hz, NHCH2CH(CH3)2), 5.27-5.32 (m, 1H, CONH-), 5.69 (d, 1H, 14.95Hz, -CH=CHCONH), 5.88 (dt, 1H, 14.25, 7.07, RCH2CH=CH-), 6.20 (dd, 1H, 10.3, 12.92Hz, -CH2CH=CHCH=CHCON-), 7.65 (dd, 1H, 11.42, 14.9Hz, -CH=CHCONH); 13C-NMR (125MHz) δ = 14.23, 20.4, 23.24, 29.15, 29.25, 29.54(2C), 32.21, 33.30, 47.53, 124.33, 129.84, 141.60, 142.74, 167.60; mass spec: m/z (E/I) 252 (3), 251 (17), 236 (10), 222 (1), 208 (4), 196 (3), 180 (16), 179 (65), 166 (9), 152 (39), 138 (7), 126 (9), 113 (21), 96 (100), 81 (93), 68 (42), 55 (86), 41 (96). The solid alkylamide was found to have a melting point of 80 – 81°C.
Cell culture: Caco-2 studies were undertaken essentially as described in Matthias et al [7]. Briefly, Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum, 2mM L-glutamine and 1% non-essential amino acids. Cells were cultured at 95% humidity and 37°C with 5% CO2 in air. Caco-2 cells were obtained from the American Type Culture Collection (Rockville, MD, USA), Transwell polycarbonate inserts were from Costar (Cambridge, MA, USA) and cell culture reagents were purchased from Gibco-BRL (Grand Island, NY, USA). Permeability experiments were performed in Hank’s balanced salt solution containing 25mM HEPES (pH 7.4) in air at 95% humidity and 37°C.
Clinical trial: As described in Matthias et al [8], eleven healthy volunteers, males and females (age range 18 - 26 years; height 157 – 188 cm; weight 48 – 95 kg; BMI 19 – 30) consented to undertake this study. The protocol was approved by the ethics committee of the Queensland Institution of Medical Research (Ethics reference number H0306-040T (P646)) and written informed consent was obtained in all cases. Subjects underwent a physical examination and a laboratory safety screen to ensure eligibility for participation in the study. They were not suffering from any recurrent illness and were not taking any other drugs with the exception of the contraceptive pill. Alcohol, caffeine and grapefruit juice were avoided for 24 hours prior to dosing. Volunteers received Echinacea orally (4 tablets, each containing 675 mg of Echinacea purpurea root and 600 mg of Echinacea angustifolia root) at approximately 0800 hours. Tablets were taken immediately after a high-fat breakfast (1 glass of orange juice, one slice of buttered toast, one fried egg, one slice of cheese, two rashers of bacon, one serving of hash brown potatoes and one glass of whole milk – the Australianised equivalent of the FDA standard high-fat breakfast) (n = 9). Blood samples were taken at set intervals until 12 hours post-dose, and were collected into tubes containing lithium heparin (125 I.U.). Plasma was separated immediately by centrifugation and stored frozen (-20°C or lower) for subsequent analysis of Echinacea components. Subjects were quietly ambulant or semi-recumbent on beds for the first 4 hours after dosing. Alkylamides were separated from the plasma and concentrated by passage through a solid phase extraction cartridge using methanol as the elution solvent.
Alkylamide analysis: Alkylamide concentrations in samples were determined by LCMS operating in positive ion SIM mode using a mobile phase of water and acetonitrile applied with a stepped gradient. Caffeic acid derivatives concentrations were determined by HPLC-PDA using a mobile phase of 50 mM phosphoric acid and acetonitrile with a stepped gradient.
Acknowledgments
The authors wish to thank Prof Istvan Toth and Dr Joanne Blanchfield for help with the Caco-2 experiments, Robert Lang for initiating synthesis of the alkylamides, Dr Russell Addison and Prof Ron Dickinson for help with analysis of clinical plasma samples and Prof Wayne Hooper and QPharm Pty Limited (Brisbane, Australia) for their help with the clinical trial. This work was funded by AUSINDUSTRY through a Biotechnology Innovation Fund Grant (No. BIF02651) and MediHerb Pty. Ltd.
References
- 1.Bräunig B., Dorn M., Knick E. Enhancement of resistance in common cold by Echinaceae purpureae radix [Echinaceae purpureae radix: zur Stärkung der körpereigenen Abwehr bei grippalen Infekten] Z. Phytother. 1992;13:7–13. [Google Scholar]
- 2.Melchart D., Walther E., Linde K., Brandmaier R., Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections. Arch. Fam. Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 3.Taylor J.A., Weber W., Standish L., Quinn H., Goesling J., McGann M., Calabrese C. Efficacy and safety of Echinacea in treating upper respiratory tract infections in children. A randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 4.Brinkeborn R.M., Shah D.V., Degenring F.H. Echinaforce® and other Echinacea fresh plant preparations in the treatment of the common cold. A randomized, placebo controlled, double-blind clinical trial. Phytomed. 1999;6:1–5. doi: 10.1016/S0944-7113(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 5.Bauer R., Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H., Farnsworth N.R., editors. Economic and Medicinal Plant Research. Vol. 5. Academic Press; London: 1991. p. 296. Chapter 8. [Google Scholar]
- 6.Bodinet C., Lindequist U., Teuscher E., Freudenstein J. Effect of orally applied herbal immunomodulator on cytokine induction and antibody response in normal and immunosuppressed mice. Phytomed. 2002;9:606–613. doi: 10.1078/094471102321616418. [DOI] [PubMed] [Google Scholar]
- 7.Matthias A., Blanchfield J.T., Penman K.G., Toth I., Lang C.-C., De Voss J.J., Lehmann R.P. Permeability studies of alkylamides and caffeic acid conjugates from Echinacea using a Caco-2 cell monolayer model. J. Clin. Pharm. Therapeut. 2004;29:7–13. doi: 10.1046/j.1365-2710.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 8.Matthias A., Addison R.S., Penman K.G., Dickinson R.G., Bone K.M., Lehmann R.P. Echinacea alkylamide bioavailability and pharmacokinetics in humans after tablet ingestion. Life Sci. 2005 doi: 10.1016/j.lfs.2005.04.009. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Matthias A., Gillam E.M.J., Penman K.G., Matovic N.J., Bone K.M., De Voss J.J., Lehmann R.P. Cytochrome P450 enzyme mediated degradation of Echinacea alkylamides by human liver. Chem.-Biol. Interact. 2005 doi: 10.1016/j.cbi.2005.04.003. (in press) [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal M., Brinckmann J., Goldberg A., editors. Herbal Medicine: Expanded Commission E monographs. Integrative Medicine Communications; Newton, MA.: 2000. Echinacea purpurea root; pp. 98–100. [Google Scholar]
- 11.Blumenthal M., Brinckmann J., Goldberg A., editors. Herbal Medicine: Expanded Commission E monographs. Integrative Medicine Communications; Newton, MA.: 2000. Echinacea angustifolia herb and root/Pallida herb; pp. 93–95. [Google Scholar]